INTRODUCTION
Germ cell tumors (GCT) are the most common tumor in males aged 18–44 years, with over 9000 cases annually in the U.S.1 Management is based on the tumor histology, staging, and risk group, with the overall survival likely approaching or exceeding 90%.2 The vast majority of GCTs originate from the gonads, with extragonadal tumors encompassing only 6% of GCTs.3
Primary mature teratomas, a form of non-seminomatous GCT (NSGCT), are relatively uncommon tumors composed of tissue representing more than one of the three germ cell layers: ectoderm, mesoderm, and endoderm. Most of these tumors arise from the gonads. Extragonadal teratomas occur along the midline, including the anterior mediastinum, retroperitoneum, sacrococcygeal region, and pineal gland.4,5 Retroperitoneal primaries are rare, accounting for 4% of primary teratomas, and are more likely found in pediatric patients.4 Surgical excision remains the standard of treatment, as teratomas are chemo-resistant, may grow locally, or can develop malignant transformation. Fortunately, prognosis tends to be excellent after excision, with reported survival rates of 100% at five years.6
Neuroendocrine tumors (NETs) of the retroperitoneum are usually metastatic from other sites.7 Primary retroperitoneal NETs are exceedingly rare, with only eight previously reported cases8 and only three prior reports of cystic teratomas containing NET components.9 We present a rare case of a 27-year-old man with a 38 cm primary retroperitoneal teratoma (RT) with well-differentiated NET and presence of erectile-type tissue.
CASE REPORT
A 27-year-old male presented with COVID-19-related symptoms. Chest imaging obtained during workup revealed a retroperitoneal mass. He reported an increase in abdominal girth, but denied pain, gastrointestinal symptoms, or weight change. Past medical history and family history were unremarkable. On exam, he had a large, firm, palpable left-sided abdominal mass. Scrotal exam was unremarkable, with no palpable testicular masses. Preoperative tumor markers were not obtained. On computed tomography (CT), the mass was 30 cm in greatest dimension, with solid and cystic areas, and located in the retroperitoneum, anterior to the left kidney and displacing the left ureter anteriorly (Figure 1). The tumor was believed to be a sarcoma prior to resection.
Figure 1.
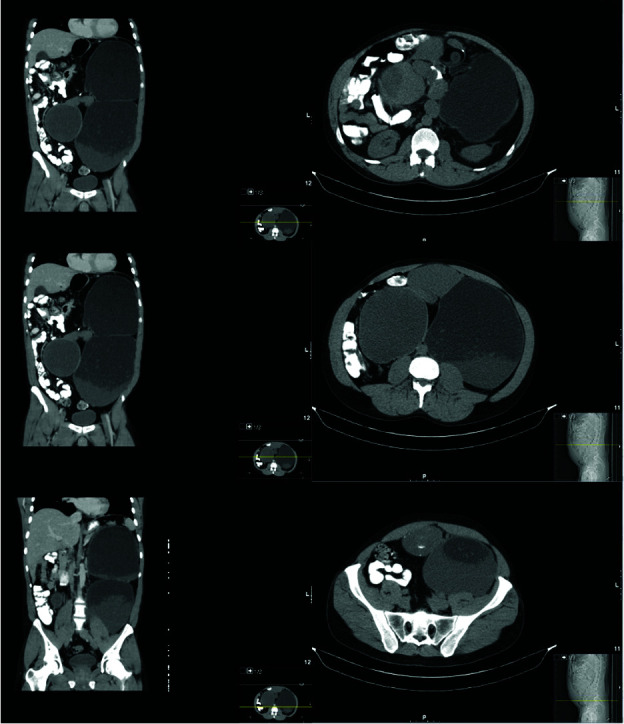
Select coronal and axial images from the computed tomography abdomen and pelvis identifying the large cystic tumor.
The patient subsequently underwent an open, transperitoneal excision of the retroperitoneal tumor. A midline incision extending from his xiphoid to his pubis was used. Despite the tumor’s proximity to the spleen and inferior mesenteric artery, all structures were able to be dissected free and spared. With the help of a ureteral stent, the left ureter was identified anterior to the mass and was also dissected free without injury. Parasitic vessels arose directly from the aorta and implanted into the medial tumor. These were effectively ligated with bipolar diathermy (Figure 2). Recovery was uneventful and he was discharged on postoperative day 3.
