ABSTRACT
Context:
Acute invasive fungal rhinosinusitis (AIFRS) is an aggressive infection affecting immunocompromised patients and carries a high morbidity and mortality. It is commonly seen in immunocompromised patients, mainly in uncontrolled diabetes, malignancy, acquired immunodeficiency syndrome, and so on. However, there has been an exponential increase in the incidence of AIFRS in relation to recent coronavirus disease 2019 (COVID-19) infection.
Aims:
We present this study to assess histomorphological features of fungal infections in the background of COVID-19 era.
Materials and Methods:
The study includes interpretation of 34 biopsies of suspected AIFRS in post COVID-19 patients. The demographic details like patients age, sex, diabetic status, COVID-19 status, and history of steroid intake were collected. All specimens were stained with hematoxylin and eosin and PAS stain. Detailed microscopic examination including the presence of fungal hyphae in the tissue, characterization of inflammatory response, presence of tissue invasion, angioinvasion, and necrosis was noted for each case.
Results:
Thirty-four biopsy specimens from various sites – nasal cavity, maxillary sinus, ethmoid sinus, and so on – were studied. The mean age of the patients with AIFRS was 52.68 years. The dominant fungi were Mucorales in 31 (91.3%), Aspergillus and Mucorales in 1 (2.9%), a combination of Mucorales and Candida identified in 1 (2.9%) case, and Candida alone in 1 case (2.9%). Bony invasion and perineural invasion were observed in 5 cases (14.7%) and 1 (2.9%) case, respectively.
Conclusion:
Histopathological examination plays an essential role in the diagnosis and appropriate management of the patients. Histopathological features including characterization of fungi, angioinvasion, and bone invasion may provide information on rare dreaded infections in post-COVID-19 patients for possible prognostic characteristics on histology.
Keywords: Acute invasive fungal rhinosinusitis, COVID-19, histopathology, mucormycosis
Introduction
The occurrence of acute invasive fungal respiratory infections in patients with coronavirus disease 2019 (COVID-19) has gained increasing attention in the recent past. Acute invasive fungal rhinosinusitis is a rare but highly co-morbid disease affecting immunosuppressed individuals.[1] It may be seen in patients with high risk of complications in the setting of COVID-19.[2-4] The risk of invasive fungal rhinosinusitis is increased in COVID-19 because of its impact in altering innate immunity and is further aggravated by widespread use of steroids/antibiotics/monoclonal antibodies.[5,6]
Acute invasive fungal rhinosinusitis (AIFRS) is a fungal infection characterized by invasion of the nasal cavity and paranasal sinuses and has a high propensity to infiltrate palate, orbit, skin, and intracranial structures with subsequent serious morbidity and mortality.[7] It is seen in immunocompromised patients, more commonly in patients with diabetes, malignancy, acquired immunodeficiency syndrome, chemotherapeutic/immunosuppressive drugs, and recently COVID-19. There has been an aberrant increase in the incidence of AIFRS in association with recent COVID-19 infections.[2-4]
COVID-19 infection is associated with an increased risk of secondary bacterial and fungal infection by reducing the number of T-lymphocytes and altering innate immunity. Alarming signs of rhino-orbital fungal infection include sinusitis, blurred vision, redness of eye, blocked nose, local pain, and blackish discoloration over bridge of nose and palate.[8,9]
Untreated rhino-sinonasal mucormycosis can develop to dangerous complications like cavernous sinus thrombosis and cerebral invasion. Therefore, the primary aspects of effective care of this fatal infection include early identification, surgical debridement, appropriate antifungal medication, and control of risk factors such as diabetes mellitus.[10,11] A sharp rise in the incidence of AIFRS in the backdrop of COVID-19 has become a matter of immediate concern. Prompt diagnosis and early intervention are crucial as the mortality rate can be as high as 50% to 80%.
Furthermore, as COVID-19 is a life-threatening infectious disease, these patients show an overexpression of inflammatory cytokines and impaired cell-mediated immunity with decreased cluster of differentiation 4 and 8 positive T helper (CD4+ T and CD8+ T) cell counts, denoting increased susceptibility to fungal co-infections.[9,10]
Histopathological examination plays a key role in diagnosing the mucormycosis and other fungal infections. The other opportunistic fungal infections reported in COVID-19 patients are oropharyngeal candidiasis and respiratory tract aspergillosis. Histomorphological findings include mycotic infiltration of blood vessels, vasculitis with thrombosis, tissue infarction, hemorrhage, and acute neutrophilic infiltrate.[1,9,10]
Therefore, the current study was initiated with the objective to examine the histopathological features of rhino-orbital/rhino-maxillary/sino-nasal fungal infections in the backdrop of the ongoing COVID-19 pandemic. This study also indicates that a high index of suspicion for AIFRS in cases of COVID-19 patients by primary care providers and physicians would lead to early diagnosis, timely intervention, and management for a better outcome.
