Abstract
The flocculus is a region of the vestibulocerebellum dedicated to the coordination of neck, head, and eye movements for optimal posture, balance, and orienting responses. Despite growing evidence of vestibular and oculomotor impairments in the aftermath of traumatic stress, little is known about the effects of chronic psychological trauma on vestibulocerebellar functioning. Here, we investigated alterations in functional connectivity of the flocculus at rest among individuals with post-traumatic stress disorder (PTSD) and its dissociative subtype (PTSD + DS) as compared to healthy controls. Forty-four healthy controls, 57 PTSD, and 32 PTSD + DS underwent 6-min resting-state MRI scans. Seed-based functional connectivity analyses using the right and left flocculi as seeds were performed. These analyses revealed that, as compared to controls, PTSD and PTSD + DS showed decreased resting-state functional connectivity of the left flocculus with cortical regions involved in bodily self-consciousness, including the temporo-parietal junction, the supramarginal and angular gyri, and the superior parietal lobule. Moreover, as compared to controls, the PTSD + DS group showed decreased functional connectivity of the left flocculus with the medial prefrontal cortex, the precuneus, and the mid/posterior cingulum, key regions of the default mode network. Critically, when comparing PTSD + DS to PTSD, we observed increased functional connectivity of the right flocculus with the right anterior hippocampus, a region affected frequently by early life trauma. Taken together, our findings point toward the crucial role of the flocculus in the neurocircuitry underlying a coherent and embodied self, which can be compromised in PTSD and PTSD + DS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12311-022-01467-4.
Keywords: Flocculus, Vestibular, PTSD, Dissociation, Functional connectivity, Bodily self-consciousness
Introduction
Increasingly, the cerebellum is recognized as contributing to cognitive and affective processes that extend beyond its role in motor performance. Here, the Universal Cerebellar Transform Hypothesis postulates that the cerebellum plays a critical role in integrating sensory, limbic, and higher-order cortical inputs, thus maintaining a homeostatic baseline and improving performance in context-dependent behavioral, emotional, and cognitive domains [91]. This theory has been critical in forming a comprehensive understanding of the diverse functions cerebellar regions play in cognition, emotion regulation, action performance, and perception. In the present report, we focus our attention on the floccular lobe and alterations in its functioning among individuals with post-traumatic stress disorder (PTSD) and its dissociative subtype (PTSD + DS).
The flocculus is located in the vestibulocerebellum (hemisphere X of the cerebellum), the most phylogenetically ancient part of the cerebellum. Through its efferent and afferent projections to and from the vestibular complex in the brainstem via the inferior cerebellar peduncle, the flocculus is involved primarily in regulating movement for optimal posture, balance, and the vestibulo-ocular eye reflex [42]. Here, the vestibulocerebellum guides eye, head, and neck movements to compensate for acceleration of the head in a linear or rotational fashion, allowing for the maintenance of a visual target fixed on the retina. The flocculus is also involved in the control of saccades (rapid eye movements occurring between two fixation points), smooth pursuit (SP, slow pursuit of a target in motion), and the optokinetic reflexive eye movements. The optokinetic reflex regulates involuntary eye movements (called nystagmus) in response to motion of the visual field [81], which is crucial for orienting oneself in the environment and for maintaining a constant point of fixation in the visual field.
Functional imaging in humans has highlighted the neural activity of the flocculus during optokinetic reflex, saccadic eye movements, and smooth pursuit [31, 117]. Optokinetic eye movements were traced in a sample of healthy volunteers via functional magnetic resonance imaging (fMRI; [86]), revealing neural activity of the flocculus during horizontal and vertical optokinetic nystagmuses (eye movement elicited by a moving environment in the visual field). Moreover, recent investigation highlights the role of the flocculus in auditory functioning and tinnitus [69], where a feedback loop has been hypothesized between the flocculus (and paraflocculus) and the auditory cortex. Along with the non-motor functions of the cerebellum [89, 92–94], ground-breaking work has revealed structural and functional connectivity of the cerebellum (flocculus included) with limbic regions, specifically with the hippocampus [5, 128], thereby suggesting a role of the cerebellum in emotion regulation and spatial and temporal processing. Finally, higher cortical structures appear functionally connected to the flocculus for eye movement modulation, including the frontal eye field (FEF), the supplementary eye field, the cingulate eye field, the parietal eye field (PEF), the parietal operculum, the middle temporal and intraparietal regions, and visual areas in the occipital cortex [81, 86, 117].
Emerging evidence suggests vestibular and oculomotor impairment in psychological disorders. Here, a recent study revealed decreased functional connectivity between the flocculus, the thalamus, and the FEF in individuals with bipolar disorder as compared to healthy subjects [24], a finding that is in line with previous research suggesting altered saccadic eye movements in uni- and bipolar depression [22]. Similarly, schizophrenia, affective disorders, and obsessive–compulsive disorder have been associated with smooth pursuit deficits [63]. A comprehensive review [40] summarized a range of oculomotor deficits relative to saccadic performance in several psychiatric conditions, including schizophrenia, mood disorders, borderline personality disorder, attention-deficit hyperactivity disorder, and obsessive–compulsive disorder. State and trait anxiety, as well as anxiety disorders, are also thought to impact negatively oculomotor reflexes and gaze [104, 126], with associated dizziness, unsteadiness, and fear of falling. Despite the fact that psychological trauma and chronic stress have been linked to altered functioning in vestibular circuits and performance [45, 87] and abnormal visual functioning, such as blurred vision in veterans [111] and oscillopsia (a visual disturbance where the visual field is perceived in motion; [114]), work examining vestibulo-ocular functioning in trauma-related disorders is scant.
Among trauma-related disorders, PTSD has a relatively high prevalence among the civilian population, with lifetime prevalence rates of 6.8% (lifetime prevalence) in the population of the USA [53] and 9.2% in Canada. PTSD is a psychiatric disorder stemming from experiencing or witnessing a traumatic event, and is characterized by symptoms of hypervigilance, reliving, negative mood and cognitions, and avoidance symptoms [4]. A subgroup of individuals with PTSD (an average of 14% among the PTSD population worldwide; [105]) presents with dissociative symptoms (PTSD + DS), including depersonalization, where the individuals experience detachment from their body or parts of their body, and derealization, where the surroundings are perceived as surreal or dream-like [58, 60, 105, 118]. Previous investigations in PTSD have revealed altered functioning of brain regions involved in vestibular and oculomotor functioning, including for the cerebellum [7, 21, 84], the vestibular circuits—encompassing the vestibular nuclei in the brainstem [45], the insula [36, 46, 50, 74], the temporo-parietal junction [75, 83, 106, 125]—the frontal eye field [84, 88], middle temporal regions [56, 76], and the parietal operculum [120]. Critically, many of these abnormalities are present at rest.
Taken together, functional neuroimaging research and clinical reports suggest a potential association between vestibular and oculomotor impairments and PTSD. Critically, however, no research has been conducted on the neural functioning of the vestibulocerebellum, including the flocculus, in PTSD and its dissociative subtype. We therefore aimed to uncover potential alterations in functional connectivity of the flocculus at rest in PTSD and PTSD + DS as compared to healthy controls. We hypothesized that, even at rest, the vestibulo-ocular circuit in PTSD would reveal specific alterations in its functional connectivity with brainstem, limbic, and cortical areas as compared to controls. In light of previous reports pointing toward a link between vestibular dysfunction and depersonalization/derealization symptoms [52, 66, 127], we also expected dissociative symptomatology in PTSD (PTSD + DS) to distinguish further the functional connectivity of the flocculus as compared to PTSD and healthy controls.
Methods
Participants
Participants were recruited via community advertisement and through mental health professionals in the London (ON) region. The final sample included 133 participants, comprising 44 healthy controls, 57 participants with a diagnosis of PTSD, and 32 participants with a diagnosis of PTSD + DS (see the “Methods” section for details on inclusion/exclusion criteria based on functional imaging quality). Part of this sample was previously analyzed in studies from our group investigating functional connectivity of different brain areas at rest [45, 47, 75, 84, 110]. The studies from which these data were drawn were approved by the Research Ethics Board of Western University, and all participants provided informed written consent. Demographic and clinical characteristics of the sample are reported in Table 1.
Table 1.
