Abstract
A 55-year-old man presented to our institution with abnormal chest X-ray shadows. Chest computed tomography (CT) showed left-sided interlobular septal thickening; thus, we suspected lymphangitis carcinomatosis and other disorders that show similar CT findings. Bronchoscopy and laboratory and imaging studies yielded no diagnostic findings. Pulmonary shadows during follow-up spontaneously improved then worsened. Thoracoscopic lung biopsy samples showed interstitial pneumonia and granulomas but the etiology of the pulmonary lesion could not be determined. At seven years after presentation, the patient's pulmonary shadows had gradually deteriorated, and he reported using topical minoxidil. His history of minoxidil use was linked to changes in the pulmonary shadows. The diagnostic delay was due to the patient's hesitancy to report drugs obtained online and the difficulty in obtaining such a history.
Keywords: Drug-induced lung disease, Minoxidil, Granuloma, Topical use, Interlobular septal thickening
1. Introduction
The lung is frequently a target of drug-related harm from various medications, with chemotherapeutic agents for cancer, anti-inflammatory drugs, antimicrobials, cardiac medications, and anticonvulsants being potential causes of lung injury. The number of drugs associated with pulmonary toxicity is steadily increasing [1] as new pharmacological agents are introduced into the therapeutic armamentarium. Drug-induced lung disease (DILD) can be challenging to recognize as its clinical, radiological, and histologic characteristics often lack specificity. Accordingly, a correct diagnosis of DILD demands a high index of suspicion. Determining whether a patient's exposure to a drug is correlated with the severity of symptoms, as well as considering physiological, laboratory, and radiological indications, aids physicians in suspecting DILD. We recently encountered a case of DILD that was associated with the use of a topical medication. The reluctance of the patient to disclose his medication use combined with atypical radiological findings contributed to the delay in the diagnosis of DILD.
2. Case presentation
In August 2014, a 55-year-old man presented with abnormal chest X-ray findings that had been detected on a medical checkup performed two months previously. He did not complain of any symptoms such as cough, sputum, and dyspnea. A chest X-ray obtained in November 2013 showed no abnormalities. He suffered cerebral infarction in April 2013 and had taken clopidogrel since then. He denied taking any other dietary supplements, health foods, or over-the-counter medicines. He had smoked one pack of cigarettes per day from 20 to 54 years of age but did not drink alcohol. His residence was made of wood and received a large amount of sunlight. As a businessman, he was never exposed to dust, birds, or pets, and he had no relevant family history.
His vital signs on examination were as follows: respiratory rate, 18/min; blood pressure, 110/62 mmHg; heart rate, 62 beats/min; body temperature, 36.6 °C; and O2 saturation by pulse oximetry, 98 % on ambient air. The superficial lymph nodes were non-palpable. Slight inspiratory fine crackles were auscultated over the left lung fields. No cardiac murmurs, extremity edema, or abnormal neurological findings were found. There were no abnormal ophthalmologic findings or skin changes.
Pulmonary function testing showed vital capacity (VC) (%predicted) of 4.38 L (105.3 %), forced VC of 4.25 L (102.2 %), and forced expiratory volume in 1 second (FEV1) of 3.61 L (122.0 %). Transthoracic echocardiography revealed no abnormal findings. His white blood cell count was 5400/μL, and his other values were as follows: hemoglobin, 13.8 g/dL; platelet count, 24.8 × 104/μL; total protein, 7.3 g/dL; albumin, 4.2 g/dL; creatinine, 0.7 mg/dL; sodium, 146 mmol/L; chloride, 110 mmol/L; potassium, 4.2 mmol/L; aspartate aminotransferase, 24 IU/L; lactate dehydrogenase, 178 IU/L; and C-reactive protein, 0.2 mg/dL. Chest X-ray showed reticular shadows and ground-glass opacities in the left lower lung field (Fig. 1a). Chest computed tomography (CT) showed interlobular septal thickening in the left lower lobe (Fig. 1b and c). No mediastinal or hilar lymphadenopathy or pleural effusion was observed.
