Abstract
Introduction
The aim of this study was to compare weight loss and gastroesophageal reflux disease (GERD) remission after one-anastomosis gastric bypass (OAGB) versus Roux-en-Y gastric bypass (RYGB) as revisional procedures after laparoscopic sleeve gastrectomy (LSG).
Methods
In PubMed, Embase, and Cochrane Library, a search was performed using the terms “Roux-en-Y gastric bypass versus one anastomosis gastric bypass,” “revisional surgery,” and “sleeve gastrectomy.” Only original articles in English language comparing OAGB and RYGB were included. No temporal interval was set. The primary outcome measure was weight loss (%TWL). The secondary endpoints were leak, bleeding, marginal ulcer, and GERD. PRISMA flowchart was used. Differences in continuous and dichotomous outcome variables were expressed as mean difference (MD) and risk difference (RD) with 95% CI, respectively. Heterogeneity was assessed by using I2 statistic.
Results
Six retrospective comparative articles were included in the present meta-analysis. Weight loss analysis showed a MD = 5.70 (95% CI 4.84–6.57) in favor of the OAGB procedure with a statistical significance (p = 0.00001) and no significant statistical heterogeneity (I2 = 0.00%). There was no significant RD for leak, bleeding, or marginal ulcer after the two revisional procedures. After conversion to OAGB, remission from GERD was 68.6% (81/118), and it was 80.6% (150/186) after conversion to RYGB with a RD = 0.10 (95% CI −0.04, 0.24), no statistical significance (p = 0.19), and high heterogeneity (I2 = 96%). De novo GERD was 6.3% (16/255) after conversional OAGB, and it was 0.5% (1/180) after conversion to RYGB with a RD = −0.23 (95% CI −0.57, 0.11), no statistical significance (p = 0.16), and high heterogeneity (I2 = 92%).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00423-023-03175-x.
Keywords: Revisional surgery, One-anastomosis gastric bypass, Roux-en-Y gastric bypass, Sleeve gastrectomy, GERD
Introduction
Laparoscopic sleeve gastrectomy (LSG) is currently the most performed bariatric procedure worldwide [1]. Despite this popularity, LSG was reported to be associated with weight regain and gastroesophageal reflux disease (GERD) in the long-term with a revision rate up to 36% [2]. Some articles have also described intestinal metaplasia (Barrett’s disease) after LSG due to the chronic exposure of the lower esophagus to reflux [3, 4]. Roux-en-Y gastric bypass (RYGB) and one-anastomosis gastric bypass (OAGB) are, respectively, the second and the third most performed interventions, and they have both been suggested as good options for failed LSG [2–5]. Specifically, RYGB is considered an efficient treatment for GERD post-LSG [6], while OAGB may provide better results in terms of further weight loss [7].
The aim of this study was to analyze and compare weight loss and GERD remission after OAGB versus RYGB as revisional procedures after LSG.
Methods
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines were followed [8].
Literature search
In PubMed, Embase, and Cochrane Library, a search was performed using the terms “Roux-en-Y gastric bypass versus one anastomosis gastric bypass,” “revisional surgery,” and “sleeve gastrectomy.” In addition, the reference lists of all retrieved articles were manually reviewed. According to Problem/Population, Intervention, Comparison, and Outcome (PICO) framework, study selection criteria were exactly defined. Only original articles in English language comparing OAGB and RYGB were included. No temporal interval was set. The primary outcome measure was weight loss. The secondary endpoints were leak, bleeding, GERD remission, and de novo reflux. The last search was performed in December 2022.
Studies selection
Two independent authors analyzed each article and performed data extraction independently. Duplicate studies were removed. In case of disagreement, further investigation was conducted by an additional author.
Statistical analysis
DataRev software (Cochrane) version 5.4.1 (the Cochrane Collaboration 2011, the Nordic Cochrane Centre, Copenhagen) was used to perform a random-effect meta-analysis with Mantel–Haenszel calculation because of the observational nature of most studies included in this analysis.
Differences in continuous and dichotomous outcome variables were expressed as mean difference and risk difference (RD) with 95% CI, respectively. Heterogeneity was assessed by using I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. Usually, values of the I2 statistic < 25% are indicative of low heterogeneity, those ranging between 25 and 75% of moderate heterogeneity, and those > 75% of high heterogeneity. I2 < 40% was considered as non-important heterogeneity. A p < 0.05 was considered statistically significant. Publication bias was assessed through visual inspection of funnel plots.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale (NOS) [9] was used as an assessment tool to evaluate case–control studies. The scale’s range varies from 0 to 9 stars, and studies with a score equal to or higher than 5 were considered to have an adequate methodological quality to be included.