Figure 2.
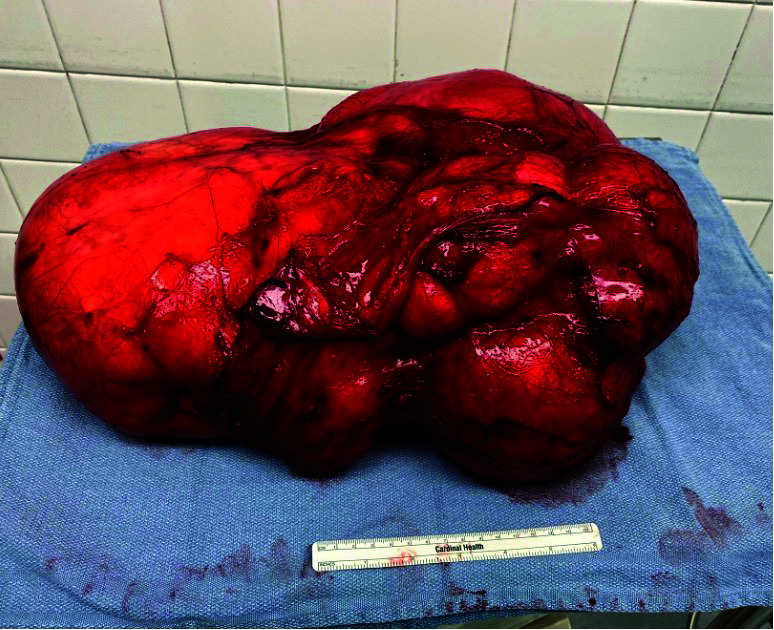
Teratoma after resection. A six-inch ruler is seen for scale.
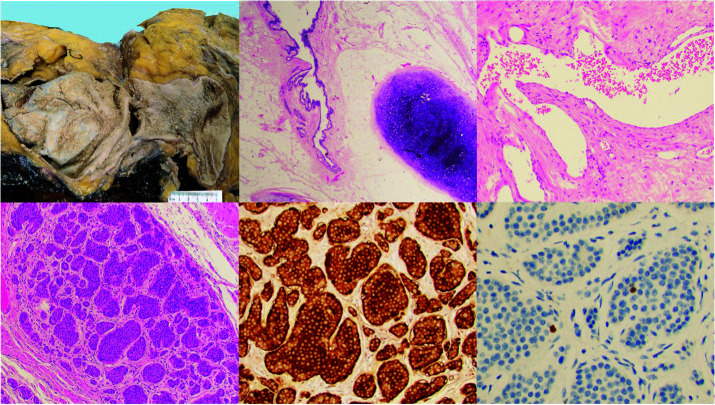
Gross examination of the excised tumor revealed a multilocular solid and cystic mass, 38 cm in largest dimension, primarily comprised of yellow adipose tissue, with cystic areas containing thick, yellow, grumous, brown-tan keratinous material. Hair, bone, and cartilage were also identified. Histologic examination revealed tissue derived from all three germ layers, with epidermal (squamous and columnar epithelium lined cysts), mesodermal (adipose, cartilage, bone, and fibrous tissue), and endodermal elements (gut epithelium). There was notably vascular tissue resembling erectile tissue identified. Two foci incidentally identified on histology were comprised of well-differentiated neuroendocrine cells that showed diffuse immunocytochemical expression of synaptophysin, chromogranin, SATB 2, and CDX2 without pancreatic polypeptide, glucagon, or somatostatin, a pattern of staining resembling NET of distal small bowel/appendiceal NET. The Ki-67 proliferation index demonstrated nuclear staining in 2% of cells (Figure 3). There was no gross or histologic evidence of a background supernumerary testis. These findings, along with the imaging findings, are consistent with well-differentiated enterochromaffin cell NET grade 1 arising in a primary retroperitoneal mature cystic teratoma.
Figure 3.
