Abstract
Mechanical properties of extracellular matrices (ECMs) regulate essential cell behaviours, including differentiation, migration and proliferation, through mechanotransduction. Studies of cell–ECM mechanotransduction have largely focused on cells cultured in 2-dimensions (2D), on top of elastic substrates with a range of stiffness. However, cells often interact with ECMs in vivo in a 3-dimensional (3D) context, and cell–ECM interactions and mechanisms of mechanotransduction can differ in 3D. The ECM exhibits various structural features as well as complex mechanical properties. In 3D, mechanical confinement by the surrounding ECM restricts changes in cell volume and shape but allows cells to generate force on the matrix through extending protrusions and regulating cell volume as well as through actomyosin-based contractility. Furthermore, cell–matrix interactions are dynamic owing to matrix remodelling. Accordingly, ECM stiffness, viscoelasticity and degradability, often play a critical role in regulating cell behaviours in 3D. Mechanisms of 3D mechanotransduction include traditional integrin-mediated pathways that sense mechanical properties and more recently described mechanosensitive ion channel-mediated pathways that sense 3D confinement, with both converging on the nucleus for downstream control of transcription and phenotype. Mechanotransduction is involved in tissues from development to cancer and is being increasingly harnessed towards mechanotherapy. Here, we discuss recent progress in our understanding of cell–ECM mechanotransduction in 3D.
eTOC
Mechanical cues from the extracellular matrix (ECM) regulate cell fate and behaviour through cell–ECM mechanotransduction. Studies of cell–ECM mechanotransduction have largely focused on cells cultured in 2D, and only recently have we begun to unravel how these processes occur in 3D — a context native to most cells in vivo.
Introduction
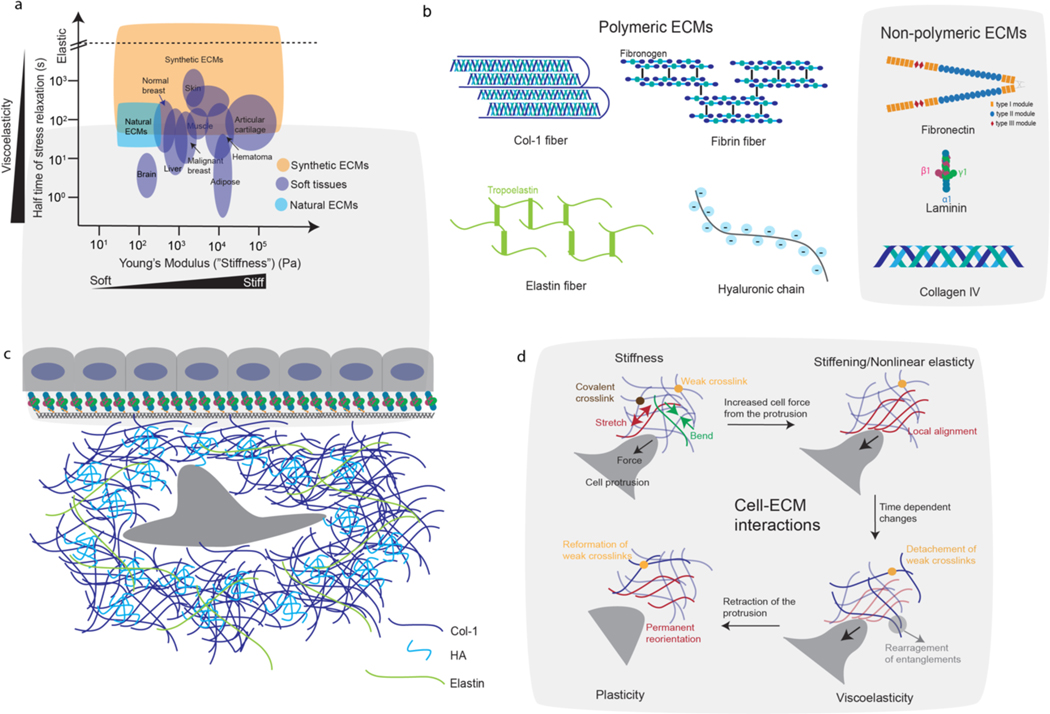
Over the last several decades, it has been established that cell intrinsic mechanisms are not sufficient to explain cell behaviours, with the microenvironment playing a critical role in many, if not all, cellular functions. Tissues consist of cells and extracellular matrix (ECM). The ECM is a scaffolding that provides mechanical support and drives biological signalling in cells in tissues and has been recognized as a key aspect of the microenvironment that regulates cell behaviours and phenotype. As cells push and pull on ECM during various biological processes, the ECM initially resists these actions, as governed by ECM stiffness [G]. Stiffness is usually described by the elastic modulus, which ranges from 100s of Pascals (Pa) to several kilopascals (kPa) in soft tissues such as brain, adipose and breast tissue, and up to 10s of kPa in muscle1,2 (Fig. 1a). As cells increase the magnitude of their pushing and pulling, the ECM resistance often increases nonlinearly or the ECM becomes stiffer, a behaviour described as nonlinear elasticity [G] 3. As cells sustain their pushing and pulling over time, the resistance of the ECM to cell-induced deformation relaxes due to stress relaxation [G], and the ECM undergoes creep [G] under loading, as defined by the ECM viscoelasticity [G]. Finally, permanent deformations can set in following release of forces by the cell due to ECM mechanical plasticity [G]. These various mechanical properties of the cells impact intracellular signalling, transcription and phenotype through feedback from the ECM, whereby cells sense and respond to mechanical cues provided by the ECM — a process known as cell–ECM mechanotransduction.
Figure 1. Tissue mechanics, ECM components, and cell-ECM mechanical interactions.
(a) Stiffness and half time of stress relaxation for various soft tissues, and the range of those properties accessible with reconstituted ECMs or synthetic ECMs (i.e. hydrogels). Data were taken from refs. 1,40,52,84,122,135,199,269. Stress relaxation half times, which provide a measure of viscoelasticity, are defined as the time for the stress to relax to half its original value in response to a constant deformation. Synthetic ECMs, including alginate hydrogels and PEG hydrogels, can be modified to mimic the physiological mechanics of soft tissues (check Box 1). (b) Schematics of structural components of major polymeric (left) and non-polymeric (right) ECMs in tissues. (c) Schematic of an epithelial monolayer with basement membrane underneath, and a stromal cell surrounded by fibrous ECMs such as col-1 and elastin in the underlying connective tissue. (d) As cells interact with ECM, these interactions are mediated by mechanical properties of the ECM including stiffness, nonlinear elasticity, viscoelasticity, and plasticity. As the cell push/pull on the ECM, the ECM may resist the cellular force through bending and stretching of the ECM fibers (left top). With increased forces from the cell, the ECM may stiffen (i.e. exhibit greater resistance) due to local alignment in fibers (right top). Over the time of force application, the ECM may undergo creep and stresses may relax due to detachment of weak crosslinks and fiber rearrangements (bottom right). Once the cell detaches from the ECM, the ECM may retain permanent deformations resulting from reformation of weak crosslinks that lock in changes in fibre position and alignment (bottom left).
Early studies of mechanotransduction focused on the impact of stiffness in 2D culture models on various processes such as cell migration, proliferation, malignancy and differentiation4–8. These established the concept of mechanotransduction and have proven to be relevant to various in vivo contexts. However, a 3D culture microenvironment is required for promoting biologically relevant behaviours in many contexts9,10. For example, a 3D microenvironment maintains a chondrogenic phenotype11, distinguishes normal mammary epithelial cells from breast cancer cells with only the normal cells forming growth arrested organotypic acinar structures12, supports fibrillar adhesions in fibroblasts that are observed in vivo9, boosts pluripotency of human embryonic stem cells13, and regulates cancer cell angiogenic capability14. Importantly, an emerging body of evidence indicates that mechanotransduction in 3D can differ from mechanotransduction in 2D. In this Review, we discuss recent advances in our understanding of cell—ECM mechanotransduction in 3D. First, we start by reviewing the mechanics of various ECMs followed by the impact of ECM mechanics on various cell behaviours such as cell spreading, migration, differentiation and other fundamental biological processes. Then, we describe the nature of cell–ECM mechanical interactions in 3D, and the corresponding mechanotransduction mechanisms mediated through cell membrane receptor proteins and the nucleus. Lastly, we end the Review by describing the role of mechanotransduction in tissue development, disease and repair as well as the potential use of these findings towards mechanotherapy.
Mechanics of ECM Components
In this section, we discuss major ECM components (Fig. 1b–c) that are implicated in tissue mechanics and cell-ECM mechanotransduction.
Collagen-1
Type-1 collagen (col-1) is ubiquitously expressed and represents the most abundant protein in humans15. Col-1 forms fibrils and then fibres that range from 50 to a few hundred nanometers in thickness and can be many microns in length. These fibres crosslink together to form col-1 networks, and reconstituted col-1 gels are often used in 3D culture studies. Architecture and crosslinking of these networks vary substantially based on tissue type. As cells bind to col-1 fibres through integrin membrane receptors, heterodimers consisting of α and b subunits, with α2β1 and 03B11β1 integrins particularly implicated16,17, these networks are central to many cell–matrix interactions.
Col-1 mechanical properties are complex and often exhibit varying levels of stiffness, nonlinear elasticity, viscoelasticity and plasticity. Individual col-1 fibrils exhibit a stiffness range of 300 MPa to 1.2 GPa, with the increase resulting from straightening and uncoiling of triple helical structures of col-1(ref. 18). Reconstituted col-1 gels are microporous and exhibit elastic moduli on the order of 10s to 100s of Pa at the micro to macro scale. Col-1 networks initially resist external mechanical loading or deformation in shear or tension through resistance of individual fibres to bending, at low strain [G] values, and then stretching of rotated and aligned fibres, at higher strains1,19 (Fig. 1d). This results in strain stiffening (nonlinear elasticity) with an almost order of magnitude increase in stiffness with strain (Fig. 1d), which depends sensitively on the length of fibres comprising the network3,20. Like most natural ECMs, col-1 networks are susceptible to degradation by proteases, particularly matrix metalloproteinases [G] (MMPs)21.
Col-1 networks are formed from a combination of weak crosslinks within fibres and between fibres, physical entanglements, and covalent crosslinks22,23. Enzymes such as lysyl oxidase [G] and advanced glycation end products [G] facilitate covalent crosslinks between col-1 fibres. Increased crosslinking density restricts bending deformation of the fibres leading to increased stiffness. Under an applied mechanical stress or strain, unbinding of weak crosslinks within fibres or between fibres can lead to fibre lengthening or fibre reorientation and flow, respectively, giving rise to time-dependent viscoelastic responses such as creep or stress relaxation, and dissipating mechanical energy22–24. Unbinding of the crosslinks leads to fibre lengthening and matrix flow, corresponding to translational movement of the fibres, which are irreversible and stabilized by reformation of weak crosslinks and therefore associated with plastic or permanent deformation (Fig. 1d). Thus, in col-1, weak bonds whose breakage allows viscoelastic creep and stress relaxation also lead to plastic deformation, thereby linking viscoelasticity to plasticity.
Fibrin
Fibrin is the major constituent of blood clots. Fibrinogen is the precursor of fibrin, which is converted to fibrin fibres in the presence of thrombin and calcium during a blood clot. Fibrin forms a 3D branched fibrous network with the network exhibiting weak physical crosslinks and covalent crosslinks, facilitated by factor XIII22,23. Cells bind to fibrin through integrin receptors, α3β2 specifically. Fibrin gels typically exhibit elastic moduli on the range of 100s of Pa to the low kPas25,26. At the macroscale, col-1 and fibrin networks show similar mechanisms for stiffness, viscoelasticity, strain stiffening and plasticity owin to structural similarities3,22. Fibrin gels are relevant for studying mechanotransduction in wound healing and also for various clinical applications.
Basement membrane
The basement membrane (BM) is a thin layer of ECM that separates epithelial and endothelial cells from the surrounding connective tissue, and also surrounds muscle and fat cells27,28. It is nanoporous and the major constituents are typically laminins, found in a layer facing cells, and type IV collagen (or col-IV), found in a layer facing the stroma, with the two layers linked by entactin. The BM has a thickness on the order of hundreds of nanometers to several microns. Curvature of the BM surrounding the epithelial cells may give rise to interesting 3D cell–ECM interactions (epithelial structures are typically 3D). Cells bind to laminins through various β1 containing integrins and α6β4 integrin. Stiffness measurements of different BMs vary from 100s of Pa to 10s of kPa, and BM exhibits nonlinear elasticity29. In tissues, cells may sense some combination of BM and col-1-rich stromal matrix mechanical properties, given the thickness of the BM30. Nonetheless, increased expression of laminin-crosslinking proteins such as Netrin-4 are associated with metastasis formation, thus indicating the role of stiffness of the BM in metastasis31. Reconstituted BM (rBM) matrices [G] comprise a homogenous nanoporous network formed from a mix of matrix proteins including laminin-111 and col-IV that is often used in 3D culture models of epithelia32.
Hyaluronic acid
Hyaluronic acid (HA) is a linear polysaccharide that is present in almost every tissue. HA forms connected or interpenetrating networks with other ECM components such as col-1, as in skin or breast, or type-II collagen and aggrecan [G], as in cartilage. Functionally, HA is a hydration molecule because of the large negative charge on its polymer chain that draws in water, and provides strong compression resistance. Cells bind to HA through CD44 [G] and RHAMM [G] receptors. While HA does not engage integrin receptors, it is implicated in mechanotransduction33 and inducing specialized microtubule rich protrusive structures known as microtentacles [G] 34. Ageing and disease conditions, such as osteoarthritis or cancer, have been shown to correlate with changes in molecular weight of HA, with higher molecular weight HA corresponding to pathology35. However, the links of molecular weight of HA to tissue mechanics and mechanotransduction are unclear. While naturally extracted HA does not form a gel, HA can be chemically modified with various crosslinking groups to form elastic or viscoelastic gels36.
Fibronectin
Fibronectin is a glycoprotein that is found in connective tissues, and often implicated in mechanotransduction37. Each fibronectin protein has two Arginine-Glycine-Aspartate (RGD) sequences [G] which bind to several β1 and β3 containing integrins17. The RGD cell adhesion peptide motif is used in many synthetic-ECM-based cell culture studies38–43. In addition to the RGD domain which binds to cells, fibronectin also has col-1 binding domains which leads to formation of fibronectin–col-1 composite networks44. Unlike col-1 and fibrin, fibronectin does not form a fibrous network by itself or in the presence of any external chemical factors. Instead, it relies on cells to mediate fibronectin assembly of individual fibronectin molecules into insoluble elastic fibres. In this case, cellular forces open up the cryptic domains in fibronectin molecules that mediate crosslinking, leading to network formation45. Fibronectin coatings are used for 2D culture studies while fibronectin-rich matrices can be formed by fibroblasts9. The RGD cell adhesion peptide motif is used in many synthetic-ECM-based cell culture studies.
Other ECM proteins implicated in mechanotransduction
Beyond the components described above, ECM consists of various other proteins, often referred to as the matrisome, with some of these components implicated in mechanotransduction. For example, tenascin-C [G] is implicated in mechanotransduction in the context of glioblastoma brain cancer46. Proteomics of breast cancer tissues revealed the novel role of other types of collagens such as col-VI and col-XII in breast cancer progression 47,48. Specifically, col-VI has been shown to enhance cancer cell invasion, while col-XII is indicated in reorganization in col-1 which may be associated with col-1 remodelling during metastases.
ECM in tissues as composite networks
While the mechanics of individual ECM components are important, tissues typically consist of multiple interacting ECM proteins, networks and cells with properties emerging from the interactions between the different components. Indeed, reconstituted col-1, fibrin, and rBM have an elastic modulus ranging from ~10s to 100s of Pa, which are much softer than many soft tissues (~kPa). Recent studies have demonstrated that composites of HA and col-1 gels exhibited enhanced stiffness and delayed strain stiffening compared to col-1-only gels49,50. Further, the presence of elastin fibres, which are linearly elastic and highly resistant to degradation51, is thought to drive elastic recovery of many tissues following bulk deformation52. Additionally, chemical interactions of proteoglycans [G] such as small leucine-rich proteoglycans and versican [G] affect col-1 organization53. However, clear mechanistic insights into the design rules by which various matrisomal proteins impact col-1 organization and mechanics remains largely unclear.
In addition to the contribution of ECM components to ECM mechanics, cell interactions with ECMs can also lead to emergent mechanical properties. For example, fibroblast contraction of nonlinear elastic col-1 gels leads to a stiffer matrix54. Alternatively, a composite of closely packed cells and col-1 exhibits compression stiffening similar to liver tissues, in contrast to compression softening of col-1 gels55. Taken together, these studies indicate the importance of understanding how ECM components interact together and with cells, as would occur in tissues, to govern the mechanical properties sensed by cells in mechanotransduction.
Tissue mimicking artificial ECMs for 3D culture
Reconstituted ECMs based on the natural ECM materials described above have several limitations in terms of their use for 3D culture studies of mechanotransduction. Many of these are much softer than soft tissues, with elastic moduli much less than 1 kPa (Fig. 1a; Supplementary Table 1). Further, stiffness, viscoelasticity, ligand density, and matrix pore size and architecture cannot be modulated independently, making it challenging to obtain definitive mechanistic insights into how these different properties influence cell biology. To address these shortcomings, engineered hydrogels with biologically relevant mechanics and signalling, and, importantly, independently tunable features, have emerged as powerful experimental platforms for 3D culture studies of mechanotransduction and led to key insights into mechanotransduction as described in the subsequent sections. Box 1 describes some of these platforms in more detail.
