Abstract
Diapause is a protective mechanism that many organisms deploy to overcome environmental adversities. Diapause extends lifespan and fertility to enhance the reproductive success and survival of the species. Although diapause states have been known and employed for commercial purposes, for example in the silk industry, detailed molecular and cell biological studies are an exciting frontier. Understanding diapause-like protective mechanisms will shed light on pathways that steer organisms through adverse conditions. One hope is that an understanding of the mechanisms that support diapause might be leveraged to extend the lifespan and/or health span of humans as well as species threatened by climate change. In addition, recent findings suggest that cancer cells that persist after treatment mimic diapause-like states, implying that these programs may facilitate cancer cell survival from chemotherapy and cause relapse. Here, we review the molecular mechanisms underlying diapause programs in a variety of organisms, and we discuss pathways supporting diapause-like states in tumor persister cells.
Keywords: chemotherapy, diapause, dormancy, germline stem cell, quiescence, stem cell self-renewal
Introduction
In the wild, animals are faced with predictable adverse conditions such as seasonal changes in temperature and humidity as well as unpredictable challenges. Since most animals, unlike humans, are unable to build and maintain optimal environments, they have evolved mechanisms to adapt or escape. For example, many animals migrate long distances to avoid harsh conditions [1]. Other animals have evolved mechanisms to cope with harsh environments such as torpor, hibernation, or reptilian brumation, amongst others. Torpor and hibernation are physiological states in which animals reduce their body temperature and metabolic rate to conserve energy. Whereas torpor occurs during periods of environmental stress or food scarcity, hibernation is a seasonal state that some mammals enter during winter months. Brumation is a similar state of dormancy or reduced metabolic activity combined with lowered body temperature found in reptiles during colder months. These states share in common that they may remain in a state of sleep or rest for an extended period, lower their body temperature, and reduce the metabolic rate to conserve energy yet differ in terms of the animal groups involved, duration, frequency, and the extent of temperature reduction. Diapause is a coping strategy triggered by environmental cues such as changes in temperature, day length, or food scarcity and is employed by a variety of organisms, from invertebrates to vertebrates including mammals. During diapause, an organism essentially ‘pauses' its normal growth, development, activity, and reproduction to conserve energy and increase its chances of survival in adverse conditions.
These kinds of defensive mechanisms are common in the wild but frequently ignored when we study common model organisms in the laboratory since we tend to rear them under uniform conditions that are usually optimized for rapid growth and reproduction. Studying this developmental plasticity in response to stressors is important if we wish to understand how they relate to development under optimal conditions and uncover protective pathways that confer resilience to environmental changes.
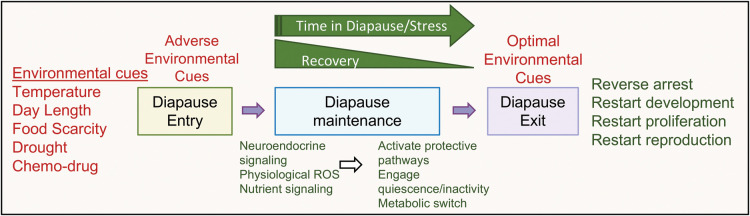
During diapause, organisms either arrest or delay development at different preferred lifecycle stages in response to regular and recurring periods of adverse environmental conditions (Figure 1). Development resumes once the conditions become favorable. To undergo diapause an organism must (1) sense the environmental cues, (2) enact changes to its physiology, (3) halt development, and (4) remain quiescent until conditions become favorable, then (5) sense the improving environmental condition, and finally (6) reverse the diapause process and restart normal development. Genes involved in any of these processes would be expected to modulate the ability to diapause and perpetuate the species.
Figure 1. Diapause/diapause-like state to survive adverse conditions.
Animals or cells sense the environmental cues or chemo-drug treatment and transform into a protective stress-resistant state to evade the harsh conditions. Once the adverse conditions reverse, they can then recover and restart the development or the proliferation. The extent of reversibility depends on the duration and intensity of the adverse conditions prevailing, and the sooner the optimal conditions arrive better the reversibility.
Understanding diapause is fundamental to harnessing the underlying mechanisms. It is of interest to determine whether these diverse responses to environmental adversity share common features or whether there may be multiple mechanisms that can confer resilience. Furthermore, studies of recovery mechanisms will uncover whether organisms reactivate normal developmental programs or employ new ones. The mechanisms may also relate to evolution, as environmental stresses may elicit phenotypic changes that can later become fixed by regulatory mutations [2]. Studying diapause contributes to a deeper understanding of ecological adaptations, offering insights into the strategies organisms employ to survive and reproduce in challenging conditions. This knowledge can have implications for fields ranging from ecology and evolution to agriculture, conservation, and medicine. Thus, diverse animal models have emerged to study the different facets of the diapause process.
Diapause across the animal kingdom
Diapause is a significant and widely distributed phenomenon in the insect world, and different species undergo diapause at distinct lifecycle stages (embryonic, juvenile, or adult) [3–5]. For example, while silkworm and mosquito diapause occurs in the embryonic stage, monarch butterflies and cotton bollworms diapause in the pupal stage, and the optimal stage for the fruit fly Drosophila melanogaster is the newly eclosed adult. In Drosophila melanogaster, the phenomenon was originally described as adult reproductive diapause due to arrested oogenesis and, more recently, spermatogenesis [6]. However, it has been variously described as a shallow diapause or as a cold-induced general stress response because the arrest to oogenesis occurs after rather than before the beginning of yolk deposition, similar to the response to stress like starvation [7]. However, detailed characterization of ovarian development uncovered differences between diapause and stresses including protein deprivation and predator exposure. These differences include early arrest of oogenesis and changes in the germline stem cell state that prolong longevity, more consistent with a dormancy [8]. The difference may lie in the details of the diapause induction conditions. When pharate adult or newly eclosed flies are cultured under short day length and at 10°C, diapause arrest is early and 100% penetrant. Shifting flies to diapause conditions days after eclosion and mating results in a less complete arrest and poorer survival. Thus true diapause may be limited to the newly eclosed virgin fly.