Results
The literature search found 55 articles. After removal of 21 duplicates, other 27 articles were excluded because they were not comparing RYGB and OAGB as revisional procedures. Seven [10–15] papers were considered eligible, but one [16] was excluded due to incomplete report of the outcome measures. PRISMA flow chart for the study selection is shown in Fig. 1. Eventually, 5 retrospective articles and 1 randomized controlled trial were included in our meta-analysis (Table 1). In total, 739 patients were included, of which 373 (50.5%) underwent OAGB and 366 (49.5%) underwent RYGB. The sample size of these studies ranged from 55 to 263 patients. The primary outcome measure was reported both as percentage of total weight loss (%TWL) and percentage of excess weight loss (%EWL) or excess BMI loss percent (%EBMIL) with a follow-up ranging from 12 to 60 months; assessment with NOS showed high-quality methodology for all the considered papers (Table 2).
Fig. 1.
PRISMA flowchart
Table 1.
Included studies and baseline characteristics
| Study (year) | Primary surgery | Revisional surgery | Patient (n) | Age (years) | Male (n) | BMI at conversion (kg/m2) | Max follow-up time (months) | BMI at follow-up (kg/m2) |
|---|---|---|---|---|---|---|---|---|
| Chiappeta (2019) | LSG | OAGB | 34 | 46.76 ± 11.48 | 11 | 45.7 ± 8 | 12 | 36.6 ± 6.3 |
| RYGB | 21 | 46.14 ± 10.8 | 2 | 36.6 ± 6.9 | 12 | 33.5 ± 5.6 | ||
| Rayman (2021) | LSG | OAGB | 144 | 42.4 ± 10.5 | 37 | 41.6 ± 5.7 | 25.5 | 31.8 ± 5.3 |
| RYGB | 119 | 44.3 ± 11.8 | 35 | 39.6 ± 5.0 | 35 | 33.3 ± 5.0 | ||
| Felsenreich (2022) | LSG | OAGB | 13 | - | - | 45.0 ± 7.3 | 15 | 31.4 ± 8.1 |
| RYGB | 45 | - | - | 38.6 ± 8.6 | 15 | 30.3 ± 8.5 | ||
| Rheinwalt (2022) | LSG | OAGB | 55 | 42 ± 1.3 | 33 | 45.5 ± 1.0 | 24 | 35 |
| RYGB | 68 | 46 ± 1.2 | 39.3 ± 1.0 kg | 24 | 31 | |||
| Wilczyński (2022) | LSG | OAGB | 47 | 45.02 ± 10.71 | 13 | 40.44 ± 5.8 | 60 | - |
| RYGB | 33 | 41.24 ± 8.906 | 6 | 38.70 ± 6.84 | 60 | - | ||
| Hany (2022) | LSG | OAGB | 80 | 42.6 ± 7.1 | 11 | 45.1 ± 8.3 | 24 | 27.4 ± 3.1 |
| RYGB | 80 | 43.4 ± 7.5 | 11 | 44.9 ± 6.6 | 24 | 27.8 ± 2.2 |
Table 2.