Fig. 1.
Chest X-ray showed reticular shadows and ground-glass opacities in the left lower lung field (a). Chest computed tomography (slice thickness: 1 mm; axial section) showed reticulation and interlobular septal thickening in the left lower lobe (b, c).
The differential diagnoses included diseases showing interlobular septal thickening, such as lymphangitis carcinomatosis, sarcoidosis, amyloidosis, and malignant lymphoma. The patient's other blood values were as follows: Krebs von den Lungen-6 (KL-6), 709 U/mL; CEA, 3.7 ng/mL; angiotensin converting enzyme, 20.6 IU/L (normal, 7.7–29.4); Immunoglobulin G, 1010 mg/dL; and IgG4, 22.5 mg/dL. No abnormalities were found on esophagogastroduodenoscopy, abdominal CT, fecal occult blood test, or urinalysis. The examination of a transbronchial lung biopsy specimen obtained by bronchoscopy showed only nonspecific inflammatory cell infiltration. The patient was followed conservatively without treatment as an outpatient; his abnormal lung shadows spontaneously improved from 2 months thereafter. The patient steadfastly denied changing or stopping any drugs and had not changed his workplace or lifestyle. We changed his clopidogrel to aspirin in 2015, and by July 2015, new reticular shadows had appeared in his left lower lobe, so we performed a thoracoscopic lung biopsy from the lateral basal segment of the left lower lobe.
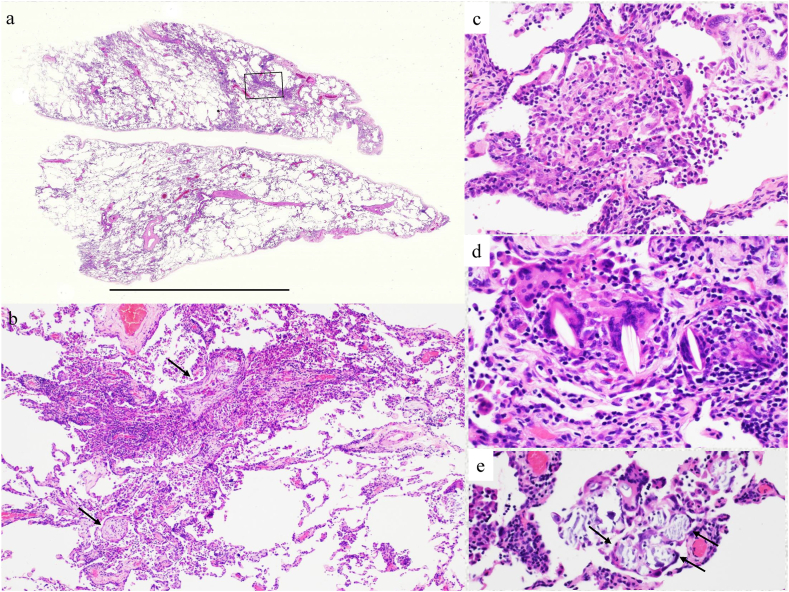
The distribution of inflammation was patchy, random, and multifocal with a monotonous phase (Fig. 2a); the primary finding was lymphocytic alveolitis with occasional intraluminal organization (arrow) (Fig. 2b), along with occasional intraluminal granulomatous lesions (e.g., poorly formed epithelioid cell granulomas of <100 μm) (Fig. 2c), cholesterol granulomas (Fig. 2d), and Schaumann bodies (Fig. 2e). There was no interlobular septum, and no well-formed epithelioid cell granulomas were observed around the blood vessels or bronchiolar walls. The pathological diagnosis was consistent with cellular non-specific interstitial pneumonia (NSIP) with granulomatous inflammation. Grocott's staining and Ziehl-Neelsen staining were negative, as were tissue cultures for acid-fast bacilli or fungi. Non-fibrotic hypersensitivity pneumonitis (HP) was unlikely because inflammation was not airway-centered, even though poorly formed epithelioid cell granulomas were present.