Description of the participants’ groups
| Demographic and clinical characteristics | PTSD group (n = 57) | PTSD + DS (n = 32) | Control group (n = 44) | p | Contr vs. PTSD p | Contr vs. PTSD + DS p | PTSD vs. PTSD + DS p |
|---|---|---|---|---|---|---|---|
| Age (mean ± SD) years | 38.32 ± 12.16 | 41.06 ± 13.09 | 34.68 ± 12.32 | 0.122 | |||
| Sex (F) frequency | 40 | 28 | 34 | 0.178 | |||
| CAPS-IV tot (mean ± SD) | 66.28 ± 12.27 (n = 35) | 79.83 ± 13.69 (n = 18) | 0.136 ± .66 (n = 44) | < .0001 | < .001 | < .001 | 0.071 |
| CAPS-IV Dep (mean ± SD; range) | 0.09 ± 0.51; 3 (n = 35) | 2.94 ± 2.51; 6 (n = 18) | 0 (n = 44) | < .001 | |||
| CAPS-IV Der (mean ± SD; range) | 0.31 ± 0.90; 3 (n = 35) | 3.33 ± 2.70; 8 (n = 18) | 0 (n = 44) | < .001 | |||
| CAPS-5 tot (mean ± SD) | 36.90 ± 8.79 (n = 22) | 41.35 ± 8.73 (n = 14) | n/a | 0.244 | |||
| CAPS-5 Dep (mean ± SD); range | 0.18 ± 0.50; 2 (n = 22) | 2.29 ± 1.14; 4 (n = 14) | n/a | < .0001 | |||
| CAPS-5 Der (mean ± SD); range | 0.23 ± 0.61; 2 (n = 22) | 1.79 ± 0.97; 3 (n = 14) | n/a | < .0001 | |||
| MDI tot score (mean ± SD) | 54.21 ± 24.77 (n = 56) | 84.9 ± 24.6 (n = 31) | 34.25 ± 4.18 (n = 43) | < .001 | < .001 | < .0001 | < .001 |
| CTQ tot score (mean ± SD) | 57.68 ± 23.82 (n = 57) | 67.74 ± 17.95 (n = 30) | 31.83 ± 9.20 (n = 43) | < .001 | < .001 | < .001 | 0.049 |
| BDI tot score (mean ± SD) | 23.3 ± 8.56 (n = 56) | 36.16 ± 13.20 (n = 31) | 1.16 ± 2.09 (n = 43) | < .0001 | < .001 | < .0001 | 0.002 |
| Post-scan STAI tot score (mean ± SD) | 5.56 ± 1.9 (n = 50) | 5.9 ± 2.16 (n = 29) | 3.62 ± 1.27 (n = 40) | < .001 | < .001 | < .001 | 0.617 |
| Post-scan CADSS tot score (mean ± SD) | 3.55 ± 1.25 (n = 56) | 4.79 ± 2.46 (n = 29) | 3.15 ± 0.42 (n = 40) | < .001 | 0.234 | < .001 | < .001 |
| Post-scan RSDI dep/der (mean ± SD) | 3.49 ± 1.22 (n = 50) | 4.84 ± 1.74 (n = 29) | 2.68 ± 0.44 (n = 40) | < .001 | < .001 | < .001 | < .001 |
Abbreviations: BDI Beck Depression Inventory, CADSS Clinician Administered Dissociative States Scale, CAPS Clinical Administered PTSD Scale, CTQ Childhood Trauma Questionnaire, DEP/DER depersonalization/derealization, MDI Multiscale Dissociation Inventory, PTSD post-traumatic stress disorder, PTSD + DS PTSD dissociative subtype, RSDI Responses to Script-Driven Imagery Scale, SD standard deviation, STAI State-Trait Anxiety Inventory
PTSD diagnosis was confirmed through administration of the Clinician Administered PTSD scale-IV (cutoff ≥ 50; [12] or the CAPS-5 [123] (see Table 1 for details). As per standard methods, inclusion in the dissociative subtype of PTSD group (PTSD + DS) required a severity ≥ 4 (frequency + intensity) for CAPS-4, or ≥ 2 (severity) for CAPS-5 on the CAPS depersonalization or derealization symptoms [84, 107, 110, 124. Psychiatric comorbidities were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) [37], and additional psychological questionnaires were administered to investigate childhood trauma using the Childhood Trauma Questionnaire (CTQ) [11], dissociative symptomatology using the Multiscale Dissociation Inventory (MDI) [19], and depression symptoms using the Beck Depression Inventory (BDI) [9]. Additional measures were administered at the end of the imaging session to evaluate state anxiety (using the State-Trait Anxiety Inventory (STAI); [101] and state dissociative symptoms (using the Clinician Administered Dissociative States Scale (CADSS) by [17], and the Responses to Script-Driven Imagery Scale (RSDI) by [49] present during the scanning session.
Exclusion criteria comprised history of head injury involving loss of consciousness, current or past history of neurological disorder, history or current psychosis, bipolar disorder, substance or alcohol use disorder, MRI incompatibility, and other relevant medical conditions. Participants included in the control sample did not meet criteria for current or past history of psychiatric disorders, as assessed by the SCID-I and CAPS.
MRI Data Acquisition
Resting-state imaging data were acquired via a whole-body 3 Tesla MRI scanner (Siemens Medical Solutions, Erlangen, Germany) either at the Lawson Health Research Institute for Imaging (Magnetom Verio/Biograph mMR; n = 46) or at the Robarts Research Institute for Functional and Metabolic Mapping (Magnetom Trio/Magnetom Prisma; n = 87) in London (ON) with the manufacturer’s 32-channel phased array head coil (please refer to Supplementary Materials for the specifics on what scanner model was utilized for each individual per group). Foam pads held the participants’ heads in position. T1-weighted anatomical images were collected using a magnetization-prepared rapid acquisition gradient echo with 1-mm isotropic resolution (TR/TE/TI = 2300 ms/2.98 ms/900 ms, FA 9°, FOV = 256 mm × 240 mm × 192 mm, acceleration factor = 4, total acquisition time = 192 s; FOV = field of view; TR = repetition time; TE = echo time; FA = flip angle).
Next, T2*-weighted functional images were collected with a standard gradient echo planar imaging (EPI) pulse sequence using 2-mm isotropic resolution. EPI volumes were acquired using the following parameters: FOV = 192 mm × 192 mm × 128 mm (94 × 94 matrix, 64 slices), TR/TE = 3,000 ms/20 ms, flip angle = 90°, for a total of 120 volumes.
As per standard methods, participants’ instructions during the 6-min resting-state acquisition were to let the mind wander while keeping their eyes closed [15, 38].
Data Analyses
Demographic and Clinical Measures
We performed Kruskal–Wallis H tests, followed by post hoc Mann–Whitney tests, to investigate significant between-group differences regarding age, CAPS, CTQ, MDI, BDI, CADSS, RSDI, and STAI scores. Sex differences were examined using Pearson’s chi-square tests between groups.
fMRI Data Preprocessing
fMRI analyses were conducted using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK) and the additional spatially unbiased infratentorial template (SUIT) toolbox (version, 3.1) was implemented in Matlab R2018b (Mathworks Inc., MA) for the investigation of the brainstem and the cerebellum.
Initially, individual functional images were visually inspected to ensure that a complete acquisition of the lower structures of the cerebellum was performed during the scan. A total of 40 subjects were excluded, resulting in 44 healthy controls, 57 PTSD, and 32 PTSD + DS being included in the analyses.
Whole-Brain Analysis
For each participant, the preprocessing pipeline included realignment to the first image, re-slicing to the mean functional image, and co-registration to the anatomical image. Image segmentation (gray and white matter, and cerebro-spinal fluid), spatial normalization to the MNI standard template, and smoothing with a 6-mm full-width at half-maximum (FWHM) Gaussian kernel followed. Additional motion correction was implemented using ART toolbox (Artifact Detection Tool version 2015–10; Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA) with 2-mm motion threshold. The obtained motion regressors were added to the six standard movement parameters during the first-level analysis [80]. Finally, smoothed images were bandpass-filtered (0.012–0.1 Hz range) using an in-house code (written by co-author J. Théberge).
Brainstem and Cerebellum
With regard to the midbrain, lower brainstem, and cerebellum, the functional and anatomical acquired volumes were normalized voxel-by-voxel to the SUIT template (version 3.1) [28, 30]. First, whole-brain anatomical images were segmented and cropped to retain only the brainstem and cerebellum. The obtained structural images were then normalized to the SUIT template using the SUIT-normalization function. Next, the whole-brain realigned and resliced functional images were normalized to the SUIT template by applying the deformation matrix as per the structural images, then cropped to retain the brainstem and cerebellum only, and resliced to a 1.5 × 1.5 × 1.5 mm3 voxel size. Finally, the obtained partial-brain functional images were smoothed to a 4-mm FWHM kernel and bandpass-filtered with a high-pass filter of 0.01 Hz and a low-pass filter of 0.8 Hz [8].
