Abstract
5-Oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) is an arachidonic acid metabolite formed by oxidation of the 5-lipoxygenase (5-LO) product 5S-hydroxy-6,8,11,14-eicosatetraenoic acid (5S-HETE) by the NADP+-dependent enzyme 5-hydroxyeicosanoid dehydrogenase. It is the only 5-LO product with appreciable chemoattractant activity for human eosinophils. Its actions are mediated by the selective OXE receptor, which is highly expressed on eosinophils, basophils, neutrophils and monocytes. Orthologs of the OXER1 gene, which encodes this receptor, are found in many species except for rodents. Intradermal injection of 5-oxo-ETE into humans and monkeys elicits eosinophil infiltration into the skin, raising the possibility that it may play a pathophysiological role in eosinophilic diseases. To investigate this and possibly identify a novel therapy we sought to prepare synthetic antagonists that could selectively block the OXE receptor. We synthesized a series of indole-based compounds bearing substituents that mimic the regions of 5-oxo-ETE that are required for biological activity, which we modified to reduce metabolism. The most potent of these OXE receptor antagonists is S-Y048, which is a potent inhibitor of 5-oxo-ETE-induced calcium mobilization (IC50, 20 pM) and has a long half-life following oral administration. S-Y048 inhibited allergen-induced eosinophil infiltration into the skin of rhesus monkeys that had been experimentally sensitized to house dust mite and inhibited pulmonary inflammation resulting from challenge with aerosolized allergen. These data provide the first evidence for a pathophysiological role for 5-oxo-ETE in mammals and suggest that potent and selective OXE receptor antagonists such as S-Y048 may be useful therapeutic agents in asthma and other eosinophilic diseases.
Keywords: 5-Oxo-ETE, OXE receptor antagonists, 5-Lipoxygenase products, Inflammation, Allergy
Graphical Abstract

1. Introduction
Arachidonic acid (AA) is a polyunsaturated fatty acid (PUFA) that is a key biological intermediate due to its conversion by a variety of enzymes to a large number of important mediators. The two major pathways of AA metabolism are initiated by the actions of cyclooxygenase, resulting in the formation of prostaglandins and thromboxanes, and 5-lipoxygenase (5-LO), which is responsible for the formation of leukotrienes. The 5-LO pathway was discovered in the late 1970s by Borgeat and Samuelsson, who showed that this enzyme oxygenates AA to 5-hydroperoxy-6,8,11,14-eicosatetraenoic acid (5S-HpETE) and then cyclizes 5S-HpETE to the unstable epoxide LTA4 (Fig. 1) [1]. LTA4 is then converted to either LTB4 by LTA4 hydrolase [2] or to LTC4 following the addition of GSH by LTC4 synthase [3]. Successive removal of γ-glutamate and glycine then leads to formation of LTD4 and LTE4 [4].
Figure 1.

Biosynthetic pathways and receptors for 5-LO products
LTB4 is a potent proinflammatory mediator, the actions of which are mediated by the BLT1 receptor, which is most highly expressed on neutrophils and to a lesser extent on other leukocytes, including monocytes, eosinophils, basophils, and lymphocytes [5]. Despite the expression of BLT1 on human eosinophils and the stimulatory effects of LTB4 on various eosinophil responses such as calcium mobilization and surface expression of CD11b [6], it is a very weak chemoattractant for these cells in humans [7–9], in contrast to its potent chemoattractant effects on rodent eosinophils [10]. Although LTB4 can also activate the BLT2 receptor, its main ligand is the cyclooxygenase product 12S-hydroxyheptadecatrienoic acid [11].
The first receptor recognized for cysLTs was the cysLT1 receptor, which is a key receptor in allergy and asthma and mediates the proinflammatory and bronchoconstrictor effects of LTD4 [12]. This receptor is a target for drugs used in the treatment of asthma, including montelukast, zafirlukast and pranlukast [5]. Subsequently, a second cysLT receptor, cysLT2, was identified and shown to have equal affinity for LTs C4 and D4 [13]. This receptor has been reported to attenuate the activation of the cysLT1 receptor by forming heterodimers [14]. GPR99 has been identified as a third cysLT receptor (cysLT3) selective for LTE4 [15], and has been reported to be involved in increased mucus production by pulmonary epithelial cells [16].
Another arm of the 5-LO pathway leads to the formation of lipoxin A4 (LXA4), which is formed in combination with 15-lipoxygenase (15-LO) and acts via the ALX receptor [17]. In contrast to all of the other 5-LO products, which are proinflammatory, LXA4 promotes the resolution of inflammation and appears to play a protective role in asthma. Administration of a metabolically stable LXA4 analog was found to block allergen-induced hyperresponsiveness and pulmonary inflammation in OVA-sensitized mice [18]. In a more recent study, LXA4 was found to increase NK cell-mediated eosinophil apoptosis and to inhibit IL-13 release by ILC2s [19].
2. Formation of 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid (5-oxo-ETE)
2.1. 5-oxo-ETE is formed by a selective dehydrogenase
We obtained evidence for the presence of an additional 5-LO product following a study on the ω-oxidation of LTB4 and its 6-trans isomers to 20-hydroxy metabolites by human neutrophils [20]. We found that in addition to hydroxylating 6-trans-LTB4 and 12-epi-6-trans-LTB4, neutrophils convert these compounds to dihydro metabolites via a 5-oxo intermediate, implicating a dehydrogenase as the initial step in their formation [21]. In contrast, LTB4, which has a 6-cis double bond and is the preferred substrate for ω-hydroxylation, was not metabolized by this pathway. Since the above 6-trans isomers are nonenzymatic degradation products of LTA4 and are biologically inactive, the existence of a selective pathway for their metabolism seemed unlikely. We therefore focused on 5S-HETE, which is an abundant 5-LO product containing a 6-trans double bond, as a potential substrate for this enzyme. We found that microsomal fractions from human neutrophils contain an NADPH-dependent dehydrogenase (5-HEDH; 5-hydroxyeicosanoid dehydrogenase) that is extremely selective for eicosanoids containing a hydroxyl group in the 5-position and in the S-configuration, followed by a 6-trans double bond [22]. The preferred substrate for this enzyme proved to be 5S-HETE, which is converted to 5-oxo-ETE.
In parallel with the above studies, Schröder and coworkers found that eosinophils convert AA to an eosinophil chemoattractant that they named “eosinophil chemotactic lipid” [8]. They subsequently showed that soybean lipoxygenase converts AA to a substance with identical properties, which they identified as 5-oxo-15-hydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid [23]. At the same time, we found that the latter substance is also formed by the oxidation of the combined 5-LO/15-LO product 5S,15S-dihydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid by neutrophil 5-HEDH [22]. These results suggested that 5-HEDH may be responsible for the formation of potent proinflammatory lipid mediators that may play important roles in inflammatory diseases such as asthma.
2.2. Regulation of 5-oxo-ETE synthesis
Although neutrophil microsomes display high 5-HEDH activity, intact neutrophils release relatively small amounts of 5-oxo-ETE when stimulated with calcium ionophore [24]. This is due to the high selectivity of 5-HEDH for NADP+, which is present within cells primarily as its reduced form, NADPH [25]. Thus, in addition to activation of cytosolic phospholipase A2 (cPLA2) and 5-LO by calcium and phosphorylation, the synthesis of 5-oxo-ETE requires the elevation of intracellular NADP+, which occurs in neutrophils following activation of the respiratory burst [26] as well as during the process of cell death [27]. Resting neutrophils convert 5S-HETE principally to 20-hydroxy-5S-HETE by the action of the cytochrome P450 enzyme LTB4 20-hydroxylase. In contrast, 5-oxo-ETE is the major metabolite of 5S-HETE in neutrophils in which NADPH oxidase has been activated by phorbol myristate acetate [26]. Activation of the respiratory burst also increases 5-oxo-ETE synthesis in monocytes [28] and eosinophils [9] (Table 1). High levels of 5-HEDH activity are also present in B lymphocytes [29], but in this case the synthesis of 5-oxo-ETE is driven by oxidative stress, which augments intracellular NADP+ levels through the GSH redox cycle. Oxidative stress also strongly promotes 5-oxo-ETE synthesis by monocytes [30].
Table 1.
Synthesis of 5-oxo-ETE by different types of cells
| AA ↓ 5oETE |
5-HETE ↓ 5oETE |
Regulation of [NADP+] | ||||
|---|---|---|---|---|---|---|
| Cell type | NOX | Ox stress | Cell death | Ref | ||
| Neutrophils | ✓ | ✓ | ✓ | ✓ | [27, 30] | |
| Monocytes | ✓ | ✓ | ✓ | ✓ | [30] | |
| Eosinophils | ✓ | ✓ | ✓ | [9] | ||
| B lymphocytes | ✓ | ✓ | ✓ | [29] | ||
| Platelets | ✓ | ✓ | [30] | |||
| Airway epithelial cells | ✓ | ✓ | [31] | |||
| Airway smooth muscle cells | ✓ | ✓ | [31] | |||
| Endothelial cells | ✓ | ✓ | [32] | |||
| Keratinocytes* | ✓ | ✓ | [87] | |||
| Dendritic cells** | ✓ | ✓ | [124] | |||
| Tumor cells*** | ✓ | ✓ | ✓ | [33] | ||
Many types of inflammatory cells can convert AA to 5-oxo-ETE because they possess both 5-LO and 5-HEDH, whereas other types of cells have been shown to convert 5S-HETE to 5-oxo-ETE because they contain 5-HEDH but not necessarily 5-LO. Depending on the cell type, NADP+ levels can be regulated by activation of NADPH oxidase (NOX), oxidative stress (OX stress), or cell death
The substrate was 5S-HODE rather than 5S-HETE
Derived from monocytes
PC3 (prostate), MCF7 (breast), and A-427 (lung) tumor cells
The inflammatory cells discussed above possess both 5-LO and 5-HEDH and can therefore synthesize 5-oxo-ETE from endogenous arachidonic acid. However, a number of other cell types that contain little or no 5-LO activity, including platelets [30], airway epithelial and smooth muscle cells [31], and vascular endothelial cells [32] also possess high 5-HEDH activity and can convert 5S-HETE (but not arachidonic acid) to 5-oxo-ETE (Table 1). These cells could therefore utilize inflammatory cell-derived 5S-HETE to synthesize 5-oxo-ETE by transcellular biosynthesis, as we have shown to occur when PC3 prostate cancer cells are cocultured with neutrophils under conditions of oxidative stress [33]. Leukotrienes can also be formed by transcellular biosynthesis [34], including the synthesis LTC4 by both vascular endothelial cells and platelets from neutrophil-derived LTA4 and the synthesis of LTB4 by erythrocytes, also from neutrophil-derived LTA4.
The optimal sites for the production of 5-oxo-ETE are likely to be inflammatory foci, where activated phagocytes having high levels of intracellular NADP+ due to activation of the respiratory burst could convert endogenous AA to 5-oxo-ETE. These cells could then release both 5-oxo-ETE and its precursor 5S-HETE, which could subsequently be converted to additional 5-oxo-ETE by nearby structural cells, including airway epithelial and smooth muscle cells as well as vascular endothelial cells. Exposure of these cells to oxidants released by inflammatory cells could increase their NADP+ levels, thus favoring the synthesis of 5-oxo-ETE. Furthermore, as infiltrating inflammatory cells begin to undergo apoptosis, their ability to synthesize 5-oxo-ETE increases dramatically, as we have shown with neutrophils [27]. This raises the possibility that 5-oxo-ETE could play an important role in chronic inflammation, in contrast to some other lipid mediators like prostaglandin D2 (PGD2), which is rapidly released from mast cells following antigen stimulation [35] and may therefore be more important in acute inflammatory responses.