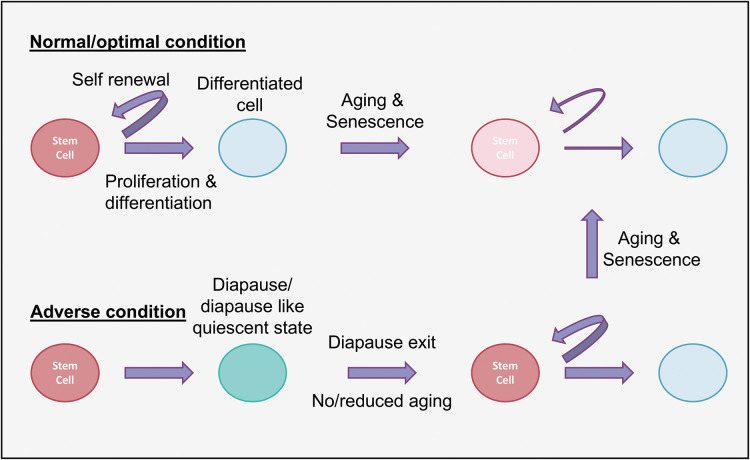
In the nematode, C. elegans diapause is known as the dauer state, which occurs when first instar larvae form dauers instead of progressing to second instar larvae. Dauer formation may be the best-studied dormancy program and has been exploited for decades for insights into normal aging and possible strategies for lifespan extension [9–12]. Reduced insulin/IGF-1 signaling leads to the activation of Foxo (Forkhead box O) transcription factors to promote dauer development, demonstrating the importance of Foxo in orchestrating a switch between growth and diapause in these tiny roundworms. The discovery that the insulin signaling pathway, which is inhibited during dauer formation, promotes aging under optimal growth conditions in organisms as diverse as worms, flies, and mammals may represent the strongest evidence that studies of diapause, even in relatively simple animals, may yield general principles of resilience and longevity. The observation that diapause preserves the long-term viability of germline stem cells, presumably to ensure fertility when favorable conditions return [8,13] raises the possibility that other types of adult stem cells may employ similar mechanisms to extend their lifespans and/or health spans during diapause (Figure 2).
Figure 2. Diapause/diapause-like state protects stem cell longevity.
Stem cells undertake a reversible quiescence and evade the adverse conditions to avoid senescence and decline to keep a youthful state. Post-diapause, the stem cells reverse the quiescence and resume proliferation and self-renewal.
Recently vertebrates like killifish [14–16] have emerged as interesting embryonic diapause models [17–21]. In killifish, diapause is a remarkable survival strategy that empowers the embryos to endure and persist in habitats prone to drying up due to temperature extremes. Transcriptome analysis of this stage revealed that diapause is an active program in which polycomb complex members repress metabolism and muscle genes by maintaining H3K27me3-mediated repressive epigenetic marks on key developmental genes [15].
Close to 130 species of mammals also undergo diapause during early embryonic development in the uterus by receiving cues from the environment such as harsh seasons or lack of sufficient nutrition like during the lactation period. In mice, diapause can occur at the blastula stage if conceived during the lactating period which is characterized by delayed implantation in the uterus [22,23]. In addition to its commercial and pest control potential, diapause may have implications for human health. At the organismal level, diapause has gained attention as a potential means to extend lifespan. Since diapause extends the lifespan in many organisms, may offer clues to slowing aging. At the cellular level, it is becoming clear that cancer persister cells mimic a diapause-like state to evade cell death from chemo- and radiation therapies [24–26]. Although different organisms undergo diapause at distinct life stages and in response to a variety of cues, the decision to enter diapause and exit in most, if not all, organisms is a response to environmental change.
Environmental sensing leading to diapause entry and exit
Diapause is typically a seasonal event and sensing the environment is fundamental for entry and exit into this program. Environmental cues such as day length and temperature are the most common diapause regulators. Day-length-sensing and tracking suggest that circadian rhythms might be involved. Consistent with that idea, the neuropeptides pigment dispersing factor (PDF) and short neuropeptide F (sNPF), which are expressed in so-called clock neurons, as well as genes controlling circadian activities like period (per), timeless (tim), eyes absent (eya), and cryptochrome (cry) affect by lowering reproductive dormancy if their function is impaired in insects [27–33]. Interestingly, similar to diapause, circadian rhythms can also modulate torpor by influencing the timing and duration of torpor [34,35].
Transient Receptor Potential (TRP) cation channels, first discovered in Drosophila [36,37] are well-established temperature sensors [38,39]. Temperature sensing regulates diapause through TRPA1 in silkworms and C. elegans [40–42]. Apart from thermosensation via sensory neurons, food scarcity and overcrowding can also induce diapause. Chemosensation plays an important role in identifying dauer pheromones while overcrowding can be sensed by touch in worms [43,44]. After sensing adverse conditions, organisms must initiate the diapause program. However, the sensors for the salient environmental cue to enter diapause are not yet known in many organisms.
Transition to diapause via neuro-endocrine signaling
After perceiving the relevant environmental cue, hormonal and neuro-endocrine signaling rapidly signals a global change from active development to the state of dormancy or diapause. Multiple neuropeptides and hormones have been shown to regulate diapause in multiple species. For instance, the neuropeptide-like diapause hormone(DH) controls embryonic diapause in silkworms [32,40,42,45]. Integrating environmental cues such as temperature and photoperiodism can be key to successful diapause. Recent reports suggest that fruit fly pigment dispersing factor (PDF) neuropeptides, expressed in the clock neurons, are temperature- and light-sensitive and in response to diapausing conditions (short-day length and low temperature), PDF levels go down which promotes diapause ovarian arrest [30,32,34].