Outcomes of the included studies
| Study (year) | Revisional surgery | Operative time (min) | Sample (n) | Leaks (n, %) | Bleeding (n, %) | Marginal ulcer (n, %) | EWL% | TWL% | T2DM resolution (%) | HTN resolution (%) | GERD on follow-up (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiappeta (2019) | OAGB | 79 ± 36 | 34 | 0 (0%) | 0 (0%) | 0 (0%) | 29 ± 13 | 15.8 ± 7.8 | 100% | 66.7% | 11.8% | 9 |
| RYGB | 98 ± 24 | 21 | 0 (0%) | 0 (0%) | 1 (4.8%) | 22 ± 18 | 10.3 ± 7.6 | 60% | 0% | 4.8% | ||
| Rayman (2021) | OAGB | - | 144 | 2 (1.4%) | 2(1.4%) | 0 (0%) | 58.7 | 32 ± 9 | - | - | 17.4% | 9 |
| RYGB | - | 119 | 1 (1.7%) | 3 (2.5%) | 0 (0%) | 44.2 | 27 ± 9 | - | - | 7.6% | ||
| Felsenreich (2022) | OAGB | - | 13 | 0 (0%) | 0 (0%) | 0 (0%) | 80.3 ± 23.7 | 39.5 ± 11.5 | - | - | 28.9% | 9 |
| RYGB | - | 45 | 0 (0%) | 0 (0%) | 0 (0%) | 79.8 ± 34.1 | 37.7 ± 14.6 | - | - | 53.8% | ||
| Rheinwalt (2022) | OAGB | 168 ± 7.2 | 55 | 2 (3.6%) | 0 (0%) | 0 (0%) | 50 | 24 ± 2.6 | 92% | 92% | 13.34% | 8 |
| RYGB | 201 ± 6.8 | 68 | 4 (5.9%) | 2 (2.9%) | 0 (0%) | 40 | 18 ± 3.0 | 100% | 89% | 11.1% | ||
| Wilczyński (2022) | OAGB | - | 47 | 0 (0%) | 1 (2.12%) | 3 (6.4.%) | 84.04 ± 18.81 | 21.81 ± 12.48 | 97.3% | 27.3% | 28.6% | 8 |
| RYGB | - | 33 | 0 (0%) | 1 (3%) | 4 (12.1%) | 72.95 ± 20.3 | 18.39 ± 11.85 | 33.3% | 30% | 60% | ||
| Hany (2022) | OAGB | 85.6 ± 18.6 | 80 | 0 (0%) | 1 (1.25%) | 0 (0%) | -* | -* | 75% | 68% | - | 8 |
| RYGB | 104.9 ± 13.7 | 80 | 0 (0%) | 1 (1.25%) | 2 (2.5%) | -* | -* | 71% | 75% | - |
NOS Newcastle–Ottawa Scale. T2SM type 2 diabetes. HTN hypertension. *Hany et al. reported weight loss as %EBMIL
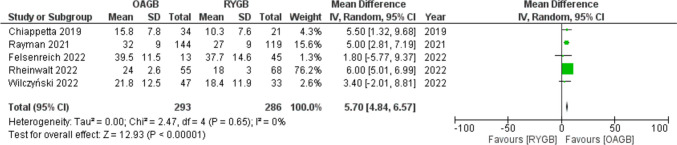
Weight loss was reported using different parameters, but percentage of total weight loss (%TWL) was used in five studies showing a MD = 5.70 (95% CI 4.84–6.57) in favor of the OAGB procedure with a statistical significance (p < 0.001) and no significant statistical heterogeneity (I2 = 0%) (Fig. 2).
Fig. 2.
Forest plot for percentage of total weight loss
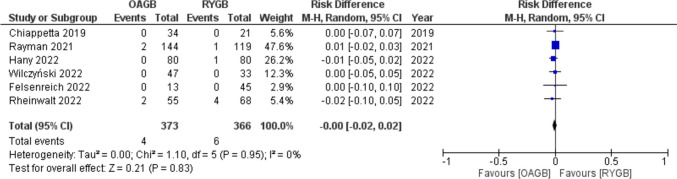
Overall leak rate after conversion to OAGB was 1% (4/373), and it was 1.6% (6/366) after revision to RYGB.
Meta-analysis showed a RD = − 0.00 (95% CI − 0.02–0.02) with no statistical significance (p = 0.83) and no significant statistical heterogeneity (I2 = 0.00%) (Fig. 3).
Fig. 3.
Forest plot for leak
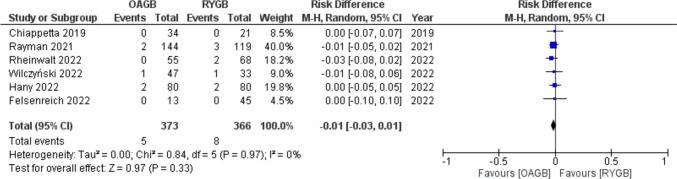
Total bleedings after revisional OAGB and RYGB were 1.3% (5/373) and 2.2% (8/366), respectively, with a RD = − 0.01 (95% CI − 0.03, 0.01) with no statistical significance (p = 0.33) and no significant statistical heterogeneity (I2 = 0.00%) (Fig. 4).
Fig. 4.