Seed-Based Connectivity Analyses
The seed regions used to perform functional connectivity analyses were generated using SPM Anatomy toolbox [33] as implemented in SPM12 and based upon the cerebellar atlas generated by Diedrichsen et al. [29]. Specifically, distinct left and right flocculus seed regions of interest (ROIs) were created (see Fig. 1).
Fig. 1.
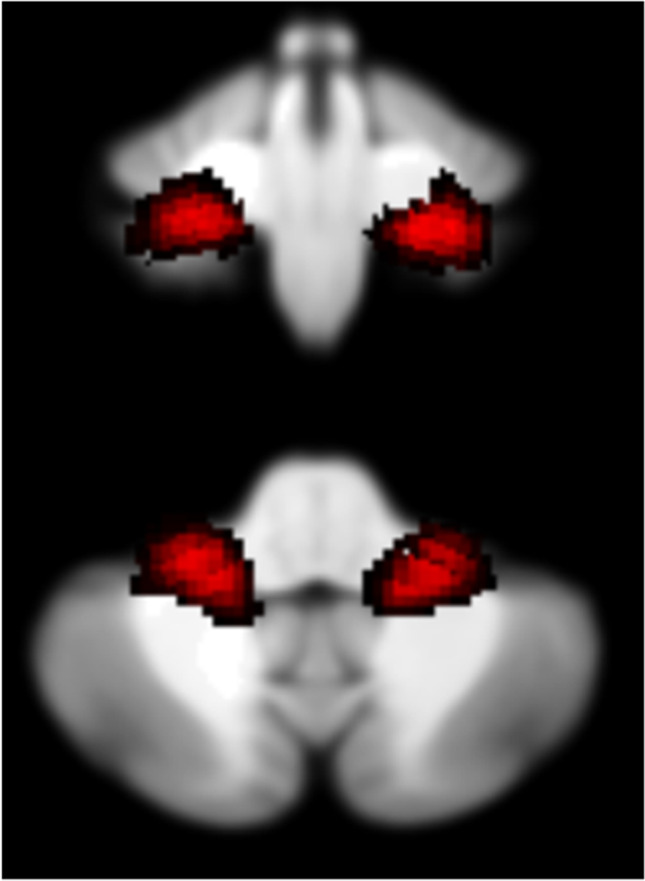
Seed regions displayed on the SUIT space in the coronal (top) and axial (bottom) planes
Each seed regions’ time course mean signal was extracted using SPM Anatomy toolbox and was subsequently input as a regressor in the first-level regression analysis per each subject, in addition to the ART regressor for motion correction. The resulting connectivity reflected a positive correlation between each seed region and any other voxel in the brain. Subsequently, the obtained individual images were used to perform a full-factorial ANOVA that included 3 groups (controls, PTSD, PTSD+DS) × 2 hemispheres (left, right). Post hoc analyses investigated interaction effects between groups for each hemisphere.
Within the SUIT maps, similar to the whole-brain connectivity analyses, each seed region time course mean signal was used as a regressor in the first-level analysis, with additional ART regressors for motion correction. Here, the results yield the functional connectivity between each seed region and each voxel within the partial brain provided by the SUIT template that included the brainstem and cerebellum exclusively. The first-level results were used to perform a full-factorial ANOVA (3 groups, controls, PTSD, and PTSD+DS, × 2 hemispheres, left and right) at the second-level analysis. Within-group analyses (simple t-tests using a significant threshold of p<.05 FWE-corrected with k≥10 at the SUIT map level) were also performed to further explore the resting-state functional connectivity (rsFC) of the flocculus within lower brain structures.
Additional regression analyses were conducted on the entire sample to investigate the association between rsFC of the right and left flocculi and the psychological measures obtained at the assessment, specifically PTSD symptom severity as measured by CAPS total (CAPS-IV and normalized CAPS-5), dissociative symptoms as measured by MDI (using the mean score obtained at the depersonalization and derealization items), childhood trauma history as measured by CTQ, depression as measured by BDI, state dissociation during the scanning session as measured by RSDI (mean scores obtained at the depersonalization and derealization items), and state anxiety during the scanning session as measured by the STAI.
All results were whole-brain corrected at the threshold of α < .05, over an initial uncorrected whole-brain threshold set at p < .005 followed by a 1000-iteration Monte Carlo simulation procedure using AlphaSim as implemented in RESTplus Toolbox version 1.8 (http://www.restfmri.net) [99]. This procedure yields minimal cluster extents that ensure a maximum false-positive rate of 5% at the whole-brain level. The individual extent thresholds were calculated per each individual T-map investigated.
An additional a priori region-of-interest analysis was used for the right and left hippocampi, given the role played by these brain structures in PTSD [65, 78, 97, 102], as well as their key role in orienting and spatial navigation [122, 128]. The ROI analysis was implemented on a 10-mm sphere with its peak at MNI ±28, −12, and −24 from Patel et al. [78]. A voxel-wise FWE threshold of p < .05 was applied with Bonferroni correction for multiple comparisons, resulting in a local significance threshold of p < .025, FWE corrected.
Results
Sociodemographic and Psychological Measures
No significant differences were found in age and sex between groups. By contrast, significant between-group differences emerged on all psychological measures, except for CAPS-5 (all healthy controls were assessed with CAPS-IV; see Table 1 for a detailed report).
rsFC of the Flocculus with the Whole Brain
The full-factorial analysis yielded a significant interaction effect (group by hemisphere) for rsFC between the flocculus and the left precentral gyrus (see Table 2).
Table 2.
Results of the resting-state functional connectivity analyses of the flocculus. (a) Full factorial results—main and interaction effects, between-group results; (b) regression analysis results
| a) Full factorial ANOVA | |||||||||
| L/R | Brain region | k | Peak Z | Peak p | x | y | z | ||
| Main effect of group | ns | ||||||||
| Main effect of hemisphere | ns | ||||||||
| Interaction effect group × hemisphere | L | Precentral gyrus | 300 | 4.54 | < 0.001 | − 38 | 2 | 32 | |
| Between-group results | |||||||||
| Seed region | Comparison | L/R | Brain region | k | Peak Z | Peak p | x | y | z |
| Left flocculus | HC > PTSD | L | Supramarginal gyrus/TPJ | 1210 | 4.54 | < 0.001 | − 56 | − 38 | 40 |
| L | Angular gyrus | 836 | 4.48 | < 0.001 | − 34 | − 64 | 46 | ||
| L | Superior parietal lobule/IPS/PEF | Subcluster | 3.93 | < 0.001 | − 22 | − 62 | 46 | ||
| PTSD > HC | ns | ||||||||
| HC > PTSD + DS | L | Supramarginal gyrus/TPJ | 267 | 3.94 | < 0.001 | − 56 | − 36 | 40 | |
| R | Precentral gyrus/middle cingulum/PCC | 252 | 3.93 | < 0.001 | 4 | − 26 | 50 | ||
| L | Angular gyrus/IPS/PEF | 621 | 3.9 | < 0.001 | − 34 | − 62 | 44 | ||
| L/R | Medial superior frontal gyrus | 281 | 3.63 | < 0.001 | 2 | 66 | 22 | ||
| L/R | Precuneus | 426 | 3.62 | < 0.001 | 0 | − 70 | 36 | ||
| PTSD + DS > HC | ns | ||||||||
| PTSD + DS > PTSD | ns | ||||||||
| PTSD > PTSD + DS | ns | ||||||||
| Right flocculus | HC > PTSD | ns | |||||||
| PTSD > HC | ns | ||||||||
| HC > PTSD + DS | ns | ||||||||
| PTSD + DS > HC | ns | ||||||||
| PTSD + DS > PTSD | R | Hippocampus* | 19 | 3.63 | < 0.013* | 34 | − 8 | − 20 | |
| PTSD > PTSD + DS | ns | ||||||||
| b) Correlation with symptoms | |||||||||
| CAPS tot | |||||||||
| Seed region | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left flocculus | Negative correlation | L | Precentral gyrus | 4715 (subcluster) | 4.42 | < 0.001 | − 56 | 12 | 34 |