Juvenile hormone(JH) is a multifunctional hormone in insects that controls metamorphosis and reproduction, and it must be down-regulated for them to enter reproductive diapause [8,46,47]. Interestingly, loss or reduction in JH can also extend the fly lifespan [48]. Allatostatin C (AstC) is a neuropeptide that plays a role in the regulation of fruit fly physiology and behavior, and low temperature-mediated down-regulation of AstC activates cholinergic AstC-receptor neurons to induce ovarian arrest possibly by inhibiting JH production [49,50].
Noradrenergic signaling through norepinephrine (NE) in vertebrates or octopamine (OA) in invertebrates can regulate oocyte quiescence in response to environmental factors like absence of mates in C. elegans or starvation in fruit flies and zebrafish [51]. Feeding is reduced in most diapausing animals, suggesting that noradrenergic signaling in combination with nutrient signaling may play a role in the diapause process.
Another stress-resisting state common among birds and heterothermic mammals (mammals that can vary their body temperature), is torpor [52,53]. While torpor may last only a few hours in small mammals and birds such as hummingbirds, awakening from it can occur in a matter of minutes. A recent report revealed that a specific subset of glutamatergic neurons in the medial and lateral preoptic area of the hypothalamus regulates torpor in mice and inhibition of this neuronal activity prevents natural torpor process [54]. It would be interesting to determine to what extent stress resistance and response pathways overlap in torpor and diapause states.
In mammals, embryo implantation at the blastocyst stage is delayed in response to diapause. Normally, the endometrial estrogen E2 induces leukemia inhibitory factor (LIF) which initiates implantation. This signaling is down-regulated during the dormant state of the blastocyst, and E2 injection can reverse the diapause arrest [55–58]. Once initiated, diapause is maintained through the perception of additional signals like nutrient availability. Absence of E2 reduces the embryo's metabolism and activity to induce quiescence.
Nutrient sensing and metabolic changes in diapause
Diapause can be influenced by nutrient-sensing signaling pathways. For instance, lack of food or overcrowding can initiate C. elegans dauer. Sensory neurons in C. elegans express G protein-coupled receptors (GPCRs) on their surfaces. These GPCRs are sensitive to environmental cues, including nutrients and odorants. Activation of GPCRs triggers the production of intracellular second messengers like cAMP or cGMP within the sensory neuron that relay the signal from the cell surface to downstream effector proteins in the cell. Nutrient sensing via neurons that express TAX-2/4 (α and β subunits of cGMP-gated channel) is necessary for worms to undergo quiescence as the loss of egl4, which encodes a protein kinase downstream of cGMP signaling hampers quiescence [59]. While nutrient deprivation can induce diapause, most organisms feed less during diapause that is triggered by other cues, leading to dramatic changes in nutrient signaling. Thus diapause involves sensing nutrients and in turn changes in feeding behavior, and subsequent responses to caloric restriction and changes in dietary composition [4,60–62].
The best-known nutrient signaling involved in diapause regulation is the insulin pathway, which is down-regulated at an organismal level in most diapause states across species [9,63–67]. Insulin is a key hormone involved in nutrient sensing and modulates metabolic responses to adjust energy expenditure according to nutrient levels. The relationship between Target of Rapamycin (TOR) signaling and diapause is an example of how cellular pathways originally associated with growth and metabolism can also play a role in regulating adaptive responses to environmental challenges. TOR signaling, which responds to amino acid levels, is down-regulated in some insect and mammalian diapause states [20,68–70]. Another nutrient signaling pathway that is down-regulated in diapause-like quiescence is the fatty acid oxidation (FAO) pathway — a metabolic process that generates energy. Embryonic stem cells are prompted to enter quiescence through the inhibition of FAO — by suppressing histone acetyltransferase MOF (Males absent on the first), which is a direct activator of FAO by H4K16ac of FAO pathway genes [71]. This report unveils a regulatory mechanism by which MOF sustains ESCs in an active state, providing a connection between epigenetic processes and metabolic pathways that govern cell behavior.
Animals in torpor or diapause can undergo periodic metabolic arousal while in dormancy by undergoing a metabolic switch from anaerobic to aerobic metabolism. A decreased level of reactive oxygen species (ROS) induces metabolic arousal and elevated ROS extends the duration of metabolic depression by regulating flux through the tricarboxylic acid cycle [72]. Another example of a metabolic changes in diapause is in the cotton bollworm pupae. During normal pupal development of cotton bollworms, the fat body dissociates into single cells, which is important for proper lipid metabolism to meet the energy needs of normal transition from pupal to adult stage. In diapause the dissociation fails, resulting in impaired lipid metabolism and leading to developmental arrest at pupal stage [73]. Thus nutrient signaling and metabolic switching are common and essential features of dormancy. In addition to these altered signaling events and the shift from active development to quiescence, protective mechanisms are activated in diapause to enhance survival despite extreme conditions.
Diapause-induced protective mechanisms
During diapause, animals alter their behavior and lower activities such as feeding, movement, and reproduction. Conditions that induce diapause are generally too harsh to support rapid development, growth, and reproduction. Protective pathways must be engaged to prevent irreversible damage [19]. The best-known protective mechanisms are reduced rates of cell division or cellular quiescence. Molecular and cell biological details of protective mechanisms are also starting to emerge. The ability to preserve reproductive capacity is essential for the perpetuation of the species. In diapausing C. elegans most of the germline cells undergo apoptosis but a few surviving cells persist and recover when favorable conditions return [13]. In Drosophila, most germline stem cells enter an unusual inactive state during diapause and resurge once the environment becomes favorable [8]. Similar protective mechanisms are observed in various organisms during diapause, ranging from C. elegans and Drosophila to mammalian embryos. Growth and metabolism are down-regulated in diapause to reduce cellular activity.