Forest plot for bleeding
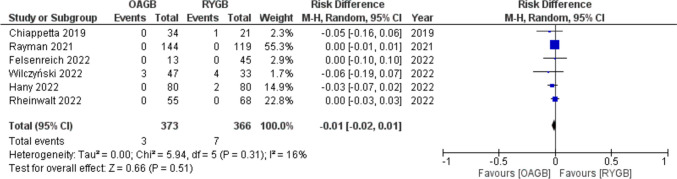
Total percentage of marginal ulcers after conversion to OAGB was 0.8% (3/373), and it was 1.9% (7/366) after revision to RYGB. Meta-analysis showed a RD = − 0.01 (95% CI − 0.02, 0.01) with no statistical significance (p = 0.51) and low heterogeneity (I2 = 16%) (Fig. 5).
Fig. 5.
Forest plot for marginal ulcer
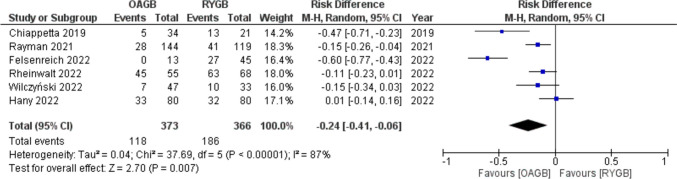
GERD was the indication for conversion for 31.6% (118/373) of patients before OAGB and for 50.8% (186/366) before RYGB. Meta-analysis of rate of preconversional GERD showed a RD = − 0.24 (95% CI − 0.41, − 0.06) with statistical significance (p = 0.007) and high heterogeneity (I2 = 87%) (Fig. 6).
Fig. 6.
Forest plot for GERD as indication for conversion
After conversion to OAGB remission from GERD was 68.6% (81/118), and it was 80.6% (150/186) after conversion to RYGB with a RD = 0.10 (95% CI − 0.04, 0.24) with no statistical significance (p = 0.19) and high heterogeneity (I2 = 96%) (Fig. 7).
Fig. 7.
Forest plot for GERD after conversion
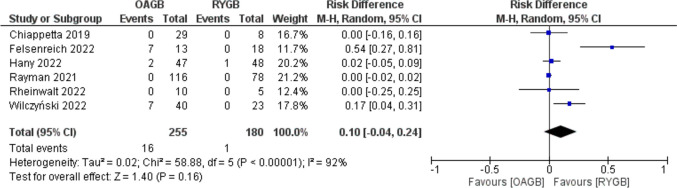
De novo GERD was 6.3% (16/255) after conversional OAGB, and it was 0.5% (1/180) after conversion to RYGB with a RD = − 0.23 (95% CI − 0.57, 0.11) with no statistical significance (p = 0.16) and high heterogeneity (I2 = 92%) (Fig. 8).
Fig. 8.
Forest plot for de novo GERD after conversion
Funnel plots inspection did not show significant bias (Supplement materials 1–6).
Discussion
LSG was initially introduced by Marceau [17] and Gagner [18] proposed as a first step of a staged procedure in patients with BMI > 60 kg/m [2]. Since postoperative outcomes demonstrated low morbidity and satisfactory weight loss, LSG became a stand-alone bariatric intervention [19]. Short-term studies (1–3 years) reported an excess weight loss (%EWL) comparable to the values of the RYGB [20]. Mid-term reports (5–7 years) have shown less successful results, with a certain percentage of weight regain [21, 22]; the SM-BOSS [23] study showed that excess BMI loss peaked at 2 years after SG (74.7%) but decreased by the end of the fifth year to 61.1%.
Recently, long-term studies have demonstrated a worrisome rate of conversion and GERD [24], especially in individuals with BMI > 50 kg/m2 [25]. Sporadic cases of vitamin deficiency after LSG have been also published [26].
A recent systematic review showed a rate of de novo GERD of 20% [27] after LSG, while a meta-analysis found that the increase of postoperative GERD was 19%, and de novo reflux occurred in 23% [28] of patients.
Despite several meta-analyses have investigated the role of OAGB and RYGB as revisional procedures after failed restrictive surgery [6, 7, 29], there is a lack of comparative studies on the role of this interventions specifically after failed LSG. Chiappetta et al. [10] first reported their single-center analysis of 55 patients showing that OAGB after failed SG was a quicker procedure with less perioperative complications. On the contrary, Rayman [12] reported that conversion of LSG to OAGB, compared to RYGB, resulted in increased weight loss with a higher rate of GERD and potential nutritional deficiencies. Instead, Felsenreich et al. [11] have recently concluded that with regard to the fact that OAGB has a low potential to cure patients from GERD symptoms after SG, RYGB is probably the best option for patients post-LSG reflux. Rheinwalt [13] also found comparable results with significantly shorter operation times for OAGB. After a follow-up of 5 years, Wilczyński[14] reported a significant remission of T2DM after OAGB when compared to RYGB after LSG. Hany et al. [15] have performed the only available controlled trial demonstrating that after 2 years, both revisional RYGB and OAGB have comparable metabolic outcomes.