| L | Supramarginal gyrus | subcluster | 4.24 | < 0.001 | − 54 | − 32 | 42 | ||
| R | Supramarginal gyrus | 376 (subcluster) | 4.24 | < 0.001 | 56 | − 36 | 34 | ||
| MDI Dep/Der | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Right flocculus | Positive correlation | R | Vermis V/VI | 331 | 3.93 | < 0.001 | 0 | − 60 | − 20 |
| L | Lingual gyrus | 244 | 3.92 | < 0.001 | − 18 | − 68 | 2 | ||
| CTQ tot | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left flocculus | Negative correlation | R | Superior frontal gyrus | 717 | 4.58 | < 0.001 | 14 | 40 | 42 |
| L | Superior frontal gyrus | 197 | 3.76 | < 0.001 | − 14 | 48 | 34 | ||
| L | Precentral gyrus | 300 | 3.73 | < 0.001 | − 58 | 10 | 30 | ||
| L | Superior parietal lobule/angular gyrus | 198 | 3.17 | < 0.001 | − 28 | − 64 | 40 | ||
| Right flocculus | Positive correlation | R | Hippocampus* | 27 | 3.62 | < 0.014* | 26 | − 12 | − 18 |
| RSDI dep/der | Correlation | L/R | Brain region | k | Z | p | x | y | z |
| Left flocculus | Negative correlation | R | Medial superior frontal gyrus | 202 | 4.35 | < 0.001 | 10 | 40 | 46 |
| L | Superior parietal lobule | 247 | 3.19 | < 0.001 | − 22 | − 68 | 46 | ||
All results are reported at p < .05 whole-brain corrected threshold. * ROI analysis reports p < .05 FWE-corrected at the peak level within the ROI. Coordinates are reported in the MNI space
Abbreviations: CAPS Clinician Administered PTSD Scale, CTQ Childhood Trauma Questionnaire, HC healthy control group, IPS intraparietal sulcus, k cluster extent, L left hemisphere, MDI Multiscale Dissociation Inventory, ns not significant results, PEF parietal eye field, PTSD post-traumatic stress disorder group, PTSD + DS PTSD dissociative subtype group, R right hemisphere, RSDI Response Script-Driven Imagery scale, TPJ temporo-parietal junction
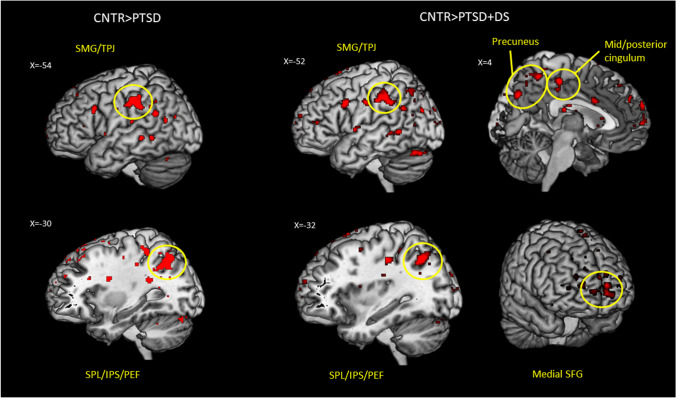
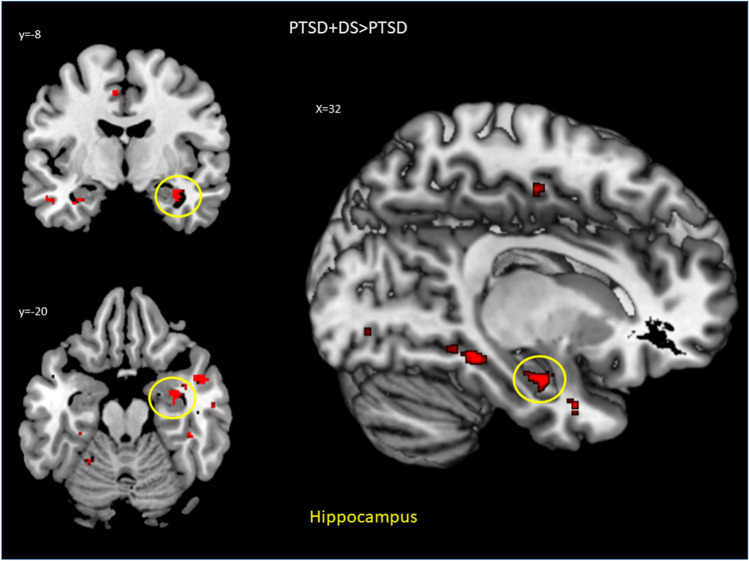
Post hoc analyses revealed that, as compared to the control group, the PTSD group displayed decreased rsFC of the left flocculus with the left supramarginal gyrus, encompassing the temporo-parietal junction (TPJ), the left superior parietal lobule (including the PEF), and the left angular gyrus. Similarly, as compared to the control group, the PTSD+DS group showed decreased rsFC of the left flocculus with the left supramarginal gyrus, including the TPJ, the right middle/posterior cingulum, the left angular gyrus, the bilateral medial superior frontal gyrus, and the bilateral precuneus. Conversely, the right flocculus showed increased rsFC with the right hippocampus in the PTSD+DS group as compared to the PTSD group (see Table 2 for a detailed report of the results, and Figs. 2 and 3). No other between-group differences emerged.
Fig. 2.
Between-group resting-state functional connectivity results for the left flocculus. Decreased rsFC of the PTSD group as compared to controls is shown on the left, decreased rsFC of the PTSD + DS group as compared to controls is shown on the right. Results are shown at a cluster extent threshold ensuring whole-brain correction at a p < .05. Abbreviations: CNTR, control group; IPS, intraparietal sulcus; PEF, parietal eye field; PTSD, post-traumatic stress disorder group; PTSD + DS, dissociative subtype of PTSD group; SFG, superior frontal gyrus; SMG, supramarginal gyrus; SPL, superior parietal lobule; TPJ, temporo-parietal junction; > , rsFC greater than
Fig. 3.
Resting-state functional connectivity results for the right flocculus in the PTSD + DS as compared to the PTSD group. Results relative to the hippocampus are shown at a peak p < .05 FWE-corrected within the ROI. Abbreviations: PTSD, post-traumatic stress disorder group; PTSD + DS, dissociative subtype of PTSD group; > , rsFC greater than
Symptom Correlations with rsFC of the Flocculus
Regression analyses revealed significant correlations between rsFC of the right and left flocculi and several clinical trait and state measures (see Table 2 for an overview of the results). Specifically, PTSD symptom severity, as measured by the CAPS total, was correlated negatively with rsFC of the left flocculus with the left precentral gyrus and with the bilateral supramarginal gyrus. Depersonalization/derealization symptoms, as measured by the MDI, correlated positively with rsFC of the right flocculus with the vermis and the left lingual gyrus. Childhood trauma severity, as measured by the CTQ, correlated negatively with rsFC of the left flocculus with the bilateral superior frontal gyrus, superior parietal lobule and angular gyrus, and the left precentral gyrus. Finally, CTQ scores correlated positively with rsFC of the right flocculus with the right hippocampus.
Finally, state dissociation (depersonalization/derealization as measured by the RSDI) at the time of scanning correlated negatively with rsFC of the left flocculus with the right medial prefrontal cortex and the left superior parietal lobule.
rsFC of the Flocculus Within the Cerebellum and Brainstem
No main or interaction effects emerged from the full factorial analysis. As a result, no post hoc comparisons were pursued. However, exploratory within-group analyses showed rsFC connectivity of the flocculus within the brainstem and cerebellum for each group separately (see Table S1).
Discussion
Our results demonstrate important differential resting-state functional connectivity of the flocculus in PTSD, PTSD+DS, and controls. The left flocculus exhibited differential rsFC in PTSD and PTSD+DS as compared to controls, specifically with brain regions involved in the default mode network, including those involved in bodily self-consciousness (supramarginal and angular gyrus, temporo-parietal junction) and medial fronto-parietal regions (precuneus, superior frontal cortex, and mid/posterior cingulum). Interestingly, the only significant difference found between the two PTSD groups centered around increased rsFC of the right flocculus with the ipsilateral hippocampus in PTSD+DS as compared to PTSD.