Box 1: In vitro materials for 3D culture: choosing the right system
Here, we provide a brief guide for choosing a 3D culture system for mechanotransduction studies, focusing on commercially available materials (see figure). Natural matrices such as collagen, reconstituted basement membrane (rBM) matrix (i.e., Matrigel or Geltrex), and fibrin are easy-to-use and broadly mimic in vivo stromal matrix, BM, and blood clot environments respectively, and are often used for 3D culture. These matrices tend to be soft, with an elastic modulus ranging from tens to hundreds of Pa, are inherently viscoelastic and degradable (Supplementary Table 1), and provide biologically relevant signalling52. Fibrin and collagen are micro-porous and have a fibrillar architecture while rBM matrix is nano-porous with a non-fibrillar architecture. Key limitations are that these materials have limited independent tunability of mechanical properties such as stiffness, viscoelasticity, fibre length and porosity, limiting their usefulness for studies of mechanotransduction. Synthetic and chemically modified natural hydrogel systems based on polyethylene glycol (PEG), alginate, agarose and hyaluronic acid provide much more tunability. Cell adhesion ligand (i.e., RGD peptide) density, stiffness, viscoelasticity and degradability can often be modulated independent of other parameters. These materials are usually nano-porous and nonfibrillar. However, these materials do not present full physiological signalling ligands and tuning some properties requires more complex chemistries than are commercially available270–273. Due to their high tunability, however, these materials are especially useful for translational applications or for mechanotransduction studies. Finally, in applications where tunability is desired but the engineered material is not sufficient to elicit the desired cell behaviour, interpenetrating networks of the engineered biomaterial with the relevant ECM matrix of interest can be considered. For example, interpenetrating networks of collagen and agarose247, rBM-matrix and alginate77,122, and collagen and alginate235 (in various proportions) offer tunability of stiffness and in some cases viscoelasticity while promoting biologically relevant behaviours. The reader is directed to various recent references for more information on choosing hydrogel platforms suitable for their needs108,274,275.
Box 2. Mechanotransduction: Lessons from 2D
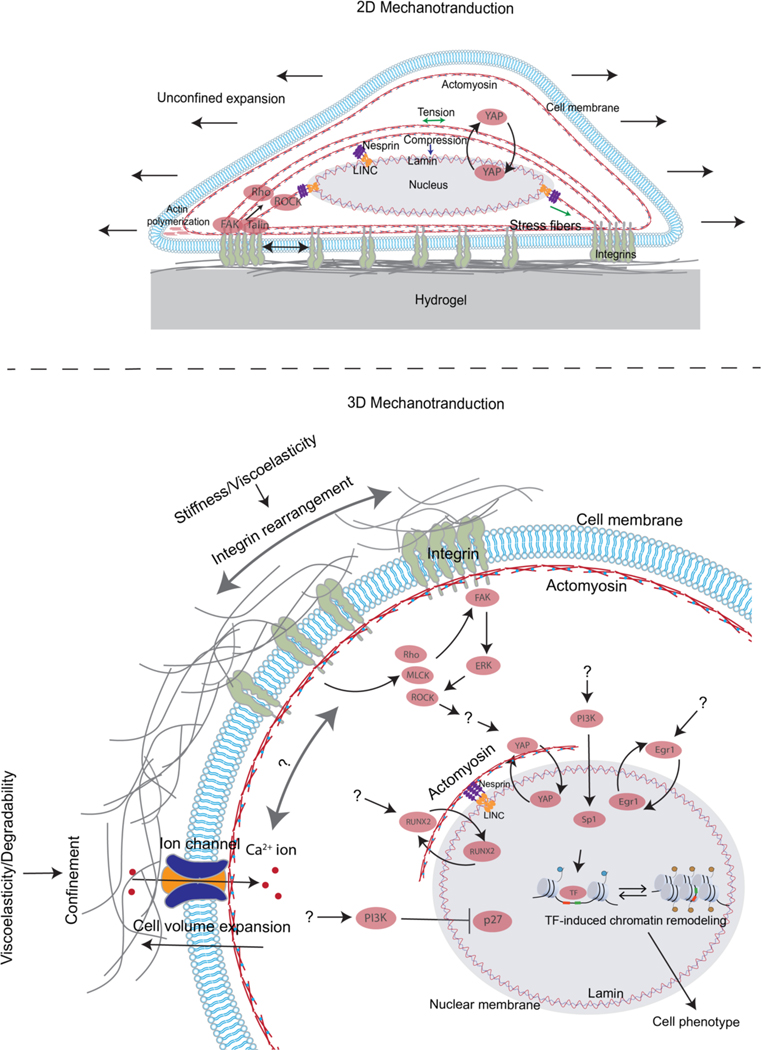
Many studies of mechanotransduction in 2D have converged upon a central mechanism by which cells sense stiffness (Fig. 3a). The predominant 2D culture mechanotransduction platform has been cells cultured on collagen1- or fibronectin-coated elastic polyacrylamide substrates with independently tunable stiffness69,90. Cells first bind to the substrate through β1 containing integrins, initiating formation of an adhesion complex, and activating actomyosin contractility, focal adhesion kinase (FAK), and the Rho pathway119. As a cell first spreads on the substrate through actin polymerization, or during processes involving actin polymerization such as filopodial extension or lamellipodial protrusion, a molecular clutch mechanism responds to stiffness276. In this mechanism, changes in stiffness mediate engagement of the molecular clutch, involving talin277, that connects polymerizing actin filaments to integrin-based adhesions, governing whether there is productive extension of the cell membrane versus retrograde flow of actin. Over time, nascent adhesions can mature into focal adhesions278, containing talin, vinculin, and FAK, which connect to clustered integrin binding ligands38 extracellularly and highly contractile actin stress fibres intracellularly279. Stress fibres connect to the lamin-containing nucleus through the linker of nucleoskeleton and cytoskeleton (LINC) complex, with higher contractile forces typically observed on stiffer substrates119. Tension across mechanosensitive proteins such as talin280, vinculin281 and lamin2 impacts their activation and binding interactions, thereby converting changes in stiffness to biological signalling. Further, tension from stress fibres causes deformation and stretching of the nucleus, which opens up pores in the nucleus, allowing translocation of the YAP transcriptional regulator into the nucleus, and subsequent activation of transcriptional pathways, regulating processes such as proliferation and differentiation282–284.
Various other mechanisms and relations have been described in 2D. There is a strong correlation between cell spreading area and ECM stiffness, and limiting cell spreading area on substrates with high stiffness can lead to mechanotransduction behaviours observed on soft substrates234,242,282. Cell adhesion ligand density, spacing of the ligands and architecture of ECM ligands can play a key role in mediating mechanotransduction63,240,285. Viscoelastic and viscoplastic substrates are amenable to ligand clustering and longer timescale of molecular clutch binding, leading to spreading, migration, and differentiation behaviors similar to that observed on stiffer elastic substrates96,238,248. By contrast, viscoelastic substrates that are not viscoplastic can lead to the opposite trend95,286. In one study, increased stiffness reduced cell volume, and modulating cell volume directly controlled mechanotransduction outcomes154. In addition to YAP, MRTF-A [G], which responds to actin polymerization, has been implicated in mediating stiffness sensing at the transcriptional level287,288. Further, stress fibres also affect chromatin accessibility through their contractility via redistribution of histone deacetylase 3 to the nucleus 289. Finally, recent studies have found distinct mechanotransduction behaviours in epithelial monolayers, finding for example that monolayers can sense shallow stiffness gradients more robustly than single cells during durotaxis71, suggesting the role of collective cell behaviour in mediating 2D cell–ECM mechanotransduction. Many excellent reviews cover 2D mechanotransduction in more detail (e.g. refs. 4,5,8).
Impact of ECM mechanics on cells
In this section, we survey some key trends between variation in specific ECM properties and their impact on various biological processes, focusing on adherent cells (Table 1 ).
Table 1: Impact of ECM mechanics on cells.
ECM properties such as stiffness, viscoelasticity, degradability, non-linear elasticity, pore size, ligand type, density and geometry regulate various cell behaviors. n/a: not applicable (ECM pore size is an important consideration in 3D but is not meaningful in 2D). “+” indicates positive correlation, “-” indicates negative correlation, “#” indicates a complex relationship and “?” indicates an unknown relationship, that is yet to be thoroughly investigated. Selected references for known impacts are included.
| Cellular process╲ECM property | Stiffness | Viscoelasticity | Degradability | Non-linear elasticity | Pore size | Ligand type & density | Geometry |
|---|---|---|---|---|---|---|---|
| Spreading | 2D63,69,234: + | 2D95,96,237,238: + | 2D: ? | 2D: ? | 2D: n/a | 2D63,97,241: # | 2D64,89,242: # |
| 3D39,60,235,236: # | 3D40,61,62,135: + | 3D42,60,103: + | 3D57: + | 3D239,240: + | 3D40,241: # | 3D65,243: # | |
|
| |||||||
| Migration | 2D68–71,191,244–246: # | 2D248: + | 2D: ? | 2D: ? | 2D: n/a | 2D241,250: # | 2D251: # |
| 3D245,247: # | 3D77,78: + | 3D42,73,249: + | 3D158,159: + | 3D73,74: + | 3D241: # | 3D252: # | |
|
| |||||||
| Differentiation | 2D56,88,90–92: # | 2D95,96: # | 2D: ? | 2D: ? | 2D: n/a | 2D90: # | 2D99,242: # |
| 3D39,40: # | 3D39,40,61: # | 3D60,103,253: # | 3D100: # | 3D239: # | 3D111: # | 3D65,254: # | |
|
| |||||||
| Division | 2D105,255: + | 2D238: + | 2D: ? | 2D: ? | 2D: n/a | 2D: ? | 2D64,257: # |
| 3D105: + | 3D106: + | 3D103,256: + | 3D: ? | 3D: ? | 3D40,41: # | 3D258: # | |
|
| |||||||
| Apoptosis | 2D38,259: - | 2D: ? | 2D: ? | 2D: ? | 2D: n/a | 2D: ? | 2D64: # |
| 3D107: - | 3D41: - | 3D: ? | 3D: ? | 3D74: - | 3D260: # | 3D: ? | |
|
| |||||||
| Tumour phenotype | 2D261,262: + | 2D: ? | 2D: ? | 2D:265: + | 2D: n/a | 2D: ? | 2D123,266: # |
| 3D118–122: + | 3D: ? | 3D263,264: + | 3D: ? | 3D73,74: # | 3D122: # | 3D124,214,266: # | |
|
| |||||||
| Morphogenesis | 2D: ? | 2D: ? | 2D: ? | 2D: ? | 2D: n/a | 2D: ? | 2D: ? |
| 3D111,115,116: # | 3D41,114,115: + | 3D110–112,267: + | 3D: ? | 3D268: # | 3D41,110,111: # | 3D65: # | |
|
| |||||||
| Matrix secretion | 2D: ? | 2D: ? | 2D: ? | 2D: ? | 2D: n/a | 2D: ? | 2D: ? |
| 3D181: + | 3D40,84,85: + | 3D82,83,86: + | 3D: ? | 3D83: # | 3D: ? | 3D: ? | |
Cell spreading
Mesenchymal and epithelial cells spread in 2D and 3D by taking different morphologies such as spherical, spindle-like, or irregular shapes with different protrusions such as lamellopodia [G], filipodia [G], and invadopodia [G]. In 2D culture, increased stiffness generally promotes cell spreading56. In 3D, cell spreading proceeds in microporous ECM that are sufficiently stiff 57, but is restricted in nanoporous ECM. Covalently crosslinked elastic hydrogels typically restrict cell spreading in 3D42,58–60. Contrastingly, viscoelastic hydrogels with sufficient stress relaxation or plasticity40,61,62, or hydrogels that undergo degradation due to hydrolytic or protease activity42, allow cell spreading. Cell-adhesion ligands, such as RGD cell adhesion peptide motifs, are required for cell spreading in both 2D and 3D, though very high ligand density diminishes cell spreading63. Finally, ECM geometry, or structural features at the scale of tens to hundreds of microns such as curvature and patterning of cell–ECM adhesion ligands, also impact cell spreading in both 2D64 and 3D contexts65,66.
Cell migration
Cell migration occurs during development, wound healing, immune trafficking, and cancer metastasis58,67. On 2D substrates of increasing stiffnesses, migration often follows a biphasic pattern with higher levels of migration at intermediate stiffness values68–70. Further, different cells sense and migrate along positive and negative gradients of substrate stiffness — a process termed durotaxis69,71,72. In 3D ECMs, various properties have been implicated in regulating cell migration. If pore size of the ECM is above roughly 3 μm in diameter, cells can migrate robustly by squeezing through the pores59,73,74, and migration can be guided by matrix architecture and alignment of fibres75. In ECMs with smaller pore sizes, cells can generate channels to migrate either using proteases to degrade the ECM42,73,76, or forces to mechanically open channels to migrate through, if the ECM exhibits sufficient matrix mechanical plasticity, a property often linked to viscoelasticity77–79. Notably, interstitial fluid flow, which is dependent on matrix porosity, has also been shown enhance cell migration in 3D80.
Matrix secretion
Cells not only degrade the ECM but also secrete nascent ECM81–84 in 3D culture. Hydrogel viscoelasticity regulates ECM production by chondrocytes84 and MSCs85, with cells forming a more interconnected cartilage-like or bone-like ECM in fast relaxing hydrogels. Similarly, ECM degradability allows chondrocytes to form a cartilage-like matrix86. Overall, cells respond to ECM properties by secreting endogenous ECMs, resulting in a feedback mechanism that fine tunes cell–ECM interactions.
Stem cell differentiation
Stem cell differentiation occurs during development and homeostasis, and controlling stem cell differentiation is a critical goal in many applications in regenerative medicine87. Early 2D cultures studies showed that substrate stiffness regulates stem cell differentiation and illustrated the concept that matching native tissue stiffness promotes differentiation down that tissue-specific pathway in vitro in some stem cell types56,88. For example, culture of mesenchymal stem or stromal cells [G] (MSCs)56 on stiff substrates, with a modulus approaching that of pre-mineralized bone, promotes osteogenic differentiation whereas culture on soft substrates, with a modulus approaching adipose tissue, promotes adipogenic differentiation2,89,90. Similar findings hold for differentiation of neural stem cells91. In other contexts, such as skeletal muscle stem cells, substrates with physiological stiffness can promote self-renewal92. Differentiation is also mediated by cell adhesion ligand density and type and viscoelasticity in 2D90,93–97. Finally, 2D ECM geometry has been shown to influence MSC differentiation98 as well as embryonic stem cell differentiation into mesoderm 99 via regulation of cell shape and cytoskeletal tension.
In 3D, some similar trends emerge, with viscoelasticity and degradation playing a more prominent role. In viscoelastic ECMs, soft substrates promote adipogenic differentiation and stiff substrates promote osteogenic differentiation of MSCs, similar to the findings from 2D39,40. Osteogenesis is enhanced with faster stress relaxation40, and stress stiffening of soft substrates can support osteogenic differentiation100. In covalently crosslinked hydrogels, degradability is required for osteogenesis60. Mechanical cues have been shown to impact fate in other stem cells populations in 3D, as well as monocyte differentiation101–104. Additionally, ECM architectural features such as fibre diameter and alignment have been shown to influence MSC differentiation66.
Cell division
Every cell arises from a cell division event, and cell division underlies development and tumour growth. In 2D, increased stiffness promotes cell proliferation38,105. In 3D, covalently crosslinked elastic matrices that are nanoporous restrict cell division, while faster stress relaxation in viscoelastic matrices, or increased matrix degradability, allows cell division and proliferation in nanoporous matrices40,103,106.
Apoptosis
Apoptosis, or programmed cell death, is necessary for proper tissue homeostasis and aberrant regulation of apoptosis is a hallmark of cancer. In 2D, soft substrates or confining geometries that limited spread area led to higher levels of apoptosis38,64. In 3D, apoptosis is triggered during cell migration through confining pores74 or through cell volume restriction in microwells in a stiffness dependent manner107, and is also modulated by matrix viscoelasticity41.
Morphogenesis
Morphogenesis is a complex multi-cellular process wherein cells self-organize to form 3D structures with specialized form and function108. While collective cell interactions are often emphasized in morphogenesis, there is increasing research emphasizing the role of 3D microenvironment, including matrix degradability and viscoelasticity as central factors regulating morphogenesis in organoid models. Organoids are multicellular structures that capture specific features of organs. rBM matrices, which are widely used for organoid culture are inherently viscoelastic and enzymatically degradable109. Degradable gels promote apicobasal polarization and lumen formation in MDCK cysts110, support viability and formation of budded intestinal organoids containing differentiated cell types via symmetry breaking mechanisms,111,112 but restrict progenitor cell phenotype in neural tubes cultures in vitro and in intestinal organoids111–113. Viscoelasticity modulates lumen formation in organoid cultures of human pluripotent stem cells (hPSCs)41, in endothelial vasculogenesis114 and during crypt budding in intestinal organoids115. Further, adhesion ligand density and type affect formation of intestinal organoids111 and lumens in MDCK cysts110 and hPSCs41. ECM geometry has recently been shown to guide intestinal organoid formation and improve reproducibility of organoids formed65. Finally, stiffness controls cell survival111 and tissue budding115 in intestinal organoids, and mimicking stiffness of native tissues enhances liver organoid formation116.
