INTRODUCTION
Bladder pain, urgency, and frequency are common symptoms that are generally seen in patients with interstitial cystitis/bladder pain syndrome (IC/BPS).1 These symptoms often arise due to excessive inflammation that disrupts the urothelial homeostasis and results in dysfunction of the bladder urothelium, which serves as a barrier to prevent injurious stimuli, toxins, or microorganisms from invading the stroma.2,3 Unfortunately, existing treatment options are palliative and do not modulate the underlying issues contributing to the symptoms. Hence, there remains an unmet clinical need for patients presenting with recalcitrant IC/BPS.
Human amniotic membrane (AM) has been used in various clinical applications, such as ocular and dermal wound healing, due to its ability to modulate inflammation and promote tissue regeneration in the wound healing process.4,5 As AM has been shown to improve wound healing in many other medical practices, likewise, it may improve urothelial wound healing in patients with recalcitrant IC/BPS. Herein, we detail the outcomes of five consecutive patients unresponsive to current therapies that were subsequently managed with intra-detrusor injection of micronized AM, i.e., amniotic bladder therapy (ABT).
METHODS
Patients were enrolled in this study with refractory IC/BPS in the absence of infection or other identifiable causes. The study was approved by the local institutional review board committee. We excluded patients with concomitant bladder outlet obstruction, prior radiotherapy, intra-vesical stones, and history of bladder and pelvic cancer. Each patient received one injection of 100 mg of commercially available micronized AM (Clarix Flo; Biotissue, Miami, FL, U.S.) diluted in 10 ml 0.9% preservative-free sodium chloride under general anesthesia. Injections were performed through a cystoscope using a 23-gauge Williams needle, intradetrusor into the lateral and posterior bladder wall, sparing the dome and the trigone.
Clinical evaluation and questionnaires were performed at pre-injection, two, four, eight, and 12 weeks, including history-taking, physical examination, serum chemistries, urinalyses, urine culture, urine cytology, postvoid residuals, and symptom assessments as measured by questionnaires of bladder pain/interstitial cystitis symptom score (BPIC-SS), overactive bladder (OAB) assessment tool, O’Leary/Sant voiding and pain indices (interstitial cystitis symptoms index and interstitial cystitis problem index) and short-form (SF)-12 health survey. Descriptive statistics for continuous variables are reported as the median values.
RESULTS
Five consecutive patients were included in the study. The five women had a median age of 43 (29–61) years and median disease duration of 8.2 (6.3–12.1) years that was recalcitrant to multiple therapies, including anticholinergics (n=5), beta-3 adrenergic agonist (n=5), tricyclic antidepressant (n=5), antihistamine (n=3), hydrodistension (n=5), pentosan polysulfate (n=5), vaginal valium (n=5), intravesical instillation (n=4), Botox injection (n=5), and neuromodulation (n=4). None of the patients had any evidence of Hunner’s lesions. Finally, all patients were discharged without neuropathic or narcotic pain medications and did not receive concomitant IC/BPS therapies. Patients were instructed to use acetaminophen or phenazopyridine as needed for any post-injection discomfort.
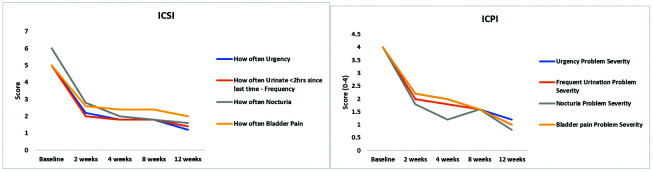
After micronized AM treatment, the ICSI score decreased from 21 at baseline to 10 at two weeks, 8 at four weeks, 8 at eight weeks, and 6 at three months, and the ICPI score decreased from 16 at baseline to 8 at two weeks, 7 at four weeks, 7 at eight weeks, and 4 at three months (Figure 1). Similarly, the BPIC-SS decreased from 38 at baseline to 20 at two weeks, 16 at four weeks, 17 at eight weeks, and 12 at three months. As noted in Figure 2, there was a reduction in the score of each individual question of the BPICSS, including bother scores. This corresponded to an improvement in their overall physical and mental quality of life: SF-12 physical health component score (PCS) increased from 19.7 at baseline to 45.5 at two weeks, 43.9 at four weeks, 47.8 at eight weeks, and 52.2 at three months. The SF-12 mental health component score (MCS) score increased from 38.2 at baseline to 43.9 at two weeks, 48.1 at four weeks, 52.6 at eight weeks, and 52.3 at three months. The OAB score decreased from 23 at baseline to 13 at two weeks, 11 at four weeks, 10 at eight weeks, and 6 at three months (Figure 3).
Figure 1.
Scores of the 4 questions of the Interstitial Cystitis Symptom Index (ICSI) and Interstitial Cystitis Problem Index ICPI before and after amniotic bladder therapy.
Figure 2.

Scores of the 8 questions of the Bladder Pain/Interstitial Cystitis Symptom Score before and after amniotic bladder therapy.
Figure 3.
