Abstract
Background:
It is theorized that the lack of a synovial lining after anterior cruciate ligament (ACL) injury and ACL reconstruction (ACLR) contributes to slow ligamentization and possible graft failure. Whether graft maturation and incorporation can be improved with the use of a scaffold requires investigation.
Purpose:
To evaluate the safety and efficacy of wrapping an ACL autograft with an amnion collagen matrix and injecting bone marrow aspirate concentrate (BMAC), quantify the cellular content of the BMAC samples, and assess 2-year postoperative patient-reported outcomes.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
A total of 40 patients aged 18 to 35 years who were scheduled to undergo ACLR were enrolled in a prospective single-blinded randomized controlled trial with 2 arms based on graft type: bone–patellar tendon–bone (BTB; n = 20) or hamstring (HS; n = 20). Participants in each arm were randomized into a control group who underwent standard ACLR or an intervention group who had their grafts wrapped with an amnion collagen matrix during graft preparation, after which BMAC was injected under the wrap layers after implantation. Postoperative magnetic resonance imaging (MRI) mapping/processing yielded mean T2* relaxation time and graft volume values at 3, 6, 9, and 12 months. Participants completed the Single Assessment Numeric Evaluation Score, Knee injury and Osteoarthritis Outcome Score, and pain visual analog scale. Statistical linear mixed-effects models were used to quantify the effects over time and the differences between the control and intervention groups. Adverse events were also recorded.
Results:
No significant differences were found at any time point between the intervention and control groups for BTB T2* (95% CI, –1.89 to 0.63; P = .31), BTB graft volume (95% CI, –606 to 876.1; P = .71), HS T2* (95% CI, –2.17 to 0.39; P = .162), or HS graft volume (95% CI, –11,141.1 to 351.5; P = .28). No significant differences were observed between the intervention and control groups of either graft type on any patient-reported outcome measure. No adverse events were reported after a 2-year follow-up.
Conclusion:
In this pilot study, wrapping a graft with an amnion collagen matrix and injecting BMAC appeared safe. MRI T2* values and graft volume of the augmented ACL graft were not significantly different from that of controls, suggesting that the intervention did not result in improved graft maturation.
Registration:
NCT03294759 (ClinicalTrials.gov identifier).
Keywords: amnion collagen matrix, anterior cruciate ligament, bone marrow aspirate concentrate
While great improvements in the technical method of anterior cruciate ligament (ACL) reconstruction (ACLR) have been made over the past decades, whether graft maturation and incorporation can be improved with biologic augmentation requires continued investigation. Histologic studies have determined that graft ligamentization (ie, the process of graft material transitioning to the ligament in histologic features) after ACLR may take anywhere from 6 to 36 months. 9 It has been suggested that incomplete graft maturation/incorporation before the return to activity and/or aggressive rehabilitation is one cause of clinical graft failure. Animal studies suggest that slower graft incorporation correlates with increased laxity and stiffness. 19 Acceleration and improvement in graft maturation and strength would significantly advance sports medicine and may eventually allow for a safer and earlier return to sports activity.
The acceptance and demand for augmentation of ACLRs with cellular therapy has outpaced the scientific evidence of efficacy. While improved tendon healing and graft integration in ACL animal models have been found with reconstructions augmented with cellular technologies, 1 recent clinical systematic review revealed only 4 studies that met the inclusion criteria.1,19,21,24 Basic scientists teach that optimization of cellular treatments for tissue regeneration requires a “regenerative triad”; that is, the use of a scaffold as well as stem cells and growth factors. For that reason, emerging models in ACL surgery have employed and shown the benefit of a scaffold wrap or a “sheet” of cells.19,21,24
The normal, uninjured human ACL is covered by a layer of synovial tissue, which contributes to the blood supply and nutrition of the native ACL. It is theorized that the lack of a synovial lining after injury and traditional ACLR contributes to slow ligamentization and possible failure of reconstructed grafts. 21 Clinical translational studies are emerging and suggest that combining a scaffold with a biologic agent has merit.11,30 A collagen membrane derived from amnion tissue (amnion matrix) has illustrated success in wound healing by acting as a barrier, scaffold, and source of growth factors. 11 Application to ACLR surgery could help reestablish the natural synovial lining of the reconstructed ACL, acting as both a barrier from the synovial fluid and providing a scaffold to contain autologous mesenchymal stem cells and growth factors contiguous with the graft.
The primary objective of this pilot study was to evaluate the safety and develop early data on the efficacy of wrapping an ACL autograft with an amnion collagen matrix and injecting bone marrow aspirate concentrate (BMAC). We hypothesized that this method of augmenting ACLR surgery would enhance graft maturation and the ligamentization process, as shown by the primary effectiveness endpoint and quantitative magnetic resonance imaging (MRI). Animal and clinical studies on MRI-derived T2* and volume measures have significantly predicted histological scores of healing ligaments, with lower T2* values and larger volumes associated with better histological scores and low T2* values specifically associated with greater cell density and collagen organization.1,11,27,30 We also aimed to quantify the patient-reported outcome measures (PROMs) as well as the cellular content of the BMAC samples.
Methods
Participants
This was a prospective, single-blinded, randomized controlled trial conducted in accordance with CONSORT (Consolidated Standards of Reporting Trials) 2010 guidelines. Institutional review board approval was obtained, and the study was posted on ClinicalTrials.gov (NCT03294759). Enrollment started in February 2017 and was completed in May 2018, and the study was conducted in the outpatient setting at the primary study institute. The follow-up was completed in May 2019, and the final data analysis was conducted by August 2022. After trial commencement, no changes were made to methods, protocols, or outcome measures.
Patients between the ages of 18 and 35 years who were scheduled to have ACLR with autologous grafts by 1 of the investigating physicians (A.W.A., S.E.J., R.V.O.) were screened for participation in the study by members of the research team. The exclusion criteria were as follows: patients with previous procedures or significant previous injuries to the same knee; patients with difficulty obtaining internet access; patients who did not have an active email address; patients who could not comprehend study documents or give informed consent; and patients who could not complete MRI examinations because of claustrophobia or anxiety. Potential participants were informed that the study may benefit them via augmented ACLR. If eligible and interested, the potential participants completed the informed consent process (enrollment). No specific advertising or recruitment materials were utilized. No compensation was given to participants in this study. Participants were also enrolled in the Surgical Outcomes System (Surgical Outcomes System; Arthrex) knee arthroscopy registry and completed all preoperative questions before the surgical date.