2.3. Formation of 5-oxo-ETE as a result of lipid peroxidation
Although the primary route for the synthesis of 5-oxo-ETE appears to be enzymatic, like most other eicosanoids, it can also be formed as a product of lipid peroxidation. Unlike isoprostanes and H(p)ETEs, 5-oxo-ETE does not have any chiral centers, so it will be biologically active irrespective of the mechanism of its formation, as long as the double bonds are in the correct configuration. Substantial amounts of 5-oxo-ETE were formed after exposure of erythrocytes to t-butyl hydroperoxide [36] as well as in model systems of chemically-induced lipid peroxidation of phospholipids [37]. 5-Oxo-ETE can also be formed by murine macrophages as a result of nonenzymatic heme-catalyzed dehydration of 5S-HpETE [38].
3. In vitro effects of 5-oxo-ETE
3.1. Effects of 5-oxo-ETE on neutrophils and eosinophils
We first investigated the effects of 5-oxo-ETE on neutrophils, in part because these were the cells in which we first identified the pathway for its formation, but also because its precursor 5S-HETE had previously been shown by O’Flaherty’s group to weakly activate these cells via what appeared to be a novel receptor [39]. We postulated that 5-oxo-ETE is the true ligand for this receptor and found it to be a potent activator of calcium mobilization and chemotaxis in neutrophils, with an EC50 that was about 100 times lower than that of 5S-HETE and 10 times more potent than 5-oxo-15S-HETE [40]. It also stimulates actin polymerization, surface expression of CD11b and adherence of these cells [41]. Although 5-oxo-ETE by itself has little [42] or no [43] effect on the production of superoxide by neutrophils, in the presence of GM-CSF [43], TNF-α or platelet-activating factor (PAF) [44] it is a potent stimulator of this response. Similarly, 5-oxo-ETE induces a modest degree of degranulation on its own, but this is dramatically enhanced in the presence of TNF-α or PAF.
The findings of Schroder’s group referred to above [23], demonstrating the powerful chemoattractant effects of 5-oxo-15S-HETE on eosinophils, suggested that the eosinophil may be a major target for 5-oxo-ETE. This proved to be the case, as 5-oxo-ETE was more potent than both 5-oxo-15S-HETE and PAF as an eosinophil chemoattractant [9] and has a variety of other effects on these cells [6, 45, 46]. In contrast, other 5-LO products, including both LTD4 and LTB4, which, despite its well documented effects on guinea pig eosinophils [7], had barely detectible chemoattractant effects on human eosinophils [9]. Interestingly, 5-oxo-ETE and LTB4 had similar potencies in stimulating surface expression of CD11b and intracellular calcium mobilization in these cells, but 5-oxo-ETE was a much better stimulator of actin polymerization and shedding of L-selectin [6]. It seems likely that the potent chemoattractant effects of 5-oxo-ETE on eosinophils are closely related to its powerful effects on actin polymerization. 5-Oxo-ETE also activates the respiratory burst in eosinophils [46] and mediates the stimulatory effects of PAF and IL-5 on the surface expression of the leukocyte activation marker CD69, whereas LTB4 has little effect on this response [47].
3.2. Synergistic interactions between 5-oxo-ETE and other eosinophil chemoattractants
A variety of mediators would be present at inflammatory sites and synergistic interactions among them are likely to be important in regulating the course of inflammation. For example, low concentrations of 5-oxo-ETE shift the concentration-response curve for PAF-induced eosinophil migration to the left [9]. Similarly, threshold concentrations of the CC chemokines eotaxin-1 (CCL11) and RANTES (CCL5) increase the chemotactic potency of 5-oxo-ETE [48]. PGD2 has also been shown to enhance 5-oxo-ETE-induced eosinophil migration [49]. Although 5-oxo-ETE alone has a relatively weak effect on eosinophil degranulation, in the presence of PAF it induced a very strong response [50]. Moreover, 5-oxo-ETE strongly enhances eosinophil degranulation induced by LTB4, C5a and fMLP.
3.3. Interactions between 5-oxo-ETE and GM-CSF
5-Oxo-ETE and GM-CSF can potentially interact at multiple levels. GM-CSF stimulates the formation of 5-LO products by increasing the release of arachidonic from inflammatory cells [51] as well as by increasing the expression of both 5-LO [52] and FLAP [53], and is therefore likely to increase the formation of 5-oxo-ETE. Conversely, we have shown that 5-oxo-ETE increases the production of GM-CSF by human monocytes and by this mechanism can increase the survival of eosinophils [54]. GM-CSF has also been shown to increase the responsiveness of eosinophils to 5-oxo-ETE as exemplified by O’Flaherty’s finding that priming with this cytokine results in a strongly enhanced degranulation response [50].
3.4. 5-Oxo-ETE promotes the transmigration of eosinophils across the basement membrane and endothelial cell monolayers
Although stimulation of eosinophil migration is critical for the tissue infiltration of these cells, they must also cross the vascular endothelium and the underlying basement membrane, a process that is dependent on secreted proteases. Laviolette’s group found that 5-oxo-ETE is a potent stimulator of the passage of eosinophils through Matrigel basement membranes [55], being more effective than both PAF and eotaxin [56]. The response to 5-oxo-ETE was enhanced by IL-5, which by itself had little effect on eosinophil transmigration [55]. The effect of 5-oxo-ETE was not due solely to its chemoattractant properties, but was also mediated by the release of MMP-9 from eosinophils as well as by the urokinase plasminogen activator receptor (uPAR) and was inhibited by antibodies against either MMP-9 or uPAR. As observed for Matrigel-coated membranes, 5-oxo-ETE also strongly promoted the passage of eosinophils through monolayers of human endothelial cells [57]. These results indicate that 5-oxo-ETE can promote the transendothelial migration of eosinophils by a combination of its chemoattractant effects and its stimulatory effects on the release or activation of tissue proteases, resulting in degradation of the extracellular matrix.
4. The 5-oxo-ETE receptor
4.1. The OXE receptor
Activation of neutrophils by 5-oxo-ETE shows a high degree of structural specificity, suggesting that it acts by a highly selective receptor [40, 44]. As noted above, 5-oxo-ETE is about 100 times more potent than its precursor 5S-HETE in activating neutrophils [40]. Moreover, pretreatment of neutrophils with 5-oxo-ETE selectively desensitizes these cells so that they no longer respond to subsequent addition of 5-oxo-ETE, but respond normally to other agonists such as LTB4. The stimulatory effect of 5-oxo-ETE on neutrophils was blocked by pertussis toxin, indicating that its actions are mediated by a G protein-coupled receptor (GPCR) [42, 43, 58]. O’Flaherty’s group subsequently identified binding sites on neutrophil plasma membranes that were highly selective for 5-oxo-ETE and sensitive to GTP, which reduced ligand binding, as expected for a GPCR [59]. The binding interaction was complicated by esterification of 5-oxo-ETE into cellular lipids, but this could be prevented by pretreatment with the acyl-CoA synthetase inhibitor triacsin C. The affinity of the neutrophil membrane binding sites for 5-oxo-ETE (Kd, 4 nM) was close to the EC50 value (2 nM) found for its effect on calcium mobilization in these cells [40].
The receptor for 5-oxo-ETE was cloned by three separate groups. With the aid of a BLAST search Hosoi and coworkers identified an intronless nucleotide sequence with homology to GPCRs in chromosome 2p21 [60]. This sequence, which they tentatively named TG1019, was predicted to code for a protein containing 423 amino acids. They then used constructs of TG1019 fused to Gαi, Gαs, or Gαq to screen a library of potential ligands for orphan receptors based on their ability to promote the binding of GTPγS. In the same year, Takeda and coworkers independently identified the same sequence, codenamed hGPCR48, as the receptor for 5-oxo-ETE [61]. In both cases, 5-oxo-ETE was by far the most potent ligand among those tested, with an EC50 of about 6 nM for stimulation of GTPγS binding to the fusion protein. The following year Jones et al identified a sequence, termed R527, that had high homology to the hydroxycarboxylic acid receptor HCAR3 (then known as HM74) [62]. R527 was nearly identical to TG1019/hGPCR44, except that it lacked the initial 39 amino acids at the amino terminus, and had 5-oxo-ETE as its preferred ligand. The receptor for 5-oxo-ETE (TG1019/hGPCR48/R527) has been designated as the OXE receptor by the IUPHAR Nomenclature Committee for Leukotriene and Lipoxin Receptors with the corresponding gene being OXER1 [63].
In humans OXER1 mRNA is highly expressed in peripheral blood leukocytes [60], particularly in eosinophils [62] and basophils [64, 65] and to a lesser extent in neutrophils, monocytes and macrophages. Among human GPCRs the OXE receptor is most closely related to the hydroxycarboxylic acid receptors HCA1, HCA2, and HCA3, with which it bears about 40% sequence identity. These receptors are encoded by the HCAR1, HCAR2 and HCAR3 genes and have as their principal ligands lactic acid, 3-hydroxybutyric acid, and 3-hydroxyoctanoic acid, respectively [66]. The HCA2 receptor is also activated by nicotinic acid. The OXE receptor is less related to other eicosanoid receptors, bearing 34% sequence identity at the protein level with the receptor for 12S-HETE (GPR31/HETER1) and 23% identity with the CysLT1 receptor for LTD4.
Many species possess orthologs of human OXER1, with the notable exception of rodents. 5-Oxo-ETE is a potent activator of eosinophils and neutrophils from both cats [67] and monkeys [68], which have OXE receptors that are about 79 and 96% identical, respectively, in sequence to the human receptor. 5-Oxo-ETE also activates zebrafish leukocytes, acting by a receptor encoded by oxer1/hcar1–4 [69]. The human protein most closely related to the protein encoded in zebrafish by the mRNA for this gene is the OXE receptor (34% sequence identity), followed by the human HCA1, HCA2 and HCA3 receptors, which have about 31% sequence identity.
4.2. Other potential receptors for 5-oxo-ETE
Although rodents lack orthologs of OXER1, both in vitro and in vivo studies with rats, mice and guinea pigs suggest that it may act in these species, presumably via a different receptor. For example, in two separate studies we found that 5-oxo-ETE induces infiltration of eosinophils into the lungs of Brown Norway rats [70, 71]. 5-Oxo-ETE has also been reported to activate certain mouse cell lines, stimulating cAMP-dependent steroidogenesis in murine MA-10 Leydig cells [72] and Gαi-dependent migration of MG5 murine microglial cells [73], although in the latter case micromolar concentrations were required. 5-Oxo-ETE was recently implicated in causing pain in some cases of irritable bowel syndrome in both humans and a mouse animal model, although the concentrations required for these effects were in the micromolar range [74]. At least in the mouse, these effects appear to be mediated by Mrgprd, a receptor found on dorsal root ganglion afferents that is also activated by β-alanine and has about 23% identity with the human OXE receptor.
5-Oxo-ETE also has some activity in the guinea pig. It contracts guinea pig bronchial smooth muscle, by a mechanism that is dependent on TXA2, since its effects are blocked by the cyclooxygenase inhibitor indomethacin as well as by the selective TP receptor antagonist SQ-29548 [75]. Interestingly, it appears to have the opposite effect on human bronchi, inducing relaxation of bronchial smooth muscle precontracted with methacholine [76]. This effect appeared to be due to activation of BKCa channels, since it was blocked by iberiotoxin, but was probably not related to the OXE receptor, since it was not blocked by pertussis toxin. Moreover, OXER1 mRNA was undetectable in human airway smooth muscle cells [62]. 5-Oxo-ETE also evokes isotonic volume reduction of guinea pig intestinal crypt epithelial cells, which is dependent on chloride secretion and involves activation of protein kinase C [77]. In contrast, jejunal villus cell volume was unaffected.