Materials and Methods
This is an observational study conducted in the Department of Pathology at a tertiary care center from February to July 2021. The bioethical approval was obtained from the institutional ethics committee (IEC-RDGMC-05/2021). The study includes biopsies from 34 patients suspected for acute invasive fungal rhinosinusitis (AIFRS) who recently recovered from COVID-19 infection and presented to the out-patient department at our tertiary health center.
The clinic-pathological data including the patient’s age, gender, diabetic status, COVID-19 status, and history of steroid intake were analyzed. Tissue samples from all suspected sites were received in formalin for histological examination. All biopsies were stained with hematoxylin and eosin (H and E). Detailed microscopic examination along with special stains, periodic acid–Schiff, was performed for further analysis.
Detailed microscopic examination including the presence of fungal hyphae in the tissue; type of inflammation - neutrophilic, mixed, chronic, or granulomatous response; presence of tissue invasion; and necrosis was noted for each case. Mucor and Aspergillus species were differentiated based on microscopic features. Broad, aseptate right-angled branching fungal hyphae were considered belonging to order Mucorales, and thin septate acute-angled branching fungal hyphae were suggestive of Aspergillus.
The biopsy specimens were studied for the following microscopic details:
Presence of fungi
Fungal morphologies delineated by H and E and PAS as broad aseptate hyphae with right-angle branching were identified as Mucorales species. The presence of mixed infection, mucormycosis and aspergillosis, and mucormycosis and Candida, was noted.
Composition of inflammatory infiltrate.
Presence of granulomatous inflammation and giant cell reactions were noted.
Presence of tissue necrosis
Tissue invasion into soft tissues and bone
Angioinvasion/perineural invasion by fungus noted.
Medical management included antifungal medicine (amphotericin B and posaconazole) and medication for control of any associated medical condition. Surgical intervention was undertaken after histological confirmation of fungal invasion. All patients underwent endoscopic debridement, while a combined approach (endoscopy and open approach) was utilized when the disease involved orbit, palate, or facial skin. Patients were considered cleared of fungus after two negative endoscopic histological results.
Results
The study comprises 34 AIFRS patients who recently recovered from COVID-19 infection. There were 27 males and 7 females (male/female ratio: 3.9:1). The ages ranged from 41 to 72 years (mean: 52.68 years). All patients were COVID-19-positive, and 30 patients were diabetics. Diabetes mellitus and hypertension were the two most common associated medical diseases noted in 30 (88.2%) and 6 (17.6%) patients, respectively. Twenty-four patients were given steroids during the treatment for COVID-19 infection [Table 1].
Table 1.
Clinicopathological features of the patients
| Number of patients | Percentage | |
|---|---|---|
| Total cases | 34 | 100% |
| Gender (male/female) | 27/7 | (79.4/20.6)% |
| Co-morbidities | ||
| Diabetes mellitus (DM) | 30 | 88.2% |
| Hypertension | 6 | 17.6% |
| Chronic kidney disease | 1 | 2.9% |
| Steroid therapy | 24 | 70.6% |
The biopsy specimen was sent from the various sites: nasal cavity (82.4%) and maxillary sinus (8.8%). Maxillary sinus was the most commonly involved paranasal sinus. Orbital disease confined to extra-conal orbital compartment with/without extra-ocular muscle involvement was noted in five of the patients. On macroscopic examination, the tissue samples were predominantly gray-white to black in color. Tissue necrosis was observed in 73.5% of cases [Table 2].
Table 2.