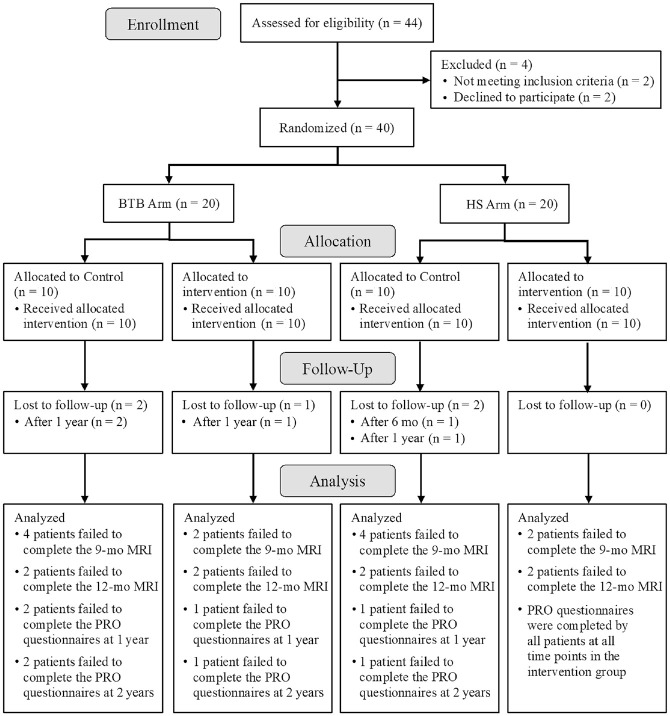
A power analysis was impossible to perform before the study because of the lack of literature involving both an amnion collagen matrix and BMAC injection as an augmentation to ACLR. A total of 44 patients were assessed for eligibility, and a final sample size of 40 participants was enrolled in the trial, which is consistent with early-phase studies of this nature. The investigation consisted of 2 study arms, with 20 participants in each arm: (1) ACLR utilizing bone–patellar tendon–bone (BTB) autografts and (2) ACLR with hamstring (HS) autografts. Patients in each arm were randomized into control (n = 10) and intervention (n = 10) groups. Figure 1 summarizes the patient inclusion process.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of patient inclusion in the study. BTB, bone–patellar tendon–bone; HS, hamstring; MRI, magnetic resonance imaging; PRO, patient-reported outcome.
Randomization
Patients in each study arm were randomized individually at a 1 to 1 ratio using a simple computer-generated randomization sequence and sequentially numbered containers, comparing traditional reconstruction (control) versus augmented reconstruction (intervention). Randomization was completed by an independent (off-study) research assistant and subsequently scheduled by the research team to undergo surgery. Three surgeons independently performed the surgical procedures (A.W.A., S.E.J., R.V.O.). The participants and research team remained blinded for the duration of the study.
Intraoperative Procedures
In the BTB autograft arm, fixation was performed with interference screws. In the HS autograft arm, fixation was performed with cortical suspensory fixation, consisting of a cortical button on the tibia and the femur. Each control group underwent the primary surgeon's standard ACL surgery, while each intervention group underwent augmented ACL surgery. Specifically, the intervention group also underwent the surgeon's standard ACL surgery with 3 additional steps to augment the ACL surgery—(1) bone marrow was harvested and concentrated to create BMAC; (2) the ACL graft was wrapped with an amnion collagen matrix; and (3) after graft implantation, the joint was dried, and the BMAC was injected under the amnion collagen matrix.
The bone marrow aspirate was harvested from the distal femur, the anterior iliac crest, or the posterior iliac crest. The performing surgeon was allowed the freedom to choose the location of the harvest. The harvest technique involved one 60-mL syringe and a traditional 11-gauge, 11-cm Jamshidi needle (Ranfac Corp). Syringes/needles were prerinsed with heparin, and 60-mL syringes were loaded with 8 mL of anticoagulant citrate dextrose solution-A. The needle was advanced into the bone marrow cavity 3 to 4 cm, aspiration was performed while withdrawing and rotating the needle; then, 60 mL was harvested. The bone marrow harvest was processed using the Arthrex Angel blood processing system (Arthrex) using the 15% hematocrit setting; 1 mL of BMAC was removed and sent for analysis immediately after the harvest. The remaining BMAC was transferred onto the field in a sterile fashion to preserve sterility of the BMAC. Control groups had a sterile bandage placed over the iliac crest without incision.
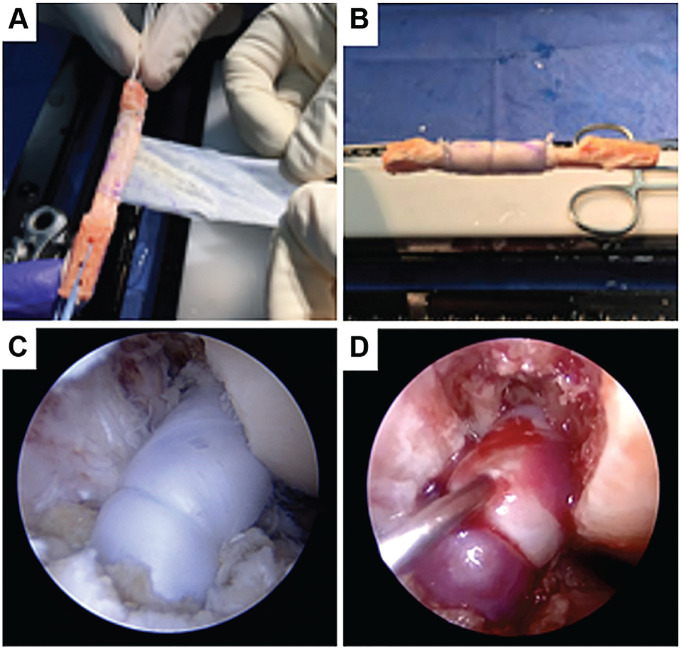
A 3 × 6–cm amnion collagen matrix (Arthrex Amnion Matrix Thick; Arthrex) was wrapped around the grafts during graft preparation (Figure 2A). The matrix length allowed for 2 to 3 wraps, creating a matrix cylinder around the graft. Using a No. 4-0, poliglecaprone-25, monofilament suture (Monocryl; Ethicon), a cerclage suture was placed at each horizontal end of the cylinder, and a running nonlocking suture was run along the vertical end of the wrap (Figure 2B). This created a watertight barrier around a 3-cm section of the intra-articular portion of the grafts. The surgeon's preferred technique was used for the implantation and fixation of the grafts (Figure 2C). After the final fixation, the joint was dried, and the remaining BMAC (2 mL on average) was injected into the watertight cylinder with a 22-gauge spinal needle (Figure 2D).
Figure 2.
(A and B). Graft preparation before implantation. (C) Graft implanted with the surgeon's standard technique. (D) Injection of biologic under membrane.
BMAC Quantification
The cellular composition of the BMAC was determined using an automated hematocytometer (Sysmex XE-5000). A complete blood cell count with differential and total nucleated cell (TNC) count was obtained, and the TNCs per milliliter of the BMAC was calculated. The proliferative potential was studied using colony-forming unit (CFU) fibroblast assays, in which 100 µL and a variable volume of BMAC to plate 106 TNCs was placed into wells of a 6-well cell culture plate in triplicate. Then, 3 mL of complete media (a mixture of 445 mL of Dulbecco's Modified Eagle Medium low glucose, 50 mL of mesenchymal stem cell–qualified fetal bovine serum, 5 mL of GlutaMax-I, and 250 µL gentamicin) was added to each well and the plate was incubated at 37°C. Standard cell culture conditions were used in a humidified atmosphere containing 5% CO2 in air (~20% O2). After 24 hours, the plates were gently washed with phosphate-buffered saline to remove nonadherent cells and leave only plastic adherent cells in the culture dish. After washing, 4 mL of complete media was added to the plates before being returned to the incubator for 10 days. The plates were then removed from the incubator, media was aspirated, and cells were fixed in ice-cold methanol for 10 minutes at 4°C. The plates were stained with crystal violet, washed 4 times with deionized water, and allowed to air dry for 24 hours. Colonies were included in the count if over 50 cells were present.