With the exception of Mrgprd in the mouse, no information is available on the identities of the receptors mediating the actions of 5-oxo-ETE in rodents. All three of the above species have genes for hydroxycarboxylic acid receptors (Hcar1 and Hcar2), with the corresponding proteins having approximately 40% sequence identity with the human OXE receptor. Therefore, it is possible that one or both of these receptors might mediate the effects of 5-oxo-ETE in rodents. So far, there is no evidence for the existence of 5-oxo-ETE receptors other than the OXE receptor in humans or other primates. As discussed below (Section 7.2), selective OXE receptor antagonists completely block the activation of neutrophils and eosinophils by 5-oxo-ETE, suggesting that at least in these cases, no other 5-oxo-ETE receptors are involved.
5. Intracellular signaling mechanisms for the OXE receptor
The actions of 5-oxo-ETE, in particular its chemoattractant effects, appear to be mediated primarily through Gi/o proteins, as most of its actions can be blocked by pertussis toxin [42, 43, 58]. Unlike the receptors for many other chemoattractant receptors, the OXE receptor does not appear to be appreciably coupled to Gq [60, 78]. The most rapid responses of granulocytes to 5-oxo-ETE are calcium mobilization and actin polymerization [6, 45, 46], which occur within the first few seconds of exposure and are blocked by pertussis toxin. Calcium mobilization is also blocked by the phospholipase C (PLC) inhibitor U73122 [79], indicating that the initial step in this process is activation of PLC, leading to the formation of inositol trisphosphate (IP3) and diacylglycerol (DAG), resulting respectively in intracellular calcium release and activation of both conventional and novel protein kinase C (PKC) isoforms (Fig. 2). The novel PKC isoform PKCδ has been implicated in the effects of 5-oxo-ETE on eosinophil motility and the release of the metalloproteinase MMP-9, which is involved in promoting the transmigration of granulocytes from the circulation into tissues [80]. There is also evidence for the involvement of the atypical PKC isoform PKCζ in eosinophil migration [80]. In addition, the novel PKC isoform, PKCε, has been reported to mediate the proliferative effect of 5-oxo-ETE on prostate cancer cells and this effect can be blocked by U73122, confirming the role of PLC in initiating this response [81].
Figure 2.

Intracellular signaling pathways coupled to the OXE receptor and cellular responses induced in granulocytes by 5-oxo-ETE.
5-Oxo-ETE has also been shown to activate phosphatidylinositol 3-kinase (PI3K), resulting in the phosphorylation of Akt [42, 79]. Inhibitors of both PI3K (LY294002) and PLC (U73122), as well as pertussis toxin, strongly inhibit 5-oxo-ETE-induced cell migration, indicating that both the PLC and the PI3K pathway are involved in this response [79]. 5-Oxo-ETE also activates the MAP kinase pathway, inducing phosphorylation of ERK-1 and ERK-2. However, in this case, there is disagreement in the literature on the sensitivity of this response to pertussis toxin [43, 79, 82], raising the possibility of additional signaling mechanisms, possibly involving the recruitment of β-arrestin [82]. 5-Oxo-ETE also activates cPLA2, inducing its phosphorylation, and this is thought to be mediated by MAP kinases [43]. Studies with selective inhibitors suggest that both ERK-1/2 and p38 are involved in the stimulatory effect of 5-oxo-ETE on CD11b expression in eosinophils, whereas ERK1/2, (along with PKCδ, as mentioned above) mediate its effect on MMP-9 secretion [80].
Consistent with the role of a Gi protein in mediating the response to activation of the OXE receptor, 5-oxo-ETE inhibits cAMP formation by adenylyl cyclase in both transfected cells [60] and neutrophils [83]. However, the functional significance of this response is unclear, as Kostenis’ group identified a biased OXE receptor antagonist, Gue1654, that blocked nearly all of the responses to 5-oxo-ETE that were tested, including cell migration, actin polymerization, calcium mobilization, and CD11b expression, but did not inhibit forskolin-stimulated cAMP formation [83]. This is presumably because Gue1654 forms a stable complex with the OXE receptor in which the αi-mediated inhibition of adenylyl cyclase is unaffected, whereas responses mediated by the βγ subunits and β-arrestin are inhibited. Thus, the OXE receptor-mediated functional responses of granulocytes to 5-oxo-ETE are mediated principally by βγ and possibly β-arrestin rather than by the αi subunit of Gi.
6. Structure-activity relationships for activation of the OXE receptor
We conducted extensive structure-activity studies to define the regions of 5-oxo-ETE that are essential for activation of the OXE receptor. In addition to the 5-oxo group, a free carboxyl group is required for potent biological activity [58]. The 5-oxo group must be conjugated with at least two double bonds (5-oxo-Δ6E,8Z) and the total chain length must be at least 18 carbons [84]. The ω-end of the molecule must be hydrophobic, as addition of a hydroxyl group at C20 by LTB4 20-hydroxylase results in dramatically reduced agonist activity [58]. Although still reducing activity by about 10-fold [85], a hydroxyl group at C15 as in 5-oxo-15S-HETE, is better tolerated. This product is formed by eosinophils by the combined actions of 5-LO, 15-LO and 5-HEDH [9, 45]. In contrast, hydroxylation of 5-oxo-ETE at C12 by platelet 12-LO, resulting in the formation of 5-oxo-12S-HETE, abolishes agonist activity, and instead results in the formation of an OXE receptor antagonist with an IC50 of about 500 nM [86].
Certain 5-oxo metabolites of PUFA other than AA are also potent OXE receptor agonists. Sebaleic acid (5Z,8Z-octadecadienoic acid), which is the major PUFA in human sebum and skin surface lipids, is converted to 5-oxo-6E,8Z-octadecadienoic acid (5-oxo-ODE) by human neutrophils [87], whereas its 8-trans isomer, 5-oxo-8-trans-ODE, is a constituent of the tubers of the South American plant Lepidium meyenii [88]. These two C18 compounds have potencies almost identical to 5-oxo-ETE in activating both human neutrophils and eosinophils [84, 87]. 5-Oxo-ODE could thus potentially contribute to granulocyte infiltration in inflammatory diseases of the skin, especially considering that sebaleic acid cannot be converted to leukotrienes. Similarly, Mead acid (5Z,8Z,11Z-eicosatrienoic acid), which can accumulate under conditions of essential fatty acid deficiency [89], is converted by neutrophils to 5-oxo-6E,8Z,11Z-eicosatrienoic acid (5-oxo-ETrE), which activates both neutrophils and eosinophils with potencies similar to 5-oxo-ETE [84]. 5-Oxo-ETrE would be the most important granulocyte chemoattractant among 5-LO products derived from Mead acid, since this PUFA is not converted to significant amounts of LTB3 due to the inhibition of LTA4 hydrolase by LTA3 [90]. Neutrophils convert the ω3 PUFA 5Z,8Z,11Z,14Z,17Z-eicosapentaenoic acid (EPA) to 5-oxo-6E,8Z,11Z,14Z,17Z-eicosapentaenoic acid (5-oxo-EPE), which is approximately 5 times less potent than 5-oxo-ETE in activating neutrophils and eosinophils [84, 91].
7. OXE receptor antagonists
The absence of OXER1 in rodents has impeded investigation into the pathophysiological role of 5-oxo-ETE because of the unavailability of gene knockout models. For this reason, the availability of selective OXE receptor antagonists is critical to establish the potential roles of this mediator in inflammatory diseases. Since the eosinophil is a major target of 5-oxo-ETE, such antagonists may be useful therapeutically as anti-inflammatory agents in allergic diseases such as asthma.
The effects of 5-oxo-ETE could also be prevented by using a 5-LO inhibitor or a FLAP antagonist, which would have the additional advantage of blocking the formation of cysLTs (Fig. 3). However, these agents would also block the formation of LXA4, which has been shown to have a protective effect in asthma and other inflammatory conditions [18]. They would also inhibit LTB4 formation, which might have somewhat unpredictable effects. There is some evidence that LTB4 plays a role in asthma, principally through effects on dendritic cells and lymphocyte subsets, rather than its classic target cell, the neutrophil [92]. Although animal experiments lend some support for such a role, clinical studies in asthmatics using a BLT1 antagonist and an LTA4 hydrolase inhibitor have been unsuccessful [93]. On the other hand, there is evidence for a beneficial role for LTB4 in host defense against both bacterial [94] and viral [95, 96] infections. Thus, an OXE receptor antagonist, either on its own or combined with a cysLT1 antagonist, might offer a more selective way to block the effects of proinflammatory 5-LO products involved in asthma without affecting the potentially beneficial effects of LXA4 and LTB4.
Figure 3. Comparison of OXE antagonists and 5-LO/FLAP inhibitors.

An OXE antagonist will block the actions of 5-oxo-ETE irrespective of its source but will not affect the beneficial actions of LXA4 and LTB4.
7.1. Naturally occurring OXE receptor antagonists
We found that stimulation of mixtures of neutrophils and platelets with calcium ionophore in the presence of phorbol myristate acetate led to the formation of 5-oxo-12S-HETE (Fig. 4) by transcellular metabolism due to the combined actions of 5-LO and 5-HEDH from neutrophils and 12-LO from platelets [86]. As noted above, this metabolite of 5-oxo-ETE was devoid of OXE agonist activity, but instead blocked neutrophil activation by 5-oxo-ETE. Although this was the first report of an OXE receptor antagonist, this compound was unsuitable for in vivo studies because of its instability and susceptibility to metabolism. Various PUFA, including 4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic acid (DHA), EPA, dihomo-γ-linolenic acid, and Mead acid were reported to antagonize the activation of the OXE receptor in transfected cells [60]. However, these fatty acids were not very potent (IC50 ~2 to 6 μM) and it is unlikely that they would be selective for the OXE receptor. Moreover, they can be converted to a variety of biologically active metabolites, including 5-oxo-ETE analogs as noted above and the DHA metabolite protectin D1 (PD1) [97]. PD1 has been reported to reduce eosinophil activation and migration in response to 5-oxo-ETE, although this effect was not selective for the OXE receptor, as the responses to other eosinophil agonists were also reduced [98].
Figure 4. Potencies of some OXE receptor antagonists.

Concentration-response curves for the inhibition of calcium mobilization in human neutrophils stimulated with 5-oxo-ETE (10 nM), added 2 min after the addition of the antagonist: initial lead compound (Δ; ref. [103]), 5-oxo-12S-HETE (♦; ref. [86]), Gue1654 (∇; ref. [103]), S-230 (▲), S-C025 (○; ref. [107]), and S-Y048 (●; ref. [108]). The effects of Gue1654 on 5-oxo-ETE-induced calcium mobilization were determined as described previously [103].
A recent study suggests that testosterone may be an endogenous OXE receptor antagonist. Although steroids classically act via nuclear receptors, there is considerable interest in extranuclear receptors located in the plasma membrane and elsewhere in the cell [99]. In an attempt to identify membrane receptors for testosterone, Kalyvianaki et al. incubated DU145 prostate cancer cells with a membrane impermeable form of testosterone (testosterone-BSA-FITC) and searched for testosterone binding proteins having the characteristics of membrane receptors [100]. They found only one such protein, which they identified as the OXE receptor, and showed that testosterone could inhibit 5-oxo-ETE-induced actin redistribution, cell migration, and inhibition of cAMP formation in DU145 cells. Testosterone could impact the actions of 5-oxo-ETE in other ways as well, as it has been shown to inhibit 5-LO activity, thereby explaining the reduced ability of human neutrophils from males to synthesize 5-LO products compared to neutrophils from females [101]. These effects of testosterone may contribute to the reduced incidence of asthma in men compared to women [102]. If 5-oxo-ETE is an important proinflammatory mediator in asthma as we suspect, its role may be diminished in men compared to women, and drugs targeting the OXE receptor may therefore be more effective in women.
7.2. Synthetic OXE receptor antagonists
To identify OXE receptor antagonists Kostenis’ group used a combination of pharmacological and in silico approaches. They screened a library of small molecules using HEK293 cells transfected with OXER1 and the promiscuous Gα16 protein and then employed computer modeling and further testing to identify Gue1654 (Fig. 4) as a biased antagonist that blocked all of the actions of 5-oxo-ETE on neutrophils and eosinophils except for inhibition of adenylyl cyclase (see Section 5).
