Abstract
A coupled oxygen evolution mechanism (COM) during oxygen evolution reaction (OER) has been reported in nickel oxyhydroxides (NiOOH)-based materials by realizing eg* band (3d electron states with eg symmetry) broadening and light irradiation. However, the link between the eg* band broadening extent and COM-based OER activities remains unclear. Here, Ni1-xFexOOH (x = 0, 0.05, 0,2) are prepared to investigate the underlying mechanism governing COM-based activities. It is revealed that in low potential region, realizing stronger eg* band broadening could facilitate the *OH deprotonation. Meanwhile, in high potential region where the photon utilization is the rate-determining step, a stronger eg* band broadening would widen the non-overlapping region between dz2 and a1g* orbitals, thereby enhancing photon utilization efficiency. Consequently, a stronger eg* band broadening could effectuate more efficient OER activities. Moreover, we demonstrate the universality of this concept by extending it to reconstruction-derived X-NiOOH (X = NiS2, NiSe2, Ni4P5) with varying extent of eg* band broadening. Such an understanding of the COM would provide valuable guidance for the future development of highly efficient OER electrocatalysts.
Subject terms: Electrocatalysis, Electrocatalysis
Coupled oxygen evolution mechanism provides an optimized electron transfer route during oxygen evolution process, but the underlying structure-activity relationship is still unclear. Here the authors investigate the role of eg* band broadening on the coupled oxygen evolution mechanism-based activities for Fe-doped NiOOH materials.
Introduction
Electrochemical water splitting, encompassing the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), constitutes a pivotal technology for addressing the intermittency issues related to renewable energy sources such as solar, wind and tidal power1–4. Among the two reactions, OER is regarded as the bottleneck due to its slow kinetics associated with multiple electron transfer steps and represents a key determinant of the overall energy conversion efficiency into chemical fuels. Thus, the development of high-performance OER electrocatalysts is of paramount importance to advance the field of sustainable energy conversion5–8. Currently, there are two widely recognized mechanisms for the OER process, i.e., the adsorbate evolution mechanism (AEM), a metal redox reaction, and the lattice oxygen oxidation mechanism (LOM), which involves oxygen redox chemistry9–12. The AEM pathway typically involves the rate-determining step (RDS) of *OOH formation, whereas in the LOM pathway, the deprotonation step serves as the RDS13–15. The existence of the RDS poses a fundamental challenge to electron transfer efficiency during OER, impeding catalytic performance enhancement. As a result, substantial research efforts have been dedicated to optimizing OER reaction pathways in pursuit of highly efficient electrocatalysts16–19.
Previously, the concept of a coupled oxygen evolution mechanism (COM) was introduced, which featured alternative metal/oxygen redox activities occurring throughout the oxygen evolution process14. The initiation of the COM mechanism was reliant upon both light irradiation and the broadening of the eg* band in nickel oxyhydroxides (NiOOH)-based materials. In contrast to the traditional AEM pathway, the COM route involved direct O-O coupling at the oxygen states, bypassing the RDS step (i.e.*,OOH formation) in AEM. Also, the deprotonation process in the COM pathway involved proton transfer occurring at the metal bands, followed by light-induced electron transfer from the (M-O) orbitals to the dz2 orbital, resembling the proton transfer in AEM deprotonation. This potentially indicated a lower energy requirement for deprotonation in COM compared to LOM. As a result, the presented electron transfer mechanism proceeded through an optimized pathway, where deprotonation occurred at the metal bands and O-O coupling took place at the oxygen states. Therefore, the COM pathway offered promising avenues for developing OER electrocatalysts with more efficient catalytic performance. However, the relationship between the extent of eg* band broadening and the resulting OER activities enhancement is not yet fully understood, particularly in terms of how the eg* band broadening influences the RDS in the COM route. These significantly impedes researchers from further optimizing the OER activities via the COM pathway.