Postoperative rehabilitation protocols followed the institution's standard for ACLR, with variation as needed, depending on the associated pathology or meniscus work.
Postoperative Follow-up
MRI Evaluation. All patients underwent postoperative MRI at 3, 6, 9, and 12 months. The primary outcome measure was quantitative MRI analysis. Each MRI utilized T2* sequences, and data were processed to acquire a mean T2* relaxation time value and volume for each graft at every time point. The steps involved in the processing have previously been described and validated. 2 These values have been shown to detect differences in ACL content, structure, and maturation.8,9 Mapping involved manually drawing a region of interest around the ACL on each sagittal image where the ACL was visible on the 3-dimensional (3D) true–fast imaging with steady-state precession (FISP) series, which was performed by a sports medicine-trained orthopaedic surgeon with MRI mapping experience (A.W.A.).2,3,18 The true-FISP series was selected, as previous authors have asserted that these sequences best highlight the difference between the ACL and surrounding structures, including the synovial fluid. 12 Segmenting was performed in OsiriX (Pixmeo) (Figure 3).
Figure 3.
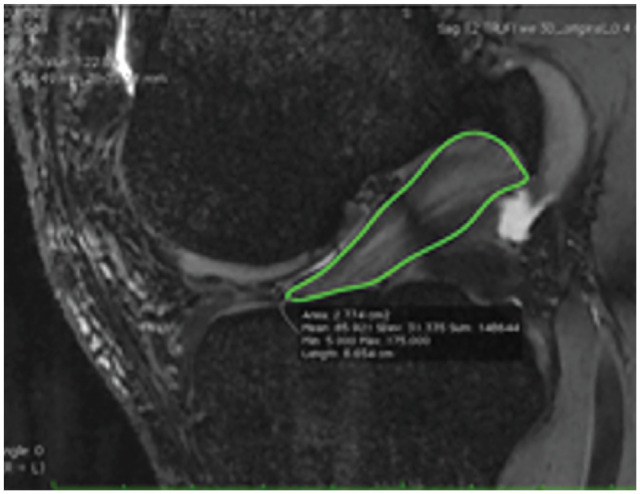
Mapping involved manually drawing a region of interest around the ACL on each image where the ACL was visible on the 3D true-FISP series of each MRI. ACL, anterior cruciate ligament; FISP, fast imaging with steady-state precession; MRI, magnetic resonance imaging; 3D, 3-dimensional.
The MRIs were deidentified and catalogued in a manner so that observations were blinded to what group each participant was assigned. A 3-parameter single exponential decay model was fit to the T2* data in each voxel of the ACL segmentation: ,
where t represents the time for each T2* value, and A and C are fitting parameters. The graft volume was calculated by summing the total number of ACL graft voxels. The segmentations defined on the 3D true-FISP could not be directly used on the T2* mapping images because they had differences in slice prescriptions and voxel sizes. Consequently, custom software written in Matlab (Mathworks) accounted for these differences while transferring the segmentations to the T2* mapping images. Customized Matlab software (Mathworks) was used to calculate the mean T2* values and standard deviation for each clinically relevant subregion.
Patient-Reported Outcome Measures. All patients were evaluated with an online PROM system (Surgical Outcome System; Arthrex). The outcome system used email prompts and 3 online questionnaires to track patient-reported outcomes at the pretreatment, 2 weeks, 6 weeks, 3 months, 6 months, 1 year, and 2 years. The 3 questionnaires were the Single Assessment Numeric Evaluation Score (SANE), the Knee injury and Osteoarthritis Outcome Score (KOOS), and a 10-point visual analog scale (VAS) for pain.32,36 For the KOOS, a composite score was created incorporating all 5 subscores into 1 weighted composite score as described in previous studies; it is this composite score that will be referred16,31 to as the KOOS5.
Statistical Analysis
All data were entered into the Research Electronic Data Capture System (REDCap, Vanderbilt University). Descriptive statistics such as means and standard deviations were reported. Also, 95% confidence intervals for the mean of the primary outcomes, PROMs, and effect sizes were calculated and presented. The effect of the intervention was quantified using mixed-effects models. The effects over time for the MRI parameters (T2* and volume) and PROMs (SANE, KOOS5, and VAS pain) were also estimated using mixed-effects models. This model is also known as a multilevel linear model or hierarchical linear model. 13 The advantage of using a linear mixed-effects model is to provide a flexible approach to handle correlated longitudinal data and outcomes that are missing completely at random.17,34 The model is presented as follows:
where yijk is the score at time tk (k = 1, …, 8) for patient j in group i (i = 0 for control), and β i (i = 0, 1, 2) are fixed effects. The group (control vs intervention) is a fixed effect, testing if a statistical difference exists between the groups’ variable mean. The random effects can include a random intercept, a random slope, or both, and ϵijk is the random error term, which can be modeled with various autocorrelation structures. All random effects and errors are assumed to follow a normal distribution. The statistical significance was considered at 5%.
Results
Overall Trial
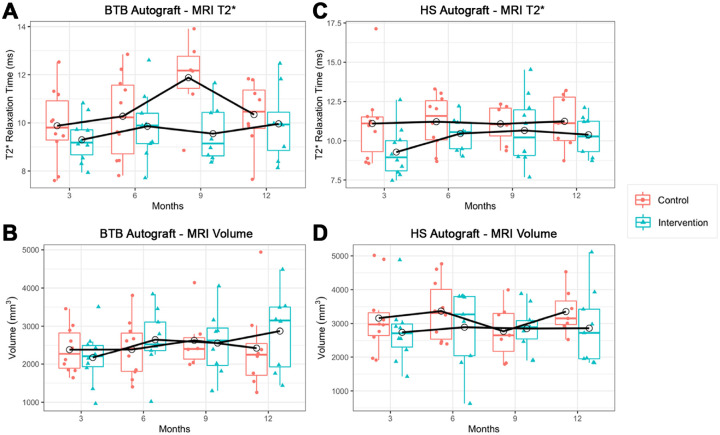
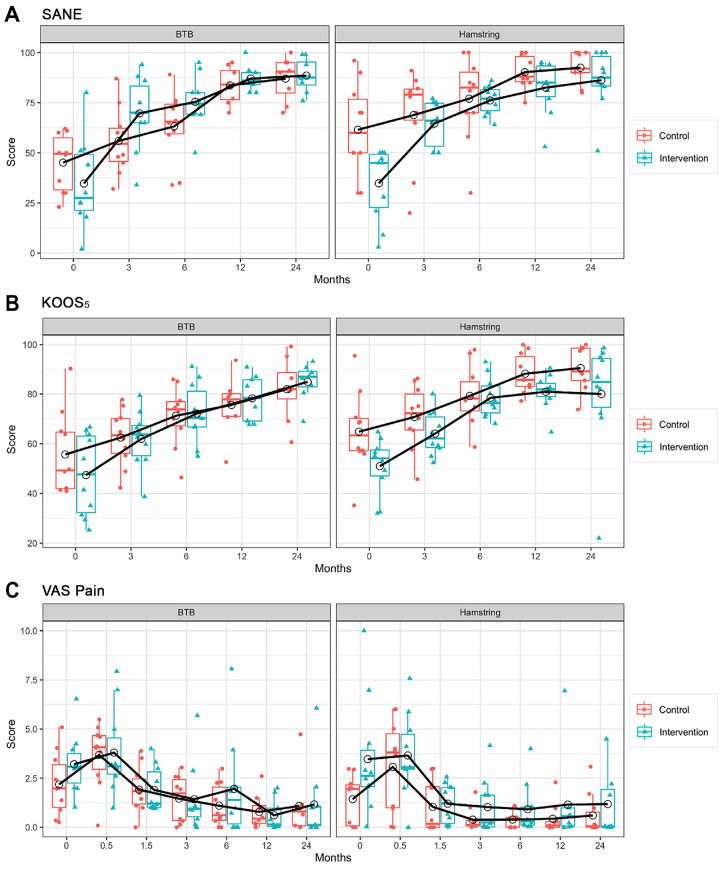
The characteristics of the 40 trial participants are included in Table 1. MRI volume and T2* relaxation time data are reported in Table 2 and Figure 4. PROM scores are included in Table 3 and Figure 5. All participants received the allocated treatment and completed the baseline questionnaires. Table 4 presents the statistical models on the primary outcomes (T2* and volume). Table 5 presents the mixed-effects models on the PROMs (SANE, KOOS5, and VAS pain). There were no postoperative complications (eg, infections, stiffness, persistent effusions) or reconstruction failures for any of the participants after a 2-year follow-up.