Finally, functional connectivity within the cerebellum and brainstem regions only (SUIT analysis) did not yield significant between-group results; however, within-group analyses point toward important directions for future research (see Supplementary Materials).
rsFC of the Flocculus with the Bodily Self-consciousness Network
As compared to controls, both PTSD groups showed decreased rsFC of the left flocculus with a cortical area encompassing the left supramarginal gyrus (SMG) and the TPJ. The SMG and TPJ have been identified as key cortical regions for the development and processing of bodily self-consciousness, an emerging quality of human consciousness that involves self-identification in terms of body-ownership, “this body belongs to me”; self-location, “this is where my body is located”; and first-person perspective, “my body is the place from where I see and interact with the world” [13, 14, 51, 95, 112]. In particular, distortions in self-location provide important foundations in the understanding of depersonalization symptoms in PTSD+DS, where individuals show the capacity to perceive their body as detached from one’s self or not their own [19, 26, 105]. We hypothesize here that a coherent experience of the self in space needs to be embodied in cortical and subcortical structures that support eye, head, and neck movement coordination, along with vestibular function, to orient oneself in space. In line with this hypothesis, in the present study, we observed altered connectivity of the flocculus to the vestibular system in PTSD and PTSD+DS. Previous data have shown altered resting-state functional connectivity of the TPJ/SMG in PTSD+DS as compared to PTSD and healthy controls within the vestibular system [45], where functional connectivity between the vestibular nuclei and the SMG correlated negatively with dissociative symptoms in PTSD at rest [45]. In addition, the left flocculus demonstrated decreased rsFC with the intraparietal sulcus in PTSD and PTSD+DS as compared to controls. The intraparietal sulcus plays a key role in multisensory integration of information regarding the space surrounding the body, namely, the peripersonal space [27, 32, 68]. Here, decreased rsFC with the left flocculus may suggest an impairment in the circuit supporting visuo-spatial processing critical to scanning the environment and gathering visual information within the peripersonal space, with downstream negative consequences on the sense of self in space. Interestingly, rsFC of the left flocculus with the left superior parietal lobule, including the intraparietal sulcus, correlated negatively with CTQ scores, indicating that the higher the severity of PTSD symptoms and childhood trauma, the lesser the rsFC of the left flocculus with cortical regions involved in peripersonal space representation. These correlational results may also have bearing on oculomotion in PTSD and PTSD+DS. The superior parietal region plays an important role in the PEF, where the PEF works in tandem with the frontal eye field and the superior colliculus to produce reflexive saccades, particularly in peripheral vision [72, 79]. Given the role of the flocculus in the production of saccades, our results suggest altered regulation of reflexive saccades in PTSD and PTSD+DS as compared to controls, with dysfunction increasing with the severity of PTSD and childhood trauma.
Interestingly, we also observed decreased functional connectivity of the left flocculus with the angular gyrus at rest in both PTSD and PTSD+DS as compared to controls. Critically, the inferior parietal lobule is a key cortical area for visuo-spatial attention and visuomotor integration [115], with previous research further revealing altered neural activity in the angular gyrus among individuals with depersonalization symptoms [98] and in PTSD [78]. In the present study, rsFC of the left flocculus with the angular gyrus correlated negatively with childhood trauma, suggesting an association of early traumatic experiences with the development of altered brain circuitry involved in spatial awareness and visuomotor integration. Given the central role of these abilities in promoting a coherent sense of self in interaction with the environment, our results are in keeping with the hypothesis that the vestibulocerebellum plays a central role in trauma-related symptomatology relating to bodily self-consciousness.
On balance, our data revealed decreased functional connectivity of the left flocculus with the cortical regions involved in eye and head movement coordination in PTSD and PTSD+DS as compared to controls, which can affect vestibular function, such as balance and gait, and thus impact bodily self-consciousness, including self-location and self-identification.
rsFC of the Flocculus with the Default Mode Network
Separate consideration is required in examining functional connectivity between the vestibulocerebellum and the default-mode network (DMN), where we have examined already the potential implications of decreased functional connectivity of the flocculus with the TPJ/SMG (cortical regions comprised in the DMN) in both PTSD groups. Notably, the PTSD+DS group also showed decreased functional connectivity between the left flocculus and two key hubs of the DMN, the medial prefrontal cortex, and the precuneus [20, 116, 119]. The DMN appears as the main brain network active during rest in healthy individuals [20, 70]. Critically, neuroimaging research has highlighted aberrant functional connectivity within the DMN in PTSD [2, 3, 15, 55, 59, 85, 113]. Specifically, a significant reduction of functional connectivity within the DMN at rest has been postulated to be associated with a disruption of the sense of self as a consequence of psychological trauma [62]. Here, our results reveal an additional lack of functional connectivity between the DMN and the flocculus in PTSD+DS as compared to controls.
How could oculomotor function relate to the DMN functions? Previous research has shown an association between autobiographical memory retrieval and oculomotion. Here, a study conducted by El Haj et al. [34] assessed eye movements during emotional autobiographical memory retrieval in healthy individuals and observed increased frequency of saccades and fixations, together with decreased fixation duration, in emotional versus neutral memory retrieval. Another study found improvement of episodic autobiographical memory retrieval through horizontal saccadic eye movements [77]. Notably, a well-known therapeutic intervention used in trauma treatment (eye movement desensitization reprocessing (EMDR); [96] successfully facilitates memory retrieval and processing of traumatic memories via the use of saccadic eye movements (for a systematic review, see [57]). Interestingly, a study investigating the neural circuitry underlying autobiographical memory retrieval during a saccadic eye movement task in PTSD as compared to controls revealed aberrant functional connectivity among fronto-parietal regions and cortical areas involved in oculomotion, including the frontal eye field and the supplementary eye field [44]. Taken together, these data suggest an association between eye movements and autobiographical memory retrieval, with the present findings supporting the hypothesis of a disruption in the functional connectivity of the neural circuitry underlying oculomotion and the DMN as a function of psychological trauma, particularly in the dissociative subtype of PTSD.
Notably, the present study also revealed a negative correlation between state depersonalization/derealization symptoms (as measured by the RSDI) and functional connectivity between the left flocculus and the medial prefrontal cortex. These results point toward an association between state dissociation at the time of scanning and disrupted functional connectivity between the vestibulocerebellum and the DMN, likely affecting processing of the sense of self in terms of self-location and self-identification, particularly among individuals with the dissociative subtype of PTSD.
Finally, as compared to controls, PTSD+DS showed decreased rsFC of a cluster encompassing the middle and posterior cingulum. The posterior cingulum plays a central role in autobiographical memory recall [20, 62, 67] and has consistently exhibited altered functional connectivity in PTSD [15, 55, 59, 82, 103]. Interestingly, the posterior cingulum is thought to serve as a key hub for integrating self-location and body ownership representation to build a coherent experience of the bodily self in space [41]. Here, our findings support further the hypothesis that the vestibulocerebellum plays a prominent role in promoting a coherent sense of self in space, including particularly self-location, where dysfunctional rsFC with the posterior cingulum may contribute to compromised bodily self-consciousness in PTSD.
Differential rsFC of the Flocculus in PTSD Versus PTSD + DS: the Role of the Hippocampus
Our results reveal increased rsFC of the right flocculus with the ipsilateral anterior hippocampus (mainly CA1 subfield in the uncus) in PTSD+DS as compared to PTSD. In addition, we observed a positive correlation between rsFC of the right flocculus with the anterior hippocampus (comprising CA4 and the dentate gyrus) and childhood trauma.
The hippocampus is thought to play a prominent role in chronic stress and PTSD [18, 35, 64, 73, 108], and high levels of stress hormones, particularly during development, are thought to reduce neurogenesis within the hippocampus, resulting in reduced hippocampal volume in adulthood [1, 16, 18, 35, 54, 108, 109].
Notably, the hippocampus has been linked to learning, memory, and visuo-spatial processing facilitating orientation in space [35, 130]. Critically, recent research reveals structural connections between the cerebellum, including the flocculus, and the hippocampus, indicating a direct cerebello-limbic pathway [5]. Here, several indirect pathways support bidirectional functional cerebellar-hippocampal connectivity [128] associated with spatial navigation and time processing tasks in humans and mice models [6, 122, 128].
Our results are therefore consistent with the hypothesis of aberrant hippocampal activity, in this case relative to rsFC with the flocculus, as a consequence of early life trauma. This hypothesis is corroborated by the observation of increased vestibulocerebellum-hippocampal functional connectivity in the dissociative subtype of PTSD, a subtype of PTSD that has been associated with higher levels of childhood trauma, where increased scanning of the environment may be necessary for survival [43, 61, 75, 83, 106, 110].
By contrast, increased flow of stress hormones coupled with the structural and functional alterations characteristic of the hippocampus in PTSD [23, 108] may lead to hyper-connectivity with the vestibulocerebellum necessary to compensate for blunted hippocampal function. The Cerebellar Universal Transform Hypothesis, which suggests that the cerebellum plays a critical role in compensating for deficits in brain function [89–91], supports this viewpoint.