Different sites and histomorphological characteristics of the biopsies in the study
| Histopathological features | No. of patients |
|---|---|
| Site of biopsy | |
| Nasal cavity | 28 (82.4%) |
| Maxillary sinus | 3 (8.8%) |
| Ethmoid sinus | 1 (2.9%) |
| Sphenoid sinus | 1 (2.9%) |
| Palate | 3 (8.8%) |
| Eye orbit | 5 (14.7%) |
| Presence of fungus | |
| Mucor | 31 (91.3%) |
| Mucor and Aspergillus | 1 (2.9%) |
| Mucor and Candida | 1 (2.9%) |
| Candida | 1 (2.9%) |
| Inflammatory infiltrate | |
| Neutrophilic | 12 (35.3%) |
| Mononuclear | 8 (23.5%) |
| Mixed | 12 (35.3%) |
| Granulomatous | 2 (5.9%) |
| Presence of Necrosis | 25 (73.5%) |
| Presence of Tissue invasion | 15 (44.1%) |
| Presence of Angioinvasion | 2 (5.9%) |
| Presence of Perineural invasion | 1 (2.9%) |
| Presence of Bone invasion | 5 (14.7%) |
Biopsies were stained with H and E and PAS stain. Histological evaluation of tissue confirmed purely Mucor species in 31 patients [Figure 1], while one patient showed both Mucor and Aspergillus [Figure 2], one patient showed both Mucor and Candida, and one patient showed Candida [Table 2]. Angioinvasion also commonly noted in specimens of maxillary sinus and nasal cavity (5.9%) [Figure 3]. Bone marrow invasion was most commonly noted in specimens of maxillary sinus [Table 2, Figure 4a and b]. Granulomatous inflammation and giant cell are seen in one case [Figure 5]. Neural invasion by Mucor fungi is also seen in one case [Figure 6].
Figure 1.

Histopathological image showing broad aseptate fungi belonging to Mucorales family (H and E stain, 400x)
Figure 2.

Histopathological image shows fruiting body of Aspergillus (PAS stain, 100x)
Figure 3.
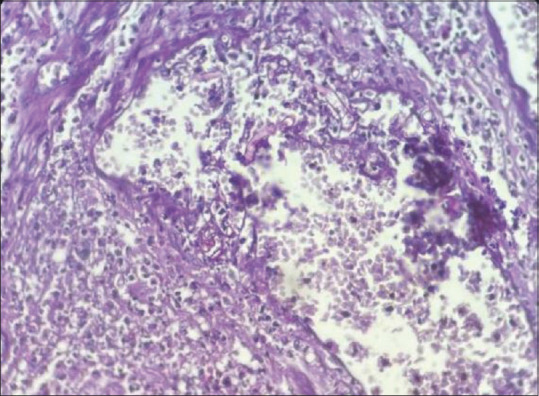
Histopathological image showing angioinvasion by Mucor fungi (H and E stain, 400x)
Figure 4.

(a and b) Histopathological images show tissue and bone invasion by fungal hyphae (H and E stain, PAS stain, 200x)
Figure 5.
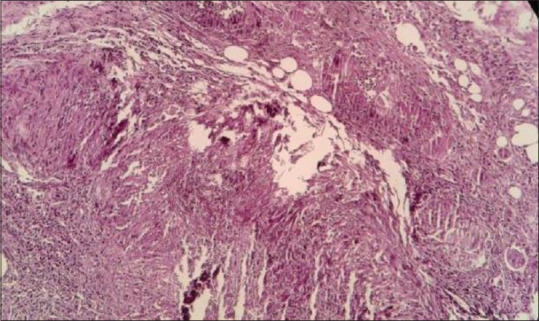
Histopathological image shows granuloma (at left-sided corner) with occasional giant cell (at right-sided corner) and a few fungal hyphae (H and E stain, 100x)
Figure 6.

Histopathological image showing neural invasion by Mucor fungi (PAS stain, 200x)
Among the total 34 cases, 26 cases were in stage 1 (having only sinonasal involvements), 5 cases in stage 2 (having sinonasal and orbital involvement.), and 3 cases in stage 3 (having sinonasal involvement with intra-cranial extension). The most common presentations in these patients were periorbital swelling (35.3%), facial swelling (20.6%), facial/periorbital pain (20.6%), and headache (23.5%).
Among 34 patients, 8 of our patients received antifungal therapy and 26 patients received a combination of surgical intervention and antifungal therapy. Liposomal amphotericin B was given in all patients. Varying degrees of endoscopic sinonasal debridement were performed in 26 of the patients. Eight patients recovered by medical management, while 24 patients recovered after surgical and medical management and 2 patients succumbed to the illness.
Discussion
COVID-19 has been affiliated with a wide range of presentations ranging from a mild cough to life-threatening pneumonia.[11] It leads to diffuse alveolar damage with severe inflammation, and simultaneously, COVID-19 increases the risk of secondary bacterial and fungal infection by altering innate immunity and decreasing T-lymphocytes. The usual sites of infection are paranasal sinuses, central nervous system, lung, gastrointestinal system, and skin.