Fig. 2.
Histological findings. a. Panoramic view, scale bar, 1 cm (hematoxylin and eosin [HE] staining; × 60). b. The box shows an organizing pneumonic lesion. c. Poorly formed epithelioid cell granuloma (HE staining; × 300). d. Cholesterol granulomas (HE staining; × 400). e. Schaumann bodies in the giant cells (HE staining; × 300).
Additional tests, including a serum cryptococcus antigen test, anti-Trichosporon antibody and anti-pigeon dropping extract antibody measurements were negative. Having no firm diagnostic results, we continued to follow the patient without additional treatment.
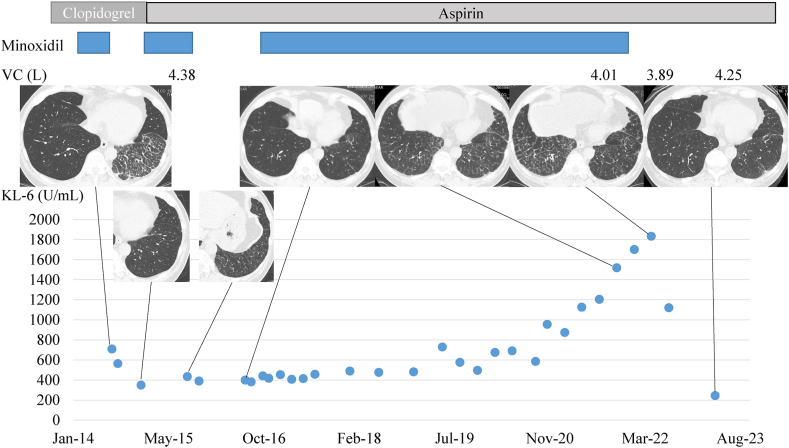
Then, from 2017 to November 2021, pulmonary reticular shadows developed and gradually increased. Mild dyspnea on effort and dry cough also developed gradually during this period. Because the lung lesions repeatedly improved and then worsened, we obtained a detailed patient history by asking concrete and direct questions such as “Are there any drugs that were started before worsening of the symptoms or chest imaging? Such drugs include those administered by any route, for example, orally, by injection, and topically. In addition, are there any drugs that you have hesitated to report to us?” At that time, the patient acknowledged his use of topical minoxidil, although he could not recall whether the amount of topical minoxidil used had changed. He fundamentally applied the drug every day, but he sometimes forgot to use the drug. When his pulmonary shadows showed improvement, he would start minoxidil, and when they worsened, he would stop using the drug. The patient's drug history and time-course of VC, CT findings, and KL-6 values are summarized in Fig. 3. Topical minoxidil was used from January 2014 until the time of his presentation in August 2014, and was stopped until January 2015, when we advised him that his pulmonary shadows on CT had improved. He restarted the drug, but by July 2015, his pulmonary shadows had worsened, and we performed a thoracoscopic lung biopsy. After this, he stopped the drug but then re-started it from August 2016 to November 2021. Changes in his VC, pulmonary shadows, and serum KL-6 values were in line with his history of using topical minoxidil. His symptoms gradually improved from a few months after cessation of minoxidil use, and his CT shadows started to improve from May 2022, at 6 months after cessation of the drug.
Fig. 3.
Timeline of drug administration, vital capacity, Krebs von den Lungen values, and chest imaging findings. The patient had been using minoxidil from January 2014 to August 2014, when he presented to our institution and had stopped taking the drug until January 2015. He then restarted the drug, but by July 2015, his pulmonary shadows had worsened. Following this, he stopped the drug but then started it again from August 2016 to November 2021. Since then, all his parameters have improved. The changes in KL-6 values matched his history of using topical minoxidil.