Cancer progression
ECM mechanics regulate emergence of a tumour phenotype117. In 2D and 3D, increasing substrate stiffness over the range detected during breast cancer progression promotes induction of a malignant phenotype in models of normal mammary epithelium118–122. This same impact has been shown in other cancers as well, as we will describe later. ECM geometry also influences cancer progression and collective invasion by controlling cell shape and subsequently, cell phenotype in both 2D123 and 3D microenvironments84,124.
Cell–matrix interactions in 3D
Here we describe key features of cell–ECM interactions and force generation in 3D (Fig. 2), which ultimately underlie 3D mechanotransduction.
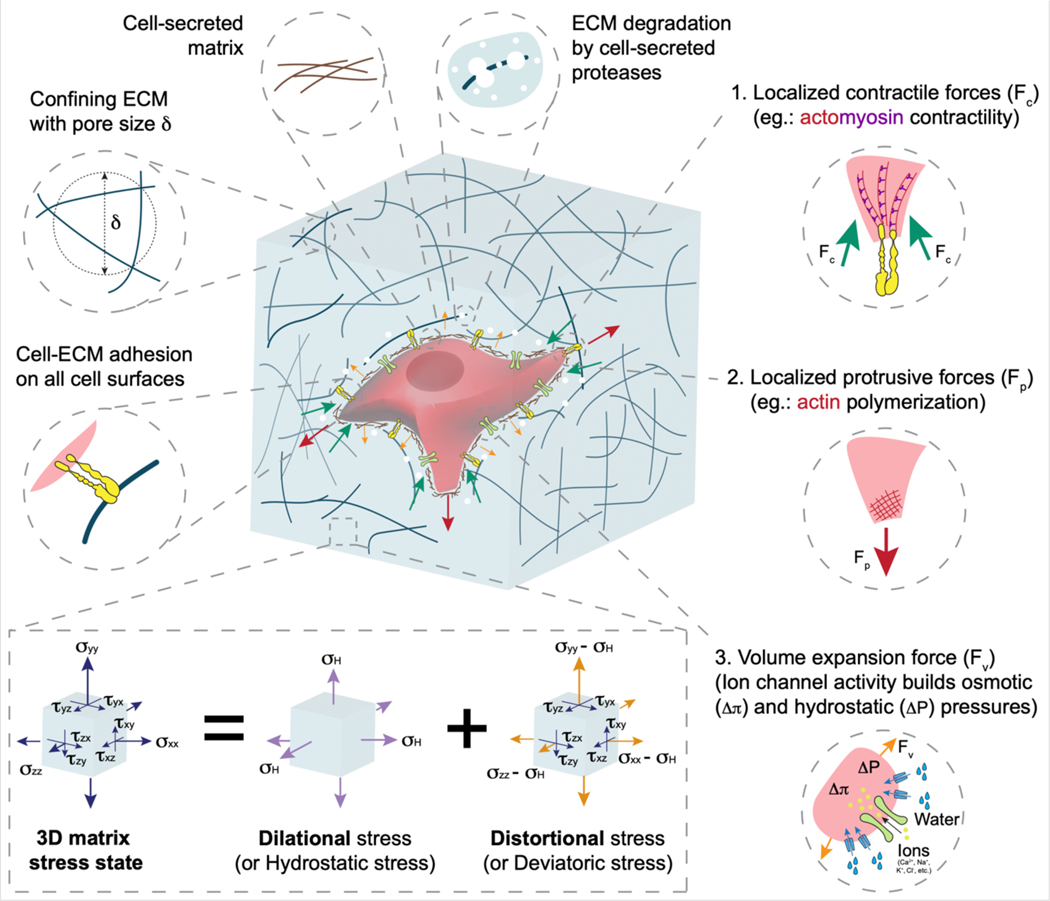
Figure 2. Cell–matrix interactions in 3D.
Cells in 3D are confined in all directions due to restrictions imposed by the matrix and can form cell–matrix adhesions on all surfaces contacting ECM. Cells exert contractile, protrusive, and volumetric forces on the matrix, generating dilational and distortional stresses in the ECM, which in turn regulate various cell behaviours (Table 1). Hydrostatic stress is volumetric or dilational stress that acts to increase or decrease the volume of an object on which it acts, without changing its shape. Deviatoric stress is distortional stress that acts to change the shape of an object on which it acts, without changing its volume. Hydrostatic and deviatoric stresses combine to produce net 3D stress fields which cells perceive. These stress fields directly deform cells and influence their behaviour including proliferation, migration and differentiation. In addition to this, such stresses change over time depending on ECM properties such as viscoelasticity, degradability and plasticity, and form a positive feedback loop with the cell-generated forces.
Cell–ECM adhesions
Cell adhesion signalling in 3D varies from that observed in 2D in several ways. Cells on 2D substrates form adhesions with ECM only on one surface, whereas cells can form adhesions in all directions in 3D, which impacts cell signalling. For example, mammary epithelial cells grow rapidly on 2D tissue culture plastic substrates, but form growth-arrested, organotypic acinar structures in 3D culture in rBM matrix12. Establishment of 3D matrix protein interactions, and not matrix per se, is sufficient for acinar morphogenesis as even on 2D rBM substrates cultured in media with the addition of soluble rBM proteins, thereby allowing cells to bind to these proteins in all directions, acini formation is observed125. How signalling activation across the entire cell surface leads to a different outcome than signalling across one bottom surface remains unclear.
Further, the structure of adhesions differ in 3D relative to 2D. Cells in most currently used 3D hydrogels (with some exceptions) typically do not form large focal adhesions [G] that are connected to robust actin stress fibres126–128. In nanoporous hydrogels, which unlike fibrillar hydrogels are more homogeneous and have nanometer-sized pores, distinct adhesive complexes are often not observed, whereas in fibrillar matrices, fibrillar adhesions [G] often form that co-localize with matrix fibres, and exhibit distinct phosphorylation of adhesion complex proteins such as paxillin and focal adhesion kinase [G] (FAK) relative to canonical focal adhesions9,126,129. By contrast, while adhesion complex formation also varies in 2D culture, substrates with sufficient stiffness and ligand density typically lead to formation of focal adhesions. Further, in 3D, cells typically lack the thick contractile actomyosin stress fibres spanning the cell length that are characteristic of 2D cell culture on stiff ligand-dense substrates126. Cells in 3D typically have an actin shell cortex at the cell membrane, however, the molecular architecture of the actin meshwork and actomyosin machinery in the cell cortex remains unclear owing to limitations in the spatial resolution of microscopy for 3D culture.
Confinement
In 2D, cells can spread out on the substrate or change their volume unrestricted, whereas in 3D, changes in volume and shape are physically resisted, or confined, by the surrounding ECM (in addition, the ECM can restrict nutrient transport)10. Level of confinement is determined by ECM pore size130 and properties such as viscoelasticity40 and degradability62. A pore size of around 3 μm in elastic gels or structures with rigid pores serves as a barrier to cell migration because the relatively stiff nucleus cannot be deformed through smaller pores73,74. However, physiological matrices are not elastic with rigid pores, but are typically viscoelastic52, exhibit mechanical plasticity52,131 and are degradable132, allowing expansion of pores by cellular forces and protease mediated degradation. For example, degradation of ECM can convert even an elastic matrix to a viscoelastic fluid-like matrix133, opening up pores for cell migration42,73,134 and promoting MSC spreading, force generation and differentiation60. Viscoelastic and viscoplastic matrices dissipate mechanical stresses, undergo matrix creep under loading, and exhibit irreversible deformations in response to force, allowing cells to generate space in the ECM, reducing confinement. As such, viscoelasticity and viscoplasticity enable cell spreading135, cell volume expansion61, cell growth for division136, proliferation of multicellular structures106, deposition of matrix extracellularly84, and cell migration independent of proteases77. Thus, in 3D, cell confinement is governed by ECM pore size, viscoelasticity and plasticity, and degradability. Natural ECMs exhibit all these properties including viscoelasticity, plasticity and degradability, allowing cells in vivo to remodel the matrix and potentially alter confinement.
Cell–ECM forces
In 2D and 3D, contractile or pulling forces generated by actomyosin machinery represent a major modality of force generation, but several additional modalities of force generation become accessible to cells in 3D. 3D traction force microscopy [G] techniques have enabled quantification of cell generated forces by measuring matrix deformations and estimating stresses using theoretical models of matrix properties137–139. These studies, and other studies using these techniques, show that cells exert contractile forces on ECMs through integrin-based adhesions in 3D. Indeed, it has long been known that fibroblasts cultured in a collagen gel will continuously contract the gel140. Owing to the differing structure of both the adhesions and the contractile actomyosin machinery, the capabilities of contractile force generation in 3D likely differ from 2D, though the precise differences remain unclear.
In 3D, cells also generate protrusive forces. Polymerization of branched actin networks generates protrusive forces141. In 3D, using adhesions or steric support of the matrix, cells apply these protrusive forces onto the ECM, in the form of structures such as invadopodia [G], filopodia [G], or lamellipodia [G]58,77,142,143. Alternatively, extension of the microtubule-based spindle generates outward protrusive forces during mitosis136,144. Any process resulting in shape change in confining 3D ECMs must necessarily generate some combination of protrusive and contractile forces.
Similarly, any process involving increased cell volume in confining 3D matrices necessarily requires force generation. Cell volume is a tightly controlled parameter that is crucial for cell survival and function145,146. Cell volume is governed by the combination of osmotic pressure and hydrostatic pressure differentials between the intracellular and extracellular space. To increase volume, cells generate osmotic pressure by increasing the concentration of ions inside the cells via activity of ion pumps and channels145,147. This draws water into cells both by diffusion across the cell membrane and transport via water channels such as aquaporins, thereby causing volume expansion145. Cell volume expansion can occur both globally and locally. For example, in 3D confining matrices, nuclear entry into thin protrusions acts as a piston148 to pressurize protrusions by opening ion channels which subsequently generates osmotic and hydrostatic pressure to expand the protrusion and expand the ECM pore to create a track allowing cell migration78. Water flux however does not necessarily indicate volume changes as directed water flow across the cell can drive cell migration149. Overall, cells generate localized contractile and protrusive forces on ECM, as well as global outward forces during cell volume expansion, in 3D.
ECM stresses in 3D
Cellular forces on ECMs in 3D result in complex mechanical stress fields, which in turn act upon other cells as well as trigger feedback mechanisms in cells applying these forces. These force fields in the matrix can be interpreted in the form of a combination of hydrostatic and deviatoric components. Hydrostatic stresses [G] are dilational in nature or act to change ECM volume, or from the cellular perspective, oppose or promote cell volume changes. For example, as tumour cells proliferate in spheroids in 3D, hydrostatic stresses are generated which mechanically oppose tumour growth150,151. Deviatoric stresses [G], on the other hand, are distortional in nature and act to change morphology while preserving volume. Such distortional stresses are necessary for matrix remodelling and fibre alignment, which in turn promote cell spreading and migration19,24. The extent of cell deformation and morphology change due to these stresses are dependent on cell mechanical properties. Cells actively regulate their cytoskeletal and nucleoskeletal proteins as well as fixed charges (the net electric charge of all intercellular components that cannot freely diffuse out of the cell) and concentration of macromolecules, determining the viscoelasticity, poroelasticity, [G] and non-linearly elasticity of the cell152,153. Such emergent mechanical properties of a cell result in a net bulk modulus which determines to what extent the cell is able to resist a change in volume and a shear modulus which determines the cell’s resistance to distortion. The cell bulk modulus is on the order of MPa to GPa, whereas the shear moduli are on the order of kPa154. Thus, volume changes in cells must be regulated actively, since the bulk modulus is so much higher than typical physiological stresses in soft tissues resulting from cell generated or externally applied forces. These moduli determine a cell’s ability to change volume and navigate confining environments. For example, nuclear stiffness governs cell migration through confining spaces74,155–157. Overall, cell mechanical properties in conjunction with matrix stresses dictate cell morphology and behaviours such as migration and differentiation in 3D.
Cell–ECM mechanical feedback
As cells interact with physiological ECMs in 3D, the interactions are dynamic as matrix properties change over time due to matrix viscoelasticity (relaxation, creep), plasticity, degradation and matrix deposition. Natural ECMs dissipate forces and flow over time due to viscoelasticity and degradability, and undergo permanent deformation due to plasticity. Further, these interactions depend on force or deformation scales (or magnitudes) due to nonlinear elasticity of the ECM, whereby matrix resistance increases with increased magnitude of force or deformation, thereby supporting the generation of higher cellular forces and forming a positive feedback loop. Thus, timescales and magnitudes of cellular forces exerted on their 3D microenvironment are critical. For example, actomyosin contractile forces applied on ECM fibres generate distortional stress and locally align fibres158. In non-linear, elastic ECMs such as collagen, this fibre alignment increases local stiffness of the matrix which in turn promotes higher force generation and increases cell stiffness revealing a positive mechanical feedback loop between cells and matrix159. Interestingly, fibroblasts exploit non-linear elasticity of the ECM and mechanical feedback loops to migrate by generating fibre alignment and higher forces at the front of a migrating cell as compared to the rear160. By contrast, in linear elastic ECMs such feedback loops are absent which prevent higher force generation and lamellipodia formation57,161. Similar feedback loops are observed between cellular pushing forces and matrix viscoelasticity or plasticity62,77,162,163. In confining nanoporous ECMs, cancer cells apply protrusive forces to deform the ECM and in sufficiently plastic ECMs, such forces lead to permanent matrix deformations, which in turn promote growth of protrusions and result in larger pores for cell migration. In addition, deposition of matrix by cells, for example MSCs secreting matrix proteins such as fibronectin, laminin and collagen, leads to formation of an endogenous ECM that the cell interacts with, and can mediate mechanotransduction82,164. Thus, dynamic cell–ECM interactions and positive cell–ECM feedback loops orchestrate cell behaviours in 3D.
Mechanisms of 3D mechanotransduction
In this section, we discuss mechanisms of 3D mechanotransduction mediated through two major classes of membrane proteins, integrins and mechanosensitive ion channels [G], and how these two pathways converge on the nucleus (Fig. 3). Many of these 3D studies were performed in hydrogels with independently tunable stiffness, stress relaxation and degradability (see Box 1).
Figure 3. 2D and 3D Mechanotransduction.
A) Cells sense substrate stiffness by exerting contractile forces on 2D substrates with stress fibres through focal adhesions, which activates various proteins such as FAK, talin, Rho and ROCK at the adhesion site. Activation of these proteins leads to adhesion maturation and stress fibre formation and contractility, which in turn transmits forces to the nucleus via the linker of nucleoskeleton and cytoskeleton (LINC) complex, resulting in changes in nuclear envelope tension and nuclear pore opening. This allows the nuclear entry of proteins such as YAP transcriptional regulator leading to downstream impact on cell phenotype. Moreover, in 2D, a cell can spread laterally without encountering any mechanical confinement. B) Cells embedded in the ECM sense stiffness and viscoelastic properties of the matrix through integrin binding, activation, and clustering, while sensing confinement, viscoelasticity and plasticity through cell volume changes and ion channel activation which leads to Ca2+ ion influx. Additionally, ECM stiffness/viscoelasticity and confinement regulate activation of various proteins, such as FAK, ROCK, MLCK, pathways, such as those involving PI3K, ERK, and Rho, and transcriptional regulators such as YAP, p27, Sp1, RUNX2, and EGR1. However, clear mechanistic links between the ECM properties and activation of these proteins, pathways, and transcription regulators remain unclear. Unknown connections in the pathways are indicated by question marks. Both mechanisms of mechanotransduction converge on the nucleus and regulate the activation of transcription factors (TFs), which are facilitated by chromatin remodelling and control cell behaviour.
Integrin-mediated mechanotransduction
Similar to their role in 2D (Box 2), integrins have been identified as central regulators of mechanotransduction in 3D, often through integrin clustering. For example, increasing stiffness of col-1 rich gels used to culture mammary epithelial cells in 3D from 100s of Pa to kPa (as is observed during breast cancer progression) — through either increased col-1 density or crosslinking, — promotes a malignant phenotype characterized by elevated proliferation and invasion into the surrounding matrix through α5 and β1 integrins and integrin clustering119,120. Integrin binding to stiff ECM in dense col-1 gels leads to activation of FAK, activated Rho signalling [G], and increased cell contractility, causing integrin clustering119,120,165. These events lead to downstream activation of the Ras-mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways, both activated during breast cancer progression, which promote the growth of a tumour and invasion165. Interestingly, an opposite trend was observed when mammary epithelial cells were cultured in interpenetrating networks of rBM and alginate, used to mimic pre-invasive breast cancer where cells are surrounded by BM; in this scenario, increased stiffness inhibited formation of clustered α6β4 integrin-containing adhesions, thereby promoting an invasive phenotype through activating Rac1 and PI3K 122. Together these data indicate that stiffness- and ligand-mediated clustering of multiple integrin types plays a critical role in 3D mechanotransduction in cancer cells and cancer progression.