Finally, a recent review of the functions of the anterior hippocampus sheds light on our findings, where Zeidmen and Maguire [129] found strong evidence for a role of the medial anterior hippocampus in spatial navigation, referred to as large-scale representation of the external environment [48, 71], especially during recall and imagination of scenes. Here, the hippocampus is thought to aid in linking elements of a scene (recalled or imagined) to produce a coherent spatial representation [129]. In our results, increased FC of the anterior hippocampus with the flocculus at rest may reveal a compensatory attempt to successfully reconstruct a scene representation or search for visuo-spatial inputs to build a more coherent scene in the individual’s mind during self-reflective processing in PTSD+DS. Indeed, depersonalization/derealization symptoms in PTSD+DS involve the perception of one’s body or the environment as unreal, distorted, scattered, and disintegrated, thus suggesting a failed integration of the sensory experience [39, 98, 100].
Here, we propose that depersonalization/derealization symptoms may be linked to disordered sensorimotor integration associated with defective memory recall (scattered recall of images/scene with pieces of sensory information not coherently integrated or misplaced in a scene representation; [10]). The vestibulocerebellum may, in turn, aid the hippocampus during memory retrieval and spatial processing, with heightened scanning of the environment to gather visuo-spatial information or recall of information to reconstruct a scene or an image of one’s self, an ability that appears to be incoherent or disintegrated in individuals with PTSD+DS.
Limitations
There is a number of limitations to this study. Firstly, the acquisition of data occurred at a single, cross-sectional, point in time; longitudinal studies are therefore warranted. Secondly, neuroimaging data were acquired using a 3-T magnetic field. A higher magnetic field and resolution may help to improve the specificity of the findings, especially in the brainstem regions. Finally, seed-based functional connectivity analysis, although standard in the field, does not allow for an examination of the directionality of functional connectivity between brain regions. No inference can therefore be made with respect to the direction of the impact of one region over another region’s activity.
Conclusion
Taken together, our results point toward an important role of the left flocculus in differentiating the neural circuits in PTSD and PTSD+DS as compared to controls at rest. In particular, as compared to controls, rsFC of the left flocculus was consistently decreased in relation to several cortical regions involved in bodily self-consciousness, including the TPJ, the SMG, the superior parietal lobule, and the angular gyrus in PTSD and its dissociative subtype. Furthermore, our findings demonstrate decreased rsFC of the flocculus with key regions of the DMN, thus further supporting the notion of aberrant functioning of the default mode network in PTSD and PTSD+DS, with associated consequences for the maintenance of a coherent sense of self. Critically, PTSD+DS appears to be uniquely associated with increased rsFC of the flocculus with the anterior hippocampus, a limbic region strongly affected by early life trauma. These results point toward the need for interventions aimed at increased vestibulocerebellum-hippocampal functional connectivity in the dissociative subtype of PTSD. Furthermore, whereas some PTSD treatments have already integrated psychological interventions using eye movements as a tool for processing traumatic events (EMDR; [96]), our findings suggest the need to further develop bottom-up clinical interventions targeting vestibulo-motor functioning and the integration of multisensory experience (see for example, Deep Brain Reorienting, [25], and Sensory Motor Arousal Regulation Treatment-SMART, [121]) in PTSD and PTSD+DS. We hypothesize that this will facilitate processing of traumatic experiences and help regain optimal cortical functioning, thus restoring the shattered self in the aftermath of trauma.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to thank Suzy Southwell and Stephanie Nevill for their contributions to recruiting and assessing participants. R.A.L is supported by the Harris-Woodman Chair in Mind-Body Medicine at Western University. M.C.M is supported by the Homewood Chair in Mental Health and Trauma at McMaster University.
Author Contribution
Daniela Rabellino and Ruth A. Lanius contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Daniela Rabellino, Janine Thome, Maria Densmore, and Jean Théberge. The first draft of the manuscript was written by Daniela Rabellino, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Canadian Institutes of Health Research (CIHR) grant no. RN287311.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aghamohammadi-Sereshki A, Coupland NJ, Silverstone PH, Huang Y, Hegadoren KM, Carter R, et al. Effects of childhood adversity on the volumes of the amygdala subnuclei and hippocampal subfields in individuals with major depressive disorder. J Psychiatry Neurosci. 2021;46:E186–E195. doi: 10.1503/jpn.200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep. 2017;19. 10.1007/s11920-017-0840-4 [DOI] [PMC free article] [PubMed]
- 3.Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, et al. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.APA. Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC: American Psychiatric Publishing; 2013. 10.1176/appi.books.9780890425596.744053.
- 5.Arrigo A, Mormina E, Anastasi GP, Gaeta M, Calamuneri A, Quartarone A, et al. Constrained spherical deconvolution analysis of the limbic network in human, with emphasis on a direct cerebello-limbic pathway. Front. Hum. Neurosci. 2014:8. 10.3389/fnhum.2014.00987 [DOI] [PMC free article] [PubMed]
- 6.Babayan BM, Watilliaux A, Viejo G, Paradis AL, Girard B, Rondi-Reig L. A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Sci Rep. 2017;7:1–16. doi: 10.1038/s41598-017-18004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldaçara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res. 2011;45:1627–1633. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Bär KJ, De la Cruz F, Schumann A, Koehler S, Sauer H, Critchley H, et al. Functional connectivity and network analysis of midbrain and brainstem nuclei. Neuroimage. 2016;134:53–63. doi: 10.1016/j.neuroimage.2016.03.071. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav Res Ther. 1997;35:785–91. [DOI] [PubMed]
- 10.Bergouignan L, Nyberg L, Ehrsson HH. Out-of-body memory encoding causes third-person perspective at recall. J Cogn Psychol. 2021 doi: 10.1080/20445911.2021.1958823. [DOI] [Google Scholar]
- 11.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27:169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 12.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 13.Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci. 2012;13:556–571. doi: 10.1038/nrn3292. [DOI] [PubMed] [Google Scholar]
- 14.Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron. 2015;88:145–166. doi: 10.1016/j.neuron.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci. 2009;34:187–94. [PMC free article] [PubMed]
- 16.Bonne O, Vythilingam M, Masatoshi I, Wood S, Neumeister A, Nugent AC, et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69:1087–1091. doi: 10.4088/JCP.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Traum Stress Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 19.Briere J. Multiscale Dissociation Inventory Professional Manual. ed. Florida: P.A. Resources Odessa. 2002.
- 20.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 21.Carletto S, Borsato T. Neurobiological correlates of post-traumatic stress disorder: a focus on cerebellum role. Eur J Trauma Dissociation. 2017;1:153–157. doi: 10.1016/j.ejtd.2017.03.012. [DOI] [Google Scholar]
- 22.Carvalho N, Laurent E, Noiret N, Chopard G, Haffen E, Bennabi D, et al. Eye movement in unipolar and bipolar depression: a systematic review of the literature. Front Psychol. 2015:6. 10.3389/fpsyg.2015.01809 [DOI] [PMC free article] [PubMed]
- 23.Chalavi S, Vissia EM, Giesen ME, Nijenhuis ERS, Draijer N, Cole JH, et al. Abnormal hippocampal morphology in dissociative identity disorder and post-traumatic stress disorder correlates with childhood trauma and dissociative symptoms. Hum Brain Mapp. 2015;36:1692–1704. doi: 10.1002/hbm.22730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chrobak AA, Bohaterewicz B, Tereszko A, Krupa A, Sobczak A, Ceglarek A, et al. Altered functional connectivity among frontal eye fields, thalamus and cerebellum in bipolar disorder. Psychiatr Pol. 2020;54:487–497. doi: 10.12740/PP/ONLINEFIRST/104445. [DOI] [PubMed] [Google Scholar]
- 25.Corrigan FM, Christie-Sands J. An innate brainstem self-other system involving orienting, affective responding, and polyvalent relational seeking: some clinical implications for a “Deep Brain Reorienting” trauma psychotherapy approach. Med Hypotheses. 2020:136. 10.1016/j.mehy.2019.109502 [DOI] [PubMed]
- 26.Dell PF. The multidimensional inventory of dissociation (MID) a comprehensive measure of pathological dissociation. J Trauma Dissociation. 2006;7:77–106. doi: 10.1300/J229v07n02_06. [DOI] [PubMed] [Google Scholar]
- 27.di Pellegrino G, Làdavas E. Peripersonal space in the brain. Neuropsychologia. 2015;66:126–133. doi: 10.1016/j.neuropsychologia.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 29.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Dieterich M, Bucher SF, Seelos KC, Brandt T. Cerebellar activation during optokinetic stimulation and saccades. Neurology. 2000;54:148–155. doi: 10.1212/wnl.54.1.148. [DOI] [PubMed] [Google Scholar]
- 32.Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science (80-). 2004;305:875–877. 10.1126/science.1097011. [DOI] [PubMed]
- 33.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 34.El Haj M, Nandrino JL, Antoine P, Boucart M, Lenoble Q. Eye movement during retrieval of emotional autobiographical memories. Acta Psychol (Amst) 2017;174:54–58. doi: 10.1016/j.actpsy.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Fanselow MS, Dang H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felmingham K, Kemp AH, Williams L, Falconer E, Olivieri G, Peduto A, et al. Dissociative responses to conscious and non-conscious fear impact underlying brain function in post-traumatic stress disorder. Psychol Med. 2008;38:1771–1780. doi: 10.1017/S0033291708002742. [DOI] [PubMed] [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, patient edition (SCID-I/P, 11/2002 revision). New York: Biometrics Research, New York State Psychiatric Institute. 2002.