In mammalian embryos, Myc or mTOR down-regulation can induce diapause in the preimplantation blastocyst stage. Micro RNAs (miRNAs) are a class of small RNA molecules that play crucial roles in post-transcriptional gene regulation. The miRNA Let-7 was initially discovered in the nematode C. elegans, which regulates the timing of developmental transitions. A family of related miRNAs in mice and roe deer were later found to induce diapause by down-regulating both Myc and mTOR pathways [20,74]. In both mammalian blastocysts and embryonic stem cells (ESCs), experimental reduction in Myc [75] and/or mTOR [19] reduces cell proliferation, inducing a reversible dormancy. The gene expression signatures of mTOR-depleted or Myc-depleted ESCs are similar to the gene expression patterns of diapausing blastocysts, suggesting that ESCs may enter a state of dormancy in response to starvation or experimental inhibition of mTOR or Myc and emerge again when the stress is removed [19,75,76].
An interesting unanswered question is how protective pathways are induced. One concept is that relatively low levels of induction of stress response pathways are protective whereas high levels of stress signaling can be harmful. Supporting this idea, increased but sub-pathological ROS levels that occur in diapause can induce DNA damage and DNA damage responses [8,15,77–80]. p53 is an important tumor suppressor, with roles in genome protection and cell cycle regulation in response to damage, and recent reports suggest that p53 protein levels are elevated during diapause in flies, worms, and mice, although the significance of this up-regulation is not yet clear [8,81,82].
The forkhead box family of transcription factors (FOX) is one of the key signaling nodes activated in responses to starvation or other stress conditions. Foxo expression or DAF-16 activation promotes diapause and dauer [64,83–85]. Foxo3 is up-regulated in blastocyst diapause in mice [86]. However, Foxa2 depletion in the mouse uterine gland causes embryonic diapause suggesting differential action of FOX transcription factors in different cell types such as in the uterus and blastocyst [57]. In general, FOX transcription factors can reduce transcription to slow down metabolism and limit cell divisions, thereby slowing growth and development, which allows time for DNA repair, all of which likely promote stress resistance.
The signaling pathways discussed above suggest that there are mechanisms acting to switch organisms between states: under optimal temperature, hydration, and nutrient availability, metabolism ramps up to promote growth and reproduction whereas when conditions are less favorable, organisms enter quiescent states and yet are poised to reactivate development if and when conditions improve. The first organisms to evolve such stress responses were likely unicellular, so it should not be surprising that similar mechanisms operate at the level of individual cells. For example, when cancer cells are exposed to chemotherapy, evidence is emerging that they can evade death by taking up a dormant state similar to that of diapause.
Persister cancer cells mimic a diapause-like state for their survival
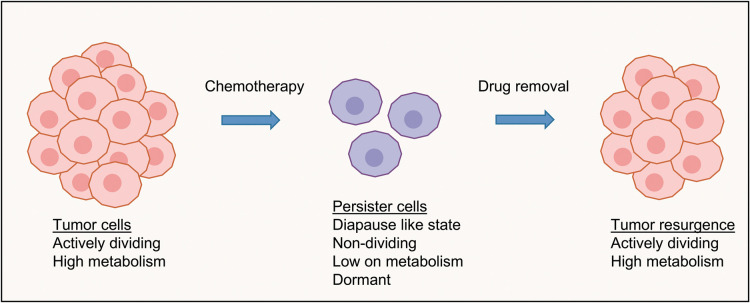
Adverse conditions can trigger diapause-like states not only in organisms but also in cells like embryonic stem cells (ESCs) (Figure 3). Cancer cells that survive chemotherapy drugs are the leading cause of relapse and death. Recently, it has been reported that drug-induced persister cells enter a diapause-like state thereby escaping the lethal effects of the drugs [24–26]. These reports show that persister cells have a transcriptional signature similar to that of a diapausing blastocyst, including down-regulating both Myc and mTOR pathways. This leads to a temporary quiescent state that allows survival during drug treatment. Once the drug is withdrawn, persister cells can exit quiescence and resume proliferation, leading to relapse.
Figure 3. Tumor cells mimic diapause-like state to persist chemotherapy.
Tumor cells take up an inactive diapause-like state to survive the chemotherapy. Once the drug is withdrawn, the persister cells can often resurge back later in life.
Due to their high proliferation rates, cancer cells experience high ROS, which is detrimental to normal cells. In cancer, oxidative stress inhibits cell division and causes metabolic changes that can allow cells to survive, resulting in persister cells [87]. Persister cells up-regulate TRPA1, which induces Ca2+ influx and prevents apoptosis by overexpressing Ca2+-mediated anti-apoptotic signaling pathways allowing cells to resist and survive chemotherapy-induced ROS. Inhibiting TRPA1-mediated redox sensing can increase chemosensitivity and reduce persister cells [87]. Another survival mechanism identified in persister cells is the intracellular cholesterol transporter protein, NPC1L1, which can promote uptake of the antioxidant vitamin E and thereby reduce oxidative stress, and enhance persister cell survival. Blocking NPC1L1 prevents tumor survival in mice after chemotherapy [88]. Similarly, increased ROS levels are detected in developmental diapause in insects like fruit flies and cotton bollworms, although it is not yet known whether protective mechanisms are induced in response. These reports suggest similarities between developmental diapause and the diapause-like state that persister cells adopt to survive in chemotherapy.
Conclusion
Diapause is a type of dormancy program initiated in response to harsh conditions in wild habitats that promote animal survival by halting growth and development until conditions improve. Cancer cells may coopt such dormancy pathways to persist through chemo- and radiation treatment. The molecular and cell biological details of normal and pathological dormancy programs are only beginning to be elucidated. How animals perceive environmental stresses and the role of neuroendocrine control of diapause programs are starting to emerge. Further in-depth mechanistic understanding of the protective pathways engaged during diapause and diapause-like states is needed. Manipulating and engaging the protective pathways has the potential to increase organismal resilience to climate change and in principle might be manipulated to control diseases like cancer and insect-borne diseases, extend stem cell longevity, reproductive span, healthspan, and even lifespan.