Integrin-mediated mechanotransduction also plays a central role in how stiffness, degradability, stress relaxation and stress stiffening regulate differentiation of stem cells. MSCs encapsulated in ionically crosslinked RGD-coupled viscoelastic alginate hydrogels with an elastic modulus in the range of 11–30 kPa differentiated into osteoblasts, in an integrin-mediated manner, whereas those cultured in lower stiffness gels underwent adipogenesis. The differentiation state was associated with integrin clustering, with maximum levels of integrin clustering associated with optimal osteogenic differentiation. Integrin clustering was driven by contractility mediated forces. Interestingly, in covalently crosslinked non-degradable HA-hydrogels, hydrogel stiffness did not impact differentiation, with adipogenesis observed from 1 kPa to 100 kPa. Instead, degradability of the HA hydrogels was required for osteogenesis, with MSCs able to exert larger integrin-mediated tractions with increasing degradability60. Further, in another study, increased rate of stress relaxation in RGD-coupled alginate hydrogels with a 20 kPa modulus promoted enhanced osteogenic differentiation of MSCs, and differentiation was associated with integrin clustering40. Importantly, cell morphology was decoupled from osteogenic differentiation in all of these studies, in contrast to the result in 2D where 2D morphologies are distinct for adipogenic, and osteogenic differentiation 56. Finally, stress stiffening in 0.2–0.4 kPa polyisocyanate hydrogels also led to integrin clustering in MSCs and shift of differentiation from adipogenesis to osteogenesis100. These studies are consistent with the concept that the extent to which the matrix can be remodelled, facilitated by either viscoelasticity or degradability, and some minimum level of tension across the integrins, mediated by the stiffness and nonlinear elasticity, are required for osteogenesis. In vasculogenesis of endothelial progenitor cells (EPCs), ECM viscoelasticity along with hypoxic environment promote the aggregation of EPCs through contractility-mediated integrin clustering114,166.
While there are similarities between 2D and 3D integrin-mediated mechanotransduction pathways, key differences have been implicated. This is suggested by the differences in adhesion structures and the actin cytoskeleton, with cells not exhibiting focal adhesions or stress fibres in most 3D conditions, as described in the previous section. Indeed, cell morphology was decoupled from osteogenic differentiation of MSCs in 3D39,40,60, in contrast to the result in 2D56, and some studies have even demonstrated integrin-mediated osteogenic differentiation in 3D is independent of contractility100,167. However, in contrast to the well-known pathways and events following integrin–ECM binding in 2D culture, there is limited available information on downstream molecular events following integrin engagement in 3D. Further, the application of the molecular clutch mechanism, broadly implicated in 2D mechanotransduction (Box 2), towards 3D contexts remains to be tested. These highlight the gaps in our understanding of integrin-mediated mechanotransduction in 3D.
Confinement sensing by cell volume regulation
Recent work has pointed towards the role of active regulation of cell volume and activation of mechanosensitive ion channels in 3D mechanotransduction, particularly in the context of sensing mechanical confinement. For example, chondrocytes increase their overall volume with increased stress relaxation of alginate hydrogels without integrin-binding ligands, and increased cell volume promoted enhanced cartilage matrix deposition and the chondrogenic phenotype84. Similarly, cell volume is shown to regulate various intracellular events such as actin organization, nuclear accumulation of histones, YAP/TAZ [G] localization, nuclear shape, and focal adhesions for MSCs cultured in 3D PDMS microwells168. Cell volume expansion is possible in degradable hydrogels or hydrogels with fast stress relaxation, but necessarily restricted in more confining elastic hydrogels. Hence, cell volume regulation has been recognized as an important mediator of 3D mechanotransduction.
Mechanosensitive ion channels, particularly TRPV4 channels, have emerged as key sensors of changes in cell volume. In MSCs cultured in RGD-coupled alginate hydrogels of different time scales of stress relaxation with same initial stiffness, faster stress relaxation leads to increased cell volumes associated with activation ofTRPV4 ion channels61. Increased calcium influx through TRPV4 activation drives nuclear localization of RUNX2, a transcription factor involved in osteogenic differentiation. Similarly, in the context of cancer cell proliferation in confining alginate hydrogels, cell growth during the G1 phase of the cell cycle activated a TRPV4–PI3K–AKT–p27 signalling axis that drove S-phase progression and proliferation106. However, increased confinement in more elastic gels blocked cell growth and activation of this pathway. Further, TRPV4 was linked to cell volume expansion and mechanical confinement-sensing in chondrocytes, and controlled phosphorylation of GSK3b (glycogen synthase kinase 3b), an enzyme associated with osteoarthritis169. In myofibroblast activation, increased stiffness induces TRPV4 activation and YAP nuclear localization suggesting a regulatory role of TRPV4 in mediating YAP nuclear shuttling and signalling170. Connecting cell volume to activation of mechanosensitive ion channels is membrane tension171. Piezo1, a mechanosensitive ion channel central to force-sensing in many contexts172, has also been implicated in some of these studies106,171, but its role in responses to ECM-mediated confinement remains less clear. Together these studies indicate that confinement regulates cell volume expansion, and as cell volume expands, membrane tension increases, activating mechanosensitive ion channels, allowing the passage of ions across the membrane, which in turn activate various signalling pathways to drive mechanotransduction.
Several key questions remain regarding this mode of mechanotransduction. For example, it is unclear what initiates cell volume expansion in many of the aforementioned studies. Moreover, it has been observed that integrins are also implicated in mechanotransduction in parallel with mechanosensitive ion channel mediated mechanotransduction. For example during MSC differentiation40,60, cells utilize integrin-binding and clustering in addition to TRPV4-mediated volume expansion to regulate their differentiation pathways, suggesting the possibility of interactions or crosstalk between integrin-mediated and ion channel-mediated pathways. In another connection, the findings on the role of mechanosensitive ion channels in cell–ECM mechanotransduction complement findings on the role of these channels in force-mediated mechanotransduction, including application of stretch and shear, indicating the potential for crosstalk between these distinct modes of mechanotransduction173,174.
Nuclear mechanotransduction
As the center of transcription, the nucleus is a key element of mechanotransduction pathways downstream of both integrin and mechanosensitive ion channel mediated routes. The membrane of the nucleus is mechanically linked to the actin cytoskeleton through linker of nucleoskeleton and cytoskeleton (LINC) complexes comprising of nesprins and SUN (also known as Sa1p in yeast and UNC-84 in C. elegans). Forces from actomyosin based contractility, which acts extracellularly on ECM in both 2D and 3D environments, also can be transmitted to the nucleus through LINC complexes leading to mechanical deformations of the nucleus175. However, a clear mechanism associating cellular forces to nuclear deformation in 3D is still unclear, in part due to lack of clarity over the actin cytoskeletal structure for cells in 3D. Below we discuss key nuclear changes observed in cells cultured in 3D and linked to mechanotransduction.
Nuclear morphologies.
2D studies have demonstrated change of nuclear morphology as a clear indicator of mechanotransduction associated with various outcomes, such as epigenetic remodelling, shuttling of transcription factors such as YAP/TAZ and cell fate changes175. However, nuclear morphologies in 3D differ significantly from those observed in 2D cell culture models. For example, projected nuclear areas and circularity are similar for mammary epithelial cells and pluripotent stem cells in vivo and in 3D culture models41,126, but significantly higher in 2D154. Further, both increased stiffness and increased degradability leads to increased nuclear wrinkling in 3D127,176, whereas increased stiffness has opposite effect on nuclear wrinkling in 2D176. Mechanosensitive ion channels in the nucleus, recently implicated in cell migration in confining microenvironments155,156, could potentially play a role in responding to morphological changes in the nucleus in 3D. Together these indicate that nuclear mechanotransduction mechanisms in 3D are likely different from 2D.
Regulation of transcription factors.
RNA-seq studies show that changes in hydrogel stiffness, stress relaxation and ligand type and density each are associated with large changes in gene expression in different cell types126,177. Whereas YAP/TAZ coregulators have been described as universal mechanotransducers in 2D culture studies, their role in 3D mechanotransduction is context dependent. Increased stiffness, faster stress relaxation, and increased degradability promote enhanced YAP/TAZ nuclear localization in MSCs in 3D40,176. However, YAP localization was decoupled from osteogenesis induced by stiffness 40,61. Further, in a 3D culture model of pre-invasive breast cancer, YAP was not required for mechanotransduction, consistent with in vivo observations126. Instead, transcription factors such as STAT3, p300, and Sp1 were implicated126,127. Contrastingly, YAP is found to play a role in 3D mechanotransduction in gastric cancer. Moreover, in 2D, YAP/TAZ localization was observed in the absence of nuclear wrinkling176, while in 3D178, nuclear wrinkling is correlated with YAP/TAZ localization. In neural stem cell differentiation, stiff ECM is associated with nuclear localization of transcription factor EGR1 in 3D but not in 2D, and this occurs in the absence of integrin-based adhesions179. In MSCs, soft ECM increases the clustering of inflammatory receptor, tumor necrosis factor-α (TNF-α), which further activates nuclear factor kB (NF-kB) and expression of chemokines and cytokines that promote monocyte recruitment and differentiation180. These set of studies highlight the role of transcription factors in mediating mechanotransduction in 3D. However, how these different transcription factors are regulated by mechanical properties of the ECM, remains a key open question.
Epigenetic regulation of chromatin accessibility.
Several recent studies have pointed to a key role of chromatin accessibility in mediating mechanotransduction. In the 3D culture model of pre-invasive breast cancer, increased ECM stiffness induced broad changes in chromatin accessibility mediated by histone deacetylases 3 and 8, with chromatin becoming much more open127. Increased chromatin accessibility then allowed binding of the Sp1 transcription factor, which led to downstream changes in gene expression that functionally mediated the malignant phenotype. In a gastric cancer study, increased ECM stiffness led to YAP translocation into the nucleus in coordination with DNA demethylation and increased chromatin accessibility of YAP promoter region, together inducing the tumorigenic phenotype178. Interestingly, this is in contrast to recent 2D studies where chromatin accessibility in fibroblasts decreased with increased stiffness, which led to compaction of chromatin promoting persistent activation of fibroblasts181. In addition to stiffness, matrix degradability affected chromatin organization in neural progenitor cells, where chromatin accessibility was increased with enhanced degradability182. Thus, chromatin accessibility has emerged as a key regulator of 3D mechanotransduction, though how this is regulated and functions in different contexts, and why effects may be different from 2D studies, remain to be determined.
Mechanotransduction in tissues
In this section, we describe some selected examples of how cell–ECM mechanotransduction is thought to play a role in tissue physiology (Fig. 4, 5), highlighting the ubiquity and importance of 3Dmechanotransduction in vivo.
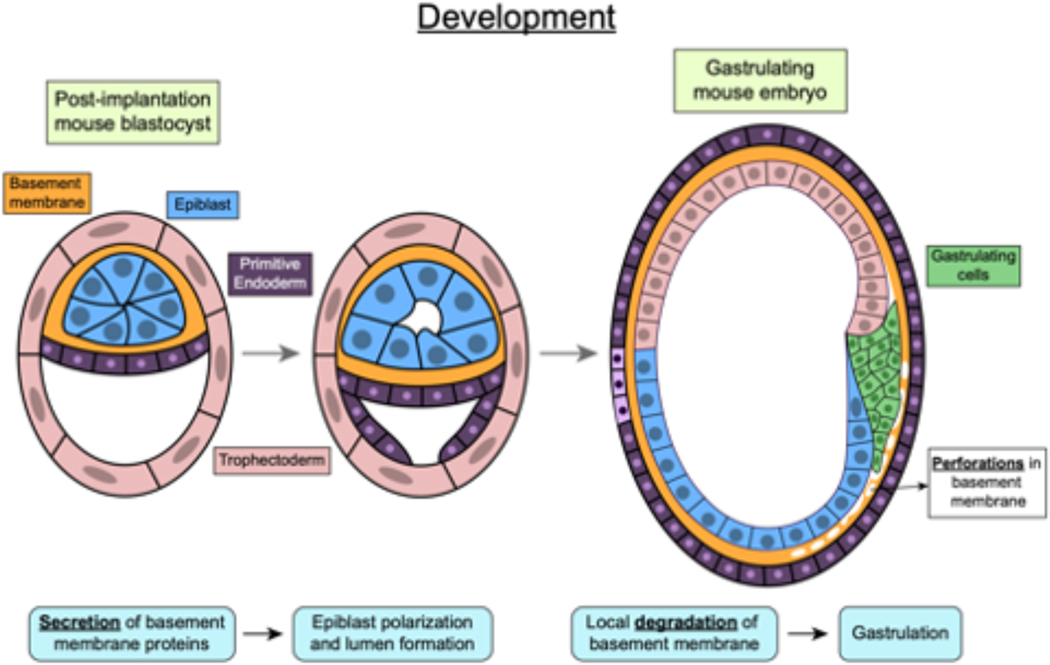
Figure 4. Mechanotransduction in development.
During development, basement membrane secretion and degradation triggers mouse epiblast lumenogenesis and gastrulation respectively. Post-implantation, trophectoderm cells in the mouse embryo, secrete a basement membrane around the epiblast which triggers polarization and lumen formation in the epiblast. Later during gastrulation, gastrulating cells, also called primitive streak cells, secrete proteases to locally degrade the basement membrane layer and enable migration.
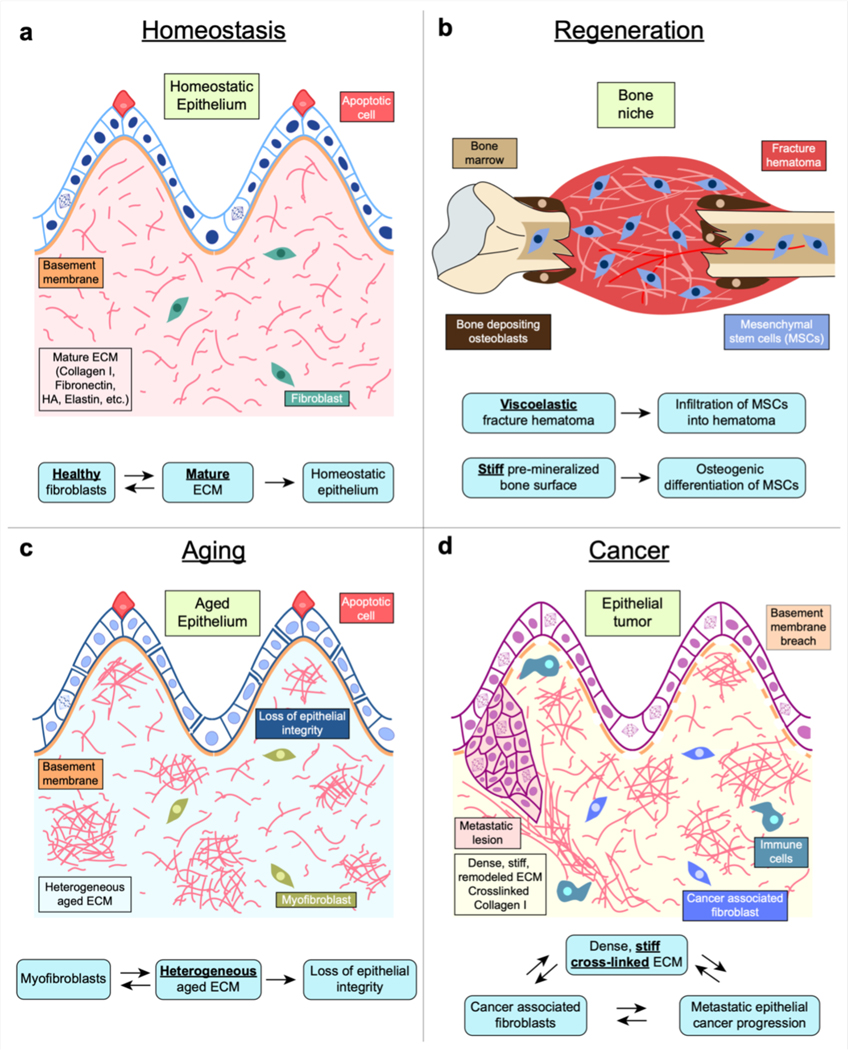
Figure 5. Mechanotransduction in tissues.
(a) In homeostasis, healthy ECM and fibroblasts help maintain normally functioning epithelium by maintaining optimal ECM mechanical properties such as stiffness and viscoelasticity. (b) Following a bone fracture, viscoelasticity of fracture hematoma promotes infiltration pf mesenchymal stem cells (MSCs) and stiff bone surface promotes differentiation of MSCs into bone-producing osteoblasts. (c) Myofibroblast differentiation and heterogeneous ECM occurring during ageing results in loss of epithelial integrity and function. As tissue fibrosis proceeds with ageing, normal fibroblasts differentiate into a myofibroblast phenotype and heterogeneously secrete and deform the ECM. Such a heterogeneous matrix promotes further myofibroblast differentiation and results in altered ECM mechanical properties. Epithelial cells sense these altered ECM properties and undergo transcriptional changes causing loss of epithelial integrity and function. (d) During cancer progression, cancer-associated fibroblasts remodel the ECM into a dense, stiff matrix. This increase in ECM stiffness, in combination with other cues and genetic changes in the cancer cells, leads to activation of a malignant phenotype in epithelial cells. These cells then undergo sustained proliferation, breach the basement membrane during invasion, migrate into the stromal matrix and eventually can metastasize.
Development
Cell–ECM interactions and mechanotransduction are implicated in multiple stages of development183,184. During peri-implantation development, BM secretion enables apicobasal polarization and formation of epiblast cavity185. During gastrulation, localized degradation of the BM mediates migration of primitive streak, establishing the body axes in mice186 (Fig. 4), whereas differences in cell stiffness drive flow of cells and internalization of the gastrulating furrow187. Fluidization and rigidity transition of tissues — whereby tissues switch from being solid-like to fluid-like and vice versa — are recurring themes for processes that shape tissues during development and have recently been linked to changes in ECM-dependent confinement 188–190. Further, migrating neural crest cells generate a stiffness gradient via N-cadherin mediated cell-cell interactions that they then follow using durotaxis191. At later stages of development, compaction of col-1–rich ECM by cell intercalation and contractility enables budding and branching of epithelia in salivary glands192 and in mesenchymal condensates (densified mesenchymal structures that deform during epithelial morphogenesis)193.