Table 1.
Characteristics of the Trial Participants a
| BTB (n = 20) | HS (n = 20) | |||
|---|---|---|---|---|
| Characteristic | Control (n = 10) | Intervention (n = 10) | Control (n = 10) | Intervention (n = 10) |
| Age, y | 29 ± 6.8 | 29.8 ± 8.2 | 25.3 ± 8.9 | 29.8 ± 8.2 |
| Sex | ||||
| Female | 5 (50) | 6 (60) | 6 (60) | 7 (70) |
| Male | 5 (50) | 4 (40) | 4 (40) | 3 (30) |
Data are presented as mean ± SD or n (%). BTB, bone–patellar tendon–bone; HS, hamstring.
Table 2.
Primary Outcomes for the Intervention and Control Groups at 3, 6, 9, and 12 Months Postoperatively a
| 3 Mo Postop | 6 Mo Postop | 9 Mo Postop | 12 Mo Postop | |
|---|---|---|---|---|
| BTB Autograft | n = 20 | n = 19 | n = 14 | n = 16 |
| Volume, mm3 | ||||
| Control | 2384.3 (1955.6-2813.1) n = 10 |
2388.9 (1848.2-2929.7) n = 10 |
2628.7 (1793.2-3464.2) n = 6 |
2424.5 (1458.8-3390.3) n = 8 |
| Intervention | 2168.9 (1669.5-2668.3) n = 10 |
2647.1 (2023.8-3270.5) n = 9 |
2558.7 (1833.9-3283.5) n = 8 |
2872.5 (1996.4-3748.5) n = 8 |
| T2*, ms | ||||
| Control | 9.9 (8.8-11) n = 10 |
10.3 (9-11.5) n = 10 |
11.9 (10.1-13.7) n = 6 |
10.3 (9.2-11.5) n = 8 |
| Intervention | 9.3 (8.6-10) n = 10 |
9.9 (8.8-11) n = 9 |
9.6 (8.6-10.5) n = 8 |
10 (8.7-11.2) n = 8 |
| HS autograft | n = 20 | n = 19 | n = 17 | n = 16 |
| Volume, mm3 | ||||
| Control | 3161.6 (2406.1-3917) n = 10 |
3369.2 (2729.2-4009.2) n = 10 |
2771.8 (2018.1-3525.4) n = 7 |
3355.6 (2735.9-3975.2) n = 7 |
| Intervention | 2742 (2082.3-3401.6) n = 10 |
2893.4 (2015.3-3771.5) n = 9 |
2853.6 (2390.6-3316.5) n = 10 |
2863 (1991.2-3734.8) n = 9 |
| T2*, ms | ||||
| Control | 11.1 (9.3-12.9) n = 10 |
11.2 (10-12.4) n = 10 |
11.1 (10-12.2) n = 7 |
11.2 (9.6-12.8) n = 7 |
| Intervention | 9.3 (8.2-10.4) n = 10 |
10.5 (9.7-11.2) n = 9 |
10.7 (9.11-12.2) n = 10 |
10.4 (9.5-11.3) n = 9 |
Data are reported as mean (95% CI) unless otherwise indicated. BTB, bone–patellar tendon–bone; HS, hamstring; Postop, postoperative.
Figure 4.
(A) T2* relaxation time in the BTB and control groups. (B) Graft volume in the BTB and control groups. (C) T2* relaxation time in the HS and control groups. (D) Graft volume in the HS and control groups. Data points represented with empty circles indicate meanswith empty circles indicate means. The black solid line shows the mean profile. BTB, bone–patellar tendon–bone; HS, hamstring; MRI, magnetic resonance imaging.
Table 3.