Our analysis has demonstrated a low-to-moderate heterogeneity among these studies with a high-quality methodology. Weight loss as TWL%, EWL%, or %EBMIL and rates of early complications (leak, bleeding) were reported in all the papers. Regardless of the used parameter, the mean weight loss after one-anastomosis gastric bypass was higher than after RYGB in all but one of the included articles; thus, the present meta-analysis confirmed the inferiority of RYGB in terms of weight loss. Only Rheinwalt [13] found that the two interventions induced comparable weight loss probably for the long biliopancreatic limb of the RYGB in this study.
Low rates of early complications (leak, bleeding) found in all the collected papers demonstrated the feasibility and safety of revisional surgery after LSG.
Regarding long-term complications, some authors have reported a higher occurrence of marginal ulcer (MU) after revisional surgery [30] especially due to the risk of retained gastric antrum syndrome (RGA) after conversion to gastric bypass [31, 32]. Conversely, in this systematic review, after a follow-up ranging from 12 to 60 months, the rate of MU was 1% both for RYGB and OAGB.
As expected, we found that a higher rate of patients with GERD after LSG was converted to RYGB rather than to OAGB, but remission from GERD was satisfactory and comparable after the two procedures. Even if de novo GERD occurred more frequently after revisional OAGB, new-onset reflux and Barrett’s disease were reported after both revisional interventions.
Strength and limitations
Although a meta-analysis [33] was recently published, the present includes two more papers (6 instead of 4) and focuses not only on weight loss but also on the safety (early complications) and on GERD symptoms after revision. The main limitation is that GERD was assessed through different diagnostic methods with a lack of information on severity of GERD, presence and size of eventual hiatal hernia, and degree of esophagitis. Moreover, several revisional procedures were performed together with a concomitant hiatoplasty, which may have influenced the results on reflux. This is particularly interesting for the treatment of patients with severe obesity suffering from GERD and/or hiatal hernia (HH). Even if from 22 to 37% of class three obesity patients have a hiatal hernia (HH) [34], these defects are preoperatively underdiagnosed or not repaired intraoperatively. Conversely, studies with long-term results have demonstrated that SG plus hiatal hernia repair (HHR) induces symptoms relief up to 60% of patients [35]. Considering that GERD itself is a major issue before and after SG, HHR should be considered mandatory for those with severe obesity and GERD undergoing sleeve gastrectomy. Eventually, we must acknowledge that weight loss is mostly influenced by the length of the biliopancreatic limb; therefore, future studies comparing OAGB and RYGB after LSG should take into account the bypassed lengths of small bowel.
Conclusion
Conversion from LSG to RYGB or OAGB is feasible and safe with a low rate of postoperative complications.
Despite weight loss is satisfactory after both procedures, OAGB provides better results. Remission from GERD is higher after RYGB but without statistical significance.
Without knowing the applied bypass length in most of the analyzed studies, OAGB might be a better option for failed LSG, while RYGB still should be preferred in case of severe GERD.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
A.V., R.P., and G.B. did the research and wrote the manuscript. P.C. helped with the research. V.P. reviewed the text.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Data availability
All data are available online since this is a systematic review of published studies.