Herein, Ni1-xFexOOH (x = 0, 0.05, 0,2) are synthesized as model materials to investigate the underlying mechanism governing the COM-based OER activities. It is revealed that Fe dopants could effectively tune the NiO6 distortion in NiOOH, resulting in varying degrees of the eg* band broadening. In the low potential region where *OH deprotonation serves as the RDS, increasing the extent of eg* band broadening could significantly facilitate the *OH deprotonation. Meanwhile, in the high potential region where light absorption becomes the RDS, a stronger eg* band broadening would lead to a wider non-overlapping region between dz2 and a1g* orbitals. This greatly facilitates photon-induced electron transfer from (M-O) to empty dz2 orbital, leading to enhanced photon utilization efficiency. Consequently, the catalyst with higher extent of eg* band broadening exhibits greater enhancement in OER activity under the COM route. Moreover, we observe a consistent relationship between the extent of eg* band broadening and COM-based OER activity in the X-NiOOH (X = NiS2, NiSe2, Ni5P4) system, where strain effects modulate eg* band broadening, highlighting the universality of this concept across diverse materials. The insights gained from this study on COM could offer valuable guidance for the development of efficient OER electrocatalysts, thus promoting the advancement of energy conversion technologies.
Results
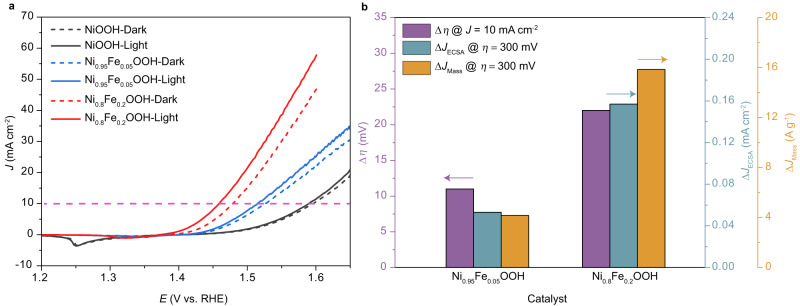
Identify COM pathway contributed OER activity promotion
It was revealed that 5% and 20% Fe could be successfully doped into the Ni(OH)2 lattice20. More content of Fe dopants i.e., 40%, 60%, 100% would lead to the aggregation of Fe cations to form Fe2O3 (Supplementary Fig. 1). Since Fe2O3 is a well-studied photocatalyst, its presence might influence the electrochemical results based on the COM. Hence, to focus on investigating the impact of Fe dopants-induced eg* band broadening on the COM, the Ni1-xFexOOH (x = 0, 0.05, 0,2) are chosen as model samples. Fe-removed 1 M KOH electrolyte is employed to minimize the influence of Fe impurity on electrochemical measurements, (the experimental process for Fe removal is described in the Methods section)21. The pH value of the purified 1 M KOH is measured to be 13.65 and the error bars represent mean ± standard error (Supplementary Fig. 2). Figure 1a shows the linear sweep voltammetry (LSV) curves of Ni1-xFexOOH (x = 0, 0.05, 0,2) under both light and dark conditions. Negative scan is conducted for the LSV measurement to avoid the influence of Ni2+/3+ redox current on the OER activity. Hence the negative current peak due to the redox from Ni3+ to Ni2+ are observed for Ni1-xFexOOH (x = 0, 0.05, 0,2) (Fig. 1a and Supplementary Fig. 3). Further detailed methodology for measuring the electrochemical activities is provided in the Methods section. Notably, Ni0.8Fe0.2OOH exhibits the highest OER activity under dark condition, followed by Ni0.95Fe0.05OOH with the second-highest activity and NiOOH with the lowest activity. For NiOOH under light irradiation, negligible current density variation is observed, which agrees well with our previous research14. Interestingly, a significant increase in current density can be observed for Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH under light condition. The overpotential drops for Ni0.8Fe0.2OOH and Ni0.95Fe0.05OOH at 10 mA cm−2 between dark and light conditions are 22 mV and 11 mV, respectively (Fig.1b). To further investigate the contribution of COM to the enhancement of OER performance, the intrinsic activities of Ni1-xFexOOH (x = 0.05, 0,2) are provided with the current density normalized to electrochemical surface area (ECSA, Supplementary Fig. 4) and loading mass. As shown in Fig.1b, the ECSA-normalized current density difference between light and dark conditions is 0.157 mA cm−2 for Ni0.8Fe0.2OOH and 0.053 mA cm−2 for Ni0.95Fe0.05OOH at an overpotential of 300 mV. Meanwhile, the loading mass-normalized current density improvement is 15.846 A g−1 for Ni0.8Fe0.2OOH and 4.159 A g−1 for Ni0.95Fe0.05OOH, respectively. Based on these results, it can be concluded that the intrinsic OER activity increment of Ni0.8Fe0.2OOH under the COM route is greater than that of Ni0.95Fe0.05OOH.