Perspectives
Organismal resilience to stress, with implications for evolution and climate change, can be better understood through diapause, which sheds light on developmental plasticity in response to adverse conditions
Not only do organisms, embryos, and stem cells undergo diapause/quiescence under adverse conditions, but cancer cells also can use the diapause-like mechanism to survive chemotherapy
An in-depth understanding of the molecular and cell biological underpinning of the diapause will help to extend healthspan and reproductive lifespan
Acknowledgements
The authors thank Maddalena Nano for proofreading the manuscript.
Abbreviations
- AstC
Allatostatin-C
- DH
diapause hormone
- ESC
embryonic stem cells
- FOX
forkhead box family of transcription factors
- JH
juvenile hormone
- LIF
leukemia inhibitory factor
- Mmp
matrix metalloproteinases
- NE
norepinephrine
- NPC1L1
Niemann-pick C1 like 1
- OA
octopamine
- ROS
reactive oxygen species
- TOR
target of rapamycin
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by NIH grant R01AG36907 to D.J.M.
Open Access
Open access for this article was enabled by the participation of University of California in an all-inclusive Read & Publish agreement with Portland Press and the Biochemical Society under a transformative agreement with UC.
References
- 1.Alerstam, T. and Bäckman, J. (2018) Ecology of animal migration. Curr. Biol. 28, R968–R972 10.1016/j.cub.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 2. https://www.jstor.org/stable/j.ctt1np9wb The Plausibility of Life: Resolving Darwin's Dilemma on JSTOR.
- 3.Tougeron, K. (2019) Diapause research in insects: historical review and recent work perspectives. Entomol. Exp. Appl. 167, 27–36 10.1111/eea.12753 [DOI] [Google Scholar]
- 4.Hahn, D.A. and Denlinger, D.L. (2007) Meeting the energetic demands of insect diapause: nutrient storage and utilization. J. Insect Physiol. 53, 760–773 10.1016/j.jinsphys.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 5.Denlinger, D.L. (2023) Insect diapause: from a rich history to an exciting future. J. Exp. Biol. 226, jeb245329 10.1242/jeb.245329 [DOI] [PubMed] [Google Scholar]
- 6.Kubrak, O.I., Kučerová, L., Theopold, U., Nylin, S. and Nässel, D.R. (2016) Characterization of reproductive dormancy in male Drosophila melanogaster. Front. Physiol. 7, 572 10.3389/fphys.2016.00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lirakis, M., Dolezal, M. and Schlötterer, C. (2018) Redefining reproductive dormancy in Drosophila as a general stress response to cold temperatures. J. Insect Physiol. 107, 175–185 10.1016/j.jinsphys.2018.04.006 [DOI] [PubMed] [Google Scholar]
- 8.Easwaran, S., Van Ligten, M., Kui, M. and Montell, D.J. (2022) Enhanced germline stem cell longevity in Drosophila diapause. Nat. Commun. 13, 711 10.1038/s41467-022-28347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apfeld, J. and Kenyon, C. (1998) Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95, 199–210 10.1016/s0092-8674(00)81751-1 [DOI] [PubMed] [Google Scholar]
- 10.Ailion, M. and Thomas, J.H. (2000) Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics 156, 1047–1067 10.1093/genetics/156.3.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu, P.J. (2007) Dauer. WormBook, 1–19 10.1895/wormbook.1.144.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassada, R.C. and Russell, R.L. (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342 10.1016/0012-1606(75)90109-8 [DOI] [PubMed] [Google Scholar]
- 13.Angelo, G. and Van Gilst, M.R. (2009) Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326, 954–958 10.1126/science.1178343 [DOI] [PubMed] [Google Scholar]
- 14.Romney, A.L.T., Davis, E.M., Corona, M.M., Wagner, J.T. and Podrabsky, J.E. (2018) Temperature-dependent vitamin D signaling regulates developmental trajectory associated with diapause in an annual killifish. Proc. Natl Acad. Sci. U.S.A. 115, 12763–12768 10.1073/pnas.1804590115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, C.-K., Wang, W., Brind'Amour, J., Singh, P.P., Reeves, G.A., Lorincz, M.C.et al. (2020) Vertebrate diapause preserves organisms long term through Polycomb complex members. Science 367, 870–874 10.1126/science.aaw2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichard, M., Cellerino, A. and Valenzano, D.R. (2015) Turquoise killifish. Curr. Biol. 25, R741–R742 10.1016/j.cub.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Renfree, M.B. and Fenelon, J.C. (2017) The enigma of embryonic diapause. Development 144, 3199–3210 10.1242/dev.148213 [DOI] [PubMed] [Google Scholar]
- 18.Ptak, G.E., Modlinski, J.A. and Loi, P. (2013) Embryonic diapause in humans: time to consider? Reprod. Biol. Endocrinol. 11, 92 10.1186/1477-7827-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulut-Karslioglu, A., Biechele, S., Jin, H., Macrae, T.A., Hejna, M., Gertsenstein, M.et al. (2016) Inhibition of mTOR induces a paused pluripotent state. Nature 540, 119–123 10.1038/nature20578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Weijden, V.A., Bick, J.T., Bauersachs, S., Rüegg, A.B., Hildebrandt, T.B., Goeritz, F.et al. (2021) Amino acids activate mTORC1 to release roe deer embryos from decelerated proliferation during diapause. Proc. Natl Acad. Sci. U.S.A. 118, e2100500118 10.1073/pnas.2100500118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Weijden, V.A., Rüegg, A.B., Bernal-Ulloa, S.M. and Ulbrich, S.E. (2021) Embryonic diapause in mammals and dormancy in embryonic stem cells with the European roe deer as experimental model. Reprod. Fertil. Dev. 33, 76 10.1071/RD20256 [DOI] [PubMed] [Google Scholar]