We used a different approach, based on structure-activity relationships, to design synthetic OXE receptor antagonists. From the structure-activity studies described in Section 6 we knew that the regions of 5-oxo-ETE that are critical for engagement of the OXE receptor included the initial 5-oxovalerate grouping (C1-C5), at least 2 double bonds conjugated with the keto group, and the terminal hydrophobic region of the molecule. Our strategy was to incorporate these groups into a rigid molecule using indole as the backbone to give conformationally-restricted molecules that would bind strongly to the receptor and fix it in an inactive conformation. Examination of a series of indoles containing 5-oxovalerate and alkyl groups in different positions led to the identification of two lead compounds containing a hexyl group in the 2-position and a 5-oxovalerate group in either the 1-position (IC50, 1.1 μM; not shown) or the 3-position (IC50, 9.8 μM) (Fig. 4) [103].
We modified the above two lead compounds with a view to improve their metabolic stability and increase their potency. Because of concern that β-oxidation of the acyl side chain might be a problem, we examined various modifications of this group that would block this metabolic pathway. Addition of a methyl group at C3 of the 5-oxovalerate group not only served this purpose, but also dramatically increased potency, but only when it was in the S-configuration [104, 105]. Further increases in potency were achieved by the addition of a chloro group at C5 of the indole and methylation of the indole nitrogen to give compound S-230, which is a potent inhibitor of 5-oxo-ETE-induced calcium mobilization (IC50, 6 nM) (Fig. 4). Similar modification of the second lead compound referred to above led to compound S-264, which had about the same antagonist potency (IC50, 7 nM). Relatively high concentrations of S-230 appeared in the blood shortly after oral administration to monkeys, but its concentration declined rapidly thereafter [68], probably due to the susceptibility of its hexyl side chain to ω2-oxidation, resulting in the formation of ω2-hydroxy-S-230 (Fig. 4), which is its major microsomal and plasma metabolite [68]. S-264 was similarly metabolized by ω2-oxidation, but also underwent partial loss of the N-acyl group, resulting in a pharmacokinetic profile that was inferior to that of S-230 [106]. For this reason, we focused on the S-230 series in further studies.
To reduce oxidation of the alkyl side chain of S-230, the hexyl side chain was replaced with a phenyl group attached to the 2-position of the indole via a polymethylene spacer, the optimal length of which was found to be 6 carbons as in compound S-C025 [107]. In addition to reducing the rate of metabolism, the presence of the phenyl group dramatically increased potency (IC50, 100 pM) (Fig. 4) and increased half-life in plasma following oral administration to monkeys. The plasma levels of S-C025 4 h after administration of a dose of 5 mg/kg were substantially higher than those of S-230, 4 h after administration of a 6-fold higher dose of the latter. In contrast to S-230, the major plasma metabolite of S-C025 (i.e. αS-hydroxy-S-C025) was formed by stereoselective hydroxylation of the methylene of the alkylphenyl group α to the indole (Fig. 4). Interestingly, this biologically-formed metabolite is itself a potent OXE receptor antagonist (IC50, 400 pM), whereas the corresponding αR-hydroxy derivative of S-C025 is over 500 times less potent [107].
Further improvements in the OXE antagonist were achieved through modification of the phenyl group. The most potent of these compounds is the meta-chloro derivative of S-C025 (i.e. S-Y048, Fig. 4), which has an IC50 of 20 pM in inhibiting calcium mobilization in neutrophils elicited by a 500-fold higher concentration of 5-oxo-ETE [108]. Although the maximal plasma level of S-Y048 following a dose of 5 mg/kg is a little less than that for S-C025, it has a longer half-life, and its concentrations are substantially higher than those of S-C025 at time points of 4 h and later. Like S-C025, the principal plasma metabolite of S-Y048 is the corresponding αS-hydroxy derivative, which is equipotent with its precursor and has a prolonged lifetime in the blood, with its levels remaining fairly constant between 4 and 24 h. This suggests that αS-hydroxy-S-Y048 could contribute significantly to the in vivo antagonist effects of S-Y048 at longer time points.
8. Pathophysiological role of 5-oxo-ETE
8.1. In vivo effects of 5-oxo-ETE
The powerful in vitro effects of 5-oxo-ETE on eosinophil migration and extracellular matrix degradation as discussed in Sections and 3.1 and 3.4 suggest that this mediator could elicit the tissue infiltration of eosinophils in vivo. To test this hypothesis, 5-oxo-ETE was administered intradermally in the forearms of both healthy and asthmatic human subjects and the numbers of inflammatory cells in the skin were counted 24 h later [109]. 5-Oxo-ETE significantly increased the numbers of macrophages (CD68+), neutrophils (elastase+), and eosinophils (MBP+) in the skin in both healthy and asthmatic subjects as well as the numbers of mast cells in the skin of asthmatic subjects, but did not significantly affect the numbers of lymphocytes (Fig. 5). Furthermore, the numbers of both eosinophils and mast cells following administration of 5-oxo-ETE were significantly higher in asthmatic subjects compared to healthy controls. Among all of the cell types evaluated, the most pronounced effect was on eosinophils in asthmatic subjects, which were present at numbers about 50-fold higher than in vehicle-treated control subjects. In subsequent studies we found that intradermal administration of 5-oxo-ETE also induces infiltration of eosinophils into both cynomolgus and rhesus monkey skin [110].
Figure 5. Recruitment of inflammatory cells into human skin in response to 5-oxo-ETE.
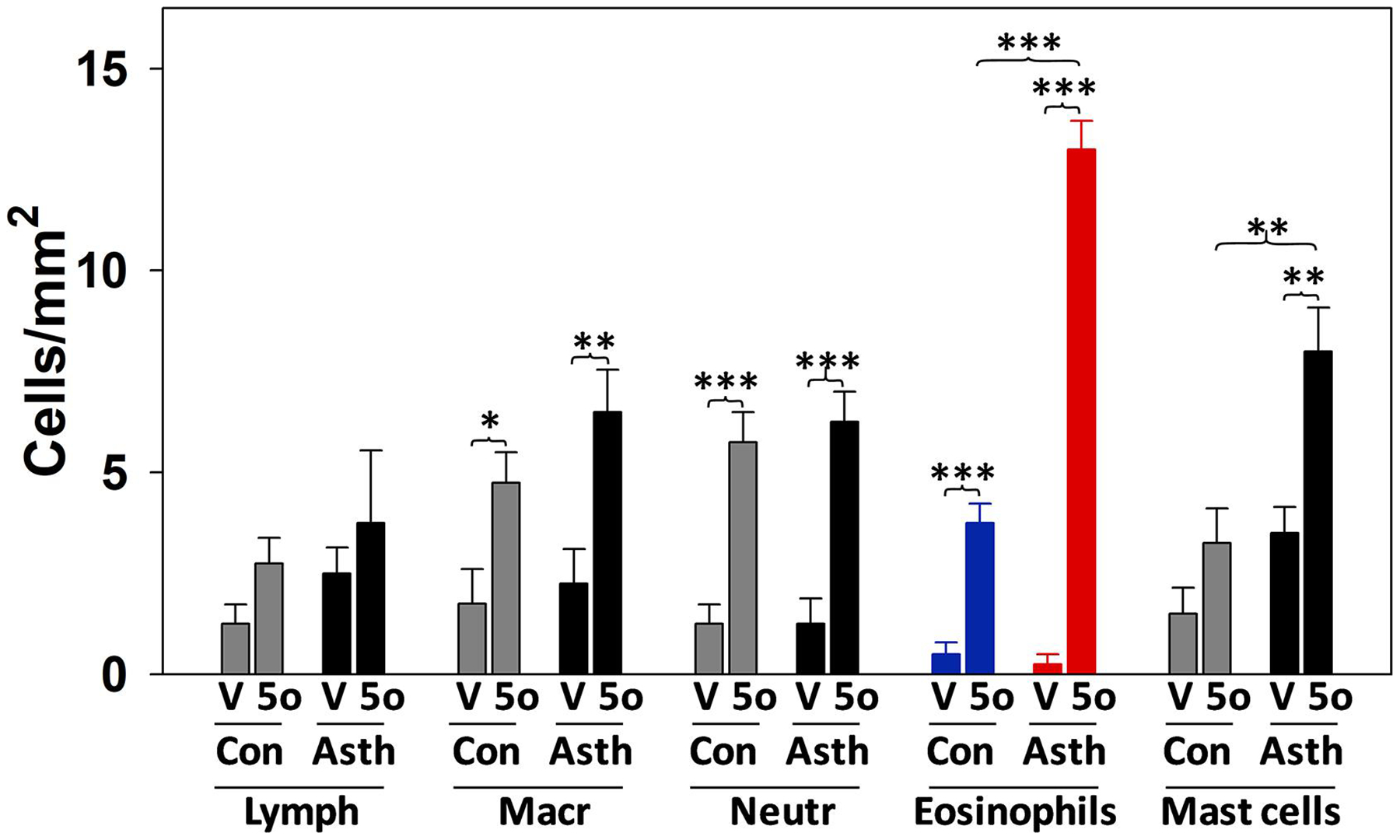
5-Oxo-ETE was injected intradermally into the forearms of subjects with mild atopic asthma (n=11) and healthy control subjects (n=10). After 24 h punch biopsy samples were obtained, and the numbers of inflammatory cells counted following immunostaining for markers specific for different cell types: T-lymphocytes (Lymph; anti-human CD3), macrophages (Macr, anti-human CD68), neutrophils (Neutr, anti-human neutrophil elastase), eosinophils (anti-human major basic protein), and mast cells (anti-human tryptase). V, vehicle; 5o, 5-oxo-ETE; Con, healthy subjects; Asth, asthmatic subjects. *, p < 0.05; **, p < 0.01; ***, p < 0.001. The data were taken from [109].
8.2. 5-Oxo-ETE levels in body fluids and tissues
There is limited information available about the levels of 5-oxo-ETE in body fluids, possibly partly because it is metabolized, principally by ω-oxidation [58] and incorporation into cellular lipids [59], and also because it is not as well known as other 5-LO products such as the leukotrienes. It has been reported that the levels of 5-oxo-ETE in the brain are increased by nearly 50-fold following experimentally-induced cerebral ischemia in rats [111]. Increased levels of 5-oxo-ETE were also recently observed in extracellular vesicles isolated from the plasma of subjects with the autoimmune disease granulomatosis with polyangiitis and this was thought to be associated with increased production of reactive oxygen species and release of double-stranded DNA from neutrophils from these patients [112].
There is also evidence of elevated 5-oxo-ETE levels in various pulmonary diseases, including asthma. Murphy’s group found that the levels of 5-oxo-ETE in lung tissue from subjects with severe pulmonary hypertension were about three times higher than in control subjects, but lower than controls in hypertensive patients who had been treated with prostacyclin [113]. 5-Oxo-ETE has also been reported to be elevated in BAL fluid from female smokers with COPD but not in male smokers with COPD [114].
Kowal et al. recently measured the levels of eicosanoids in exhaled breath condensate from a group of asthmatic subjects who were sensitive to house dust mite allergen (HDM) [115]. HDM challenge resulted in increased levels of 5-oxo-ETE > LTD4 > 8-iso-PGF2α in exhaled breath condensate of those subjects who exhibited a bronchoconstrictor response to allergen. Furthermore, the relative change in 5-oxo-ETE levels was positively correlated with the severity of the subsequent late asthmatic response. Increased levels of 5-oxo-ETE were also observed in BAL fluid following allergen challenge of HDM-sensitized mice [116]. 5-Oxo-ETE may also play a role in the development of nasal polyps, as it is formed by epithelial cells from this tissue and increases the levels of eosinophil cationic protein in organ cultures derived from nasal polyps [117]. These studies raise the possibility that 5-oxo-ETE may be an important mediator in eosinophilic diseases such as atopic dermatitis, allergic rhinitis, and asthma.