- 38.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frewen PA, Lanius RA. Trauma-Related Altered States of Consciousness (TRASC): exploring the 4-D Model. J Trauma Dissociation. 2014;15:37–41. doi: 10.1080/15299732.2013.873377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guterstam A, Björnsdotter M, Gentile G, Ehrsson HH. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr Biol. 2015;25:1416–1425. doi: 10.1016/j.cub.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 42.Haines DE, Mihailoff GA. The cerebellum. In: Fundamental neuroscience for basic and clinical applications. 5th ed. 2018 Elsevier. Chapter 27 Pages 394–412.e1. 10.1016/B978-0-323-39632-5.00027-X.
- 43.Harricharan S, McKinnon MC, Lanius RA. How processing of sensory information from the internal and external worlds shape the perception and engagement with the world in the aftermath of trauma: implications for PTSD. Front. Neurosci. 2021:15. 10.3389/fnins.2021.625490. [DOI] [PMC free article] [PubMed]
- 44.Harricharan S, McKinnon MC, Tursich M, Densmore M, Frewen P, Théberge J, et al. Overlapping frontoparietal networks in response to oculomotion and traumatic autobiographical memory retrieval: implications for eye movement desensitization and reprocessing. Eur J Psychotraumatol. 2019a:10. 10.1080/20008198.2019.1586265 [DOI] [PMC free article] [PubMed]
- 45.Harricharan S, Nicholson AA, Densmore M, Théberge J, McKinnon MC, Neufeld RWJ, et al. Sensory overload and imbalance: resting-state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia. 2017;106:169–178. doi: 10.1016/j.neuropsychologia.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Harricharan S, Nicholson AA, Thome J, Densmore M, McKinnon MC, Théberge J, et al. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2019b:1–23. 10.1111/psyp.13472. [DOI] [PubMed]
- 47.Harricharan S, Rabellino D, Frewen PA, Densmore M, Théberge J, McKinnon MC, et al. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 2016:6. 10.1002/brb3.579. [DOI] [PMC free article] [PubMed]
- 48.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104(5):1726–31. 10.1073/pnas.061056110. [DOI] [PMC free article] [PubMed]
- 49.Hopper JW, Frewen PA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD : symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. doi: 10.1002/jts. [DOI] [PubMed] [Google Scholar]
- 50.Hughes KC, Shin LM. Functionl neuroimaging studies of post-traumatic stress disorder. Expert Rev Neurother. 2011;11:275–285. doi: 10.1586/ern.10.198.Functional. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ionta S, Heydrich L, Lenggenhager B, Mouthon M, Fornari E, Chapuis D, et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron. 2011;70:363–374. doi: 10.1016/j.neuron.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Jáuregui Renaud K. Vestibular function and depersonalization/derealization symptoms. Multisens Res. 2015;28:637–651. doi: 10.1163/22134808-00002480. [DOI] [PubMed] [Google Scholar]
- 53.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of dsm-iv disorders in the national comorbidity survey replication. Arch Gen Psych. 2005; 62(6):593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed]
- 54.Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety. 2016;33:592–605. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- 56.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 57.Landin-Romero R, Moreno-Alcazar A, Pagani M, Amann BL. How does eye movement desensitization and reprocessing therapy work? A systematic review on suggested mechanisms of action. Front Psychol. 2018;9. 10.3389/fpsyg.2018.01395. [DOI] [PMC free article] [PubMed]
- 58.Lanius RA. Trauma-related dissociation and altered states of consciousness: a call for clinical, treatment, and neuroscience research. Eur J Psychotraumatol. 2015;6:1–9. doi: 10.3402/ejpt.v6.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 60.Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D. The dissociative subtype of posttraumatic stress disorder: rationale, clinical and neurobiological evidence, and implications. Depress Anxiety. 2012;29:701–708. doi: 10.1002/da.21889. [DOI] [PubMed] [Google Scholar]
- 61.Lanius RA, Rabellino D, Boyd JE, Harricharan S, Frewen PA, McKinnon MC. The innate alarm system in PTSD: conscious and subconscious processing of threat. Curr Opin Psychol. 2016;14:109–115. doi: 10.1016/j.copsyc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Lanius RA, Terpou BA, McKinnon MC. The sense of self in the aftermath of trauma: lessons from the default mode network in posttraumatic stress disorder. Eur J Psychotraumatol. 2020;11. 10.1080/20008198.2020.1807703. [DOI] [PMC free article] [PubMed]
- 63.Lencer R, Trillenberg P, Trillenberg-Krecker K, Junghanns K, Kordon A, Broocks A, et al. Smooth pursuit deficits in schizophrenia, affective disorder and obsessive - compulsive disorder. Psychol Med. 2004;34:451–460. doi: 10.1017/S0033291703001314. [DOI] [PubMed] [Google Scholar]
- 64.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 2018;83:244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez C, Elzière M. Out-of-body experience in vestibular disorders – a prospective study of 210 patients with dizziness. Cortex. 2018;104:193–206. doi: 10.1016/j.cortex.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 67.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–76. 10.1016/S0306-4522(01)00108-7. [DOI] [PubMed]
- 68.Makin TR, Holmes NP, Zohary E. Is that near my hand? Multisensory representation of peripersonal space in human intraparietal sulcus. J Neurosci. 2007;27:731–740. doi: 10.1523/JNEUROSCI.3653-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mennink LM, van Dijk JMC, van Dijk P. The cerebellar (para)flocculus: a review on its auditory function and a possible role in tinnitus. Hear Res. 2020;398:108081. doi: 10.1016/j.heares.2020.108081. [DOI] [PubMed] [Google Scholar]
- 70.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Mullally SL, Intraub H, Maguire EA. Attenuated boundary extension produces a paradoxical memory advantage in amnesic patients. Curr Biol. 2012;22:261–268. doi: 10.1016/j.cub.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naito Y, Tateya I, Hirano S, Inoue M, Funabiki K, Toyoda H, et al. Cortical correlates of vestibulo-ocular reflex modulation: a PET study. Brain. 2003;126:1562–1578. doi: 10.1093/brain/awg165. [DOI] [PubMed] [Google Scholar]
- 73.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RWJ, Théberge J, et al. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res - Neuroimaging. 2016;250:61–72. doi: 10.1016/j.pscychresns.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Olivé I, Densmore M, Harricharan S, Théberge J, McKinnon MC, Lanius R. Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39:563–574. doi: 10.1002/hbm.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, et al. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry 2001;50:246–53. 10.1016/s0006-3223(00)00920-3. [DOI] [PubMed]
- 77.Parker A, Parkin A, Dagnall N. Effects of saccadic bilateral eye movements on episodic and semantic autobiographical memory fluency. Front Hum Neurosci. 2013;7:1–10. doi: 10.3389/fnhum.2013.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82:463–71. 10.1152/jn.1999.82.1.463. [DOI] [PubMed]
- 80.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2141–2154. doi: 10.1016/j.neuroimage.2011.10.018.Spurious. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purves D. Neuroscience. 5. SUnderland, MA: Sinauer Associates; 2012. [Google Scholar]
- 82.Qin L, Wang Z, Sun Y-W, Wan J-Q, Su S-S, Zhou Y, et al. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 83.Rabellino D, Boyd JE, Mckinnon MC, Lanius RA. The innate alarm system: a translational approach. In: Fink GR, editor. Stress: physiology, biochemistry, and pathology. Chapter 17, pages 197–212; Elsevier 2019. 10.1016/B978-0-12-813146-6.00017-5.