- 22.Fenelon, J.C. and Renfree, M.B. (2018) The history of the discovery of embryonic diapause in mammals. Biol. Reprod. 99, 242–251 10.1093/biolre/ioy112 [DOI] [PubMed] [Google Scholar]
- 23.Deng, L., Li, C., Chen, L., Liu, Y., Hou, R. and Zhou, X. (2018) Research advances on embryonic diapause in mammals. Anim. Reprod. Sci. 198, 1–10 10.1016/j.anireprosci.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Dhimolea, E., de Matos Simoes, R., Kansara, D., Al'Khafaji, A., Bouyssou, J., Weng, X.et al. (2021) An embryonic diapause-like adaptation with suppressed Myc activity enables tumor treatment persistence. Cancer Cell 39, 240–256.e11 10.1016/j.ccell.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehman, S.K., Haynes, J., Collignon, E., Brown, K.R., Wang, Y., Nixon, A.M.L.et al. (2021) Colorectal cancer cells enter a diapause-like DTP state to survive chemotherapy. Cell 184, 226–242.e21 10.1016/j.cell.2020.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, Y.-H. and Zhu, H. (2021) A malignant case of arrested development: cancer cell dormancy mimics embryonic diapause. Cancer Cell 39, 142–144 10.1016/j.ccell.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo, S., Anguiano, M., Tabuloc, C.A. and Chiu, J.C. (2023) Seasonal cues act through the circadian clock and pigment-dispersing factor to control EYES ABSENT and downstream physiological changes. Curr. Biol. 33, 675–687.e5 10.1016/j.cub.2023.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauranen, H., Kinnunen, J., Hiillos, A.-L., Lankinen, P., Hopkins, D., Wiberg, R.A.W.et al. (2019) Selection for reproduction under short photoperiods changes diapause-associated traits and induces widespread genomic divergence. J. Exp. Biol. 222, jeb205831 10.1242/jeb.205831 [DOI] [PubMed] [Google Scholar]
- 29.Abrieux, A., Xue, Y., Cai, Y., Lewald, K.M., Nguyen, H.N., Zhang, Y.et al. (2020) EYES ABSENT and TIMELESS integrate photoperiodic and temperature cues to regulate seasonal physiology in Drosophila. Proc. Natl Acad. Sci. U.S.A. 117, 15293–15304 10.1073/pnas.2004262117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasebe, M. and Shiga, S. (2022) Clock gene-dependent glutamate dynamics in the bean bug brain regulate photoperiodic reproduction. PLoS Biol. 20, e3001734 10.1371/journal.pbio.3001734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy, D., Cusumano, P., Andreatta, G., Anduaga, A.M., Hermann-Luibl, C., Reinhard, N.et al. (2019) Peptidergic signaling from clock neurons regulates reproductive dormancy in Drosophila melanogaster. PLoS Genet. 15, e1008158 10.1371/journal.pgen.1008158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui, W.-Z., Qiu, J.-F., Dai, T.-M., Chen, Z., Li, J.-L., Liu, K.et al. (2021) Circadian clock gene period contributes to diapause via GABAeric-diapause hormone pathway in Bombyx mori. Biology (Basel) 10, 842 10.3390/biology10090842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunders, D.S. (2020) Dormancy, diapause, and the role of the circadian system in insect photoperiodism. Annu. Rev. Entomol. 65, 373–389 10.1146/annurev-ento-011019-025116 [DOI] [PubMed] [Google Scholar]
- 34.van der Vinne, V., Bingaman, M.J., Weaver, D.R. and Swoap, S.J. (2018) Clocks and meals keep mice from being cool. J. Exp. Biol. 221, jeb179812 10.1242/jeb.179812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Revel, F.G., Herwig, A., Garidou, M.-L., Dardente, H., Menet, J.S., Masson-Pévet, M.et al. (2007) The circadian clock stops ticking during deep hibernation in the European hamster. Proc. Natl Acad. Sci. U.S.A. 104, 13816–13820 10.1073/pnas.0704699104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montell, C., Jones, K., Hafen, E. and Rubin, G. (1985) Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230, 1040–1043 10.1126/science.3933112 [DOI] [PubMed] [Google Scholar]
- 37.Montell, C. and Rubin, G.M. (1989) Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2, 1313–1323 10.1016/0896-6273(89)90069-x [DOI] [PubMed] [Google Scholar]
- 38.Caterina, M.J., Schumacher, M.A., Tominaga, M., Rosen, T.A., Levine, J.D. and Julius, D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 39.Jordt, S.-E., McKemy, D.D. and Julius, D. (2003) Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 13, 487–492 10.1016/S0959-4388(03)00101-6 [DOI] [PubMed] [Google Scholar]
- 40.Sato, A., Sokabe, T., Kashio, M., Yasukochi, Y., Tominaga, M. and Shiomi, K. (2014) Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc. Natl Acad. Sci. U.S.A. 111, E1249–E1255 10.1073/pnas.1322134111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao, R., Zhang, B., Dong, Y., Gong, J., Xu, T., Liu, J.et al. (2013) A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 152, 806–817 10.1016/j.cell.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama, T., Saito, S., Shimoda, M., Kobayashi, M., Takasu, Y., Sezutsu, H.et al. (2021) Comparisons in temperature and photoperiodic-dependent diapause induction between domestic and wild mulberry silkworms. Sci. Rep. 11, 8052 10.1038/s41598-021-87590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golden, J.W. and Riddle, D.L. (1982) A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218, 578–580 10.1126/science.6896933 [DOI] [PubMed] [Google Scholar]
- 44.Park, J., Oh, H., Kim, D.-Y., Cheon, Y., Park, Y.-J., Hwang, H.et al. (2021) CREB mediates the C. elegans dauer polyphenism through direct and cell-autonomous regulation of TGF-β expression. PLoS Genet. 17, e1009678 10.1371/journal.pgen.1009678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita, O. (1996) Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J. Insect Physiol. 42, 669–679 10.1016/0022-1910(96)00003-0 [DOI] [Google Scholar]