AIFRS is an aggressive form of fungal infection, with a high rate of morbidity and mortality. The rapid progression and dissemination are attributed to the angio-invasive nature of the fungi. Due to its invasive nature, the infection may also invade the palate, skin, orbit, and intra-cranial structures.[12,13] Clinical presentation depends on the site involved starting with nasal congestion and progressing onto frontal headache, facial numbness, ocular pain, blurry vision, and diplopia, indicating the involvement of orbital and cerebral areas.[14,15] Therefore, early diagnosis and appropriate management are essential to prevent life-threatening complications.
Various studies are being undertaken to acquire knowledge about the new and long-term manifestations of the COVID-19. Therefore, the primary health care providers must be aware of the possibility of invasive fungal infection in such COVID patients with a history of diabetes and other co-morbidities. A high index of suspicion with early diagnosis of acute invasive fungal sinusitis among COVID-19 patients and early management with antifungal therapy and surgical debridement is essential for better outcome.
In a study by Ganesan et al., the mean age was 51.68 ± 10.7 years and there was a predominance of males (83.33%) affected with mucormycosis, which is similar to our study.[16] Diabetes was detected in 88.2% of the patients in our study, which was similar to study by Jain et al.[17] Arora et al. studied the histopathological features of COVID-associated rhino cerebral mucormycosis, and the median age of the subjects was 57 years. The majority of these patients were male; steroids had been used in 45% of the cases, and diabetes mellitus was the predisposing factor in 98% of the cases. The results of the present study closely match those of the above study in accordance to age, sex, and predisposing factor being similar.[18]
The main fungal pathogens for co-infection in severe COVID-19 patients are Mucor, Aspergillus, and Candida. Hence, surveillance for opportunistic fungal pathogens is essential for severely ill and co-morbid patients. Mucormycosis is a rapidly progressive fungal infection and may prove fatal if early diagnosis and treatment are not administered to such patients.
In the study by Ganesan et al., the dominant fungi identified in the specimens were Mucorales in 58 samples (96.67%) and Aspergillus along with Mucorales identified in 12 samples (20%), where as a combination of Mucorales and Candida was noted in 8 cases (13.33%).[16]
Histopathologic examination remains one of the major diagnostic tools because it permits rapid presumptive identification of fungal organisms. Bone marrow invasion and necrosis of bone are common in sinonasal mucormycosis. Perineural/neural invasion is frequently seen in rhino orbital mucormycosis. Histopathology plays a major role as it not only distinguishes the presence of the fungus in the specimen from a culture contaminant but also is indispensible to define whether there is blood vessel invasion in the debrided tissue.
Cornely et al. have described the histopathological picture of mucormycosis and summarized that the acute lesions show hemorrhagic necrosis, angioinvasion, coagulative necrosis, neutrophilic infiltration, and perineural invasion, while the chronic lesion shows a granulomatous inflammation with giant cells along with a deeply eosinophilic material surrounding the pathogen, the Splendore Hoeppli phenomenon.[19]
Tissue necrosis was observed in 71.67% of cases in the study by Ganesan et al., which is similar to our study.[16] In our study, the presence of perineural fungal invasion was evaluated in all cases; however, only one case (n = 1) showed the presence of fungal hyphae within it. This could be because neural tissue was seen in very few cases in our study, possibly owing to the necrosis in our samples.
In a study by Arora et al., the presence of acute inflammation, necrosis with no cellular response, and granulomatous reaction were seen in 2, 16, 4 of the samples, respectively.[18] Granulomatous inflammation is seen in two of the patients in our study. The rare granulomatous response seen in post-COVID-19 mucormycosis could again be due to immune dysregulation as a result of steroid therapy. However, the use of steroids affects the phagocytic ability of macrophages and increases the risk of infection.[20,21]
In the study by Ganesan et al., the predominant type of inflammatory response observed was mixed suppurative (73.33%), followed by acute (21.7%), and the least was chronic type (5%); however, 23% of the cases showed granulomatous inflammation with fungi. Interestingly, the majority of these cases were reported in the late month of May and June when the pandemic infection was showing a downtrend and thus could be the cause for many cases with granulomatous inflammation.[16]
Limitations
This study was a histopathology-based research, and also, ours is a single-center laboratory-based observational study with a limited number of samples from post-COVID-19 patients. More larger studies may be undertaken to compare histopathologic features with prognosis, morbidity, and mortality in COVID-19 patients.
Conclusion
India has witnessed an upsurge of fungal rhinosinusitis during COVID-19 pandemic era more in comparison to the other parts of the world. Uncontrolled diabetes and extensive use of steroids in COVID-19 management can also suppress immunity, allowing emergence of opportunistic fungal infections which might deteriorate the patients. Its association with invasive sinusitis caused by mucormycosis is detrimental and must be looked with suspicion.