PROMs at Baseline and Postoperatively a
| Baseline | 2 Wk Postop | 6 Wk Postop | 3 Mo Postop | 6 Mo Postop | 12 Mo Postop | 24 Mo Postop | |
|---|---|---|---|---|---|---|---|
| BTB Autograft | n = 20 | n = 20 | n = 20 | n = 20 | n = 20 | n = 17 | n = 16/17 b |
| SANE (0-100) | |||||||
| Control | 45.1 (34.8 to 55.4) n = 10 |
— | — | 55.9 (44.1 to 67.7) n = 10 |
63.2 (50.7 to 75.7) n = 10 |
83.6 (76.0 to 91.2) n = 8 |
87 (77.9 to 96.1) n = 8 |
| Intervention | 34.7 (18.7 to 50.7) n = 10 |
— | — | 69.7 (56.8 to 82.6) n = 10 |
75.4 (66.3 to 84.5) n = 10 |
87 (82.2 to 91.8) n = 9 |
88.5 (81.5 to 95.5) n = 8 |
| KOOS5 (0-100) | |||||||
| Control | 55.7 (43.7 to 67.6) n = 10 |
— | — | 62.5 (54.3 to 70.6) n = 10 |
71.2 (62.7 to 79.8) n = 10 |
75.7 (65.8 to 85.4) n = 8 |
81.9 (71.4 to 92.5) n = 8 |
| Intervention | 47.4 (35.5 to 59.2) n = 10 |
— | — | 61.9 (53.8 to 70.1) n = 10 |
72.1 (63.5 to 80.7) n = 10 |
78.4 (71.4 to 85.1) n = 9 |
84.9 (78.6 to 91.2) n = 8 |
| VAS pain (0-10) | |||||||
| Control | 2.2 (1 to 3.3) n = 10 |
3.7 (2.5 to 4.8) n = 10 |
1.9 (1.1 to 2.7) n = 10 |
1.5 (0.6 to 2.3) n = 10 |
1.1 (0.3 to 1.9) n = 10 |
0.8 (0 to 1.5) n = 8 |
1.1 (−0.2 to 2.4) n = 8 |
| Intervention | 3.2 (2.1 to 4.3) n = 10 |
3.8 (2.2 to 5.4) n = 10 |
1.9 (1.1 to 2.7) n = 10 |
1.4 (0.2 to 2.6) n = 10 |
2 (0.2 to 3.7) n = 10 |
0.6 (0 to 1.2) n = 9 |
1.2 (−0.3 to 2.7) n = 9 |
| HS autograft | n = 20 | n = 20 | n = 20 | n = 20 | n = 20 | n = 20/18 b | n = 19/20 b |
| SANE (0-100) | |||||||
| Control | 61.5 (44.9 to 78.1) n = 10 |
— | — | 68.8 (52.2 to 85.4) n = 10 |
77 (61.8 to 92.2) n = 10 |
90.2 (84.4 to 96) n = 10 |
92.4 (86.3 to 98.6) n = 9 |
| Intervention | 34.8 (21.8 to 47.8) n = 10 |
— | — | 64.6 (56.7 to 72.5) n = 10 |
76.1 (71 to 81.2) n = 10 |
82.5 (73.1 to 91.9) n = 10 |
86.2 (75.6 to 96.8) n = 10 |
| KOOS5 (0-100) | |||||||
| Control | 64.8 (53.2 to 76.5) n = 10 |
— | — | 70.8 (61.7 to 79.8) n = 10 |
79.3 (70.7 to 87.8) n = 10 |
88.2 (82.5 to 93.9) n = 10 |
90.4 (83.5 to 97.3) n = 9 |
| Intervention | 50.9 (42.9 to 59) n = 10 |
— | — | 64.1 (57.8 to 70.4) n = 10 |
78.5 (72.6 to 84.3) n = 10 |
80.9 (75.9 to 86) n = 10 |
80 (63.8 to 96.2) n = 10 |
| VAS pain (0-10) | |||||||
| Control | 1.4 (0.5 to 2.3) n = 10 |
3.1 (1.4 to 4.8) n = 10 |
1 (0.1 to 2) n = 10 |
0.4 (0 to 0.8) n = 10 |
0.4 (0.1 to 0.7) n = 10 |
0.4 (−0.2 to 1) n = 9 |
0.7 (−0.1 to 1.3) n = 10 |
| Intervention | 3.5 (1.3 to 5.6) n = 10 |
3.7 (2.1 to 5.2) n = 10 |
1.2 (0.5 to 1.9) n = 10 |
1 (0 to 2) n = 10 |
0.9 (0 to 1.8) n = 10 |
1.2 (−0.5 to 2.8) n = 9 |
1.2 (−0 to 2.4) n = 10 |
Data are reported as mean (95% CI) unless otherwise indicated. Dashes indicate that a PROM was not taken at a particular timepoint. BTB, bone–patellar tendon–bone; HS, hamstring; KOOS5, Knee injury and Osteoarthritis Outcome Score; Postop, postoperative; PROM, patient-reported outcome measure; SANE, Single Assessment Numeric Evaluation Score; VAS, visual analog scale.
Different subsample sizes at the follow-up visits are because of the decreased PROM compliancy over time.
Figure 5.
(A) The SANE versus time in all groups. (B) The KOOS5 versus time in all groups. (C) The VAS versus time in all groups. Data points represented with empty circles indicate means, and the horizontal lines within the boxes represent the medians. The black solid line shows the mean profile. KOOS5, Composite Knee injury and Osteoarthritis Outcome Score; SANE, Single Assessment Numeric Evaluation Score; VAS, visual analog scale.
Table 4.
Results of Linear Mixed-Effects Models for Primary Outcomes (MRI T2* and Graft Volume) a
| BTB Autograft: T2* | BTB Autograft: Volume | HS Autograft: T2* | HS Autograft: Volume | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate (95% CI) |
P | Estimate (95% CI) |
P | Estimate (95% CI) |
P | Estimate (95% CI) |
P | |
| (Intercept) | 9.90 (8.99 to 10.82) |
<.001 | 2209.1 (1684.5 to 2733.6) |
<.001 | 10.63 (9.54 to 11.73) |
<.001 | 3187.13 (2636.1 to 3738.2) |
<.001 |
| Time: 6 mo | 0.52 (−0.05 to 1.09) |
.074 | 217.8 (14.7 to 421.0) |
.036 | 0.59 (−0.16 to 1.33) |
.119 | 118.49 (−223.3 to 460.2) |
.489 |
| Time: 9 mo | 0.77 (0.13 to 1.40) |
.019 | 144.5 (−150 to 439.1) |
.328 | 0.61 (−0.35 to 1.57) |
.205 | 39.16 (−194.7 to 273) |
.738 |
| Time: 12 mo | 0.62 (0.01 to 1.22) |
.046 | 308.1 (−35.9 to 652.1) |
.078 | 0.52 (−0.30 to 1.34) |
.207 | 1.19 (−462.5 to 464.9) |
.996 |
| Group: intervention | −0.63 (−1.89 to 0.63) |
.309 | 135.1 (−606 to 876.1) |
.706 | −0.89 (−2.17 to 0.39) |
.162 | −395.81 (−11,141.1 to 351.5) |
.280 |
BTB autograft: 20 patients, 69 MRIs; HS autograft: 20 patients, 72 MRIs. Bold P values indicate statistical significance (P < .05). BTB, bone–patellar tendon–bone; HS, hamstring; MRI, magnetic resonance imaging.
Table 5.
Results of Linear Mixed-Effects Models for PROMs a
| BTB Autograft: SANE | BTB Autograft: KOOS5 | BTB Autograft: VAS Pain | ||||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| (Intercept) | 38.38 (28.60 to 48.17) |
<.001 | 51.37 (42.73 to 60.02) |
<.001 | 2.66 (1.74 to 3.58) |
<.001 |
| Time: 2 wk | — | — | — | — | 1.05 (0.02 to 2.08) |
.046 |
| Time: 6 wk | — | — | — | — | −0.78 (−1.55 to −0.01) |
.047 |
| Time: 3 mo | 22.90 (12.42 to 33.38) |
<.001 | 10.68 (3.25 to 18.11) |
.005 | −1.25 (−2.07 to −0.44) |
.003 |
| Time: 6 mo | 29.40 (19.85 to 38.95) |
<.001 | 20.17 (13.21 to 27.12) |
<.001 | −1.17 (−2.17 to −0.17) |
.023 |
| Time: 12 mo | 44.63 (35.99 to 53.27) |
<.001 | 24.83 (17.87 to 31.79) |
<.001 | −1.81 (−2.56 to −1.05) |
<.001 |
| Time: 24 mo | 47.35 (38.67 to 56.04) |
<.001 | 31.74 (24.70 to 38.79) |
<.001 | −1.37 (−2.46 to −0.29) |
.014 |
| Group: intervention | 3.03 (−5.36 to 11.42) |
.457 | 0.28 (−8.79 to 9.35) |
.949 | 0.07 (−0.85 to 0.98) |
.881 |
| HS Autograft: SANE | HS Autograft: KOOS5 | HS Autograft: VAS Pain | ||||
| Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| (Intercept) | 51.79 (40.65 to 62.93) |
<.001 | 61.33 (54.62 to 68.04) |
<.001 | 1.94 (0.97 to 2.92) |
<.001 |
| Time: 2 wk | — | — | — | — | 0.85 (−0.29 to 1.99) |
.142 |
| Time: 6 wk | — | — | — | — | −1.31 (−2.59 to −0.03) |
.044 |
| Time: 3 mo | 18.55 (6.26 to 30.84) |
.004 | 9.53 (3.08 to 15.97) |
.004 | −1.78 (−3.02 to −0.53) |
.006 |
| Time: 6 mo | 28.40 (17.10 to 39.70) |
<.001 | 20.97 (15.27 to 26.68) |
<.001 | −1.75 (−2.94 to −0.56) |
.004 |
| Time: 12 mo | 38.20 (28.61 to 47.79) |
<.001 | 26.66 (21.73 to 31.59) |
<.001 | −1.68 (−2.77 to −0.58) |
.003 |
| Time: 24 mo | 41.25 (31.43 to 51.06) |
<.001 | 26.99 (19.09 to 34.90) |
<.001 | −1.57 (−2.69 to −0.45) |
.006 |
| Group: intervention | −7.28 (−16.71 to 2.14) |
.122 | −6.88 (−14.06 to 0.29) |
.059 | 1.04 (−0.03 to 2.12) |
.057 |
Dashes indicate that a PROM was not taken at a particular timepoint. Bold P values indicate statistical significance (P < .05). BTB, bone–patellar tendon–bone; HS, hamstring; KOOS5, Composite Knee injury and Osteoarthritis Outcome Score; PROM, patient-reported outcome measure; SANE, Single Assessment Numeric Evaluation Score; VAS, visual analog scale.