Based on established criteria [2], we made a diagnosis of DILD. The patient stopped taking minoxidil after November 2021, and by April 2023, his VC had recovered to 4.25 L, his KL-6 value was within the normal range, and his pulmonary shadows on CT had improved.
3. Discussion
Although DILD accounts for only 3 % of all interstitial lung disease (ILD) [3], it is interesting because its symptoms can reverse following drug withdrawal. As the first diagnostic step, physicians should suspect the possibility of DILD. Frequently, the relationship between drug exposure and the time course and magnitude of respiratory signs and symptoms helps the physician to distinguish DILD from ILD of other causes. Thus, obtaining an accurate drug history from patients with suspected DILD is essential but not always easy. Although the history of prescribed drugs can be easily investigated from a pharmacy's medication records, it is not always possible to discover self-administered drugs. Despite the well-publicized risks of taking medications without medical supervision, this practice is increasingly common. Currently, medications can be easily purchased online, even those that traditionally require a prescription. Such drugs can be easily overlooked if patients do not report them, and this can easily complicate the clinical course, as it did in our patient. In our patient and a previously reported case [4], the determination of the diagnosis of DILD was delayed due to patient hesitancy to report the self-administration of unprescribed drugs.
The topical minoxidil product our patient used included minoxidil, propylene glycol, ethanol, and other chemicals. Chest disorders stemming from minoxidil use include retention of pleural effusion and pericardial effusion [5]. We found only one published case of DILD due to minoxidil in a patient taking oral minoxidil who developed bilateral ground-glass opacities [4]. DILD due to propylene glycol and topical ethanol has not been reported. Therefore, we diagnosed our patient as having DILD due to minoxidil.
Minoxidil is now much more commonly used to treat alopecia and is taken orally or topically on the skin. On reflection, our interview regarding the patient's drug history may have been insufficient because we had not included questions about the route of drug administration until November 2021. As almost any route of drug administration can cause ILD, obtaining a wide-ranging drug history that considers all such routes is essential.
DILD is generally distributed on chest imaging in a bilateral, non-segmental pattern [6]. Although interlobular septal thickening was initially found unilaterally, our patient subsequently showed bilateral shadows. When lung injury or impairment of respiratory motion due to adhesions occurs unilaterally, pulmonary shadows due to DILD tend to develop in the contralateral healthy lung; however, our patient did not show such a condition, and the cause of the laterality found in our patient was unclear.
CT patterns of DILD are typically classified into diffuse alveolar damage, HP, organizing pneumonia, eosinophilic pneumonia, NSIP, and other patterns [7]. Although the laterality of shadows was remarkable on the CT imaging in our patient, it could suggest an NSIP pattern. As interlobular septal thickening was prominent, we suspected lymphangitis carcinomatosis. We cannot conclude that the findings are typical of DILD caused by topical drug use because the imaging pattern of DILD due to topical drug use is not fully known. When drugs are administered topically, they are considered to reach the lungs by two pathways. One is that drugs directly enter the vasculature from the skin and are transported via the venous bloodstream. The other pathway is that drugs are absorbed and enter the lymphatics, are transported to the right or left venous angle, and are disseminated to the lung. In our patient, minoxidil was applied to the head and then reach the lungs by the above pathways without passing through the liver, which is the primary site for drug metabolism. Characteristics of chest imaging may be affected by the site of application such as the upper or lower body, right or left side (skin and skin appendages), substrates that make absorption of drugs easy, and metabolization before reaching the lungs. Further case reports need to be accumulated to better understand this phenomenon.