Homeostasis and regeneration
In adults, mechanotransduction-regulated behaviours maintain homeostasis and normal activity. Fibroblasts in stromal tissues maintain or modulate a state of tensional force by contracting against their surrounding col-1-rich stromal ECM to maintain mechanical homeostasis, a state of mechanical equilibrium194,195. Differentiation or maintenance of stemness in stem cells, or maintenance of normal phenotypes in differentiated cells, are often supported by stiffness, and in some cases viscoelasticity, of the niche56,84,88,92. When mechanisms maintaining normal tissue function become disrupted, diseases such as fibrosis and cancer, both discussed in the following sub-sections, can occur196, as can other diseases such as osteoarthritis169, polycystic kidney disease197 and aneurysms198.
Mechanotransduction is also implicated in regenerative processes. For example, the hematoma that follows a bone fracture is highly viscoelastic, and such viscoelasticity has been found to be necessary for infiltration of MSCs and promoting osteogenesis of the MSCs in vivo40,78,199 (Fig. 5b).
Ageing and fibrosis
Ageing disrupts tissue properties and alters homeostatic mechanosensation further reinforcing disease phenotypes200. Skin BM exhibits characteristic thinning with increasing age200, whereas BMs in the retina and blood-brain barrier thicken 201. In stromal matrices that fill up soft tissues and are rich in type-1 collagen, buildup of advanced glycation end products and increased non-enzymatic crosslinking of col-1 results in pockets of stiff matrix while matrix metalloproteinase-driven matrix degradation softens other regions of the stroma, resulting in highly heterogeneous collagen networks with low solubility200 resulting in fibroblast senescence202 and reduced motility203. These changes alter epithelial and mesenchymal cell phenotypes, and result in positive feedback between changes in cell phenotype and ECM structure which increase the risk of diseases such as fibrosis and cancer200,204,205.
Tissue fibrosis involving excessive deposition of ECM and aberrant tissue mechanics and is implicated in many deaths206. Persistent activation of fibroblasts to a myofibroblast phenotype is responsible for poor prognosis of fibrotic diseases, and is driven by altered mechanosensing181. As opposed to regenerative wound healing, fibrosis leads to scar formation wherein excess secretion57 and alteration of collagen architecture207 leads to differentiation of fibroblasts to myofibroblasts, which in turn reinforce the fibrotic niche208 (Fig. 5c). Fibrosis of the bone marrow, or myelofibrosis, increases marrow stiffness and reduces stress relaxation, promoting monocyte differentiation towards dendritic cells, thus promoting a pro-inflammatory microenvironment and disease progression104.
Cancer
Although at the root of cancer are mutations that activate oncogenes and inactivate tumour suppressor genes, as well as changes in gene copy number resulting from genomic instability, there has been increased recognition that the tumour microenvironment, including ECM mechanics, plays a key role in restraining or promoting tumour progression101,209. In breast cancer, enhanced mammographic density, associated with increased ECM stiffness, has been a well-known risk factor for disease progression210,211. Increased ECM stiffness is associated with increased col-1 density and elevated crosslinking of the col-1, and likely results from the activity of cancer associated fibroblasts and tumor associated macrophages212,213. Increased ECM stiffness promotes a more proliferative and invasive phenotype in breast cancer118–122,126 (Fig. 5d). Matrix mechanical plasticity and degradation of the stromal matrix mediate formation of collagen tracks that carcinoma cells utilize to migrate away from the tumour73,77,214. Similarly, increased stiffness and more fibrillar col-1 has been linked to other cancers including pancreatic ductal adenocarcinoma215,216, glioma brain cancer46, colorectal cancer217, lung cancer218, hepatocellular carcinoma219 and cutaneous squamous cell carcinoma220. Changes in viscoelasticity are also associated with breast cancer progression221, brain cancer222, and liver cancer223, and likely other cancers, and also regulate tumour spheroid growth in vitro106; however the functional significance of these changes in viscoelasticity to disease progression in vivo are unclear.
Conclusions and perspectives
Mechanotransduction of cells in 3D impacts various cellular behaviors, which play a key role in many aspects of tissue physiology from development to disease. 2D culture models are sufficient for capturing critical aspects of mechanotransduction in vivo in some contexts; in other contexts, 3D culture is required. In 3D culture, it has become clear that stiffness, viscoelasticity, plasticity, and degradability are key parameters regulating cell behaviours (Table 1). Confinement has emerged as a key aspect of the mechanical microenvironment in 3D. Mechanosensitive ion channel-mediated sensing of confinement can, at least in some cases, complement integrin-mediated mechanotransduction pathways to regulate nuclear morphologies, chromatin accessibility and transcription factor activity, which in turn regulate gene expression and cell phenotype.
Despite these major insights, key gaps in our knowledge of cell–matrix mechanotransduction in 3D remain, as we have highlighted throughout the Review. Beyond integrins and mechanosensitive ion channels, it remains relatively unclear how mechanical cues are transduced in the 3D context. Specifically, subcellular cytoskeletal structures, downstream mechanotransduction molecules, the specific pathways, chromatin remodelling enzymes and events and set of transcription factors that mediate mechanotransduction remain to be uncovered. Promisingly, there are a large set of tools emerging that can potentially be applied to 3D culture. Spatiotemporal control of local hydrogel properties224 and single cell microencapsulations225 provide more tailored control of local cell microenvironments in 3D. Super resolution imaging techniques226 suitable for 3D can reveal the structure of integrin-based adhesions, the actin cytoskeleton, and the actomyosin machinery of cells in 3D, as well as actin adaptor proteins such as talin and vinculin, and the spatiotemporal dynamics of Rho GTPase activation as indicated by FRET [G] based sensors227. Further, genome-wide assays such as RNA-seq and single-cell RNA-seq for gene expression or ATAC-seq [G] for chromatin accessibility, and genome-wide CRISPR screens tailored for 3D assays228 can identify mechanotransduction regulators unique to 3D.
In addition to the need for deeper mechanistic insights, there is also a critical requirement for studies on in vivo relevance. This has been done in a number of contexts often by associating measured tissue mechanics with biological signatures (i.e. gene expression or transcription factor activation) predicted from 3D culture studies, or by perturbing key mechanotransduction regulators in vivo, such as FAK or YAP, and examining the downstream effect. Clever approaches to directly modulate stiffness and viscoelasticity in vivo, or perturb novel downstream regulators, could further increase confidence in the relevance of the causal, mechanistic insights reported in in vitro studies. Furthermore, although the role of mechanotransduction pathways in development and cancer have been studied heavily and are becoming increasingly clear, the role of mechanotransduction behaviours in other contexts including homeostasis, regenerative processes, ageing, various other diseases, and in immune cell activity require significant additional effort.
These future efforts in mechanotransduction are likely to be very impactful. Beyond providing a fundamental understanding of cell and tissue physiology, these studies can advance the rapidly emerging area of mechanotherapy, which holds great promise for medicine. In a recent mice study229, fibrosis induced by stiff (~MPa) silicone implants, which are used in breast mastectomy, was reduced by encapsuling the stiff implants in soft silicone implants (~kPa). Relatedly, another study230 had the objective to reduce fibrosis associated with skin grafting. Targeting mechanotransduction using a small molecule FAK inhibitor, the fibrotic nature of the skin was substantially reduced with increased healing of wounds. In the context of ovarian fibrosis, targeting fibrotic col-1 using antifibrosis drugs restored ovulation in mice231.
ECM stiffness and degradability have been the clinical targets in the context of cancer232. However, results have been mixed. In the context of stiffness, inhibition of lysyl oxidases (NCT00195091), β1 integrin mediated mechanotransduction (NCT02683824), and FAK (NCT03727880) are undergoing clinical trials. In the context of ECM degradation, MMP inhibition was not effective in preventing cancer progression, potentially owing to off target effects as well as alternative mechanisms of invasion of cancer cells, not relying on ECM proteolysis. MMP inhibition also has severe side effects232. Future efforts could potentially target other downstream effectors of mechanotransduction as well as viscoelasticity and viscoplasticity in combination with degradation.
Injecting cells within biomaterial carriers into injured or diseased tissues is a common approach in regenerative medicine233. Varying stiffness, degradability and viscoelasticity have shown to optimize the in vivo healing response. For example, promoting viscoelasticity of ECM in vivo was shown to increase bone formation by MSCs199. Interestingly, most biomaterials used in translational applications tend to be viscoelastic or degradable52. These highlight the importance of mechanotransduction in guiding the selection and use of biomaterials in these applications.
Supplementary Material
Acknowledgements
We apologize for not being able to cite all the relevant publications in this manuscript due to space and reference limitations. O.C. acknowledges support from a National Institutes of Health National Cancer Institute grant (R37 CA214136), a National Science Foundation CAREER award (CMMI 1846367), and National Science Foundation grant MCB 2148041.
Glossary
- Advanced glycation end products (AGEs)
AGEs are proteins or lipids that are glycated when exposed to sugars, and are a biomarker implicated in many diseases such as diabetes and atherosclerosis
- Aggrecan
Aggrecan is the major proteoglycan in the articular cartilage which provides hydration to the cartilage
- Arginine-Glycine-Aspartate (RGD) sequences
A three peptide cell-matrix adhesion motif derived from ECM proteins such as fibronectin and vitronection that serves as a binding site for integrins such as αvβ3, α5β1 and αIIbβ3
- ATAC-seq (Assay for Tranposases-Accessible Chromating using Sequencing)
A genome wide assay that identifies accesible DNA regions/genome wide chromatin accessibility
- Biglycan
Biglycan consists of a protein core and two glycosaminoglycan (GAG) chains, and is found in connective tissues
- Creep
Creep, a behavior of viscoelastic materials, is the time-dependent deformation or strain of a material under constant force/stress
- Deviatoric stresses
Distortional mechanical stresses that act to change the shape of an object on which they act, without changing its volume
- FRET
Fluorescent Resonance Energy Transfer (FRET) involves energy transfer between two light-sensitive molecules, and the efficiency of this energy transfer is inversely proportional to the sixth power of the distance between the molecules. This can be used to study small changes in distances between molecules
- Fibrillar adhesions
Fibrillar adhesions are cell-ECM adhesions whose shapes are elliptical in nature, often forming along fibrous ECM
- Filipodia
Actin-rich protrusions that are long and thin, and can be highly dynamic
- Focal adhesion kinase (FAK)
A receptor tyrosine kinase protein that localizes to focal complexes at cell-ECM adhesion sites and plays a crucial role in the several intergin-dependent mechanotransductive pathways
- Focal adhesions
Large cell–matrix adhesions typically formed by cells cultured on stiff 2D substrates, characterized by clustered integrin receptors, localization of proteins such as paxilin, talin, vinculin, phosphorylated FAK and thick actomyosin stress fibres, mediating strong cell-substrate adhesion
- Hydrostatic stresses
Volumetric or dilational stresses that act to increase or decrease the volume of an object on which they act, without changing its shape
- Invadapodia
Actin-rich structures that are present at the basal surface of cells, and thought to degrade and apply forces to ECM
- Lamellipodia
Thin sheet-like protrusions composedof a branched network of actin filaments. Extension of lamellipodia at the leading edge is often implicated in driving cell migration
- Lysyl oxidase (LOX)
An enzyme that converts lysine molecules into highly reactive aldehydes that form crosslinks in ECMs such as col-1 and elastin
- Nonlinear elasticity
Elasticity is the ability of a material to retain its initial shape/configuration following the application and release of external deformation or loading. In nonlinear elasticity, stress is nonlinearly related to strain even at small strains. By contrast, in linear elastic materials, stress of a material is linearly related to the strain until the material starts to yield
- Matrix metalloproteinases (MMPs)
Cell-secreted enzymes that are capable of proteolytically degrading various ECM components
- Mechanical Plasticity
A material property that defines the extent to which it undergoes permanent or irreversible deformation following the application and release of external deformation or loading
- Mechanosensitive ion channels
Ion channels that open or close in response to cell membrane stretch or tension. TRPV4 and Piezo1 are examples of mechanosensitive ion channels implicated in mechanotransduction
- Mesenchymal stem or stromal cells (MSCs)
Multipotent stem or stromal cells that are found in the bone marrow, which have been reported to differentiate into osteoblasts, adipocytes, chondrocytes, myocytes and neurons
- Microtentacles
Microtubule-based membrane protrusions which are often obseved in detached circulating tumor cells
- MRTF-A
Myocardin related transcription factor A. A transcription factor that plays a key role in mediating smooth muscle cell differentiation
- Poroelasticity
A material property that describes the interaction between fluid flow and solid deformations within the porous material. Cells and ECMs are usually poroelastic in nature
- Proteoglycans
Supramolecules that posses protein as a core and a side chain of sugars
- Reconstituted BM (rBM) matrices
Commercially available matrices such as Matrigel or Geltrex, that are derived from Engelbreth–Holm–Swarm (EHS) tumor, a mouse sarcoma, and are commonly used for in vitro cell culture and contain laminin, collagen IV, entactin, perlecan and other components
- RHAMM
Receptor for hyaluronin-mediated motility (RHAMM) is a protein which bounds to hyaluronan
- Rho signalling
A cell signalling pathway involved in regulation of wide variety of cell processes such as cell morphology, survival, proliferation, and adhesion
- Stiffness
Stiffness describes the resistance to deformation of a specific structure, which is dependent on the Young’s modulus and geometry of the structure. Hence, stiffness is regarded as property of a specific structure while Young’s modulus is an inherent property of the material
- Strain
Strain is a measure of localized deformation in a material, with uniaxial strain typically defined as the ratio of deformation to the original length of the material
- Stress
A measure of force per unit area, which has units of Pascals (Pa)
- Stress relaxation
A behaviour of viscoelastic materials referring to the time-dependent change in stress in a material under constant deformation
- Tenascin-C
A multimodular ECM glycoprotein which is expressed in various tissues during development, disease, or injury
- Traction force microscopy
A technique that takes experimentally measured the force-induced displacement field of a substrate with known mechanical properties and uses these to computationally determine the cell–ECM forces applied to the substrate
- Versican
A large ECM proteoglycan that is found in various tissues such as blood vessels, and skin
- Viscoelasticity
Describes materials that exhibit some behaviours characteristic of elastic solids and some of viscous liquids, and is characterized by a time-dependent mechanical response (i.e. creep or stress relaxation)
- YAP/TAZ
Transcriptional coactivators that shuttle between the nucleus and cytoplasm and play an important role in mediating mechanotransduction, particularly in 2D. When translocated to the nucleus, YAP/TAZ do not directly bind to DNA but regulate gene expression through their binding to transcription factors of the TEAD family
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s415XX-XXX-XXXX-X
Peer review information
Nature Reviews Molecular Cell Biology thanks Kenneth Yamada, Jennifer 1799 Young, who co-reviewed Martin Kiwanuka, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1.Levental I, Georges PC & Janmey PA Soft biological materials and their impact on cell function. Soft Matter 3, 299–306, doi: 10.1039/B610522J (2007). [DOI] [PubMed] [Google Scholar]
- 2.Swift J. et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 341, 1240104, doi:doi: 10.1126/science.1240104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC & Janmey PA Nonlinear elasticity inbiological gels. Nature 435, 191–194, doi: 10.1038/nature03521 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Discher Dennis E, Janmey P. & Wang Y -l. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 310, 1139–1143, doi: 10.1126/science.1116995 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Vogel V. & Sheetz M. Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology 7, 265–275, doi: 10.1038/nrm1890 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Wozniak MA & Chen CS Mechanotransduction in development: a growing role for contractility. Nature Reviews Molecular Cell Biology 10, 34–43, doi: 10.1038/nrm2592 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuFort CC, Paszek MJ & Weaver VM Balancing forces: architectural control of mechanotransduction. Nature Reviews Molecular Cell Biology 12, 308–319, doi: 10.1038/nrm3112 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kechagia JZ, Ivaska J. & Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nature Reviews Molecular Cell Biology 20, 457–473, doi: 10.1038/s41580-019-0134-2 (2019). [DOI] [PubMed] [Google Scholar]
- 9. Cukierman E, Pankov R, Stevens DR & Yamada KM Taking Cell-Matrix Adhesions to the Third Dimension. Science 294, 1708–1712, doi:doi: 10.1126/science.1064829 (2001). This paper demonstrated the key differences in structure and composition of cell-ECM adhesions for fibroblasts between 2D culture, 3D culture, and tissues.