BTB Autograft Trial
The control group included 5 men and 5 women, with a mean (±SD) age of 29 ± 6.8 years (range, 18-40 years), and the intervention group included 6 men and 4 women, with a mean age of 29.8 ± 8.2 years (range, 18-41 years) (see Table 1). Analysis of the BMAC product demonstrated 85.24 ± 66.18 TNC (k/μL) (range, 3.57-374.9 TNC [k/μL]) and 422.03 ± 531.92 CFU/mL (range, 0-2673.75 CFU/mL). No significant difference was found between reported patient characteristics and bone marrow concentrate (BMC) products (P > .05).
Repeat MRI studies were completed at 3, 6, 9, and 12 months. The total number of MRI observations was 69 out of 80, representing an 86% capture rate (see Table 2 for details). Completion rates were as follows: 100% at 3 months, 95% (100% control, 90% intervention) at 6 months, 70% (60% control, 80% intervention) at 9 months, and 80% (80% control, 80% intervention) at 12 months.
The variability of the T2* in both groups changed over time, especially at the 6-month time point for the control group. No significant differences were found in T2* values between groups. The time × group interaction was not significant. Therefore, it was removed from all models (Table 4). For the entire cohort (intervention + control), the volume was statistically significantly higher at 9 (P = .02) and 12 (P = .046) months compared with 3 months. Comparing control versus intervention groups, there was no evidence of significant difference between the 2 groups (95% CI, –1.89 to 0.63; P = .31).
No significant differences were found in the mean volume between groups. The volume variability in the intervention group increased over time, while that of the control group stayed stable. Overall, no significant differences were detected when comparing control versus intervention groups (95% CI, –606 to 876.1; P = .71).
KOOS5 total survey observations were 93 out of 100, representing a 93% capture rate; 100% at 0, 3, and 6 months; 85% (80% control and 90% intervention) at 12 months; and 80% at 24 months. SANE observations were 93 out of 100, with a 93% capture rate; 100% at 0, 3, and 6 months, 85% (80% control and 90% intervention) at 12 months, and 80% at 24 months. VAS observations were 134 out of 140 for a 96% capture rate: 100% at 0, 0.5, 1.5, 3, and 6 months and 95% (80% control, 90% intervention) at 12 and 24 months.
The mean KOOS5 score increased from the baseline for both groups. For KOOS scores, there was a statistically significant increase in the values at all time points compared with the baseline (P < .01) (see Table 3). There was no significant difference between the mean KOOS5 scores for the control and intervention groups (95% CI, –8.8 to 9.4; P = .95). The mean SANE score increased from the baseline for both groups. For SANE scores, the effect of time was statistically significant (P < .001). No significant difference was detected between the mean SANE scores for the control and intervention groups (95% CI, –5.4 to 11.4; P = .46). The mean VAS scores demonstrated an initial transient increase at the postoperative 2-week time point, followed by a decrease from the baseline in the 2 groups from 6 weeks to the 24-month follow-up (P < .001). No significant difference was observed between the means of VAS scores for the control and intervention groups (95% CI, –0.9 to 1; P = .88).
HS Autograft Trial
The control group included 6 men and 4 women, with a mean age of 25.3 ± 8.9 years (range, 18-43 years), and the intervention group included 7 men and 3 women, with a mean age of 29.8 ± 8.2 years (range, 18-33 years) (see Table 1). Analysis of the bone marrow aspirate product demonstrated 66.45 ± 35.5 TNC (k/μL) (range, 11.23-148.57 TNC [k/μL]) and 106.84 ± 170.51 CFU/mL (range, 0-881.9 CFU/mL). No significant difference was found between reported patient characteristics and BMC products.
Repeated MRI studies were completed at 3, 6, 9, and 12 months. The total number of MRI observations was 72 out of 80, representing a 90% capture rate (see Table 2 for details). Completion rates were as follows: 100% at 3 months, 95% (100% control, 90% intervention) at 6 months, 85% (70% control, 100% intervention) at 9 months, and 80% (70% control, 90% intervention) at 12 months.
Linear mixed-effects models were employed to estimate the difference between the intervention and control groups on the primary outcomes and the PROMs. Comparing the control and the intervention groups, no significant difference in the mean volume values was found (95% CI, –11,141.1 to 351.5; P = .28). The change in the mean volume over time was not statistically significant. The control group showed a steady mean T2* over 12 months, and the intervention group showed an increase in the mean T2* from 3 to 12 months. Comparing control versus intervention groups, there was no evidence of significant difference between the 2 groups (95% CI, –2.17 to 0.39; P = .162). There were no significant changes in the mean T2* over time (see Table 4).
KOOS5 total survey observations were 99 out of 100, representing a 99% capture rate, 100% at 0, 3, 6, and 12 months and 95% (90% control and 100% intervention) at 24 months. SANE total survey observations were 99 out of 100, representing a 99% capture rate: 100% at 0, 3, 6, and 12 months and 95% (90% control and 100% intervention) at 24 months. VAS pain observations were 138 out of 140 for a 99% capture rate: 100% at 0, 0.5 (2 weeks), 1.5 (6 weeks), 3, 6, and 24 months and 95% (90% control, 90% intervention) at 12 months.
The mean KOOS5 score increased significantly from the baseline for both groups (P < .001) (see Table 3). There was no significant difference between the mean KOOS5 scores for the control and intervention groups (95% CI, –14.1 to 0.3; P = .06). The mean SANE score increased from the baseline for both groups. For the SANE scores, the increase over time was statistically significant (P < .001). There was no significant difference between the mean SANE scores for the control and intervention groups (95% CI, –16.7 to 2.1; P = .12). The mean VAS scores demonstrated an initial transient increase at the postoperative 2-week time point (P = .14), followed by a decrease from the baseline in both groups from 6 weeks to the 24-month follow-up (P < .05). There was no significant difference between the mean VAS scores for the control and intervention groups (95% CI, –0.03 to 2.1; P = .06).