Top left: Gross photograph of the tumor demonstrates predominantly solid composition with large cystic areas (5 cm ruler for scale). Top middle: H&E stain showing a mature cystic teratoma with epithelial and mesenchymal elements. Top right: 40× H&E stain showing a focus of erectile-type vascular tissue. Bottom: 20× H&E showing incidental focus of well differentiated neuroendocrine tumors with diffuse expression of chromogranin and a low Ki67 proliferation index.
Postoperative tumor markers were within normal limits (alpha fetoprotein [AFP], beta-human chorionic gonadotropin [bHCG]). Postoperative scrotal ultrasound was negative. Plasma 5-HIAA was obtained due to NET finding and within normal limits. Tumor board recommendations included surveillance imaging in four months with CT of the chest/abdomen/pelvis and scrotal ultrasound, with repeat tumor markers, including 5-HIAA. At his one-year postoperative visit, the patient reported occasional facial flushing and diarrhea, although his 5-HIAA remained within the normal range, as well as anejaculation. Imaging was negative for disease recurrence. Scrotal ultrasound demonstrated a moderate left hydrocele and mild left varicoceles but no masses. AFP and bHGB remained unelevated.
DISCUSSION
We present a 27-year-old man with an incidentally found primary mature RT with well-differentiated NET. A broad differential for retroperitoneal mass should include malignant vs. benign causes, such as GCT, liposarcomas, and epidermoid cysts. Of note, NETs of the retroperitoneum are most commonly metastatic from the pancreas or the gastrointestinal tract.10 Ideally, patients would have preoperative tumor markers and a scrotal ultrasound, as GCT origin is the most likely diagnosis for a RP mass in this age group.
Primary mature RT with concomitant NET is rare, with only three previouly reported cases.9 Carcinoid syndrome is not a feature of retroperitoneal NETs11 and mass effect is not detected until the tumor is very large.12 Prior cases report tumor size ranging from 3.5–21cm,8 making ours the largest at 38 cm. The presence of erectile tissue is even rarer than the NET component. There are few reports of teratomas containing well-differentiated erectile tissue arising in the abdomen or from the spine, but no reports of primary RT containing erectile tissue.13,14
Primary RT management is surgical excision, as teratomas are chemotherapy resistant. This contrasts with cases with a testicular primary, where patients would undergo orchiectomy, followed by chemotherapy and post-chemotherapy retroperitoneal lymph node dissection (PC-RPLND). Small case series have suggested that chemotherapy may be omitted in select men with pure teratoma NSGCT on orchiectomy with clinical stage II disease on imaging.15 Meticulous surgical dissection in primary or PC-RPLND, including the division of lumbar arteries and veins, mobilization of the great vessels, and complete removal of lymphatic tissue within predefined borders, is crucial to minimize risk of in-field recurrence.16 This patient underwent a mass excision without complete vascular mobilization and removal of retroperitoneal lymphatic tissue for a retroperitoneal teratoma. Given the surgical approach for this case, the need for a more thorough anatomical lymphadenectomy must be considered or, at minimum, more frequent and longer surveillance for local recurrence.
Postoperative care includes monitoring possible side effects from resection and for residual disease. Potential complications from surgical resection are likely dependent on proximity to adjacent structures, given that it does not tend to infiltrate nearby structures.5 This patient is currently anejaculatory, potentially explained by the tumor proximity to the sympathetic chain; this is also a common adverse effect after standard template RPLND. Preoperative counselling and sperm banking when appropriate should be considered, especially when masses are of such size.
The patient reports episodic flushing and diarrhea, which may be an indication of residual NET, although his 5-HIAA continues to be normal. There is no standard surveillance schedule after excision of a primary RT. Currently, he is receiving cross-sectional imaging, scrotal ultrasounds, and testicular serum tumor markers every four months for the first year, with the notable addition of 5-HIAA given the NET component.
CONCLUSIONS
To our knowledge, there have only been three previous reports of cystic teratomas containing NET components and no previous case of primary RT with erectile tissue reported. Therefore, we present the first case of primary RT with concomitant NET and erectile tissue, as well as the largest recorded at 38 cm.