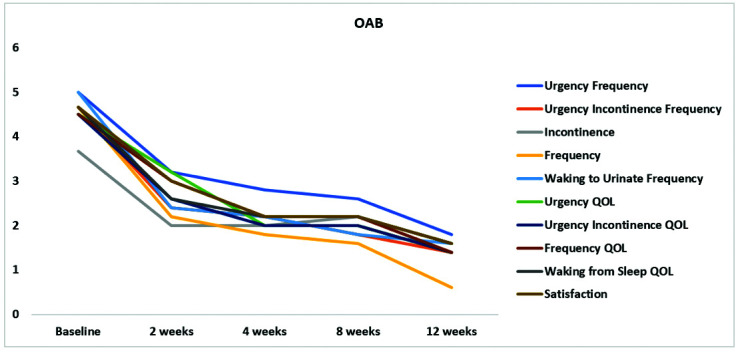
Scores of the 10 questions of the overactive bladder before and after amniotic bladder therapy. QOL: quality of life.
No adverse events related to micronized AM injections, such as urinary tract infection or acute urinary retention, occurred throughout the current study and no patient required readmission for management of post-procedural pain.
DISCUSSION
Persistent cycles of inflammation and recurrent injury to the luminal bladder have been shown to be associated with IC/BPS. Use of micronized AM in the bladder (i.e., ABT) of patients with these symptoms is based on the primary rationale that a better treatment effect might be achieved by inhibiting inflammation, reducing the excitability of bladder nerves, repairing damaged urothelium, and enhancing tissue regeneration. This idea is supported by recent preclinical studies in which instilling AM extracts into rats’ urinary bladder with lipopolysaccharide-induced urinary bladder inflammation could improve bladder urothelial pathologic changes and rehabilitate the bladder urothelium.6
There has been no prior clinical investigation to treat IC/BPS with micronized AM intra-detrusor injection; however micronized AM has proven utility in various musculoskeletal applications, including osteoarthritis, plantar fasciitis, tendinitis, and neuropathy recovery.7–10 Similarly, our data demonstrates significantly improved voiding symptoms and bladder pain in patients for at least three months, but longer followup is crucial for the evaluation of clinical durability.
CONCLUSIONS
Our work is a pilot study and the first to evaluate intradetrusor micronized AM in patients suffering from IC/BPS. Based on the findings previously discussed, we believe this preliminary study requires further evaluation to confirm the usefulness of ABT in patients with recalcitrant IC/BPS, including a randomized placebo-controlled trial with a larger cohort of patients.
KEY MESSAGES
■ This is the first clinical investigation regarding treatment of IC/BPS with micronized amnionic membrane intra-detrusor injection.
■ We have demonstrated there is improved voiding symptoms and bladder pain in patients for at least three months following micronized amniotic membrane intra-detrusor injection.
■ We plan to expand on this preliminary study and conduct further investigation to confirm the usefulness of amniotic bladder therapy in patients with IC/BPS.
Footnotes
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Clemens JQ, Erickson DR, Varela NP, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2022;208:34–42. doi: 10.1097/JU.0000000000002756. [DOI] [PubMed] [Google Scholar]
- 2.Keay S. Cell signaling in interstitial cystitis/painful bladder syndrome. Cell Signal. 2008;20:2174–9. doi: 10.1016/j.cellsig.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Shie JH, Liu HT, Kuo HC. Increased cell apoptosis of urothelium mediated by inflammation in interstitial cystitis/painful bladder syndrome. Urology. 2012;79:484.e7–13. doi: 10.1016/j.urology.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004;2:177–87. doi: 10.1016/S1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 5.Tseng SC. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: Insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2016;57:orsfh1. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CH, Tung MC, Lin YS, et al. Dehydrated human amnion-chorion membrane extracts can ameliorate interstitial cystitis in rats by down-regulating inflammatory cytoknes and protein coding genes: A preclinical study. Life. 2022;12:1693. doi: 10.3390/life12111693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanselman AE, Tidwell JE, Santrock RD. Cryptopreserved human amniotic membrane injection for plantar fascitis: A randomized, controlled, double-blind pilot study. Foot Ankle Int. 2015;36:151–8. doi: 10.1177/1071100714552824. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos R. Injectable amniotic membrane/umbilical cord particulate for facet joint syndrome: A retrospective, single-center study. J Back Musculoskelet Rehabil. 2022;35:559–64. doi: 10.3233/BMR-200330. [DOI] [PubMed] [Google Scholar]
- 9.Chin MJ, laviolette K. Amniotic membrane / umbilical cord particulate injection for achilles tendinopathy with or without a partial tear. J Foot Ankel Surg. 2022;2:100169. doi: 10.1016/j.fastrc.2022.100169. [DOI] [Google Scholar]
- 10.Buksh AB, Del Pozzi Ultrasound-guided injections of amniotic membrane/umbilical cord particulate for painful neuropathy of the lower extremity. Cogent Medicine. 2020;7:1724067. doi: 10.1080/2331205X.2020.1724067. [DOI] [Google Scholar]