- 46.Zhou, W.-Z., Wu, Y.-F., Yin, Z.-Y., Guo, J.-J. and Li, H.-Y. (2022) Juvenile hormone is an important factor in regulating Aspongopus chinensis dallas diapause. Front. Physiol. 13, 873580 10.3389/fphys.2022.873580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, Y.-Y., Chen, J.-J., Liu, M.-Y., He, W.-W., Reynolds, J.A., Wang, Y.-N.et al. (2022) Enhanced degradation of juvenile hormone promotes reproductive diapause in the predatory ladybeetle Coccinella septempunctata. Front. Physiol. 13, 877153 10.3389/fphys.2022.877153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutfilz, C. (2022) Endocrine regulation of lifespan in insect diapause. Front. Physiol. 13, 825057 10.3389/fphys.2022.825057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meiselman, M.R., Alpert, M.H., Cui, X., Shea, J., Gregg, I., Gallio, M.et al. (2022) Recovery from cold-induced reproductive dormancy is regulated by temperature-dependent AstC signaling. Curr. Biol. 32, 1362–1375.e8 10.1016/j.cub.2022.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, C., Kim, A.J., Rivera-Perez, C., Noriega, F.G. and Kim, Y.-J. (2022) The insect somatostatin pathway gates vitellogenesis progression during reproductive maturation and the post-mating response. Nat. Commun. 13, 969 10.1038/s41467-022-28592-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim, J., Hyun, M., Hibi, M. and You, Y.-J. (2021) Maintenance of quiescent oocytes by noradrenergic signals. Nat. Commun. 12, 6925 10.1038/s41467-021-26945-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heldmaier, G., Ortmann, S. and Elvert, R. (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329 10.1016/j.resp.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 53.Geiser, F. (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274 10.1146/annurev.physiol.66.032102.115105 [DOI] [PubMed] [Google Scholar]
- 54.Hrvatin, S., Sun, S., Wilcox, O.F., Yao, H., Lavin-Peter, A.J., Cicconet, M.et al. (2020) Neurons that regulate mouse torpor. Nature 583, 115–121 10.1038/s41586-020-2387-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deng, W. and Wang, H. (2022) Efficient cell chatting between embryo and uterus ensures embryo implantation†. Biol. Reprod. 107, 339–348 10.1093/biolre/ioac135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lufkin, H., Flores, D., Raider, Z., Madhavan, M., Dawson, M., Coronel, A.et al. (2022) Pre-implantation mouse embryo movement under hormonally altered conditions. Mol. Hum. Reprod. 29, gaac043 10.1093/molehr/gaac043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuo, M., Yuan, J., Kim, Y.S., Dewar, A., Fujita, H., Dey, S.K.et al. (2022) Targeted depletion of uterine glandular Foxa2 induces embryonic diapause in mice. eLife 11, e78277 10.7554/eLife.78277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pozzi, S., Bowling, S., Apps, J., Brickman, J.M., Rodriguez, T.A. and Martinez-Barbera, J.P. (2019) Genetic deletion of Hesx1 promotes exit from the pluripotent state and impairs developmental diapause. Stem Cell Rep 13, 970–979 10.1016/j.stemcr.2019.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You, Y., Kim, J., Raizen, D.M. and Avery, L. (2008) Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7, 249–257 10.1016/j.cmet.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eustice, M., Konzman, D., Reece, J.M., Ghosh, S., Alston, J., Hansen, T.et al. (2022) Nutrient sensing pathways regulating adult reproductive diapause in C. elegans. PLoS ONE 17, e0274076 10.1371/journal.pone.0274076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster, A.K., Jordan, J.M., Hibshman, J.D., Chitrakar, R. and Baugh, L.R. (2018) Transgenerational effects of extended dauer diapause on starvation survival and gene expression plasticity in caenorhabditis elegans. Genetics 210, 263–274 10.1534/genetics.118.301250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brankatschk, M., Gutmann, T., Knittelfelder, O., Palladini, A., Prince, E., Grzybek, M.et al. (2018) A temperature-dependent switch in feeding preference improves Drosophila development and survival in the cold. Dev. Cell 46, 781–793.e4 10.1016/j.devcel.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 63.Kubrak, O.I., Kučerová, L., Theopold, U. and Nässel, D.R. (2014) The sleeping beauty: how reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE 9, e113051 10.1371/journal.pone.0113051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sim, C. and Denlinger, D.L. (2008) Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl Acad. Sci. U.S.A. 105, 6777–6781 10.1073/pnas.0802067105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams, K.D., Busto, M., Suster, M.L., So, A.K.-C., Ben-Shahar, Y., Leevers, S.J.et al. (2006) Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc. Natl Acad. Sci. U.S.A. 103, 15911–15915 10.1073/pnas.0604592103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hondo, E. and Stewart, C.L. (2005) Profiling gene expression in growth-arrested mouse embryos in diapause. Genome Biol. 6, 202 10.1186/gb-2004-6-1-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura, K.D., Tissenbaum, H.A., Liu, Y. and Ruvkun, G. (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946 10.1126/science.277.5328.942 [DOI] [PubMed] [Google Scholar]