8.3. Pathophysiological roles of 5-oxo-ETE in animal disease models
Although elevated levels of 5-oxo-ETE have been detected in body fluids or tissues in various diseases or disease models as discussed above, such studies do not provide conclusive evidence for the involvement of 5-oxo-ETE or the OXE receptor in these conditions. To provide stronger evidence for a pathophysiological role for 5-oxo-ETE the use of receptor or enzyme knockdown or selective antagonists or enzyme inhibitors would be required. As noted above, this has been hampered by the lack of an ortholog of the OXE receptor in rodents. To date, only two published studies have addressed the direct involvement of 5-oxo-ETE in animal models of disease.
Enyedi et al. discovered an important role for 5-oxo-ETE in wound healing in zebrafish, which have a receptor, encoded by the oxer1/hcar1–4 gene, which has about 40% identity with the human OXE receptor [69]. Wounding of the tail fin of larval zebrafish in hypotonic medium results in the rapid recruitment of leukocytes as part of the wound healing process. This response was inhibited by depletion of cPLA2 and restored by addition of AA, 5S-HETE or 5-oxo-ETE to the medium. Leukocyte recruitment was also inhibited by knocking down oxer1/hcar1–4 with a translation-blocking morpholino, which also blocked the response to 5-oxo-ETE added to the medium. In contrast, wound-induced leukocyte infiltration was not affected by inhibition or knockdown of LTA4 hydrolase, the enzyme required for the formation of LTB4. These results suggest that exposure of zebrafish cells to hypotonic medium as a result of wounding results in cell swelling and the cPLA2-mediated release of AA, followed by its conversion to 5-oxo-ETE, resulting in the recruitment of leukocytes to the wound site.
Because of our interest in the potential role of 5-oxo-ETE in asthma and other allergic diseases we sought to test our OXE receptor antagonists in animal models. As noted above, rodent models were not possible due to the absence of the targeted receptor. We therefore initially investigated the possibility of using the cat, as this species possesses an ortholog of the OXE receptor and is particularly susceptible to asthma [118]. We found that BAL cells from asthmatic cats synthesize 5-oxo-ETE, which we also detected in BAL fluid [67]. 5-Oxo-ETE is a potent stimulator of actin polymerization in feline eosinophils and also induces leukocyte migration. However, compound 230 was several hundred-fold less potent in blocking activation of the feline OXE receptor compared to the human receptor. Similar results were subsequently obtained with the more potent antagonist S-C025. This is presumably due to differences between the two receptors, which differ by about 21% in their sequences.
8.4. Effects of OXE receptor antagonists on allergen-induced tissue eosinophilia in monkeys
Because of the low potency of our OXE receptor antagonists in the cat we decided to focus on the monkey, which has an OXE receptor with about 96% sequence identity with the human receptor. Monkey leukocytes synthesize 5-oxo-ETE, which is a potent stimulator of calcium mobilization, leukocyte migration, and actin polymerization in eosinophils from this species. Most importantly, OXE receptor antagonists block the activation of monkey eosinophils by 5-oxo-ETE with a potency almost identical to that observed with human cells [68].
To determine whether OXE receptor antagonists can inhibit allergen-induced eosinophilia we initially conducted a pilot experiment using racemic 230, one of our earlier antagonists, and wild-caught cynomolgus monkeys which had an acquired sensitivity to Ascaris suum through prior environmental exposure. Intradermal injection of Ascaris extract elicited eosinophil infiltration into the skin in all 3 animals tested, although the magnitude of the response was quite variable among individual monkeys. Administration of 230 (3 × 30 mg/kg; 8 h apart) by oral gavage reduced the numbers of eosinophils in the skin in all three animals tested, but this difference was not statistically significant because of the small number of animals and the high degree of inter-animal variability.
In an attempt to reduce the variability among monkeys, subsequent in vivo studies were carried out using captive-bred rhesus monkeys that had been experimentally sensitized to HDM, a well-established model developed at the California National Primate Research Center at the University of California, Davis [119]. These animals were sensitized by weekly subcutaneous injections of HDM followed by challenge with intradermal HDM after treatment with either vehicle or S-Y048 (2 x either 5 or 10 mg/kg), administered by nasogastric intubation [110]. The two doses of S-Y048 gave nearly identical results, inhibiting eosinophil infiltration into the skin in 5 out of the 6 animals in the study, with an overall inhibition of about 50%. This is the first evidence supporting a role for 5-oxo-ETE as a proinflammatory mediator in allergic responses.
To determine whether 5-oxo-ETE could also be involved in allergen-induced pulmonary inflammation we conducted a subsequent study using rhesus monkeys sensitized to HDM by a combination of subcutaneous injection as described above followed by biweekly exposure to aerosolized HDM (Cossette et al., manuscript in preparation). Following sensitization, animals were challenged with aerosolized HDM after administration of either S-Y048 or vehicle. S-Y048 significantly reduced the numbers of eosinophils, neutrophils and macrophages in BAL fluid 24 h after challenge as well as the numbers of eosinophils and neutrophils in lung tissue. Interestingly, the numbers of mucus-containing cells in the bronchi were also significantly reduced by S-Y048 as evaluated by staining with Alcian blue/periodic acid-Schiff and antibodies to MUC2 and MUC5AC.
9. Conclusions
5-Oxo-ETE is a 5-LO product formed from AA along with cys-LTs and LTB4. However, its synthesis is more tightly controlled than leukotrienes, as it requires not only activation of cPLA2 and 5-LO, but also elevation of the intracellular level of the obligatory 5-HEDH cofactor NADP+, which is normally present at very low concentrations in comparison to its reduced counterpart NADPH. Thus, conditions that promote the oxidation of NADPH to NADP+, including activation of the respiratory burst in phagocytic cells [26], oxidative stress [30], and cell death [27], all of which are associated with inflammation, favor the formation of 5-oxo-ETE (Fig. 6). This suggests that the synthesis of 5-oxo-ETE may be more prominent in prolonged inflammation, prior to resolution, rather than in the immediate acute response to cell stimulation, where other mediators such as PGD2 (rapidly released from mast cells) and LTs may predominate. For example, we found that when neutrophils are stimulated with calcium ionophore they initially produce more LTB4 than 5-oxo-ETE, but at longer time points (up to 24 h) the amounts of 5-oxo-ETE are much greater than those of LTB4 [27]. 5-Oxo-ETE can stimulate GM-CSF synthesis by monocytes [54] and this may result in synergy between these two mediators, as GM-CSF can potentially increase the synthesis of 5-oxo-ETE by activating cPLA2 [51] and increasing the expression of 5-LO and FLAP [52, 53], enhance the responsiveness of granulocytes to 5-oxo-ETE [50], and prolong eosinophil survival [120].
Figure 6. Regulation of synthesis and actions of 5-oxo-ETE.

The synthesis of 5-oxo-ETE requires activation of 5-LO as well as increased availability of the obligatory 5-HEDH cofactor NADP+ under conditions that are found in inflammation. 5-Oxo-ETE can promote inflammation by eliciting the tissue infiltration of inflammatory cells and also by stimulating the release of GM-CSF from monocytes, which could potentially result in positive feedback loops to increase the synthesis and response to 5-oxo-ETE.
Our recent studies with OXE receptor antagonists in monkeys provide the first evidence for a pathophysiological role for 5-oxo-ETE in mammals. The use of the monkey as an animal model has the advantage that the results of these studies are highly relevant to human disease. However, the high cost of experiments with monkeys severely restricts their scope, making it impossible to test a wide range of conditions. Thus, the range of antagonist doses that we were able to test was very limited, and it is possible that lower or higher doses might have been more effective. In the skin and lung studies described above the animals were exposed acutely to a single dose of allergen and the numbers of inflammatory cells were determined 24 h later. It would be interesting to examine the effects of repeated dosing with antagonist over a period of days or weeks in a chronic model of allergic inflammation.
Although the initial skin study discussed above focused on allergen-induced eosinophil infiltration, our unpublished findings on allergen-induced pulmonary inflammation suggest that 5-oxo-ETE may also target neutrophils and monocytes/macrophages, raising the possibility that it could play a role in endotypes of severe asthma displaying high numbers of neutrophils [121]. The significant inhibitory effects of S-Y048 on the numbers of mucin-containing cells in the bronchi were unanticipated and are quite interesting. Further work is clearly required to explain the mechanism for these effects and to determine whether 5-oxo-ETE acts directly on epithelial cells or indirectly, for example by stimulating the release of a cytokines or growth factor from inflammatory cells. For example, coculture of eosinophils with epithelial cells in the presence of GM-CSF and IL-3 has been reported to lead to an increase in the synthesis of MUC5AC by epithelial cells [122].
Targeting the OXE receptor with S-Y048 or related selective antagonists would be a novel therapeutic approach for the treatment of asthma and other allergic diseases. The effects of 5-oxo-ETE could also be prevented by using 5-LO or FLAP inhibitors, but this approach would not be specific. Although it would benefit from the concomitant inhibition of cysLT synthesis, the formation of LTB4 and LXA4 would also be prevented, which may not be beneficial because of the role of the former in host defense [94] and the latter in the resolution of inflammation [123]. OXE receptor antagonists could be used either on their own or in combination with current asthma medication such as glucocorticoids or cysLT1 antagonists, which act by different mechanisms, thus potentially resulting in synergy between these drugs.
Acknowledgements
Work conducted in the authors laboratories was supported by the Canadian Institutes of Health Research (WSP: Grants MOP-6254 and PP2-133388), the American Asthma Foundation (JR: Grant 12–0049), and the National Heart, Lung, and Blood Institute (JR: Grant R01HL081873) and by AmorChem (Montreal, QC). The Meakins-Christie Laboratories-MUHC-RI are supported in part by a Centre grant from Le Fond de la Recherche en Santé du Québec as well as by the J. T. Costello Memorial Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations
- S-C025
(S)-5-(5-chloro-1-methyl-2-(6-phenylhexyl)-1H-indol-3-yl)-3-methyl-5-oxopentanoic acid
- 5(S)-HETE
5S-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid
- 5(S)-HpETE
5S-hydroperoxy-6E,8Z,11Z,14Z-eicosatetraenoic acid
- PGD2
prostaglandin D2
- 5-oxo-15S-HETE
5-oxo-15S-hydroxy-6E,8Z,11Z,13E-eicosatetraenoic acid
- 5-oxo-ETE
5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid
- LT
Leukotriene
- S-230
(S)-5-(5-chloro-2-hexyl-1-methyl-1H-indol-3-yl)-3-methyl-5-oxopentanoic acid
- S-Y048
(S)-5-(5-chloro-2-(6-(3-chlorophenyl)hexyl)-1-methyl-1H-indol-3-yl)-3-methyl-5-oxopentanoic acid
- AA
arachidonic acid
- OXE receptor
oxoeicosanoid receptor
- LXA4
lipoxin A4
- 5-HEDH
5-hydroxyeicosanoid dehydrogenase
- PAF
platelet-activating factor
- 12S-HETE
12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid
- PKC
protein kinase C
- 5-oxo-12S-HETE
5-oxo-12S-hydroxy-6E,8Z,10E,14Z-eicosatetraenoic acid
- 5-oxo-ETrE
5-oxo-6E,8Z,11Z-eicosatrienoic acid
- EPA
5Z,8Z,11Z,14Z,17Z-eicosapentaenoic acid
- DHA
4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic acid
- HDM
house dust mite allergen
- BAL
bronchoalveolar lavage
- S-264
(S)-5-(6-chloro-2-hexyl-1H-indol-1-yl)-3-methyl-5-oxopentanoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest
W.S.P. and J.R. have been granted a patent covering S-230 and have applied for a patent covering S-C025 and S-Y048.