- 10.Baker BM & Chen CS Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. Journal of Cell Science 125, 3015–3024, doi: 10.1242/jcs.079509 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Der Mark K, Gauss V, Von Der Mark H. & MÜLler P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267, 531–532, doi: 10.1038/267531a0 (1977). [DOI] [PubMed] [Google Scholar]
- 12.Petersen OW, Rønnov-Jessen L, Howlett AR & Bissell MJ Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences 89, 9064–9068, doi: 10.1073/pnas.89.19.9064 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerecht S. et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proceedings of the National Academy of Sciences 104, 11298–11303, doi: 10.1073/pnas.0703723104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischbach C. et al. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proceedings of the National Academy of Sciences 106, 399–404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fratzl P. in Collagen: Structure and Mechanics (ed Fratzl Peter) 1–13 (Springer US, 2008). [Google Scholar]
- 16.Jokinen J. et al. Integrin-mediated Cell Adhesion to Type I Collagen Fibrils. Journal of Biological Chemistry 279, 31956–31963, doi: 10.1074/jbc.M401409200 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Humphries JD, Byron A. & Humphries MJ Integrin ligands at a glance. Journal of Cell Science 119, 3901–3903, doi: 10.1242/jcs.03098 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautieri A, Vesentini S, Redaelli A. & Buehler MJ Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Letters 11, 757–766, doi: 10.1021/nl103943u (2011). [DOI] [PubMed] [Google Scholar]
- 19.Vader D, Kabla A, Weitz D. & Mahadevan L. Strain-Induced Alignment in Collagen Gels. PLOS ONE 4, e5902, doi: 10.1371/journal.pone.0005902 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proestaki M, Ogren A, Burkel B. & Notbohm J. Modulus of Fibrous Collagen at the Length Scale of a Cell. Experimental Mechanics 59, 1323–1334, doi: 10.1007/s11340-018-00453-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotary K, Allen E, Punturieri A, Yana I. & Weiss SJ Regulation of Cell Invasion and Morphogenesis in a Three-Dimensional Type I Collagen Matrix by Membrane-Type Matrix Metalloproteinases 1, 2, and 3. Journal of Cell Biology 149, 1309–1323, doi: 10.1083/jcb.149.6.1309 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Münster S. et al. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proceedings of the National Academy of Sciences 110, 12197–12202, doi: 10.1073/pnas.1222787110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam S, Hu KH, Butte MJ & Chaudhuri O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proceedings of the National Academy of Sciences 113, 5492–5497, doi: 10.1073/pnas.1523906113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ban E. et al. Mechanisms of Plastic Deformation in Collagen Networks Induced by Cellular Forces. Biophysical Journal 114, 450–461, doi: 10.1016/j.bpj.2017.11.3739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collet J-P, Shuman H, Ledger Robert E, Lee S. & Weisel John W. The elasticity of an individual fibrin fiber in a clot. Proceedings of the National Academy of Sciences 102, 9133–9137, doi: 10.1073/pnas.0504120102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown André EX, Litvinov Rustem I, Discher Dennis E, Purohit Prashant K. & Weisel John W. Multiscale Mechanics of Fibrin Polymer: Gel Stretching with Protein Unfolding and Loss of Water. Science 325, 741–744, doi: 10.1126/science.1172484 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yurchenco PD Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harbor Perspectives in Biology 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang J. & Chaudhuri O. Beyond proteases: Basement membrane mechanics and cancer invasion. Journal of Cell Biology 218, 2456–2469, doi: 10.1083/jcb.201903066 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Zheng Y, Han YL, Cai S. & Guo M. Nonlinear elasticity of biological basement membrane revealed by rapid inflation and deflation. Proceedings of the National Academy of Sciences 118, e2022422118, doi:doi: 10.1073/pnas.2022422118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stowers RS et al. Extracellular Matrix Stiffening Induces a Malignant Phenotypic Transition in Breast Epithelial Cells. Cellular and Molecular Bioengineering 10, 114–123, doi: 10.1007/s12195-016-0468-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reuten R. et al. Basement membrane stiffness determines metastases formation. Nature Materials 20, 892–903, doi: 10.1038/s41563-020-00894-0 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Kleinman HK & Martin GR Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology 15, 378–386, doi: 10.1016/j.semcancer.2005.05.004 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Chopra A. et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials 35, 71–82, doi: 10.1016/j.biomaterials.2013.09.066 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf KJ et al. A mode of cell adhesion and migration facilitated by CD44-dependent microtentacles. Proceedings of the National Academy of Sciences 117, 11432–11443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf KJ & Kumar S. Hyaluronic Acid: Incorporating the Bio into the Material. ACS Biomaterials Science & Engineering 5, 3753–3765, doi: 10.1021/acsbiomaterials.8b01268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdick JA & Prestwich GD Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Materials 23, H41–H56, doi: 10.1002/adma.201003963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogel V. MECHANOTRANSDUCTION INVOLVING MULTIMODULAR PROTEINS: Converting Force into Biochemical Signals. Annual Review of Biophysics and Biomolecular Structure 35, 459–488, doi: 10.1146/annurev.biophys.35.040405.102013 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Kong Hyun J, Polte Thomas R, Alsberg E. & Mooney David J. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences 102, 4300–4305, doi: 10.1073/pnas.0405873102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huebsch N. et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials 9, 518–526, doi: 10.1038/nmat2732 (2010). Using hydrogels to tune stiffness indepdendent of ligand density, the authors demonstrated that ECM stiffness directs stem cell fate in 3D through integrin clustering.
- 40. Chaudhuri O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials 15, 326–334, doi: 10.1038/nmat4489 (2016). By developing hydrogels of same initial stiffness but independently tunable stress relaxation and using these for 3D cell culture, this paper showed that stress relaxation (viscoelasticity) impacted cell spreading, proliferation, and differentiation of MSCs.
- 41.Indana D, Agarwal P, Bhutani N. & Chaudhuri O. Viscoelasticity and Adhesion Signaling in Biomaterials Control Human Pluripotent Stem Cell Morphogenesis in 3D Culture. Advanced Materials 33, 2101966, doi: 10.1002/adma.202101966 (2021). [DOI] [PubMed] [Google Scholar]
- 42. Raeber GP, Lutolf MP & Hubbell JA Molecularly Engineered PEG Hydrogels: A Novel Model System for Proteolytically Mediated Cell Migration. Biophysical Journal 89, 1374–1388, doi: 10.1529/biophysj.104.050682 (2005). This study used polyethylene glycol (PEG) based hydrogels engineered to be susceptible to proteolytic degradetion by cell-secreted matrix metalloproteinases (MMPs) to show that increased matrix degradability promotes cell migration.
- 43.Rowley JA, Madlambayan G. & Mooney DJ Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53, doi: 10.1016/S0142-9612(98)00107-0 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Kubow KE et al. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nature Communications 6, 8026, doi: 10.1038/ncomms9026 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baneyx G, Baugh L. & Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proceedings of the National Academy of Sciences 99, 5139–5143, doi: 10.1073/pnas.072650799 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miroshnikova YA et al. Tissue mechanics promote IDH1-dependent HIF1α–tenascin C feedback to regulate glioblastoma aggression. Nature Cell Biology 18, 1336–1345, doi: 10.1038/ncb3429 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wishart AL et al. Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Science Advances 6, eabc3175, doi: 10.1126/sciadv.abc3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papanicolaou M. et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nature Communications 13, 4587, doi: 10.1038/s41467-022-32255-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai VK et al. Swelling of Collagen-Hyaluronic Acid Co-Gels: An In Vitro Residual Stress Model. Annals of Biomedical Engineering 44, 2984–2993, doi: 10.1007/s10439-016-1636-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Burla F, Tauber J, Dussi S, van der Gucht J. & Koenderink GH Stress management in composite biopolymer networks. Nature Physics 15, 549–553, doi: 10.1038/s41567-019-0443-6 (2019). These papers (Lai et al., and Burla et al.) show the emergent properties of composite materials consisting of type-1 collagen and HA.
- 51.Cocciolone AJ et al. Elastin, arterial mechanics, and cardiovascular disease. American Journal of Physiology-Heart and Circulatory Physiology 315, H189–H205, doi: 10.1152/ajpheart.00087.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ & Shenoy VB Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546, doi: 10.1038/s41586-020-2612-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen D. et al. Distinct effects of different matrix proteoglycans on collagen fibrillogenesis and cell-mediated collagen reorganization. Scientific Reports 10, 19065, doi: 10.1038/s41598-020-76107-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han Yu L. et al. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proceedings of the National Academy of Sciences 115, 4075–4080, doi: 10.1073/pnas.1722619115 (2018). In this paper, using optical tweezers, the authors demonstrated that cells use their contractile forces to mechanically remodel their local microenvironments into stiffer environments.
- 55. van Oosten ASG et al. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 573, 96–101, doi: 10.1038/s41586-019-1516-5 (2019). This paper demonstrated that a composite of ECM and densly packed cells give rise to compression stiffening, whereas pure ECM exhibited compression softening, highlighting the role of cells in tissue mechanics.
- 56.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, 677–689, doi: 10.1016/j.cell.2006.06.044 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Davidson MD et al. Engineered Fibrous Networks To Investigate the Influence of Fiber Mechanics on Myofibroblast Differentiation. ACS Biomaterials Science & Engineering 5, 3899–3908, doi: 10.1021/acsbiomaterials.8b01276 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Yamada KM & Sixt M. Mechanisms of 3D cell migration. Nature Reviews Molecular Cell Biology 20, 738–752, doi: 10.1038/s41580-019-0172-9 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Paul CD, Mistriotis P. & Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nature Reviews Cancer 17, 131–140, doi: 10.1038/nrc.2016.123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khetan S. et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature Materials 12, 458–465, doi: 10.1038/nmat3586 (2013). In this seminal paper, by changing hydrogel degradability with same initial stiffness, the authors demonstrated that hydrogel degradability-mediated cell traction direct stem cell fate in 3D micronenvironments.
- 61. Lee H. p., Stowers R. & Chaudhuri O. Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nature Communications 10, 529, doi: 10.1038/s41467-019-08465-x (2019). This study identified a mechanosensitive ion channel mediated mechanism mesenchymal stem cells employ to sense matrix. Fast stress relaxation promoted reciprocal cell volume expansion and activation of TRPV4 mechanosensitive ion channels to drive nuclear translocation of RUNX2 and osteogenic differentiation.
- 62.Yang B. et al. Enhanced mechanosensing of cells in synthetic 3D matrix with controlled biophysical dynamics. Nature Communications 12, 3514, doi: 10.1038/s41467-021-23120-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engler A. et al. Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophysical Journal 86, 617–628, doi: 10.1016/S0006-3495(04)74140-5 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Christopher S, Mrksich M, Huang S, Whitesides George M. & Ingber Donald E. Geometric Control of Cell Life and Death. Science 276, 1425–1428, doi: 10.1126/science.276.5317.1425 (1997). [DOI] [PubMed] [Google Scholar]
- 65.Gjorevski N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021, doi:doi: 10.1126/science.aaw9021 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M. et al. Regulating Mechanotransduction in Three Dimensions using Sub-Cellular Scale, Crosslinkable Fibers of Controlled Diameter, Stiffness, and Alignment. Advanced Functional Materials 29, 1808967, doi: 10.1002/adfm.201808967 (2019). [DOI] [Google Scholar]
- 67.Bodor DL, Pönisch W, Endres RG & Paluch EK Of Cell Shapes and Motion: The Physical Basis of Animal Cell Migration. Developmental Cell 52, 550–562, doi: 10.1016/j.devcel.2020.02.013 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Peyton SR & Putnam AJ Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cellular Physiology 204, 198–209, doi: 10.1002/jcp.20274 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Pelham Robert J. & Wang Y -l. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences 94, 13661–13665, doi: 10.1073/pnas.94.25.13661 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardel ML et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. Journal of Cell Biology 183, 999–1005, doi: 10.1083/jcb.200810060 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sunyer R. et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 353, 1157–1161, doi: 10.1126/science.aaf7119 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Isomursu A. et al. Directed cell migration towards softer environments. Nature Materials 21, 1081–1090, doi: 10.1038/s41563-022-01294-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf K. et al. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. Journal of Cell Biology 201, 1069–1084, doi: 10.1083/jcb.201210152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harada T. et al. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. Journal of Cell Biology 204, 669–682, doi: 10.1083/jcb.201308029 (2014). These two papers, Wolf et al., 2013 and Harada et al., 2014, show that matrix pore size regulates cell migration with cells unable to migrate through pores smaller than 3μm in diameter in elastic non-degradable gels.
- 75.Fraley SI et al. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Scientific reports 5, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trappmann B. et al. Matrix degradability controls multicellularity of 3D cell migration. Nature communications 8, 1–8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wisdom KM et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nature Communications 9, 4144, doi: 10.1038/s41467-018-06641-z (2018). Using nanoporous hydrogels with tunable mechanical plasticity, this paper discovered that cells can migrate through nanoporous matrices independent of proteases if the matrix exhibit sufficient matrix mechanical plasticity.
- 78.Lee H. p. et al. The nuclear piston activates mechanosensitive ion channels to generate cell migration paths in confining microenvironments. Science Advances 7, eabd4058, doi:doi: 10.1126/sciadv.abd4058 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petrie RJ, Harlin HM, Korsak LIT & Yamada KM Activating the nuclear piston mechanism of 3D migration in tumor cells. Journal of Cell Biology 216, 93–100, doi: 10.1083/jcb.201605097 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Polacheck WJ, German AE, Mammoto A, Ingber DE & Kamm RD Mechanotransduction of fluid stresses governs 3D cell migration. Proceedings of the National Academy of Sciences 111, 2447–2452, doi: 10.1073/pnas.1316848111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blache U, Stevens MM & Gentleman E. Harnessing the secreted extracellular matrix to engineer tissues. Nature Biomedical Engineering 4, 357–363, doi: 10.1038/s41551-019-0500-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loebel C, Mauck RL & Burdick JA Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nature Materials 18, 883–891, doi: 10.1038/s41563-019-0307-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferreira SA et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nature Communications 9, 4049, doi: 10.1038/s41467-018-06183-4 (2018). These papers, Loebel et al. and Ferreira et al., demonstrated the role of nascent proteins secreted by cells in 3D as a key regulator of various mechanosensing behaviors such as cell spread area, YAP/TAZ nuclear translocation, and osteogenic differentiation.
- 84.Lee H. p., Gu L, Mooney DJ, Levenston ME & Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nature Materials 16, 1243–1251, doi: 10.1038/nmat4993 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borelli AN et al. Stress Relaxation and Composition of Hydrazone-Crosslinked Hybrid Biopolymer-Synthetic Hydrogels Determine Spreading and Secretory Properties of MSCs. Advanced Healthcare Materials n/a, 2200393, doi: 10.1002/adhm.202200393 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sridhar BV et al. Development of a Cellularly Degradable PEG Hydrogel to Promote Articular Cartilage Extracellular Matrix Deposition. Advanced Healthcare Materials 4, 702–713, doi: 10.1002/adhm.201400695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vining KH & Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews Molecular Cell Biology 18, 728–742, doi: 10.1038/nrm.2017.108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engler AJ et al. Myotubes differentiate optimally on substrates with tissue-like stiffness : pathological implications for soft or stiff microenvironments. Journal of Cell Biology 166, 877–887, doi: 10.1083/jcb.200405004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu J. et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature Methods 7, 733–736, doi: 10.1038/nmeth.1487 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen JH et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature Materials 13, 979–987, doi: 10.1038/nmat4051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saha K. et al. Substrate Modulus Directs Neural Stem Cell Behavior. Biophysical Journal 95, 4426–4438, doi: 10.1529/biophysj.108.132217 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilbert PM et al. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science 329, 1078–1081, doi:doi: 10.1126/science.1191035 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ye K. et al. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Letters 15, 4720–4729, doi: 10.1021/acs.nanolett.5b01619 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Yang C. et al. Spatially patterned matrix elasticity directs stem cell fate. Proceedings of the National Academy of Sciences 113, E4439–E4445, doi:doi: 10.1073/pnas.1609731113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Charrier EE, Pogoda K, Wells RG & Janmey PA Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nature Communications 9, 449, doi: 10.1038/s41467-018-02906-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993, doi: 10.1016/j.biomaterials.2011.04.003 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Stanton AE, Tong X. & Yang F. Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomaterialia 96, 310–320, doi: 10.1016/j.actbio.2019.06.048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kilian KA, Bugarija B, Lahn BT & Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences 107, 4872–4877, doi: 10.1073/pnas.0903269107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muncie JM et al. Mechanical Tension Promotes Formation of Gastrulation-like Nodes and Patterns Mesoderm Specification in Human Embryonic Stem Cells. Developmental Cell 55, 679–694.e611, doi: 10.1016/j.devcel.2020.10.015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das RK, Gocheva V, Hammink R, Zouani OF & Rowan AE Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nature Materials 15, 318–325, doi: 10.1038/nmat4483 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Hayward M-K, Muncie JM & Weaver VM Tissue mechanics in stem cell fate, development, and cancer. Developmental Cell 56, 1833–1847, doi: 10.1016/j.devcel.2021.05.011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao Y, Sang J. & Fu J. On human pluripotent stem cell control: The rise of 3D bioengineering and mechanobiology. Biomaterials 52, 26–43, doi: 10.1016/j.biomaterials.2015.01.078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Madl CM et al. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nature Materials 16, 1233–1242, doi: 10.1038/nmat5020 (2017). This paper demonstrated that increased ECM degradability in 3D allows cell-cell contact formation which helps to maintain nueral progenitor cell stemness, in the absence of cytoskeletal tension generation.