- 68.Hussein, A.M., Wang, Y., Mathieu, J., Margaretha, L., Song, C., Jones, D.C.et al. (2020) Metabolic control over mTOR-dependent diapause-like state. Dev. Cell 52, 236–250.e7 10.1016/j.devcel.2019.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu, F., Li, K., Cai, W., Zhao, J., Zou, Y. and Hua, H. (2017) Knockdown of TOR causing ovarian diapause in a genetically stable brachypterous strain of Nilaparvata lugens. Arch. Insect Biochem. Physiol. 95, e21400 10.1002/arch.21400 [DOI] [PubMed] [Google Scholar]
- 70.Miki, T., Shinohara, T., Chafino, S., Noji, S. and Tomioka, K. (2020) Photoperiod and temperature separately regulate nymphal development through JH and insulin/TOR signaling pathways in an insect. Proc. Natl Acad. Sci. U.S.A. 117, 5525–5531 10.1073/pnas.1922747117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khoa, L.T.P., Tsan, Y.-C., Mao, F., Kremer, D.M., Sajjakulnukit, P., Zhang, L.et al. (2020) Histone acetyltransferase MOF blocks acquisition of quiescence in ground-state ESCs through activating fatty acid oxidation. Cell Stem Cell 27, 441–458.e10 10.1016/j.stem.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen, C., Mahar, R., Merritt, M.E., Denlinger, D.L. and Hahn, D.A. (2021) ROS and hypoxia signaling regulate periodic metabolic arousal during insect dormancy to coordinate glucose, amino acid, and lipid metabolism. Proc. Natl Acad. Sci. U.S.A. 118, e2017603118 10.1073/pnas.2017603118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jia, Q. and Li, S. (2023) Mmp-induced fat body cell dissociation promotes pupal development and moderately averts pupal diapause by activating lipid metabolism. Proc. Natl Acad. Sci. U.S.A. 120, e2215214120 10.1073/pnas.2215214120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu, W.M., Cheng, R.R., Niu, Z.R., Chen, A.C., Ma, M.Y., Li, T.et al. (2020) Let-7 derived from endometrial extracellular vesicles is an important inducer of embryonic diapause in mice. Sci. Adv. 6, eaaz7070 10.1126/sciadv.aaz7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scognamiglio, R., Cabezas-Wallscheid, N., Thier, M.C., Altamura, S., Reyes, A., Prendergast, Á.Met al. (2016) Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164, 668–680 10.1016/j.cell.2015.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hussein, A.M., Balachandar, N., Mathieu, J. and Ruohola-Baker, H. (2022) Molecular regulators of embryonic diapause and cancer diapause-like state. Cells 11, 2929 10.3390/cells11192929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su, X., Su, Z. and Xu, W. (2022) ROS elevate HIF-1α phosphorylation for insect lifespan through the CK2-MKP3-p38 pathway. Biochim. Biophys. Acta Mol. Cell Res. 1870, 119389 10.1016/j.bbamcr.2022.119389 [DOI] [PubMed] [Google Scholar]
- 78.Zhang, X.-S., Wang, T., Lin, X.-W., Denlinger, D.L. and Xu, W.-H. (2017) Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc. Natl Acad. Sci. U.S.A. 114, E7832–E7840 10.1073/pnas.1711042114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang, X., Du, W., Zhang, J., Zou, Z. and Ruan, C. (2020) High-throughput profiling of diapause regulated genes from Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). BMC Genomics 21, 864 10.1186/s12864-020-07285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang, X.-X., Geng, S.-L., Zhang, X.-S. and Xu, W.-H. (2020) P-S6K is associated with insect diapause via the ROS/AKT/S6K/CREB/HIF-1 pathway in the cotton bollworm, Helicoverpa armigera. Insect Biochem. Mol. Biol. 120, 103262 10.1016/j.ibmb.2019.103262 [DOI] [PubMed] [Google Scholar]
- 81.Godthi, A., Das, S., Cruz-Corchado, J., Deonarine, A., Misel-Wuchter, K., Issuree, P.D.et al. (2022) Neuronal IL-17 controls C. elegans developmental diapause through p53/CEP-1. BioRxiv 2022-11 10.1101/2022.11.22.517560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ter Huurne, M., Peng, T., Yi, G., van Mierlo, G., Marks, H. and Stunnenberg, H.G. (2020) Critical role for P53 in regulating the cell cycle of ground state embryonic stem cells. Stem Cell Rep. 14, 175–183 10.1016/j.stemcr.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duan, T.-F., Li, L., Wang, H.-C. and Pang, B.-P. (2022) MicroRNA miR-2765-3p regulates reproductive diapause by targeting FoxO in Galeruca daurica. Insect Sci. 30, 279–292 10.1111/1744-7917.13089 [DOI] [PubMed] [Google Scholar]
- 84.Zhang, X.-S., Wang, Z.-H., Li, W.-S. and Xu, W.-H. (2022) Foxo induces pupal diapause by decreasing TGFβ signaling. Proc. Natl Acad. Sci. U.S.A. 119, e2210404119 10.1073/pnas.2210404119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhattacharya, A., Aghayeva, U., Berghoff, E.G. and Hobert, O. (2019) Plasticity of the electrical connectome of C. elegans. Cell 176, 1174–1189.e16 10.1016/j.cell.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fenelon, J.C., Shaw, G., Frankenberg, S.R., Murphy, B.D. and Renfree, M.B. (2017) Embryo arrest and reactivation: potential candidates controlling embryonic diapause in the tammar wallaby and mink†. Biol. Reprod. 96, 877–894 10.1093/biolre/iox019 [DOI] [PubMed] [Google Scholar]
- 87.Takahashi, N., Chen, H.-Y., Harris, I.S., Stover, D.G., Selfors, L.M., Bronson, R.T.et al. (2018) Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell 33, 985–1003.e7 10.1016/j.ccell.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang, Z., Qin, S., Chen, Y., Zhou, L., Yang, M., Tang, Y.et al. (2022) Inhibition of NPC1L1 disrupts adaptive responses of drug-tolerant persister cells to chemotherapy. EMBO Mol. Med. 14, e14903 10.15252/emmm.202114903 [DOI] [PMC free article] [PubMed] [Google Scholar]