Footnotes
COMPETING INTERESTS: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
REFERENCES
- 1.Ghazarian AA, Kelly SP, Altekruse SF, et al. Future of testicular germ cell tumor incidence in the United States. Forecast through 2026. Cancer. 2017;123:2320–8. doi: 10.1002/cncr.30597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilligan T, Lin DW, Aggarwal R, et al. Testicular cancer, Version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:1529–54. doi: 10.6004/jnccn.2019.0058. [DOI] [PubMed] [Google Scholar]
- 3.Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumors in the United States, 1973–2007. Int J Androl. 2012;35:616–25. doi: 10.1111/j.1365-2605.2011.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosfeld JL, Billmire DF. Teratomas in infancy and childhood: Current problems in cancer. Curr Probl Cancer. 1985;9:1–53. doi: 10.1016/S0147-0272(85)80031-3. [DOI] [PubMed] [Google Scholar]
- 5.Gatcombe HG, Assikis V, Kooby D, et al. Primary retroperitoneal teratomas: A review of the literature. J Surg Oncol. 2004;86:107–13. doi: 10.1002/jso.20043. [DOI] [PubMed] [Google Scholar]
- 6.Pinson CW, Remine SG, Fletcher WS, et al. Long-term results with primary retroperitoneal tumors. Arch Surg. 1989;124:1168–73. doi: 10.1001/archsurg.1989.01410100070012. [DOI] [PubMed] [Google Scholar]
- 7.Hainsworth JD, Johnson DH, Greco FA. Poorly differentiated neuroendocrine carcinoma of unknown primary site. Ann Intern Med. 1988;109:364–71. doi: 10.7326/0003-4819-109-5-364. [DOI] [PubMed] [Google Scholar]
- 8.Shi D, Dong GQ, Shen KR, et al. Primary cystic and solid neuroendocrine tumor of the retroperitoneum. Medicine. 2021;100:e24054. doi: 10.1097/MD.0000000000024054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Djokic M, Hadzialjevic B, Rankovic B, et al. Neuroendocrine tumor arising within mature cystic teratoma of the pancreas: Literature review and case report. Curr Oncol. 2022;29:4717–24. doi: 10.3390/curroncol29070374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehal A, Kim S, Ali A, et al. Primary epithelial neuroendocrine tumors of the retroperitoneum. Perm J. 2015;19:71–5. doi: 10.7812/TPP/15-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polikarpova SB, Lubimova NV, Ogereliev AS, et al. Clinical and biochemical aspects of the carcinoid syndrome in neuroendocrine tumors of the abdominal and retroperitoneal organs and its impact for the disease prognosis. Bull Exp Biol Med. 2009;148:803–6. doi: 10.1007/s10517-010-0821-7. [DOI] [PubMed] [Google Scholar]
- 12.Scott AL, Abbassi-Ghadi N, Archer CM, et al. Neuroendocrine carcinoma arising within a retroperitoneal mature teratoma. Ann R Coll Surg Engl. 2010;92:W5–8. doi: 10.1308/147870810X12699662980952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thway K, Berney D, Hayes AJ, et al. Giant intra-abdominal mature cystic teratoma (dermoid cyst) in an adult man, with male genitourinary tissue including prostatic and penile elements. Hum Pathol. 2016;54:1–7. doi: 10.1016/j.humpath.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Vaishya S, Pandey P. Unusual spinal teratoma with an accessory penis on the back. Childs Nerv Syst. 2006;22:440–3. doi: 10.1007/s00381-005-1241-2. [DOI] [PubMed] [Google Scholar]
- 15.Cary C, Kern SQ, Jacob JM, et al. Management of metastatic pure teratoma in chemotherapy naive patients with testicular primaries. Am J Clin Oncol. 2020;43:690–3. doi: 10.1097/COC.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 16.Pedrosa JA, Masterson TA, Rice KR, et al. Reoperative retroperitoneal lymph node dissection for metastatic germ cell tumors: Analysis of local recurrence and predictors of survival. J Urol. 2014;191:1777–82. doi: 10.1016/j.juro.2014.01.010ears. 6 . [DOI] [PubMed] [Google Scholar]