- 104.Vining KH et al. Mechanical checkpoint regulates monocyte differentiation in fibrotic niches. Nature Materials 21, 939–950, doi: 10.1038/s41563-022-01293-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Klein EA et al. Cell-Cycle Control by Physiological Matrix Elasticity and In Vivo Tissue Stiffening. Current Biology 19, 1511–1518, doi: 10.1016/j.cub.2009.07.069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nam S. et al. Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27Kip1 signaling axis. Science Advances 5, eaaw6171, doi: 10.1126/sciadv.aaw6171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dudaryeva OY et al. 3D Confinement Regulates Cell Life and Death. Advanced Functional Materials 31, 2104098, doi: 10.1002/adfm.202104098 (2021). [DOI] [Google Scholar]
- 108.Kratochvil MJ et al. Engineered materials for organoid systems. Nature Reviews Materials 4, 606–622, doi: 10.1038/s41578-019-0129-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rezakhani S, Gjorevski N. & Lutolf MP Extracellular matrix requirements for gastrointestinal organoid cultures. Biomaterials 276, 121020, doi: 10.1016/j.biomaterials.2021.121020 (2021). [DOI] [PubMed] [Google Scholar]
- 110.Enemchukwu NO et al. Synthetic matrices reveal contributions of ECM biophysical and biochemical properties to epithelial morphogenesis. Journal of Cell Biology 212, 113–124, doi: 10.1083/jcb.201506055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gjorevski N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564, doi: 10.1038/nature20168 (2016). [DOI] [PubMed] [Google Scholar]
- 112. Cruz-Acuña R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nature Cell Biology 19, 1326–1335, doi: 10.1038/ncb3632 (2017). These two papers (Gjorevski and Cruz-Acuna) demonstrate the use of 3D culture in engineered hydrogels for guiding morphogenesis of stem cells, highlighting the role of hydrogel degradability.
- 113.Ranga A. et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proceedings of the National Academy of Sciences 113, E6831–E6839, doi: 10.1073/pnas.1603529113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Blatchley MR, Hall F, Wang S, Pruitt HC & Gerecht S. Hypoxia and matrix viscoelasticity sequentially regulate endothelial progenitor cluster-based vasculogenesis. Science Advances 5, eaau7518, doi:doi: 10.1126/sciadv.aau7518 (2019). These papers, Blatchley et al. and Indana et al. (ref. 41), demonstrate the use of 3D culture in engineered hydrogels for guiding morphogenesis of stem cells, highlighting the role of hydrogel viscoelasticity.
- 115.Chrisnandy A, Blondel D, Rezakhani S, Broguiere N. & Lutolf MP Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nature Materials 21, 479–487, doi: 10.1038/s41563-021-01136-7 (2022). [DOI] [PubMed] [Google Scholar]
- 116.Sorrentino G. et al. Mechano-modulatory synthetic niches for liver organoid derivation. Nature Communications 11, 3416, doi: 10.1038/s41467-020-17161-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kai F, Drain AP & Weaver VM The Extracellular Matrix Modulates the Metastatic Journey. Developmental Cell 49, 332–346, doi: 10.1016/j.devcel.2019.03.026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Provenzano PP et al. Collagen density promotes mammary tumor initiation and progression. BMC Medicine 6, 11, doi: 10.1186/1741-7015-6-11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254, doi: 10.1016/j.ccr.2005.08.010 (2005). In this multifaceted paper that utilized both 2D and 3D cell culture studies, the authors show that increased stiffness and collagen density, as occurs during breast cancer progression, promotes a malignant phenotype in even normal mammary epithelial cells through β1 integrin clustering, Rho, and FAK activation
- 120.Levental KR et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 139, 891–906, doi: 10.1016/j.cell.2009.10.027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wei SC et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nature Cell Biology 17, 678–688, doi: 10.1038/ncb3157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chaudhuri O. et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Materials 13, 970–978, doi: 10.1038/nmat4009 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Tse JM et al. Mechanical compression drives cancer cells toward invasive phenotype. Proceedings of the National Academy of Sciences 109, 911–916, doi: 10.1073/pnas.1118910109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim J. et al. Geometric Dependence of 3D Collective Cancer Invasion. Biophysical Journal 118, 1177–1182, doi: 10.1016/j.bpj.2020.01.008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Debnath J. & Brugge JS Modelling glandular epithelial cancers in three-dimensional cultures. Nature Reviews Cancer 5, 675–688 (2005). [DOI] [PubMed] [Google Scholar]
- 126. Lee JY et al. YAP-independent mechanotransduction drives breast cancer progression. Nature Communications 10, 1848, doi: 10.1038/s41467-019-09755-0 (2019). In contrast to the universal role of YAP in mediating mechanotransduction on 2D substrates, this paper demonstrated that increased stiffness promotes malignancy in mammary epithelial cells independent of YAP in 3D culture, which is consistent with in vivo analysis of breast cancer, and connected this observation to the lack of focal adhesions, stress fibers, and stretched nuclei in 3D.
- 127. Stowers RS et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nature Biomedical Engineering 3, 1009–1019, doi: 10.1038/s41551-019-0420-5 (2019). In this paper, the auhthors demonstrated changes in chromatin accesibility in breast cance cells with change in ECM stiffness in 3D, and these changes allow binding of the SP1 transcription factor to promote a malignant phenotype, revealing that chromatin state is a critical mediator of mechanotranduction.
- 128.Long H, Vos BE, Betz T, Baker BM & Trappmann B. Nonswelling and Hydrolytically Stable Hydrogels Uncover Cellular Mechanosensing in 3 D. Advanced Science 9, 2105325, doi: 10.1002/advs.202105325 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lou J, Stowers R, Nam S, Xia Y. & Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials 154, 213–222 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Caliari SR, Vega SL, Kwon M, Soulas EM & Burdick JA Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 103, 314–323, doi: 10.1016/j.biomaterials.2016.06.061 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nam S, Lee J, Brownfield Doug G. & Chaudhuri O Viscoplasticity Enables Mechanical Remodeling of Matrix by Cells. Biophysical Journal 111, 2296–2308, doi: 10.1016/j.bpj.2016.10.002 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qazi TH et al. Programming hydrogels to probe spatiotemporal cell biology. Cell Stem Cell, doi: 10.1016/j.stem.2022.03.013 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schultz KM, Kyburz KA & Anseth KS Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proceedings of the National Academy of Sciences 112, E3757–E3764 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sabeh F, Shimizu-Hirota R. & Weiss SJ Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. Journal of Cell Biology 185, 11–19, doi: 10.1083/jcb.200807195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McKinnon DD, Domaille DW, Cha JN & Anseth KS Biophysically Defined and Cytocompatible Covalently Adaptable Networks as Viscoelastic 3D Cell Culture Systems. Advanced Materials 26, 865–872, doi: 10.1002/adma.201303680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nam S. & Chaudhuri O. Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nature Physics 14, 621–628, doi: 10.1038/s41567-018-0092-1 (2018). [DOI] [Google Scholar]
- 137.Legant WR et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nature Methods 7, 969–971, doi: 10.1038/nmeth.1531 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Franck C, Maskarinec SA, Tirrell DA & Ravichandran G. Three-Dimensional Traction Force Microscopy: A New Tool for Quantifying Cell-Matrix Interactions. PLOS ONE 6, e17833, doi: 10.1371/journal.pone.0017833 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Steinwachs J. et al. Three-dimensional force microscopy of cells in biopolymer networks. Nature Methods 13, 171–176, doi: 10.1038/nmeth.3685 (2016). [DOI] [PubMed] [Google Scholar]
- 140.Bell E, Ivarsson B. & Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences 76, 1274–1278, doi: 10.1073/pnas.76.3.1274 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fletcher DA & Mullins RD Cell mechanics and the cytoskeleton. Nature 463, 485–492, doi: 10.1038/nature08908 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Papalazarou V. & Machesky LM The cell pushes back: The Arp2/3 complex is a key orchestrator of cellular responses to environmental forces. Current Opinion in Cell Biology 68, 37–44, doi: 10.1016/j.ceb.2020.08.012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gaertner F. et al. WASp triggers mechanosensitive actin patches to facilitate immune cell migration in dense tissues. Developmental Cell 57, 47–62.e49, doi: 10.1016/j.devcel.2021.11.024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nam S, Lin Y-H, Kim T. & Chaudhuri O. Cellular Pushing Forces during Mitosis Drive Mitotic Elongation in Collagen Gels. Advanced Science 8, 2000403, doi: 10.1002/advs.202000403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hoffmann EK, Lambert IH & Pedersen SF Physiology of Cell Volume Regulation in Vertebrates. Physiological Reviews 89, 193–277, doi: 10.1152/physrev.00037.2007 (2009). [DOI] [PubMed] [Google Scholar]
- 146.Ginzberg Miriam B, Kafri R. & Kirschner M. On being the right (cell) size. Science 348, 1245075, doi: 10.1126/science.1245075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Adar Ram M. & Safran Samuel A. Active volume regulation in adhered cells. Proceedings of the National Academy of Sciences 117, 5604–5609, doi: 10.1073/pnas.1918203117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Petrie Ryan J, Koo H. & Yamada Kenneth M. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 345, 1062–1065, doi: 10.1126/science.1256965 (2014). In contrast to the conventional thinking that nuclues acts a limiting factor for cell migration in 3D, these papers, from Petrie et al., 2014 and Lee et al., 2021 (Ref. 78) drives the nucleus like a piston into the protrusion. This action activates mechanosensitive ion channels to allow an influx of ions that increases osmotic pressure, which then outcompetes hydrostatic pressure to drive protrusion expansion, generating a path for migration in confining 3D microenvironments.
- 149.Stroka KM et al. Water permeation drives tumor cell migration in confined microenvironments. Cell 157, 611–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ & Jain RK Solid stress inhibits the growth of multicellular tumor spheroids. Nature Biotechnology 15, 778–783, doi: 10.1038/nbt0897-778 (1997). [DOI] [PubMed] [Google Scholar]
- 151.Nia HT et al. Solid stress and elastic energy as measures of tumour mechanopathology. Nature Biomedical Engineering 1, 0004, doi: 10.1038/s41551-016-0004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kasza KE et al. The cell as a material. Current Opinion in Cell Biology 19, 101–107, doi: 10.1016/j.ceb.2006.12.002 (2007). [DOI] [PubMed] [Google Scholar]
- 153.Jung W, Li J, Chaudhuri O. & Kim T. Nonlinear Elastic and Inelastic Properties of Cells. Journal of Biomechanical Engineering 142, doi: 10.1115/1.4046863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guo M. et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proceedings of the National Academy of Sciences 114, E8618–E8627, doi: 10.1073/pnas.1705179114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lomakin AJ et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 370, eaba2894, doi: 10.1126/science.aba2894 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Venturini V. et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 370, eaba2644, doi:doi: 10.1126/science.aba2644 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Heo S-J et al. Nuclear softening expedites interstitial cell migration in fibrous networks and dense connective tissues. Science Advances 6, eaax5083, doi:doi: 10.1126/sciadv.aax5083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Doyle AD, Carvajal N, Jin A, Matsumoto K. & Yamada KM Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nature Communications 6, 8720, doi: 10.1038/ncomms9720 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hall Matthew S. et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proceedings of the National Academy of Sciences 113, 14043–14048, doi: 10.1073/pnas.1613058113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Doyle AD, Sykora DJ, Pacheco GG, Kutys ML & Yamada KM 3D mesenchymal cell migration is driven by anterior cellular contraction that generates an extracellular matrix prestrain. Developmental Cell 56, 826–841.e824, doi: 10.1016/j.devcel.2021.02.017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Petrie RJ, Gavara N, Chadwick RS & Yamada KM Nonpolarized signaling reveals two distinct modes of 3D cell migration. Journal of Cell Biology 197, 439–455, doi: 10.1083/jcb.201201124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gong Z. et al. Recursive feedback between matrix dissipation and chemo-mechanical signaling drives oscillatory growth of cancer cell invadopodia. Cell Reports 35, 109047, doi: 10.1016/j.celrep.2021.109047 (2021). In this study, authors used a chemo-mechanical model to study cell-matrix interactions and found protrusive forces generated by cells through invadopodia, deform the matrix and in sufficiently plastic matrices, such forces lead to permanent matrix deformations, which in turn promote growth of protrusions and result in larger pores for cell migration.
- 163.Gong Z. et al. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proceedings of the National Academy of Sciences 115, E2686–E2695, doi: 10.1073/pnas.1716620115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Blache U. et al. Notch-inducing hydrogels reveal a perivascular switch of mesenchymal stem cell fate. EMBO reports 19, e45964, doi: 10.15252/embr.201845964 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Provenzano PP, Inman DR, Eliceiri KW & Keely PJ Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage. Oncogene 28, 4326–4343, doi: 10.1038/onc.2009.299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei Z, Schnellmann R, Pruitt HC & Gerecht S. Hydrogel Network Dynamics Regulate Vascular Morphogenesis. Cell Stem Cell 27, 798–812.e796, doi: 10.1016/j.stem.2020.08.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Parekh SH et al. Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials 32, 2256–2264, doi: 10.1016/j.biomaterials.2010.11.065 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bao M, Xie J, Piruska A. & Huck WTS 3D microniches reveal the importance of cell size and shape. Nature Communications 8, 1962, doi: 10.1038/s41467-017-02163-2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Agarwal P. et al. A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nature Biomedical Engineering 5, 1472–1484, doi: 10.1038/s41551-021-00691-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Batan D. et al. Hydrogel cultures reveal Transient Receptor Potential Vanilloid 4 regulation of myofibroblast activation and proliferation in valvular interstitial cells. The FASEB Journal 36, e22306, doi: 10.1096/fj.202101863R (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wong SW et al. Controlled Deposition of 3D Matrices to Direct Single Cell Functions. Advanced Science 7, 2001066, doi: 10.1002/advs.202001066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Murthy SE, Dubin AE & Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nature Reviews Molecular Cell Biology 18, 771–783, doi: 10.1038/nrm.2017.92 (2017). [DOI] [PubMed] [Google Scholar]
- 173.Zhao R. et al. Cell sensing and decision-making in confinement: The role of TRPM7 in a tug of war between hydraulic pressure and cross-sectional area. Science Advances 5, eaaw7243, doi: 10.1126/sciadv.aaw7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yankaskas CL et al. The fluid shear stress sensor TRPM7 regulates tumor cell intravasation. Science Advances 7, eabh3457, doi: 10.1126/sciadv.abh3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kalukula Y, Stephens AD, Lammerding J. & Gabriele S. Mechanics and functional consequences of nuclear deformations. Nature Reviews Molecular Cell Biology, doi: 10.1038/s41580-022-00480-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cosgrove BD et al. Nuclear envelope wrinkling predicts mesenchymal progenitor cell mechano-response in 2D and 3D microenvironments. Biomaterials 270, 120662, doi: 10.1016/j.biomaterials.2021.120662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Darnell M. et al. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proceedings of the National Academy of Sciences 115, E8368–E8377, doi: 10.1073/pnas.1802568115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jang M. et al. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nature Biomedical Engineering 5, 114–123, doi: 10.1038/s41551-020-00657-x (2021). [DOI] [PubMed] [Google Scholar]
- 179. Baek J. et al. Egr1 is a 3D matrix-specific mediator of mechanosensitive stem cell lineage commitment. Science Advances 8, eabm4646, doi:doi: 10.1126/sciadv.abm4646 (2022). This paper demonstrated upregulation of Egr1 which mediates neural stem cell differentiation is unque to 3D, not 2D, highlighting Egr1 as 3D-specific stem cell mechanoregulatory factor.
- 180.Wong SW, Lenzini S, Cooper MH, Mooney DJ & Shin J-W Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking. Science Advances 6, eaaw0158, doi: 10.1126/sciadv.aaw0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walker CJ et al. Nuclear mechanosensing drives chromatin remodelling in persistently activated fibroblasts. Nature Biomedical Engineering 5, 1485–1499, doi: 10.1038/s41551-021-00709-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Madl CM, LeSavage BL, Khariton M. & Heilshorn SC Neural Progenitor Cells Alter Chromatin Organization and Neurotrophin Expression in Response to 3D Matrix Degradability. Advanced Healthcare Materials 9, 2000754, doi: 10.1002/adhm.202000754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stooke-Vaughan GA & Campàs O. Physical control of tissue morphogenesis across scales. Current Opinion in Genetics & Development 51, 111–119, doi: 10.1016/j.gde.2018.09.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Serwane F. et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nature Methods 14, 181–186, doi: 10.1038/nmeth.4101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bedzhov I. & Zernicka-Goetz M. Self-Organizing Properties of Mouse Pluripotent Cells Initiate Morphogenesis upon Implantation. Cell 156, 1032–1044, doi: 10.1016/j.cell.2014.01.023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kyprianou C. et al. Basement membrane remodelling regulates mouse embryogenesis. Nature 582, 253–258, doi: 10.1038/s41586-020-2264-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rauzi M. et al. Embryo-scale tissue mechanics during Drosophila gastrulation movements. Nature Communications 6, 8677, doi: 10.1038/ncomms9677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hannezo E. & Heisenberg C-P Rigidity transitions in development and disease. Trends in Cell Biology 32, 433–444, doi: 10.1016/j.tcb.2021.12.006 (2022). [DOI] [PubMed] [Google Scholar]
- 189.Kim S, Pochitaloff M, Stooke-Vaughan GA & Campàs O. Embryonic tissues as active foams. Nature Physics 17, 859–866, doi: 10.1038/s41567-021-01215-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Priya R. et al. Tension heterogeneity directs form and fate to pattern the myocardial wall. Nature 588, 130–134, doi: 10.1038/s41586-020-2946-9 (2020). [DOI] [PubMed] [Google Scholar]
- 191. Shellard A. & Mayor R. Collective durotaxis along a self-generated stiffness gradient in vivo. Nature 600, 690–694, doi: 10.1038/s41586-021-04210-x (2021). This paper demonstrated that cells collectively generate stiffness gradients in vivo that results in efficient collective cell migration.