There are several pathological manifestations of DILD: chronic interstitial pneumonia, diffuse alveolar damage, organizing pneumonia, eosinophilic pneumonia, HP, pulmonary hemorrhage, pulmonary hypertension, pulmonary edema, pulmonary veno-occlusive disease, and granulomatous inflammation [8]. Further, many drugs can cause more than one histopathologic type of lung reaction in the same or different patients. Our patient showed granulomatous inflammation and a chronic interstitial pneumonia (cellular NSIP) pattern. Pulmonary granulomatous inflammation results from an adverse reaction to methotrexate, interferon-α or -β, sirolimus, and BCG (Bacille de Calmette et Guérin) therapy. They must be differentiated from foreign body granulomas, which are centered on the airway in patients with chronic aspiration (as a reaction to food or disintegrants in pharmaceutical tablets) and in the arterioles of drug abusers. Our patient's granuloma distribution was not airway- or arteriole-centered, so such diseases and HP were not strongly suspected. DILD due to the administration of a topical drug is reportedly associated with granuloma formation, with some incidences reported as sarcoidosis due to tattoos [9]. Unfortunately, interlobular septum was not seen, and the thickening of structures, including the lymphatic pathways, were unremarkable in our patient's pulmonary tissue samples, although they were notable on CT. This discrepancy may be due to biopsy tissue being sampled from an area where such histologic findings were not remarkable.
The initial treatment of DILD begins with stopping the causative drugs and potentially administering corticosteroid after considering the patient's respiratory condition and any damage to pulmonary tissues. Our patient's pulmonary shadows improved with the cessation of minoxidil. Thus, the unnecessary administration of corticosteroids was avoided.
4. Conclusion
We reported a case of DILD due to self-administration of topical minoxidil. In our patient, chest imaging showing unilateral disease and prominent interlobular septal thickening confused the diagnosis. Obtaining an accurate drug history, regardless of the route of administration or route of purchase of a drug, is important, as is obtaining a drug history even if patients are sometimes hesitant to report it.
Funding/support
None declared.
Declaration of competing interest
No conflict
References
- 1.Rosenow E.C., 3rd The spectrum of drug-induced pulmonary disease. Ann. Intern. Med. 1972;77(6):977–991. doi: 10.7326/0003-4819-77-6-977. [DOI] [PubMed] [Google Scholar]
- 2.Camus P., Fanton A., Bonniaud P., Camus C., Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71(4):301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 3.Thomeer M., Demedts M., Vandeurzen K., VRGT Working Group on Interstitial Lung Diseases Registration of interstitial lung diseases by 20 centres of respiratory medicine in Flanders. Acta Clin. Belg. 2001;56:163–172. doi: 10.1179/acb.2001.026. [DOI] [PubMed] [Google Scholar]
- 4.Takekosh D., Nemoto T., Saito S., et al. Minoxidil-induced lung disease, masquerading as hypersensitivity pneumonitis. Respir Med Case Rep. 2023;43 doi: 10.1016/j.rmcr.2023.101861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin W.B., Spodick D.H., Zins G.R. Pericardial disorders occurring during open-label study of 1,869 severely hypertensive patients treated with minoxidil. J. Cardiovasc. Pharmacol. 1980;2(Suppl 2):S217–S227. doi: 10.1097/00005344-198000022-00016. [DOI] [PubMed] [Google Scholar]
- 6.The Japanese Respiratory Society . 2018. The JRS Guidelines for the Management of Drug-Induced Lung Disease. Tokyo; p. 20. [Google Scholar]
- 7.Webb W.R., Muller N.L., Naidich D.P., editors. High-resolution CT of the Lung. fifth ed. Wolters Kluwer; Baltimore: 2015. Drug-induced lung disease and radiation pneumonitis; pp. 397–410. [Google Scholar]
- 8.Myers J.L., El-Zammar O. In: Katzenstein and Askin's Surgical Pathology of Non-neoplastic Lung Disease. fourth ed. Katzenstein A.L., editor. Saunders Elsevier; Philadelphia: 2006. Pathology of drug-induced lung disease; pp. 85–125. [Google Scholar]
- 9.Okuma T., Sato Y., Onodera K. A case of diffuse, granulomatous interstitial pneumonia due to tattoo. Nihon Kyobu Shikkan Gakkai-Shi. 1983;21(12):1213–1221. (In Japanese) [PubMed] [Google Scholar]