References
- [1].Borgeat P, Samuelsson B, Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids, Proc. Natl. Acad. Sci. USA 76 (1979) 3213–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Borgeat P, Samuelsson B, Transformation of arachidonic acid by rabbit polymorphonuclear leukocytes. Formation of a novel dihydroxyeicosatetraenoic acid, J. Biol. Chem 254 (1979) 2643–2646. [PubMed] [Google Scholar]
- [3].Murphy RC, Hammarström S, Samuelsson B, Leukotriene C: a slow-reacting substance from murine mastocytoma cells., Proc. Natl. Acad. Sci. USA 76 (1979) 4275–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haeggström JZ, Leukotriene biosynthetic enzymes as therapeutic targets, J Clin Invest 128(7) (2018) 2680–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yokomizo T, Nakamura M, Shimizu T, Leukotriene receptors as potential therapeutic targets, J Clin Invest 128(7) (2018) 2691–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Powell WS, Gravel S, Halwani F, 5-oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of L-selectin shedding, surface expression of CD11b, actin polymerization, and calcium mobilization in human eosinophils, Am J Respir Cell Mol Biol 20(1) (1999) 163–70. [DOI] [PubMed] [Google Scholar]
- [7].Sun FF, Crittenden NJ, Czuk CI, Taylor BM, Stout BK, Johnson HG, Biochemical and Functional Differences between Eosinophils from Animal Species and Man, J Leukocyte Biol 50(2) (1991) 140–150. [DOI] [PubMed] [Google Scholar]
- [8].Morita E, Schröder JM, Christophers E, Identification of a novel and highly potent eosinophil chemotactic lipid in human eosinophils treated with arachidonic acid, J. Immunol 144 (1990) 1893–1900. [PubMed] [Google Scholar]
- [9].Powell WS, Chung D, Gravel S, 5-Oxo-6,8,11,14-Eicosatetraenoic Acid Is a Potent Stimulator of Human Eosinophil Migration, Journal of Immunology 154(8) (1995) 4123–4132. [PubMed] [Google Scholar]
- [10].Coeffier E, Joseph D, Vargaftig BB, Ltb4, a Potent Chemotactic Factor for Purified Guinea-Pig Eosinophils - Interference of Paf-Acether Antagonists, International Journal of Immunopharmacology 13(2–3) (1991) 273–280. [DOI] [PubMed] [Google Scholar]
- [11].Okuno T, Iizuka Y, Okazaki H, Yokomizo T, Taguchi R, Shimizu T, 12(S)-Hydroxyheptadeca-5Z, 8E, 10E-trienoic acid is a natural ligand for leukotriene B4 receptor 2, J. Exp. Med 205(4) (2008) 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lynch KR, O’neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL Jr., Ford-Hutchinson AW, Caskey CT, Evans JF, Characterization of the human cysteinyl leukotriene CysLT1 receptor, Nature 399(6738) (1999) 789–793. [DOI] [PubMed] [Google Scholar]
- [13].Heise CE, O’Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Zeng ZZ, Liu QY, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O’Neill GP, Metters KM, Lynch KR, Evans JF, Characterization of the human cysteinyl leukotriene 2 receptor, Journal of Biological Chemistry 275(39) (2000) 30531–30536. [DOI] [PubMed] [Google Scholar]
- [14].Jiang Y, Borrelli L, Kanaoka Y, Bacskal BJ, Boyce JA, CysLT(2) receptors interact with CysLT(1) receptors and down-modulate cysteinyl leukotriene-dependent mitogenic responses of mast cells, Blood 110(9) (2007) 3263–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kanaoka Y, Maekawa A, Austen KF, Identification of GPR99 Protein as a Potential Third Cysteinyl Leukotriene Receptor with a Preference for Leukotriene E-4 Ligand, Journal of Biological Chemistry 288(16) (2013) 10967–10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bankova LG, Lai JY, Yoshimoto E, Boyce JA, Austen KF, Kanaoka Y, Barrett NA, Leukotriene E-4 elicits respiratory epithelial cell mucin release through the G-protein-coupled receptor, GPR99, Proc. Natl. Acad. Sci. USA 113(22) (2016) 6242–6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DWP, Rovati GE, Shimizu T, Yokomizo T, Brink C, The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo, Pharmacological Reviews 58(3) (2006) 463–487. [DOI] [PubMed] [Google Scholar]
- [18].Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN, Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4, Nat.Med 8(9) (2002) 1018–1023. [DOI] [PubMed] [Google Scholar]
- [19].Barnig C, Cernadas M, Dutile S, Liu XL, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD, Lipoxin A(4) Regulates Natural Killer Cell and Type 2 Innate Lymphoid Cell Activation in Asthma, Sci Transl Med 5(174) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Powell WS, Properties of leukotriene B4 20-hydroxylase from polymorphonuclear leukocytes, J. Biol. Chem 259 (1984) 3082–3089. [PubMed] [Google Scholar]
- [21].Powell WS, Gravelle F, Metabolism of 6-trans isomers of leukotriene B4 to dihydro products by human polymorphonuclear leukocytes, J. Biol. Chem 263 (1988) 2170–2177. [PubMed] [Google Scholar]
- [22].Powell WS, Gravelle F, Gravel S, Metabolism of 5(S)-hydroxy-6,8,11,14-eicosatetraenoic acid and other 5(S)-hydroxyeicosanoids by a specific dehydrogenase in human polymorphonuclear leukocytes, J. Biol. Chem 267(27) (1992) 19233–41. [PubMed] [Google Scholar]
- [23].Schwenk U, Morita E, Engel R, Schröder JM, Identification of 5-oxo-15-hydroxy-6,8,11,13-eicosatetraenoic acid as a novel and potent human eosinophil chemotactic eicosanoid, J. Biol. Chem 267 (1992) 12482–12488. [PubMed] [Google Scholar]
- [24].Powell WS, Zhang Y, Gravel S, Effects of phorbol myristate acetate on the synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid by human polymorphonuclear leukocytes, Biochemistry 33 (1994) 3927–3933. [DOI] [PubMed] [Google Scholar]
- [25].Erlemann KR, Cossette C, Grant GE, Lee GJ, Patel P, Rokach J, Powell WS, Regulation of 5-hydroxyeicosanoid dehydrogenase activity in monocytic cells, Biochem.J 403(1) (2007) 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Powell WS, Gravelle F, Gravel S, Phorbol myristate acetate stimulates the formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by human neutrophils by activating NADPH oxidase, J. Biol. Chem 269 (1994) 25373–25380. [PubMed] [Google Scholar]
- [27].Graham FD, Erlemann KR, Gravel S, Rokach J, Powell WS, Oxidative stress-induced changes in pyridine nucleotides and chemoattractant 5-lipoxygenase products in aging neutrophils, Free Radic.Biol.Med 47(1) (2009) 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Y, Styhler A, Powell WS, Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid by human monocytes and lymphocytes, J. Leukoc. Biol 59(6) (1996) 847–854. [DOI] [PubMed] [Google Scholar]
- [29].Grant GE, Gravel S, Guay J, Patel P, Mazer BD, Rokach J, Powell WS, 5-oxo-ETE is a major oxidative stress-induced arachidonate metabolite in B lymphocytes, Free Radic.Biol.Med 50(10) (2011) 1297–1304. [DOI] [PubMed] [Google Scholar]
- [30].Erlemann KR, Rokach J, Powell WS, Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells, J. Biol. Chem 279 (2004) 40376–40384. [DOI] [PubMed] [Google Scholar]
- [31].Erlemann KR, Cossette C, Gravel S, Lesimple A, Lee GJ, Saha G, Rokach J, Powell WS, Airway epithelial cells synthesize the lipid mediator 5-oxo-ETE in response to oxidative stress, Free Radic.Biol.Med 42(5) (2007) 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Erlemann KR, Cossette C, Gravel S, Stamatiou PB, Lee GJ, Rokach J, Powell WS, Metabolism of 5-hydroxy-6,8,11,14-eicosatetraenoic acid by human endothelial cells, Biochem.Biophys.Res.Commun 350(1) (2006) 151–156. [DOI] [PubMed] [Google Scholar]
- [33].Grant GE, Rubino S, Gravel S, Wang X, Patel P, Rokach J, Powell WS, Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid, Carcinogenesis 32(6) (2011) 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Folco G, Murphy RC, Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses, Pharmacological Reviews 58(3) (2006) 375–388. [DOI] [PubMed] [Google Scholar]
- [35].Murray JJ, Tonnel AB, Brash AR, Roberts LJ, Gosset P, Workman R, Capron A, Oates JA, Release of prostaglandin D2 into human airways during acute antigen challenge, N Engl J Med 315(13) (1986) 800–804. [DOI] [PubMed] [Google Scholar]
- [36].Hall LM, Murphy RC, Activation of human polymorphonuclear leukocytes by products derived from the peroxidation of human red blood cell membranes., Chem.Res.Toxicol 11(9) (1998) 1024–1031. [DOI] [PubMed] [Google Scholar]
- [37].Khaselev N, Murphy RC, Peroxidation of arachidonate containing plasmenyl glycerophosphocholine: facile oxidation of esterified arachidonate at carbon-5, Free Radic.Biol.Med 29(7) (2000) 620–632. [DOI] [PubMed] [Google Scholar]
- [38].Hevko JM, Bowers RC, Murphy RC, Synthesis of 5-oxo-6,8,11,14-eicosatetraenoic acid and identification of novel omega-oxidized metabolites in the mouse macrophage, J Pharmacol Exp Ther 296(2) (2001) 293–305. [PubMed] [Google Scholar]
- [39].O’Flaherty JT, Jacobson D, Redman J, Mechanism involved in the mobilization of neutrophil calcium by 5-hydroxyeicosatetraenoate, J. Immunol 140 (1988) 4323–4328. [PubMed] [Google Scholar]
- [40].Powell WS, Gravel S, MacLeod RJ, Mills E, Hashefi M, Stimulation of human neutrophils by 5-oxo-6,8,11,14- eicosatetraenoic acid by a mechanism independent of the leukotriene B4 receptor, J. Biol. Chem 268 (1993) 9280–9286. [PubMed] [Google Scholar]
- [41].Powell WS, Gravel S, Halwani F, Hii CS, Huang ZH, Tan AM, Ferrante A, Effects of 5-oxo-6,8,11,14-eicosatetraenoic acid on expression of CD11b, actin polymerization and adherence in human neutrophils, J. Immunol 159 (1997) 2952–2959. [PubMed] [Google Scholar]
- [42].Norgauer J, Barbisch M, Czech W, Pareigis J, Schwenk U, Schröder JM, Chemotactic 5-oxo-icosatetraenoic acids activate a unique pattern of neutrophil responses - analysis of phospholipid metabolism, intracellular Ca2+ transients, actin reorganization, superoxide-anion production and receptor up-regulation, Eur.J Biochem 236(3) (1996) 1003–1009. [DOI] [PubMed] [Google Scholar]
- [43].O’Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW, 5-Oxo-eicosanoids and hematopoietic cytokines cooperate in stimulating neutrophil function and the mitogen-activated protein kinase pathway, J. Biol. Chem 271(30) (1996) 17821–8. [DOI] [PubMed] [Google Scholar]
- [44].O’Flaherty JT, Cordes JF, Lee SL, Samuel M, Thomas MJ, Chemical and biological characterization of oxo-eicosatetraenoic acids, Biochim.Biophys.Acta 1201(3) (1994) 505–515. [DOI] [PubMed] [Google Scholar]