Declarations
Ethics approval
This report does not describe any study with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angrisani L, Santonicola A, Iovino P, et al. Erratum to: Bariatric surgery and endoluminal procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27(9):2290–2292. doi: 10.1007/s11695-017-2773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felsenreich DM, Langer FB, Kefurt R, et al. Weight loss, weight regain, and conversions to Roux-en-Y gastric bypass: 10-year results of laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2016;12(9):1655–1662. doi: 10.1016/j.soard.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 3.Genco A, Soricelli E, Casella G, et al. Gastroesophageal reflux disease and Barrett’s esophagus after laparoscopic sleeve gastrectomy: a possible, underestimated long-term complication. Surg Obes Relat Dis. 2017;13(4):568–574. doi: 10.1016/j.soard.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, sleeve dilation, and Barrett’s esophagus after laparoscopic sleeve gastrectomy: long-term follow-up. Obes Surg. 2017;27(12):3092–3101. doi: 10.1007/s11695-017-2748-9. [DOI] [PubMed] [Google Scholar]
- 5.Iannelli A, Debs T, Martini F, Benichou B, Ben Amor I, Gugenheim J. Laparoscopic conversion of sleeve gastrectomy to Roux-en-Y gastric bypass: indications and preliminary results. Surg Obes Relat Dis. 2016;8:1533–1538. doi: 10.1016/j.soard.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Parmar CD, Mahawar KK, Boyle M. Conversion of sleeve gastrectomy to Roux-en-Y gastric bypass is effective for gastro-oesophageal reflux disease but not for further weight loss. Obes Surg. 2017;27(7):1651–1658. doi: 10.1007/s11695-017-2542-8. [DOI] [PubMed] [Google Scholar]
- 7.Kermansaravi M, Shahmiri SS, DavarpanahJazi AH, et al. One anastomosis/mini-gastric bypass (OAGB/MGB) as revisional surgery following primary restrictive bariatric procedures: a systematic review and meta-analysis. Obes Surg. 2021;1:370–383. doi: 10.1007/s11695-020-05079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ Br Med J. 2015;349:7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Felsenreich DM, Steinlechner K, Langer FB, et al. Outcome of sleeve gastrectomy converted to Roux-en-Y gastric bypass and one-anastomosis gastric bypass. Obes Surg. 2022;32(3):643–651. doi: 10.1007/s11695-021-05866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiappetta S, Stier C, Scheffel O, et al. Mini/one anastomosis gastric bypass versus Roux-en-Y gastric bypass as a second step procedure after sleeve gastrectomy-a retrospective cohort study. Obes Surg. 2019;29(3):819–827. doi: 10.1007/s11695-018-03629-y. [DOI] [PubMed] [Google Scholar]
- 12.Rayman S, Assaf D, Azran C, et al. Sleeve gastrectomy failure-revision to laparoscopic one-anastomosis gastric bypass or Roux-n-Y gastric bypass: a multicenter study. Obes Surg. 2021;31(7):2927–2934. doi: 10.1007/s11695-021-05334-9. [DOI] [PubMed] [Google Scholar]
- 13.Rheinwalt KP, Schipper S, Plamper A, et al. Roux-en-Y versus one anastomosis gastric bypass as redo-operations following sleeve gastrectomy: a retrospective study. World J Surg. 2022;46(4):855–864. doi: 10.1007/s00268-021-06424-6. [DOI] [PubMed] [Google Scholar]
- 14.Wilczyński M, Spychalski P, Proczko-Stepaniak M, et al. Comparison of the long-term outcomes of RYGB and OAGB as conversion procedures after failed LSG — a case–control study. J Gastrointest Surg. 2022;26:2255–2265. doi: 10.1007/s11605-022-05395-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hany M, Zidan A, Elmongui E, et al. Revisional Roux-en-Y gastric bypass versus revisional one-anastomosis gastric bypass after failed sleeve gastrectomy: a randomized controlled trial. Obes Surg. 2022;11:3491–3503. doi: 10.1007/s11695-022-06266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auricchio P, Tanay E, Kieninger C, et al. Re-do surgery after sleeve gastrectomy: a single center comparison between Roux-en-Y gastric bypass and one anastomosis gastric bypass. Surgeries. 2022;3(2):126–133. doi: 10.3390/surgeries3020014. [DOI] [Google Scholar]
- 17.Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic diversion with a new type of gastrectomy. Obes Surg. 1993;3(1):29–35. doi: 10.1381/096089293765559728. [DOI] [PubMed] [Google Scholar]
- 18.Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13(6):861–864. doi: 10.1381/096089203322618669. [DOI] [PubMed] [Google Scholar]