- 192.Wang S, Matsumoto K, Lish SR, Cartagena-Rivera AX & Yamada KM Budding epithelial morphogenesis driven by cell-matrix versus cell-cell adhesion. Cell 184, 3702–3716.e3730, doi: 10.1016/j.cell.2021.05.015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hughes AJ et al. Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Developmental Cell 44, 165–178.e166, doi: 10.1016/j.devcel.2017.12.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Webster Kevin D., Ng Win P. & Fletcher, Daniel A. Tensional Homeostasis in Single Fibroblasts. Biophysical Journal 107, 146–155, doi: 10.1016/j.bpj.2014.04.051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends in Cell Biology 13, 264–269, doi: 10.1016/S0962-8924(03)00057-6 (2003). [DOI] [PubMed] [Google Scholar]
- 196.Jaalouk DE & Lammerding J. Mechanotransduction gone awry. Nature Reviews Molecular Cell Biology 10, 63–73, doi: 10.1038/nrm2597 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choudhury MI et al. Kidney epithelial cells are active mechano-biological fluid pumps. Nature Communications 13, 2317, doi: 10.1038/s41467-022-29988-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qian W. et al. Microskeletal stiffness promotes aortic aneurysm by sustaining pathological vascular smooth muscle cell mechanosensation via Piezo1. Nature Communications 13, 512, doi: 10.1038/s41467-021-27874-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Darnell M. et al. Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Advanced Healthcare Materials 6, 1601185, doi: 10.1002/adhm.201601185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Phillip JM, Aifuwa I, Walston J. & Wirtz D. The Mechanobiology of Aging. Annual Review of Biomedical Engineering 17, 113–141, doi: 10.1146/annurev-bioeng-071114-040829 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khalilgharibi N. & Mao Y. To form and function: on the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biology 11, 200360, doi:doi: 10.1098/rsob.200360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brun C. et al. Phenotypic and functional changes in dermal primary fibroblasts isolated from intrinsically aged human skin. Experimental Dermatology 25, 113–119, doi: 10.1111/exd.12874 (2016). [DOI] [PubMed] [Google Scholar]
- 203.Phillip JM et al. Fractional re-distribution among cell motility states during ageing. Communications Biology 4, 81, doi: 10.1038/s42003-020-01605-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Humphrey JD & Schwartz MA Vascular Mechanobiology: Homeostasis, Adaptation, and Disease. Annual Review of Biomedical Engineering 23, 1–27, doi: 10.1146/annurev-bioeng-092419-060810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hall CM, Moeendarbary E. & Sheridan GK Mechanobiology of the brain in ageing and Alzheimer’s disease. European Journal of Neuroscience 53, 3851–3878, doi: 10.1111/ejn.14766 (2021). [DOI] [PubMed] [Google Scholar]
- 206.Wynn TA Fibrotic disease and the TH1/TH2 paradigm. Nature Reviews Immunology 4, 583–594, doi: 10.1038/nri1412 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seo BR et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proceedings of the National Academy of Sciences 117, 11387–11398, doi:doi: 10.1073/pnas.1919394117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Freeberg MAT et al. Mechanical Feed-Forward Loops Contribute to Idiopathic Pulmonary Fibrosis. The American Journal of Pathology 191, 18–25, doi: 10.1016/j.ajpath.2020.09.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bissell MJ & Hines WC Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature Medicine 17, 320–329, doi: 10.1038/nm.2328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Piersma B, Hayward M-K & Weaver VM Fibrosis and cancer: A strained relationship. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1873, 188356, doi: 10.1016/j.bbcan.2020.188356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Acerbi I. et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology 7, 1120–1134, doi: 10.1039/C5IB00040H (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Calvo F. et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature Cell Biology 15, 637–646, doi: 10.1038/ncb2756 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Maller O. et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nature Materials 20, 548–559, doi: 10.1038/s41563-020-00849-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Provenzano PP et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine 4, 38, doi: 10.1186/1741-7015-4-38 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Laklai H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nature Medicine 22, 497–505, doi: 10.1038/nm.4082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jiang H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Medicine 22, 851–860, doi: 10.1038/nm.4123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Baker AM, Bird D, Lang G, Cox TR & Erler JT Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 32, 1863–1868, doi: 10.1038/onc.2012.202 (2013). [DOI] [PubMed] [Google Scholar]
- 218.Peng DH et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene 36, 1925–1938, doi: 10.1038/onc.2016.358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chang TT, Thakar D. & Weaver VM Force-dependent breaching of the basement membrane. Matrix Biology 57–58, 178–189, doi: 10.1016/j.matbio.2016.12.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mittapalli VR et al. Injury-Driven Stiffening of the Dermis Expedites Skin Carcinoma Progression. Cancer Research 76, 940–951, doi: 10.1158/0008-5472.CAN-15-1348 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Balleyguier C. et al. Value of whole breast magnetic resonance elastography added to MRI for lesion characterization. NMR in Biomedicine 31, e3795, doi: 10.1002/nbm.3795 (2018). [DOI] [PubMed] [Google Scholar]
- 222.Streitberger K-J et al. High-Resolution Mechanical Imaging of Glioblastoma by Multifrequency Magnetic Resonance Elastography. PLOS ONE 9, e110588, doi: 10.1371/journal.pone.0110588 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Garteiser P. et al. MR elastography of liver tumours: value of viscoelastic properties for tumour characterisation. European Radiology 22, 2169–2177, doi: 10.1007/s00330-012-2474-6 (2012). [DOI] [PubMed] [Google Scholar]
- 224.DeForest CA & Tirrell DA A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nature materials 14, 523–531 (2015). [DOI] [PubMed] [Google Scholar]
- 225.Mao AS et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nature Materials 16, 236–243, doi: 10.1038/nmat4781 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Chen B-C et al. Lattice light-sheet microscopy: Imaging molecules to embryos at high spatiotemporal resolution. Science 346, 1257998, doi: 10.1126/science.1257998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Welch CM, Elliott H, Danuser G. & Hahn KM Imaging the coordination of multiple signaling activities in living cells. Nature Reviews Molecular Cell Biology 12, 749–756, doi: 10.1038/nrm3212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Han K. et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 580, 136–141, doi: 10.1038/s41586-020-2099-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Noskovicova N. et al. Suppression of the fibrotic encapsulation of silicone implants by inhibiting the mechanical activation of pro-fibrotic TGF-β. Nature Biomedical Engineering 5, 1437–1456, doi: 10.1038/s41551-021-00722-z (2021). [DOI] [PubMed] [Google Scholar]
- 230.Chen K. et al. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Science Translational Medicine 14, eabj9152, doi: 10.1126/scitranslmed.abj9152. [DOI] [PubMed] [Google Scholar]
- 231.Umehara T. et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Science Advances 8, eabn4564, doi: 10.1126/sciadv.abn4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Winkler J, Abisoye-Ogunniyan A, Metcalf KJ & Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nature Communications 11, 5120, doi: 10.1038/s41467-020-18794-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Madl CM, Heilshorn SC & Blau HM Bioengineering strategies to accelerate stem cell therapeutics. Nature 557, 335–342, doi: 10.1038/s41586-018-0089-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yeung T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motility 60, 24–34, doi: 10.1002/cm.20041 (2005). [DOI] [PubMed] [Google Scholar]
- 235.Branco da Cunha C. et al. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35, 8927–8936, doi: 10.1016/j.biomaterials.2014.06.047 (2014). [DOI] [PubMed] [Google Scholar]
- 236.Stowers RS, Allen SC & Suggs LJ Dynamic phototuning of 3D hydrogel stiffness. Proceedings of the National Academy of Sciences 112, 1953–1958, doi:doi: 10.1073/pnas.1421897112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cameron AR, Frith JE, Gomez GA, Yap AS & Cooper-White JJ The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials 35, 1857–1868, doi: 10.1016/j.biomaterials.2013.11.023 (2014). [DOI] [PubMed] [Google Scholar]
- 238.Chaudhuri O. et al. Substrate stress relaxation regulates cell spreading. Nature Communications 6, 6365, doi: 10.1038/ncomms7365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Huebsch N. et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nature Materials 14, 1269–1277, doi: 10.1038/nmat4407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Trappmann B. et al. Extracellular-matrix tethering regulates stem-cell fate. Nature Materials 11, 642–649, doi: 10.1038/nmat3339 (2012). [DOI] [PubMed] [Google Scholar]
- 241.Hakkinen KM, Harunaga JS, Doyle AD & Yamada KM Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices. Tissue Eng Part A 17, 713–724, doi: 10.1089/ten.TEA.2010.0273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.McBeath R, Pirone DM, Nelson CM, Bhadriraju K. & Chen CS Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell 6, 483–495, doi: 10.1016/S1534-5807(04)00075-9 (2004). [DOI] [PubMed] [Google Scholar]
- 243.Kassianidou E. et al. Extracellular Matrix Geometry and Initial Adhesive Position Determine Stress Fiber Network Organization during Cell Spreading. Cell Reports 27, 1897–1909.e1894, doi: 10.1016/j.celrep.2019.04.035 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ulrich TA, de Juan Pardo EM & Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69, 4167–4174, doi: 10.1158/0008-5472.Can-08-4859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Raab M. et al. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. Journal of Cell Biology 199, 669–683, doi: 10.1083/jcb.201205056 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Plotnikov Sergey V., Pasapera Ana M., Sabass, B. & Waterman, Clare M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 151, 1513–1527, doi: 10.1016/j.cell.2012.11.034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ulrich TA, Jain A, Tanner K, MacKay JL & Kumar S. Probing cellular mechanobiology in three-dimensional culture with collagen–agarose matrices. Biomaterials 31, 1875–1884, doi: 10.1016/j.biomaterials.2009.10.047 (2010). [DOI] [PubMed] [Google Scholar]
- 248.Adebowale K. et al. Enhanced substrate stress relaxation promotes filopodia-mediated cell migration. Nature Materials 20, 1290–1299, doi: 10.1038/s41563-021-00981-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Trappmann B. et al. Matrix degradability controls multicellularity of 3D cell migration. Nature Communications 8, 371, doi: 10.1038/s41467-017-00418-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hu X. et al. Control cell migration by engineering integrin ligand assembly. Nature Communications 13, 5002, doi: 10.1038/s41467-022-32686-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Garbett D. et al. T-Plastin reinforces membrane protrusions to bridge matrix gaps during cell migration. Nature Communications 11, 4818, doi: 10.1038/s41467-020-18586-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Reversat A. et al. Cellular locomotion using environmental topography. Nature 582, 582–585, doi: 10.1038/s41586-020-2283-z (2020). [DOI] [PubMed] [Google Scholar]
- 253.Kloxin AM, Kasko AM, Salinas CN & Anseth KS Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 324, 59–63, doi:doi: 10.1126/science.1169494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nikolaev M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578, doi: 10.1038/s41586-020-2724-8 (2020). [DOI] [PubMed] [Google Scholar]
- 255.Mih JD, Marinkovic A, Liu F, Sharif AS & Tschumperlin DJ Matrix stiffness reverses the effect of actomyosin tension on cell proliferation. Journal of Cell Science 125, 5974–5983, doi: 10.1242/jcs.108886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Han WM et al. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Science Advances 4, eaar4008, doi:doi: 10.1126/sciadv.aar4008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vitiello E. et al. Acto-myosin force organization modulates centriole separation and PLK4 recruitment to ensure centriole fidelity. Nature Communications 10, 52, doi: 10.1038/s41467-018-07965-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Moriarty RA & Stroka KM Physical confinement alters sarcoma cell cycle progression and division. Cell Cycle 17, 2360–2373, doi: 10.1080/15384101.2018.1533776 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Qin R. et al. Tumor Suppressor DAPK1 Catalyzes Adhesion Assembly on Rigid but Anoikis on Soft Matrices. Frontiers in Cell and Developmental Biology 10, doi: 10.3389/fcell.2022.959521 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Farrelly N, Lee Y-J, Oliver J, Dive C. & Streuli CH Extracellular Matrix Regulates Apoptosis in Mammary Epithelium through a Control on Insulin Signaling. Journal of Cell Biology 144, 1337–1348, doi: 10.1083/jcb.144.6.1337 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Indra I. et al. An in vitro correlation of mechanical forces and metastatic capacity. Physical Biology 8, 015015, doi: 10.1088/1478-3975/8/1/015015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kraning-Rush CM, Califano JP & Reinhart-King CA Cellular Traction Stresses Increase with Increasing Metastatic Potential. PLOS ONE 7, e32572, doi: 10.1371/journal.pone.0032572 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.van den Berg MCW et al. Proteolytic and Opportunistic Breaching of the Basement Membrane Zone by Immune Cells during Tumor Initiation. Cell Reports 27, 2837–2846.e2834, doi: 10.1016/j.celrep.2019.05.029 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wolf K. et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nature Cell Biology 9, 893–904, doi: 10.1038/ncb1616 (2007). [DOI] [PubMed] [Google Scholar]
- 265.Shi Q. et al. Rapid disorganization of mechanically interacting systems of mammary acini. Proceedings of the National Academy of Sciences 111, 658–663, doi:doi: 10.1073/pnas.1311312110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lee J, Abdeen AA, Wycislo KL, Fan TM & Kilian KA Interfacial geometry dictates cancer cell tumorigenicity. Nature Materials 15, 856–862, doi: 10.1038/nmat4610 (2016). [DOI] [PubMed] [Google Scholar]
- 267.Hushka EA, Yavitt FM, Brown TE, Dempsey PJ & Anseth KS Relaxation of Extracellular Matrix Forces Directs Crypt Formation and Architecture in Intestinal Organoids. Advanced Healthcare Materials 9, 1901214, doi: 10.1002/adhm.201901214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wang WY et al. Direct comparison of angiogenesis in natural and synthetic biomaterials reveals that matrix porosity regulates endothelial cell invasion speed and sprout diameter. Acta Biomaterialia 135, 260–273, doi: 10.1016/j.actbio.2021.08.038 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang CCB, Hung CT & Mow VC An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. Journal of Biomechanics 34, 75–84, doi: 10.1016/S0021-9290(00)00137-8 (2001). [DOI] [PubMed] [Google Scholar]
- 270.Charbonier F, Indana D. & Chaudhuri O. Tuning Viscoelasticity in Alginate Hydrogels for 3 D Cell Culture Studies. Current Protocols 1, e124, doi: 10.1002/cpz1.124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gjorevski N. & Lutolf MP Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nature Protocols 12, 2263–2274, doi: 10.1038/nprot.2017.095 (2017). [DOI] [PubMed] [Google Scholar]
- 272.Kloxin AM, Tibbitt MW & Anseth KS Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nature Protocols 5, 1867–1887, doi: 10.1038/nprot.2010.139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Serban MA, Scott A. & Prestwich GD Use of Hyaluronan-Derived Hydrogels for Three-Dimensional Cell Culture and Tumor Xenografts. Current Protocols in Cell Biology 40, 10.14.11–10.14.21, doi: 10.1002/0471143030.cb1014s40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Caliari SR & Burdick JA A practical guide to hydrogels for cell culture. Nature Methods 13, 405–414, doi: 10.1038/nmeth.3839 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Correa S. et al. Translational Applications of Hydrogels. Chemical Reviews 121, 11385–11457, doi: 10.1021/acs.chemrev.0c01177 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chan Clarence E. & Odde David J. Traction Dynamics of Filopodia on Compliant Substrates. Science 322, 1687–1691, doi: 10.1126/science.1163595 (2008). [DOI] [PubMed] [Google Scholar]
- 277.Elosegui-Artola A. et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nature Cell Biology 18, 540–548, doi: 10.1038/ncb3336 (2016). [DOI] [PubMed] [Google Scholar]
- 278.Kanchanawong P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584, doi: 10.1038/nature09621 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kumar S. et al. Viscoelastic Retraction of Single Living Stress Fibers and Its Impact on Cell Shape, Cytoskeletal Organization, and Extracellular Matrix Mechanics. Biophysical Journal 90, 3762–3773, doi: 10.1529/biophysj.105.071506 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.del Rio A. et al. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science 323, 638–641, doi: 10.1126/science.1162912 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Grashoff C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266, doi: 10.1038/nature09198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Dupont S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183, doi: 10.1038/nature10137 (2011). In this paper, the authors revealed the role of the YAP/TAZ transcription co-regulators in as central regulators of mechanotransduction in 2D culture, mediating cell spreading, proliferation and apoptosis, and differentiation in response to stiffness.
- 283.Elosegui-Artola A. et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 171, 1397–1410.e1314, doi: 10.1016/j.cell.2017.10.008 (2017). [DOI] [PubMed] [Google Scholar]
- 284.Shiu J-Y, Aires L, Lin Z. & Vogel V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nature Cell Biology 20, 262–271, doi: 10.1038/s41556-017-0030-y (2018). [DOI] [PubMed] [Google Scholar]
- 285.Oria R. et al. Force loading explains spatial sensing of ligands by cells. Nature 552, 219–224, doi: 10.1038/nature24662 (2017). [DOI] [PubMed] [Google Scholar]
- 286.Hui E, Gimeno KI, Guan G. & Caliari SR Spatiotemporal Control of Viscoelasticity in Phototunable Hyaluronic Acid Hydrogels. Biomacromolecules 20, 4126–4134, doi: 10.1021/acs.biomac.9b00965 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hadden William J. et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proceedings of the National Academy of Sciences 114, 5647–5652, doi: 10.1073/pnas.1618239114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Jain N. & Vogel V. Spatial confinement downsizes the inflammatory response of macrophages. Nature Materials 17, 1134–1144, doi: 10.1038/s41563-018-0190-6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jain N, Iyer KV, Kumar A. & Shivashankar GV Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proceedings of the National Academy of Sciences 110, 11349–11354, doi: 10.1073/pnas.1300801110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.