- [45].Schwenk U, Schröder JM, 5-Oxo-eicosanoids are potent eosinophil chemotactic factors - functional characterization and structural requirements, J. Biol. Chem 270 (1995) 15029–15036. [DOI] [PubMed] [Google Scholar]
- [46].Czech W, Barbisch M, Tenscher K, Schopf E, Schroder JM, Norgauer J, Chemotactic 5-oxo-eicosatetraenoic acids induce oxygen radical production, Ca2+-mobilization, and actin reorganization in human eosinophils via a pertussis toxin-sensitive G-protein, J Invest Dermatol 108(1) (1997) 108–12. [DOI] [PubMed] [Google Scholar]
- [47].Urasaki T, Takasaki J, Nagasawa T, Ninomiya H, Pivotal role of 5-lipoxygenase in the activation of human eosinophils: platelet-activating factor and interleukin-5 induce CD69 on eosinophils through the 5-lipoxygenase pathway, J. Leukoc. Biol 69(1) (2001) 105–112. [PubMed] [Google Scholar]
- [48].Powell WS, Ahmed S, Gravel S, Rokach J, Eotaxin and RANTES enhance 5-oxo-6,8,11,14-eicosatetraenoic acid-induced eosinophil chemotaxis, J Allergy Clin Immunol 107(2) (2001) 272–8. [DOI] [PubMed] [Google Scholar]
- [49].Schratl P, Sturm EM, Royer JF, Sturm GJ, Lippe IT, Peskar BA, Heinemann A, Hierarchy of eosinophil chemoattractants: role of p38 mitogen-activated protein kinase, Eur J Immunol 36(9) (2006) 2401–9. [DOI] [PubMed] [Google Scholar]
- [50].O’Flaherty JT, Kuroki M, Nixon AB, Wijkander J, Yee E, Lee SL, Smitherman PK, Wykle RL, Daniel LW, 5-Oxo-eicosatetraenoate is a broadly active, eosinophil-selective stimulus for human granulocytes, J. Immunol 157(1) (1996) 336–342. [PubMed] [Google Scholar]
- [51].DiPersio JF, Billing P, Williams R, Gasson JC, Human granulocyte-macrophage colony-stimulating factor and other cytokines prime human neutrophils for enhanced arachidonic acid release and leukotriene B4 synthesis., J. Immunol 140(12) (1988) 4315–4322. [PubMed] [Google Scholar]
- [52].Pouliot M, McDonald PP, Khamzina L, Borgeat P, McColl SR, Granulocyte-Macrophage Colony-Stimulating Factor Enhances 5-Lipoxygenase Levels in Human Polymorphonuclear Leukocytes, J. Immunol 152 (1994) 851–858. [PubMed] [Google Scholar]
- [53].Pouliot M, McDonald PP, Borgeat P, McColl SR, Granulocyte Macrophage Colony-Stimulating Factor Stimulates the Expression of the 5-Lipoxygenase-Activating Protein (FLAP) in Human Neutrophils, J. Exp. Med 179 (1994) 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stamatiou PB, Chan CC, Monneret G, Ethier D, Rokach J, Powell WS, 5-oxo-6,8,11,14-eicosatetraenoic acid stimulates the release of the eosinophil survival factor granulocyte/macrophage colony-stimulating factor from monocytes, J. Biol. Chem 279(27) (2004) 28159–64. [DOI] [PubMed] [Google Scholar]
- [55].Guilbert M, Ferland C, Bosse M, Flamand N, Lavigne S, Laviolette M, 5-oxo-6,8,11,14-eicosatetraenoic acid induces important eosinophil transmigration through basement membrane components - Comparison of normal and asthmatic eosinophils, Am. J. Respir. Cell Mol. Biol 21(1) (1999) 97–104. [DOI] [PubMed] [Google Scholar]
- [56].Ferland C, Guilbert M, Davoine F, Flamand N, Chakir J, Laviolette M, Eotaxin promotes eosinophil transmigration via the activation of the plasminogen-plasmin system, J. Leukoc. Biol 69(5) (2001) 772–778. [PubMed] [Google Scholar]
- [57].Dallaire MJ, Ferland C, Page N, Lavigne S, Davoine F, Laviolette M, Endothelial cells modulate eosinophil surface markers and mediator release, Eur Respir J 21(6) (2003) 918–24. [DOI] [PubMed] [Google Scholar]
- [58].Powell WS, MacLeod RJ, Gravel S, Gravelle F, Bhakar A, Metabolism and biologic effects of 5-oxoeicosanoids on human neutrophils, J Immunol 156(1) (1996) 336–42. [PubMed] [Google Scholar]
- [59].O’Flaherty JT, Taylor JS, Thomas MJ, Receptors for the 5-oxo class of eicosanoids in neutrophils, J. Biol. Chem 273(49) (1998) 32535–32541. [DOI] [PubMed] [Google Scholar]
- [60].Hosoi T, Koguchi Y, Sugikawa E, Chikada A, Ogawa K, Tsuda N, Suto N, Tsunoda S, Taniguchi T, Ohnuki T, Identification of a novel eicosanoid receptor coupled to Gi/o, J. Biol. Chem 277 (2002) 31459–31465. [DOI] [PubMed] [Google Scholar]
- [61].Takeda S, Yamamoto A, Haga T, Identification of a G protein-coupled receptor for 5-oxo-eicosatetraenoic acid, Biomedical Research-Tokyo 23(2) (2002) 101–108. [Google Scholar]
- [62].Jones CE, Holden S, Tenaillon L, Bhatia U, Seuwen K, Tranter P, Turner J, Kettle R, Bouhelal R, Charlton S, Nirmala NR, Jarai G, Finan P, Expression and characterization of a 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid receptor highly expressed on human eosinophils and neutrophils, Mol. Pharmacol 63(3) (2003) 471–7. [DOI] [PubMed] [Google Scholar]
- [63].Bäck M, Powell WS, Dahlén SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE, Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7, Brit J Pharmacol 171(15) (2014) 3551–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Iikura M, Suzukawa M, Yamaguchi M, Sekiya T, Komiya A, Yoshimura-Uchiyama C, Nagase H, Matsushima K, Yamamoto K, Hirai K, 5-Lipoxygenase products regulate basophil functions: 5-Oxo-ETE elicits migration, and leukotriene B4 induces degranulation, J. Allergy Clin. Immunol 116(3) (2005) 578–585. [DOI] [PubMed] [Google Scholar]
- [65].Sturm GJ, Schuligoi R, Sturm EM, Royer JF, Lang-Loidolt D, Stammberger H, Amann R, Peskar BA, Heinemann A, 5-oxo-6,8,11,14-eicosatetraenoic acid is a potent chemoattractant for human basophils, J Allergy Clin Immunol 116(5) (2005) 1014–1019. [DOI] [PubMed] [Google Scholar]
- [66].Offermanns S, Free Fatty Acid (FFA) and Hydroxy Carboxylic Acid (HCA) Receptors, Annu Rev Pharmacol 54 (2014) 407–434. [DOI] [PubMed] [Google Scholar]
- [67].Cossette C, Gravel S, Reddy CN, Gore V, Chourey S, Ye Q, Snyder NW, Mesaros CA, Blair IA, Lavoie JP, Reinero CR, Rokach J, Powell WS, Biosynthesis and actions of 5-oxoeicosatetraenoic acid (5-oxo-ETE) on feline granulocytes, Biochem Pharmacol 96(3) (2015) 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cossette C, Chourey S, Ye Q, Nagendra Reddy C, Gore V, Gravel S, Slobodchikova I, Vuckovic D, Rokach J, Powell WS, Pharmacokinetics and metabolism of selective oxoeicosanoid (OXE) receptor antagonists and their effects on 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE)-induced granulocyte activation in monkeys, J. Med. Chem 59(22) (2016) 10127–10146. [DOI] [PubMed] [Google Scholar]
- [69].Enyedi B, Kala S, Nikolich-Zugich T, Niethammer P, Tissue damage detection by osmotic surveillance, Nat Cell Biol 15(9) (2013) 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Stamatiou P, Hamid Q, Taha R, Yu W, Issekutz TB, Rokach J, Khanapure SP, Powell WS, 5-Oxo-ETE induces pulmonary eosinophilia in an integrin-dependent manner in Brown Norway rats., J.Clin.Invest 102(12) (1998) 2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Almishri W, Cossette C, Rokach J, Martin JG, Hamid Q, Powell WS, Effects of prostaglandin D2, 15-deoxy-Δ12,14-prostaglandin J2, and selective DP1 and DP2 receptor agonists on pulmonary infiltration of eosinophils in Brown Norway rats, J Pharmacol Exp Ther 313(1) (2005) 64–69. [DOI] [PubMed] [Google Scholar]
- [72].Cooke M, Di CH, Maloberti P, Cornejo MF, Expression and function of OXE receptor, an eicosanoid receptor, in steroidogenic cells, Mol.Cell Endocrinol 371(1–2) (2013) 71–78. [DOI] [PubMed] [Google Scholar]
- [73].Fukano Y, Okino N, Furuya S, Ito M, A label-free impedance-based whole cell assay revealed a new G protein-coupled receptor ligand for mouse microglial cell migration, Biochem. Biophys. Res. Commun 478(2) (2016) 624–630. [DOI] [PubMed] [Google Scholar]
- [74].Bautzova T, Hockley JRF, Perez-Berezo T, Pujo J, Tranter MM, Desormeaux C, Barbaro MR, Basso L, Le Faouder P, Rolland C, Malapert P, Moqrich A, Eutamene H, Denadai-Souza A, Vergnolle N, Smith ES, Hughes DI, Barbara G, Dietrich G, Bulmer DC, Cenac N, 5-oxoETE triggers nociception in constipation-predominant irritable bowel syndrome through MAS-related G protein-coupled receptor D, Sci Signal 11(561) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mercier F, Morin C, Cloutier M, Proteau S, Rokach J, Powell WS, Rousseau E, 5-Oxo-ETE regulates tone of guinea pig airway smooth muscle via activation of Ca2+ pools and Rho-kinase pathway, Am.J.Physiol Lung Cell Mol.Physiol 287(4) (2004) L631–L640. [DOI] [PubMed] [Google Scholar]
- [76].Morin C, Sirois M, Echave V, Gomes MM, Rousseau E, Relaxing effects of 5-oxo-ETE on human bronchi involve BK Ca channel activation, Prostaglandins Other Lipid Mediat. 83(4) (2007) 311–319. [DOI] [PubMed] [Google Scholar]
- [77].MacLeod RJ, Lembessis P, Hamilton JR, Powell WS, 5-Oxo-6,8,11,14-eicosatetraenoic acid stimulates isotonic volume reduction of guinea pig jejunal crypt epithelial cells, J Pharmacol Exp Ther 291(2) (1999) 511–516. [PubMed] [Google Scholar]
- [78].O’Flaherty JT, Taylor JS, Kuroki M, The coupling of 5-oxo-eicosanoid receptors to heterotrimeric G proteins, J. Immunol 164(6) (2000) 3345–3352. [DOI] [PubMed] [Google Scholar]
- [79].Hosoi T, Sugikawa E, Chikada A, Koguchi Y, Ohnuki T, TG1019/OXE, a Galpha(i/o)-protein-coupled receptor, mediates 5-oxo-eicosatetraenoic acid-induced chemotaxis, Biochem.Biophys.Res.Commun 334(4) (2005) 987–995. [DOI] [PubMed] [Google Scholar]
- [80].Langlois A, Chouinard F, Flamand N, Ferland C, Rola-Pleszczynski M, Laviolette M, Crucial implication of protein kinase C (PKC)-delta, PKC-zeta, ERK-1/2, and p38 MAPK in migration of human asthmatic eosinophils, J Leukoc Biol 85(4) (2009) 656–63. [DOI] [PubMed] [Google Scholar]
- [81].Sarveswaran S, Ghosh J, OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells, Cancer Lett. 336(1) (2013) 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Konya V, Blattermann S, Jandl K, Platzer W, Ottersbach PA, Marsche G, Gutschow M, Kostenis E, Heinemann A, A Biased Non-Galphai OXE-R Antagonist Demonstrates That Galphai Protein Subunit Is Not Directly Involved in Neutrophil, Eosinophil, and Monocyte Activation by 5-Oxo-ETE, J Immunol 192(10) (2014) 4774–4782. [DOI] [PubMed] [Google Scholar]
- [83].Blättermann S, Peters L, Ottersbach PA, Bock A, Konya V, Weaver CD, Gonzalez A, Schroder R, Tyagi R, Luschnig P, Gab J, Hennen S, Ulven T, Pardo L, Mohr K, Gutschow M, Heinemann A, Kostenis E, A biased ligand for OXE-R uncouples Gα and Gβγ signaling within a heterotrimer, Nat. Chem. Biol 8(7) (2012) 631–638. [DOI] [PubMed] [Google Scholar]