- 19.Gumbs AA, Gagner M, Dakin G, Pomp A. Sleeve gastrectomy for morbid obesity. Obes Surg. 2007;17(7):962–969. doi: 10.1007/s11695-007-9151-x. [DOI] [PubMed] [Google Scholar]
- 20.Leyba JL, Aulestia SN, Llopis SN. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the treatment of morbid obesity. A prospective study of 117 patients. Obes Surg. 2011;2:212–6. doi: 10.1007/s11695-010-0279-8. [DOI] [PubMed] [Google Scholar]
- 21.Hirth DA, Jones EL, Rothchild KB, Mitchell BC, Schoen JA. Laparoscopic sleeve gastrectomy: long-term weight loss outcomes. Surg Obes Relat Dis. 2015;11(5):1004–7. doi: 10.1016/j.soard.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Jain M, Tantia O, Goyal G, Chaudhuri T, Khanna S, Poddar A, Majumdar K, Gupta S. LSG vs MGB-OAGB: 5-year follow-up data and comparative outcome of the two procedures over long term-results of a randomised control trial. Obes Surg. 2021;31(3):1223–1232. doi: 10.1007/s11695-020-05119-6. [DOI] [PubMed] [Google Scholar]
- 23.Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: the SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musella M, Berardi G, Velotti N, et al. Ten-year results of laparoscopic sleeve gastrectomy: retrospective matched comparison with laparoscopic adjustable gastric banding-is there a significant difference in long term? Obes Surg. 2021;12:5267–5274. doi: 10.1007/s11695-021-05735-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitiello A, Berardi G, Velotti N, et al. Should sleeve gastrectomy be considered only as a first step in super obese patients? 5-year results from a single center. Surg Laparosc Endosc Percutan Tech. 2020;31(2):203–207. doi: 10.1097/SLE.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 26.Milone M, Di Minno MND, Lupoli R, et al. Wernicke encephalopathy in subjects undergoing restrictive weight loss surgery: a systematic review of literature data. Eur Eat Disorders Rev. 2014;22:223–229. doi: 10.1002/erv.2292. [DOI] [PubMed] [Google Scholar]
- 27.Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250–267. doi: 10.1016/j.amjsurg.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 28.Yeung KTD, Penney N, Ashrafian L, Darzi A, Ashrafian H. Does Sleeve Gastrectomy Expose the Distal Esophagus to Severe Reflux? A Systematic Review and Meta-analysis. Ann Surg. 2020;271(2):257–265. doi: 10.1097/SLA.0000000000003275. [DOI] [PubMed] [Google Scholar]
- 29.Parmar CD, Bryant C, Luque-de-Leon E, et al. One anastomosis gastric bypass in morbidly obese patients with BMI ≥ 50 kg/m2: a systematic review comparing it with Roux-En-Y gastric bypass and sleeve gastrectomy. OBES SURG. 2019;29:3039–3046. doi: 10.1007/s11695-019-04034-9. [DOI] [PubMed] [Google Scholar]
- 30.Clapp B, Hahn J, Dodoo C, et al. Evaluation of the rate of marginal ulcer formation after bariatric surgery using the MBSAQIP database. Surg Endosc. 2019;33(6):1890–1897. doi: 10.1007/s00464-018-6468-6. [DOI] [PubMed] [Google Scholar]
- 31.Gibril F, Lindeman RJ, Abou-Saif A, et al. Case report: retained gastric antrum syndrome. Dig Dis Sci. 2001;46:610–617. doi: 10.1023/A:1005667719847. [DOI] [PubMed] [Google Scholar]
- 32.Abou Hussein B, Al Marzouqi O, Khammas A. Anastomotic gastro-jejunal ulcer perforation following one anastomosis gastric bypass: clinical presentation and options of management—case series and review of literature. OBES SURG. 2020;30:2423–2428. doi: 10.1007/s11695-020-04423-5. [DOI] [PubMed] [Google Scholar]
- 33.Dantas ACB, Branco LT, Tustumi F, de Oliveira DRCF, Pajecki D, Santo MA. One-anastomosis gastric bypass versus Roux-en-Y gastric bypass as revisional surgery after sleeve gastrectomy: a systematic review and meta-analysis. Obes Surg. 2022;32(12):4082–4088. doi: 10.1007/s11695-022-06326-z. [DOI] [PubMed] [Google Scholar]
- 34.Sakata S, Grove PM, Stevenson AR. Effect of 3-dimensional vision on surgeons using the da vinci robot for laparoscopy: more than meets the eye. JAMA Surg. 2016;151:793–794. doi: 10.1001/jamasurg.2016.0412. [DOI] [PubMed] [Google Scholar]
- 35.Angrisani L, Santonicola A, Borrelli V, et al. Sleeve gastrectomy with concomitant hiatal hernia repair in obese patients: long-term results on gastroesophageal reflux disease. Surg Obes Relat Dis. 2020;16(9):1171–1177. doi: 10.1016/j.soard.2020.04.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available online since this is a systematic review of published studies.