Histopathological examination is a major diagnostic tool in the diagnosis of AIFRS as it not only distinguishes the presence of the fungus in the specimen but also helps to identify whether there is blood vessel invasion, bone invasion, or perineural invasion in the debrided tissue.
Key points
Clinical suspicion by primary health care providers would lead to early diagnosis of acute invasive fungal sinusitis among COVID-19 patients by debridement of tissue and histopathological evaluation. Histopathological evaluation may also give an insight into the prognostic indicators, morbidity, and mortality in these patients. Early diagnosis along with early management with antifungal therapy and surgical debridement is essential for better outcome and higher survival.
List of abbreviations
AIFRS Acute invasive fungal rhinosinusitis
H and E Hematoxylin and Eosin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis:A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135:442–7. doi: 10.1017/S0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouad YA, Abdelaziz TT, Askoura A, Saleh MI, Mahmoud MS, Ashour DM. Spike in rhino-orbital-cerebral mucormycosis cases presenting to a tertiary care center during the COVID-19 pandemic. Front Med (Lausanne) 2021;8:645270. doi: 10.3389/fmed.2021.645270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Aziz M, Azab N. Acute invasive fungal rhinosinusitis and Coronavirus disease 2019. J Craniofac Surg. 2021;32:e827–30. doi: 10.1097/SCS.0000000000008231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel-Aziz M, Azab N, Abdel-Aziz NM, Abdel-Aziz DM. Mucormycosis:A potential head and neck problem in COVID-19 patients. Laryngoscope Investig Otolaryngol. 2022;7:67–9. doi: 10.1002/lio2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M. Bacterial and fungal coinfection in individuals with coronavirus:A rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–68. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangneux JP, Bougnoux ME, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID-19:We should be prepared. J Mycol Med. 2020;30:100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashour MM, Abdelaziz TT, Ashour DM, Askoura A, Saleh MI, Mahmoud MS. Imaging spectrum of acute invasive fungal rhino-orbital-cerebral sinusitis in COVID-19 patients:A case series and a review of literature. J Neuroradiol. 2021;48:319–24. doi: 10.1016/j.neurad.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Therakathu J, Prabhu S, Irodi A, Sudhakar SV, Yadav VK, Rupa V. Imaging features of rhinocerebral mucromycosis:A study of 43 patients. The Egyptian Journal of Radiology and Nuclear Medicine. 2018;49:447–52. [Google Scholar]
- 9.Singh VP, Bansal C, Kaintura M. Sinonasal mucormycosis:A to Z. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 3):1962–71. doi: 10.1007/s12070-018-1384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagano L, Valentini CG, Fianchi L, Caira M. The role of neutrophils in the development and outcome of zygomycosis in haematological patients. Clin Microbiol Infect. 2009;15(Suppl 5):33–6. doi: 10.1111/j.1469-0691.2009.02977.x. [DOI] [PubMed] [Google Scholar]
- 13.Walther G, Wagner L, Kurzai O. Updates on the taxonomy of mucorales with an emphasis on clinically important taxa. J Fungi (Basel) 2019;5:106. doi: 10.3390/jof5040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahota R, Gambhir R, Anand S, Dixit A. Rhinocerebral mucormycosis:Report of a rare case. Ethiop J Health Sci. 2017;27:85–90. doi: 10.4314/ejhs.v27i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin E, Moua T, Limper AH. Pulmonary mucormycosis:Clinical features and outcomes. Infection. 2017;45:443–8. doi: 10.1007/s15010-017-0991-6. [DOI] [PubMed] [Google Scholar]
- 16.Ganesan N, Sivanandam S. Histomorphological features of mucormycosis with rise and fall of COVID-19 pandemic. Pathol Res Pract. 2022;236:153981. doi: 10.1016/j.prp.2022.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain K, Surana A, Choudhary TS, Vaidya S, Nandedkar S, Purohit M. Clinical and histology features as predictor of severity of mucormycosis in post-COVID-19 patients:An experience from a rural tertiary setting in Central India. SAGE Open Med. 2022;10:20503121221074785. doi: 10.1177/20503121221074785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora R, Goel R, Khanam S, Kumar S, Shah S, Singh S. Rhino-orbito-cerebral-mucormycosis during the COVID-19 second wave in 2021-A preliminary report from a single hospital. Clin Ophthalmol. 2021;15:3505–14. doi: 10.2147/OPTH.S324977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B. Global guideline for the diagnosis and management of mucormycosis:An initiative of the european confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19:e405–21. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM. The epidemiology and clinical manifestations of mucormycosis:A systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019:A meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]