Discussion
The most important finding of this study is that augmenting ACL autograft reconstruction with a collagen amnion matrix and BMAC was feasible and had no postoperative complications (infections, stiffness, persistent effusions) or reconstruction failures observed in 40 patients after a 2-year follow-up. However, our main hypothesis was rejected. This study did not demonstrate any advantage of the intervention on graft maturation or PROMs. Furthermore, MRI values for T2* in the BTB (P = .31) and HS (P = .16) groups demonstrated decreased mean and median T2* at all time points compared with the controls, although not statistically significant. An additional outcome of interest was reconstructed graft volume and volume change over time. For the HS arm, neither the effect size between the intervention and the control nor the change in volume over time were statistically significant. When looking at the BTB group, an encouraging finding was the change in volume over time was significant at 6 months compared with 3 months (P = .036). The primary intent of this study was to develop the concept further by investigating safety and developing pilot data for further study. This early clinical study provides information for further clinical study design in this space.
The use of MRI to evaluate graft maturation has preclinical solid (animal) as well as clinical support. Low T2* values were explicitly associated with greater cell density and collagen organization. 7 The biomechanical properties of the ACL, including yield load, stiffness, and maximum load, have also been correlated with T2* measurements. Weiler et al 35 investigated 1.5-T, contrast-enhanced MRI scans with histology and biomechanical properties after ACLR in a sheep model. High signal intensity values were associated with low load to failure, stiffness, and tensile strength values. 35 Previous studies regarding the MRI methods used in this study have validated reliability and produced normal values for native ACL, demonstrating that T2, T2* relaxation values, and ACL volume do not vary with age. The T2* values of the intervention groups in this study, with a range of 9.3 to 10.4, were consistently lower than previously determined normative data, suggesting that reconstructed grafts may be denser than the normal ACL.
Clinical studies have also supported using MRI measures to assess graft ligamentization. A study concluded that significant changes over time in the graft T1ρ and T2 relaxation times are consistent with descriptions of in vivo ligamentization. 22 A similar study comparing MRI measures to PROMs and physical examination findings at 2 years concluded that MRI is sensitive enough to capture biomechanical ligamentization of graft tissue while PROMs and physical examination are not. 23 In contrast, previous study suggests that physical examinations lack sensitivity in detecting critical graft mechanical properties of the postoperative ACLR as it progresses through ligamentization.6,14 Not surprisingly, this study demonstrated no significant difference in PROMs, KOOS5, SANE, and VAS, for the BTB and HS arms. Therefore, noninvasive MRI evaluation of the postoperative ACLR can provide valuable insight into the state of the reconstruction, demonstrating a correlation with signal intensity and graft maturity.5-8,15,23,29,33,35 Although the PROMs used in this study may have a place for detecting detrimental effects and identifying loss of function, they may demonstrate increased sensitivity and be better suited for use at the lower end of physical function.
Studies evaluating biologic interventions to augment ACLR have progressed from preclinical to clinical applications. A recent systematic review of comparative studies assessing the use of platelet-rich plasma (PRP) to augment ACLR found 11 studies. 10 Four studies reported a statistically significant difference toward faster graft maturation, 2 found trends, and 1 found no difference. For PROMs, 1 study showed better clinical outcomes with PRP use, and 5 studies showed no benefits. A review of studies evaluating cellular augmentation found 4 studies—2 randomized clinical trials; 1 cohort study with a matched historical control group; and 1 case series. 1 Cellular therapies varied and included BMAC, collagenase/centrifuge processed adipose, marrow stimulation combined with PRP, and cells cultured from allograft bone marrow aspirate. The BMC and adipose tissue study results did not support their use. The marrow stimulation technique combined with repairs led to promising clinical results. Allograft cultured cells improved PROMs and postoperative radiographic findings. Two previous clinical studies are worth particular mention, Radice et al 30 evaluated 50 patients in a multicenter, single-blinded study comparing PRP loaded onto an absorbable gelatin sponge and sutured onto either the HS or BTB autografts. They observed graft maturation using postoperative MRI scans and found 48% faster maturation in the intervention group. In addition to augmenting ACLR, clinicians have evaluated augmenting ACL repair. Murray et al25-27 have developed and clinically evaluated a suture repair combined with a resorbable, protein-based matrix scaffold loaded with whole blood and placed in the gap between the torn ends of an ACL injury. In a prospective randomized controlled trial with 100 patients, noninferior PROMs, anterior-posterior knee laxity, and superior HS muscle strength were found compared with a control autograft at 2 years. 4 When considering clinical technique development, additional costs must be considered. The technique studied in this study would add time and expense to ACLR. These added expenses cannot be justified currently based on this study. However, amniotic membranes may still be valuable in wound healing—including bone and soft tissue.20,28
Limitations
The biggest limitation of this study is the small sample size, which creates the possibility of type 2 errors. Other limitations of this study include incomplete MRI data and the fact that MRI serves as a surrogate measure for ligamentization. Increased validity could be found using second-look needle arthroscopy with biopsy. Another limitation is that bone marrow aspirate was harvested from the distal femur, anterior iliac crest, or posterior iliac crest instead of 1 location; this contributed to variation in the cellular content of the BMC. Blinding with a sham dressing was likely ineffective when the harvest was from 1 of the 3 crests; importantly, this would not affect the objective MRI values. A confounding variable in this study is the allowance of bone marrow aspirate harvest from multiple sites. This limits conclusions about the benefit of the addition of the BMAC to the final graft construct, as harvesting bone marrow from varying locations produces variable products.
Conclusion
In this pilot study, wrapping a graft with an amnion collagen matrix and injecting BMAC appeared safe. MRI T2* values and graft volume of the augmented ACLRs were not significantly different from that of controls, suggesting that the intervention did not result in improved graft maturation.
Footnotes
Final revision submitted May 22, 2023; accepted June 6, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding was received from Arthrex (grant US-01087). A.W.A. has received nonconsulting fees from Arthrex, CGG Medical, and Smith & Nephew; consulting fees from Arthrex and Bioventus; education payments from Arthrex, CGG Medical, Bioventus, and Mid-Atlantic Surgical Systems; and royalties from Arthrex. S.E.J. has received royalties from Arthrex; education payments from CGG Medical; nonconsulting fees from Arthrex and CGG Medical; and education payments from Arthrex and CGG Medical. R.V.O. has received education payments and nonconsulting fees from Arthrex; and consulting fees from DePuy Synthes. E.A.B. has received grant support from Arthrex and education payments from Smith & Nephew, MVP Orthopedics, and Arthrex. J.R.A. has received nonconsulting fees from Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Baptist Hospital–Pensacola (ref No. 1017674-1).