- 84.Rabellino D, Densmore M, Théberge J, McKinnon MC, Lanius RA. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018b:39. 10.1002/hbm.24081 [DOI] [PMC free article] [PubMed]
- 85.Rabellino D, Tursich M, Frewen PA, Daniels JK, Densmore M, Théberge J, et al. Intrinsic connectivity networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr Scand. 2015;132:365–378. doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- 86.Ruehl RM, Hinkel C, Bauermann T, Eulenburg P Zu. Delineating function and connectivity of optokinetic hubs in the cerebellum and the brainstem. Brain Struct Funct 2017;222:4163–4185. 10.1007/s00429-017-1461-8. [DOI] [PubMed]
- 87.Saman Y, Bamiou DE, Gleeson M, Dutia MB. Interactions between stress and vestibular compensation - a review. Front Neurol. 2012:1–8. 10.3389/fneur.2012.00116. [DOI] [PMC free article] [PubMed]
- 88.Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, et al. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE. 2013;8:e58150. 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed]
- 89.Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214. doi: 10.1016/S0911-6044(00)00011-7. [DOI] [Google Scholar]
- 90.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/appi.neuropsych.16.3.367. [DOI] [PubMed] [Google Scholar]
- 91.Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337–364. doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- 92.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum - insights from the clinic. The Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 93.Schutter DJLG. Human cerebellum in motivation and emotion. In: Manto M, Gruol DL, Schmamhann JD, Koibuchi N, Rossi F, eds. Handbook of the Cerebellum and Cerebellar Disorders. Dordrecht: Springer 2013;1771–1782. 10.1007/978-94-007-1333-8.
- 94.Schutter DJLG, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4:290–294. doi: 10.1080/14734220500348584. [DOI] [PubMed] [Google Scholar]
- 95.Serino A, Noel JP, Galli G, Canzoneri E, Marmaroli P, Lissek H, et al. Body part-centered and full body-centered peripersonal space representations. Sci Rep. 2015;5:1–14. doi: 10.1038/srep18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shapiro F. Eye movement desensitization: a new treatment for post-traumatic stress disorder. J Behav Ther Exp Psychiatry. 1989;20:211–217. doi: 10.1016/0005-7916(89)90025-6. [DOI] [PubMed] [Google Scholar]
- 97.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 98.Sierra M, David AS. Depersonalization: a selective impairment of self-awareness. Conscious Cogn. 2011;20:99–108. doi: 10.1016/j.concog.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 99.Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spiegel D, Lewis-Fernández R, Lanius R, Vermetten E, Simeon D, Friedman M. Dissociative disorders in DSM-5. Annu Rev Clin Psychol. 2013;9:299–326. doi: 10.1146/annurev-clinpsy-050212-185531. [DOI] [PubMed] [Google Scholar]
- 101.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 102.Sripada RK, Garfinkel SN, Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front Hum Neurosci. 2013;7:672. doi: 10.3389/fnhum.2013.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Pyschosomatic Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33.Neural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Staab JP. The influence of anxiety on ocular motor control and gaze. Curr Opin Neurol. 2014;27:118–124. doi: 10.1097/WCO.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 105.Stein DJ, Koenen KC, Friedman MJ, Hill E, McLaughlin KA, Petukhova M, et al. Dissociation in posttraumatic stress disorder: evidence from the world mental health surveys. Biol Psychiatry. 2013;73:302–312. doi: 10.1016/j.biopsych.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steuwe C, Daniels JK, Frewen PA, Densmore M, Theberge J, Lanius RA. Effect of direct eye contact in women with PTSD related to interpersonal trauma: psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Res Neuroimaging. 2015;232:162–167. doi: 10.1093/scan/nss105. [DOI] [PubMed] [Google Scholar]
- 107.Steuwe C, Lanius RA, Frewen PA. Evidence for a dissociative subtype of PTSD by latent profile and confirmatory factor analyses in a civilian sample. Depress Anxiety. 2012;29:689–700. doi: 10.1002/da.21944. [DOI] [PubMed] [Google Scholar]
- 108.Teicher, M. H., Anderson, C. M., and Polcari, A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus,and subiculum. Proc. Natl. Acad. Sci. U. S. A. 109. 10.1073/pnas.1115396109 [DOI] [PMC free article] [PubMed]
- 109.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 110.Thome J, Densmore M, Koppe G, Terpou B, Théberge J, McKinnon MC, et al. Back to the basics: resting state functional connectivity of the reticular activation system in PTSD and its dissociative subtype. Chronic Stress. 2019;3:247054701987366. doi: 10.1177/2470547019873663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trachtman JN. Post-traumatic stress disorder and vision. Optometry. 2010;81:240–252. doi: 10.1016/j.optm.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 112.Tsakiris M, Costantini M, Haggard P. Neuropsychologia The role of the right temporo-parietal junction in maintaining a coherent sense of one ’ s body. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 113.Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, Lanius RA. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- 114.Tym, R., Beaumont, P., Lioulios, T. (2009). Two persisting pathophysiological visual phenomena following psychological trauma and their elimination with rapid eye movements: a possible refinement of construct PTSD and its visual state marker. Traumatology (Tallahass. Fla). 15, 23–33. 10.1177/1534765609335521.
- 115.Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, et al. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voogd J, Schraa-Tam CKL, Van Der Geest JN, De Zeeuw CI. Visuomotor cerebellum in human and nonhuman primates. Cerebellum. 2012;11:392–410. doi: 10.1007/s12311-010-0204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wabnitz P, Gast U, Catani C. Differences in trauma history and psychopathology between PTSD patients with and without co-occurring dissociative disorders. Eur J Psychotraumatol. 2013;4:1–10. doi: 10.3402/ejpt.v4i0.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang S, Tepfer LJ, Taren AA, Smith DV. Functional parcellation of the default mode network: a large-scale meta-analysis. Sci Rep 2020;10. 10.1038/s41598-020-72317-8 [DOI] [PMC free article] [PubMed]
- 120.Wang T, Liu J, Zhang J, Zhan W, Li L, Wu M, et al. Altered resting-state functional activity in posttraumatic stress disorder : a quantitative meta- analysis. Sci Rep. 2016;6:1–14. doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Warner E, Westcott A, Cook A, Finn H. Transforming truma in children and adolescents: an embodied approach to somatic regulation, trauma processing, and attachment-building. Berkeley, CA: North Atlantic Books; 2020. [Google Scholar]
- 122.Watson TC, Obiang P, Torres-Herraez A, Watilliaux A, Coulon P, Rochefort C, et al. Anatomical and physiological foundations of cerebello-hippocampal interaction. Elife. 2019;8. 10.7554/eLife.41896.001. [DOI] [PMC free article] [PubMed]
- 123.Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM. The clinician-administered PTSD scale for DSM-5 (CAPS-5). 2013. The CAPS-5 is available from the National Center for PTSD at www.ptsd.va.gov. Accessed 9/2/2022.
- 124.Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Postrtraumatic Stress Disorder Scale. Psychol Assess. 1999;11:124–133. doi: 10.1037/1040-3590.11.2.124. [DOI] [Google Scholar]
- 125.Whalley MG, Kroes MCW, Huntley Z, Rugg MD, Davis SW, Brewin CR. An fMRI investigation of posttraumatic flashbacks. Brain Cogn. 2013;81:151–159. doi: 10.1016/j.bandc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yardley L, Watson S, Britton J, Lear S, Bird J. Effects of anxiety arousal and mental stress on the vestibulo-ocular reflex. Acta Otolaryngol. 1995;115:597–602. doi: 10.3109/00016489509139373. [DOI] [PubMed] [Google Scholar]
- 127.Yen Pik Sang F, Jáuregui-Renaud K, Green DA, Bronstein AM, Gresty MA. Depersonalisation/derealisation symptoms in vestibular disease. J Neurol Neurosurg Psychiatry. 2006;77:760–766. 10.1136/jnnp.2005.075473 [DOI] [PMC free article] [PubMed]
- 128.Yu W, Krook-magnuson E, Clark RE, Krook-magnuson E. Cognitive collaborations : bidirectional functional connectivity between the cerebellum and the hippocampus. Front Systmes Neurosci. 2015;9:1–10. doi: 10.3389/fnsys.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17:173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng F, Cui D, Zhang L, Zhang S, Zhao Y, Liu X, et al. The volume of hippocampal subfields in relation to decline of memory recall across the adult lifespan. Front Aging Neurosci. 2018:10. 10.3389/fnagi.2018.00320 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.