- [84].Patel P, Cossette C, Anumolu JR, Gravel S, Lesimple A, Mamer OA, Rokach J, Powell WS, Structural requirements for activation of the 5-oxo-6E,8Z, 11Z,14Z-eicosatetraenoic acid (5-oxo-ETE) receptor: identification of a mead acid metabolite with potent agonist activity, J Pharmacol Exp Ther 325(2) (2008) 698–707. [DOI] [PubMed] [Google Scholar]
- [85].Patel P, Anumolu JR, Powell WS, Rokach J, 5-Oxo-15-HETE: Total synthesis and bioactivity, Bioorg.Med.Chem.Lett 21(6) (2011) 1857–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Powell WS, Gravel S, Khanapure SP, Rokach J, Biological inactivation of 5-oxo-6,8,11,14-eicosatetraenoic acid by human platelets, Blood 93(3) (1999) 1086–1096. [PubMed] [Google Scholar]
- [87].Cossette C, Patel P, Anumolu JR, Sivendran S, Lee GJ, Gravel S, Graham FD, Lesimple A, Mamer OA, Rokach J, Powell WS, Human neutrophils convert the sebum-derived polyunsaturated fatty acid Sebaleic acid to a potent granulocyte chemoattractant, J. Biol. Chem 283(17) (2008) 11234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Muhammad I, Zhao J, Dunbar DC, Khan IA, Constituents of Lepidium meyenii ‘maca’, Phytochemistry 59(1) (2002) 105–110. [DOI] [PubMed] [Google Scholar]
- [89].Mead JF, Slaton WH, Metabolism of Essential Fatty Acids .3. Isolation of 5,8,11-Eicosatrienoic Acid from Fat-Deficient Rats, Journal of Biological Chemistry 219(2) (1956) 705–709. [PubMed] [Google Scholar]
- [90].Evans JF, Nathaniel DJ, Zamboni RJ, Ford-Hutchinson AW, Leukotriene A3. A poor substrate but a potent inhibitor of rat and human neutrophil leukotriene A4 hydrolase, J. Biol. Chem 260(20) (1985) 10966–10970. [PubMed] [Google Scholar]
- [91].Powell WS, Gravel S, Gravelle F, Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils, J. Lipid Res 36(12) (1995) 2590–2598. [PubMed] [Google Scholar]
- [92].Gelfand EW, Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma, Semin Immunol 33(C) (2017) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bhatt L, Roinestad K, Van T, Springman EB, Recent advances in clinical development of leukotriene B4 pathway drugs, Semin Immunol 33(C) (2017) 65–73. [DOI] [PubMed] [Google Scholar]
- [94].Bailie MB, Standiford TJ, Laichalk LL, Coffey MJ, Strieter R, Peters-Golden M, Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities., J. Immunol 157(12) (1996) 5221–5224. [PubMed] [Google Scholar]
- [95].Le Bel M, Gosselin J, Leukotriene B-4 Enhances NOD2-Dependent Innate Response against Influenza Virus Infection, Plos One 10(10) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pernet E, Downey J, Vinh DC, Powell WS, Divangahi M, Leukotriene B4-type I interferon axis regulates macrophage-mediated disease tolerance to influenza infection, Nat Microbiol 4(8) (2019) 1389–1400. [DOI] [PubMed] [Google Scholar]
- [97].Serhan CN, Petasis NA, Resolvins and protectins in inflammation resolution, Chem.Rev 111(10) (2011) 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, Takihara T, Tomomatsu K, Suzuki Y, Oguma T, Sayama K, Arai H, Betsuyaku T, Arita M, Asano K, Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma, J.Allergy Clin.Immunol 131(2) (2013) 353–360. [DOI] [PubMed] [Google Scholar]
- [99].Levin ER, Hammes SR, Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors, Nat Rev Mol Cell Bio 17(12) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kalyvianaki K, Gebhart V, Peroulis N, Panagiotopoulou C, Kiagiadaki F, Pediaditakis I, Aivaliotis M, Moustou E, Tzardi M, Notas G, Castanas E, Kampa M, Antagonizing effects of membrane-acting androgens on the eicosanoid receptor OXER1 in prostate cancer, Scientific Reports 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pergola C, Dodt G, Rossi A, Neunhoeffer E, Lawrenz B, Northoff H, Samuelsson B, Radmark O, Sautebin L, Werz O, ERK-mediated regulation of leukotriene biosynthesis by androgens: A molecular basis for gender differences in inflammation and asthma, Proc. Natl. Acad. Sci. USA 105(50) (2008) 19881–19886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].de Marco R, Locatelli F, Sunyer J, Burney P, Surve ECRH, Differences in incidence of reported asthma related to age in men and women - A retrospective analysis of the data of the European Respiratory Health Survey, Am J Resp Crit Care 162(1) (2000) 68–74. [DOI] [PubMed] [Google Scholar]
- [103].Gore V, Gravel S, Cossette C, Patel P, Chourey S, Ye Q, Rokach J, Powell WS, Inhibition of 5-oxo-6,8,11,14-eicosatetraenoic acid-induced activation of neutrophils and eosinophils by novel indole OXE receptor antagonists, J. Med. Chem 57(2) (2014) 364–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ye QJ, Chourey S, Wang R, Chintam NR, Gravel S, Powell WS, Rokach J, Structure-activity relationship study of beta-oxidation resistant indole-based 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) receptor antagonists, Bioorg. Med. Chem. Lett 27(20) (2017) 4770–4776. [DOI] [PubMed] [Google Scholar]
- [105].Reddy CN, Ye QJ, Chourey S, Gravel S, Powell WS, Rokach J, Stereoselective synthesis of two highly potent 5-oxo-ETE receptor antagonists, Tetrahedron Lett. 56(49) (2015) 6896–6899. [Google Scholar]
- [106].Reddy CN, Alhamza H, Chourey S, Ye Q, Gore V, Cossette C, Gravel S, Slobodchikova I, Vuckovic D, Rokach J, Powell WS, Metabolism and pharmacokinetics of a potent N-acylindole antagonist of the OXE receptor for the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) in rats and monkeys, Eur. J. Pharm. Sci 115 (2018) 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chourey S, Ye Q, Reddy CN, Wang R, Cossette C, Gravel S, Slobodchikova I, Vuckovic D, Rokach J, Powell WS, Novel Highly Potent and Metabolically Resistant Oxoeicosanoid (OXE) Receptor Antagonists That Block the Actions of the Granulocyte Chemoattractant 5-Oxo-6,8,11,14-Eicosatetraenoic Acid (5-oxo-ETE), J Med Chem 61(14) (2018) 5934–5948. [DOI] [PubMed] [Google Scholar]
- [108].Ye Q, Chourey S, Reddy CN, Wang R, Cossette C, Gravel S, Slobodchikova I, Vuckovic D, Rokach J, Powell WS, Novel highly potent OXE receptor antagonists with prolonged plasma lifetimes that are converted to active metabolites in vivo in monkeys, Br J Pharmacol 177(2) (2020) 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Muro S, Hamid Q, Olivenstein R, Taha R, Rokach J, Powell WS, 5-Oxo-6,8,11,14-eicosatetraenoic acid induces the infiltration of granulocytes into human skin, J Allergy Clin Immunol 112(4) (2003) 768–774. [DOI] [PubMed] [Google Scholar]
- [110].Miller LA, Cossette C, Chourey S, Ye Q, Reddy CN, Rokach J, Powell WS, Inhibition of allergen-induced dermal eosinophilia by an oxoeicosanoid receptor antagonist in non-human primates, Br J Pharmacol 177(2) (2020) 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Farias SE, Basselin M, Chang L, Heidenreich KA, Rapoport SI, Murphy RC, Formation of eicosanoids, E2/D2 isoprostanes, and docosanoids following decapitation-induced ischemia, measured in high-energy-microwaved rat brain, J. Lipid Res 49(9) (2008) 1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Surmiak M, Gielicz A, Stojkov D, Szatanek R, Wawrzycka-Adamczyk K, Yousefi S, Simon HU, Sanak M, LTB4 and 5-oxo-ETE from extracellular vesicles stimulate neutrophils in granulomatosis with polyangiitis[S], Journal of Lipid Research 61(1) (2020) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, Voelkel NF, Oxidative Stress in Severe Pulmonary Hypertension, Am.J Respir.Crit Care Med 169(6) (2004) 764–769. [DOI] [PubMed] [Google Scholar]
- [114].Balgoma D, Yang MX, Sjodin M, Snowden S, Karimi R, Levanen B, Merikallio H, Kaarteenaho R, Palmberg L, Larsson K, Erle DJ, Dahlen SE, Dahlen B, Skold CM, Wheelock AM, Wheelock CE, Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD, Eur. Respir. J 47(6) (2016) 1645–1656. [DOI] [PubMed] [Google Scholar]
- [115].Kowal K, Gielicz A, Sanak M, The effect of allergen-induced bronchoconstriction on concentration of 5-oxo-ETE in exhaled breath condensate of house dust mite-allergic patients, Clin Exp Allergy 47(10) (2017) 1253–1262. [DOI] [PubMed] [Google Scholar]
- [116].Kolmert J, Pineiro-Hermida S, Hamberg M, Gregory JA, Lopez IP, Fauland A, Wheelock CE, Dahlen SE, Pichel JG, Adner M, Prominent release of lipoxygenase generated mediators in a murine house dust mite-induced asthma model, Prostag Oth Lipid M 137 (2018) 20–29. [DOI] [PubMed] [Google Scholar]
- [117].Lin L, Chen Z, Tang XY, Dai F, Wei JJ, Sun GB, 5-Oxo-ETE from Nasal Epithelial Cells Upregulates Eosinophil Cation Protein by Eosinophils in Nasal Polyps in vitro, Int Arch Allergy Imm 177(2) (2018) 107–115. [DOI] [PubMed] [Google Scholar]
- [118].Dye JA, McKiernan BC, Rozanski EA, Hoffmann WE, Losonsky JM, Homco LD, Weisiger RM, Kakoma I, Bronchopulmonary disease in the cat: historical, physical, radiographic, clinicopathologic, and pulmonary functional evaluation of 24 affected and 15 healthy cats, J Vet Intern Med 10(6) (1996) 385–400. [DOI] [PubMed] [Google Scholar]
- [119].Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, Wong VJ, Joad JP, Pinkerton KB, Wu R, Evans MJ, Hyde DM, Plopper CG, Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae), Am J Pathol 158(1) (2001) 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, Kim YH, Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways, Eur.Respir.J 12(4) (1998) 872–878. [DOI] [PubMed] [Google Scholar]
- [121].Svenningsen S, Nair P, Asthma Endotypes and an Overview of Targeted Therapy for Asthma, Front Med 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Burgel PR, Lazarus SC, Tam DCW, Ueki IF, Atabai K, Birch M, Nadel JA, Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation, Journal of Immunology 167(10) (2001) 5948–5954. [DOI] [PubMed] [Google Scholar]
- [123].Dalli J, Serhan CN, Identification and structure elucidation of the pro-resolving mediators provides novel leads for resolution pharmacology, Br. J. Pharmacol 176(8) (2019) 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zimpfer U, Dichmann S, Termeer CC, Simon JC, Schroder JM, Norgauer J, Human dendritic cells are a physiological source of the chemotactic arachidonic acid metabolite 5-oxoeicosatetraenoic acid, Inflamm.Res 49(11) (2000) 633–638. [DOI] [PubMed] [Google Scholar]