References
- 1. Anz A. Cellular augmentation of ACL surgery is not currently evidence based: a systematic review of clinical studies. Arthroscopy. In press. [DOI] [PubMed] [Google Scholar]
- 2. Anz AW, Edison J, Denney TS, et al. 3-T MRI mapping is a valid in vivo method of quantitatively evaluating the anterior cruciate ligament: rater reliability and comparison across age. Skeletal Radiol. 2020;49(3):443-452. [DOI] [PubMed] [Google Scholar]
- 3. Anz AW, Lucas EP, Fitzcharles EK, Surowiec RK, Millett PJ, Ho CP. MRI T2 mapping of the asymptomatic supraspinatus tendon by age and imaging plane using clinically relevant subregions. Eur J Radiol. 2014;83(5):801-805. doi: 10.1016/j.ejrad.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 4. Barnett SC, Murray MM, Badger GJ, et al. Earlier resolution of symptoms and return of function after bridge-enhanced anterior cruciate ligament repair as compared with anterior cruciate ligament reconstruction. Orthop J Sports Med. 2021;9(11):23259671211052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beveridge JE, Machan JT, Walsh EG, et al. Magnetic resonance measurements of tissue quantity and quality using T2* relaxometry predict temporal changes in the biomechanical properties of the healing ACL. J Orthop Res. 2018;36(6):1701-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biercevicz AM, Akelman MR, Fadale PD, et al. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am J Sports Med. 2015;43(3):693-699. doi: 10.1177/0363546514561435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41(3):560-566. doi: 10.1177/0363546512472978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biercevicz AM, Murray MM, Walsh EG, Miranda DL, Machan JT, Fleming BC. T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. 2014;32(4):492-499. doi: 10.1002/jor.22563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Claes S, Verdonk P, Forsyth R, Bellemans J. The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft? A systematic review of the literature. Am J Sports Med. 2011;39(11):2476-2483. [DOI] [PubMed] [Google Scholar]
- 10. Davey MS, Hurley ET, Withers D, Moran R, Moran CJ. Anterior cruciate ligament reconstruction with platelet-rich plasma: a systematic review of randomized control trials. Arthroscopy. 2020;36(4):1204-1210. [DOI] [PubMed] [Google Scholar]
- 11. DiDomenico LA, Orgill DP, Galiano RD, et al. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. 2016;4(10):e1095. doi: 10.1097/GOX.0000000000001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duc SR, Pfirrmann CW, Koch PP, Zanetti M, Hodler J. Internal knee derangement assessed with 3-minute three-dimensional isovoxel true FISP MR sequence: preliminary study. Radiology. 2008;246(2):526-535. [DOI] [PubMed] [Google Scholar]
- 13. Finch WH, Bolin JE, Kelley K. Multilevel Modeling Using R. CRC Press; 2019. [Google Scholar]
- 14. Fleming BC, Fadale PD, Hulstyn MJ, et al. The effect of initial graft tension after anterior cruciate ligament reconstruction: a randomized clinical trial with 36-month follow-up. Am J Sports Med. 2013;41(1):25-34. doi: 10.1177/0363546512464200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44(16):2843-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363(4):331-342. [DOI] [PubMed] [Google Scholar]
- 17. Hedeker D, Gibbons RD. Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2(1):64. [Google Scholar]
- 18. Ho CP, Surowiec RK, Ferro FP, et al. Subregional anatomical distribution of T2 values of articular cartilage in asymptomatic hips. Cartilage. 2014;5(3):154-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jang K-M, Lim HC, Jung WY, Moon SW, Wang JH. Efficacy and safety of human umbilical cord blood–derived mesenchymal stem cells in anterior cruciate ligament reconstruction of a rabbit model: new strategy to enhance tendon graft healing. Arthroscopy. 2015;31(8):1530-1539. [DOI] [PubMed] [Google Scholar]
- 20. Johri S, Verma P, Tikku AP, Bains R, Kohli N. Effect of amniotic membrane and platelet-rich fibrin membrane on bone healing post endodontic surgery: an ultrasonographic, randomized controlled study. J Tissue Eng Regen Med. 2022;16(12):1208-1222. doi: 10.1002/term.3362 [DOI] [PubMed] [Google Scholar]
- 21. Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lansdown DA, Xiao W, Zhang AL, et al. Quantitative imaging of anterior cruciate ligament (ACL) graft demonstrates longitudinal compositional changes and relationships with clinical outcomes at 2 years after ACL reconstruction. J Orthop Res. 2020;38(6):1289-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H, Tao H, Cho S, Chen S, Yao Z, Chen S. Difference in graft maturity of the reconstructed anterior cruciate ligament 2 years postoperatively: a comparison between autografts and allografts in young men using clinical and 3.0-T magnetic resonance imaging evaluation. Am J Sports Med. 2012;40(7):1519-1526. doi: 10.1177/0363546512443050 [DOI] [PubMed] [Google Scholar]
- 24. Mifune Y, Matsumoto T, Takayama K, et al. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials. 2013;34(22):5476-5487. [DOI] [PubMed] [Google Scholar]
- 25. Murray MM, Fleming BC, Badger GJ, et al. Bridge-enhanced anterior cruciate ligament repair is not inferior to autograft anterior cruciate ligament reconstruction at 2 years: results of a prospective randomized clinical trial. Am J Sports Med. 2020;48(6):1305-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray MM, Kalish LA, Fleming BC, et al. Bridge-enhanced anterior cruciate ligament repair: two-year results of a first-in-human study. Orthop J Sports Med. 2019;7(3):2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Niami F, Molavynejad S, Hemmati AA, et al. Evaluation of the effect of a gel made with amniotic fluid formulation on the healing of diabetic foot ulcers: a triple-blind clinical trial. Front Public Health. 2022;10:1025391. doi: 10.3389/fpubh.2022.1025391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niki Y, Yasuoka T, Kobayashi S, et al. Feasibility of T1rho and T2 map magnetic resonance imaging for evaluating graft maturation after anatomic double-bundle anterior cruciate ligament reconstruction. J Orthop Surg Res. 2019;14(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radice F, Yanez R, Gutierrez V, Rosales J, Pinedo M, Coda S. Comparison of magnetic resonance imaging findings in anterior cruciate ligament grafts with and without autologous platelet-derived growth factors. Arthroscopy. 2010;26(1):50-57. doi: 10.1016/j.arthro.2009.06.030 [DOI] [PubMed] [Google Scholar]
- 31. Roos EM, Engelhart L, Ranstam J, et al. ICRS recommendation document: patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage. 2011;2(2):122-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. [DOI] [PubMed] [Google Scholar]
- 33. Rose M, Crawford D. Allograft maturation after reconstruction of the anterior cruciate ligament is dependent on graft parameters in the sagittal plane. Orthop J Sports Med. 2017;5(8):2325967117719695. doi: 10.1177/2325967117719695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rubin LH, Witkiewitz K, Andre JS, Reilly S. Methods for handling missing data in the behavioral neurosciences: don't throw the baby rat out with the bath water. J Undergrad Neurosci Educ. 2007;5(2):A71. [PMC free article] [PubMed] [Google Scholar]
- 35. Weiler A, Peters G, Maurer J, Unterhauser FN, Sudkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29(6):751-761. doi: 10.1177/03635465010290061401 [DOI] [PubMed] [Google Scholar]
- 36. Williams GN, Taylor DC, Gangel TJ, Uhorchak JM, Arciero RA. Comparison of the single assessment numeric evaluation method and the Lysholm score. Clin Orthop Relat Res. 2000;373:184-192. [DOI] [PubMed] [Google Scholar]