Fig. 1. Electrochemical characterization of Ni1-xFexOOH (x = 0, 0.05, 0.2) for the OER under dark and light condition.
a LSV polarization curves of Ni1-xFexOOH (x = 0, 0.05, 0.2) based on a backward scan conducted at a scan rate of 0.1 mV s−1 under dark (dash line) and light (solid line) condition (without iR-correction). b Comparison of the overpotential drop () at a current density (J) of 10 mA cm−2 (purple color), the current density improvement normalized to ECSA () at an overpotential of 300 mV (cyan color), and the current density improvement normalized to loading mass () at an overpotential of 300 mV (orange color), respectively for Ni1-xFexOOH (x = 0.05, 0.2).
In our previous investigation, we have established that the COM predominantly governs the electrochemical process in low potential region, where there is an ample supply of photons to initiate electron transfer from (M-O) to dz2, prompting geometric transition from NiO6 octahedra to NiO4 square planar structures14. In high potential region, the limited photon utilization would hinder further contribution of COM to the OER activity, thus serving as the new RDS (Fig. 2a). Therefore, it is of great importance to firstly investigate the two possible RDS in low potential region and high potential region, respectively. The deprotonation of *OH in the COM route closely parallels that in the AEM route, with the primary distinction being the absence of electron transfer during the deprotonation step in the COM pathway14. As such, the *OH deprotonation ability in the AEM route could be used to estimate that in the COM route. Therefore, pulse-voltammetry measurements are conducted to investigate the *OH deprotonation ability of Ni1-xFexOOH (x = 0.05, 0,2) within the low potential range (Supplementary Fig. 5, detailed protocol is described in the Methods section). As shown in Fig. 2b, the total stored charge QECSA varies linearly with the applied potential for both Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH. During OER process, the oxidative charge is accumulated on the surface of catalysts via the deprotonation step22. Hence, the slopes of these fitted lines are calculated to elucidate the rate of oxidative charge to the potential, thereby reflecting the *OH deprotonation ability20. The slope value for Ni0.8Fe0.2OOH is 24.257, which is significantly higher than the slope value of 7.450 for Ni0.95Fe0.05OOH. This discrepancy suggests that the *OH deprotonation ability of Ni0.8Fe0.2OOH in the COM route is much stronger than that of Ni0.95Fe0.05OOH.
Fig. 2. Analysis of *OH deprotonation and photon utilization efficiency for Ni1-xFexOOH (x = 0.05, 0.2).
a Schematic illustration of the rate-determining step in low potential region and high potential region under COM route. b Charge versus potential for Ni1-xFexOOH (x = 0.05, 0.2) from pulse-voltammetry (without iR-correction). c Tafel slope for Ni1-xFexOOH (x = 0.05, 0.2) in high potential region. d Photon utilization efficiency for Ni1-xFexOOH (x = 0.05, 0.2) at 1.57 V vs. RHE.
Subsequently, we delve into the analysis of photon utilization efficiency. Within the high potential range, the photon utilization to trigger the conversion from NiO6 octahedron to NiO4 square planar would gradually become the RDS. In this work, a simulated solar source matching AM 1.5 G was used as the light source with a light irradiation intensity of 100 mW cm−2. As the potential increases, the COM would become limited due to insufficient photon, leading to the co-existence of COM and AEM route during OER process. Hence, further increasing the applied potential, the promotion of OER activity would be mainly contributed by AEM rather than COM. This could be understood from Tafel slope analysis for Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH in the high potential region, revealing identical slope values under both light and dark conditions (Fig. 2c). Based on the COM route, two photons would participate into the evolution of one oxygen molecule, concomitant with the transfer of four electrons to the external circuit. Therefore, we could estimate the number of participated photons based on the enhanced OER current between light and dark conditions in the high potential region. The photon utilization efficiency hence can be derived by normalizing the number of participated photons to the whole number of photons under 100 mW cm−2 light irradiation (Detailed calculation information is provided in the Methods section and Supplementary Fig. 6). Here, the photon utilization efficiency for Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH is compared at the potential of 1.57 V vs. RHE. The calculated photon utilization efficiency is 0.584% for Ni0.8Fe0.2OOH, which is nearly 3.3 times than that of Ni0.95Fe0.05OOH, showing an efficiency of 0.175% (Fig. 2d).
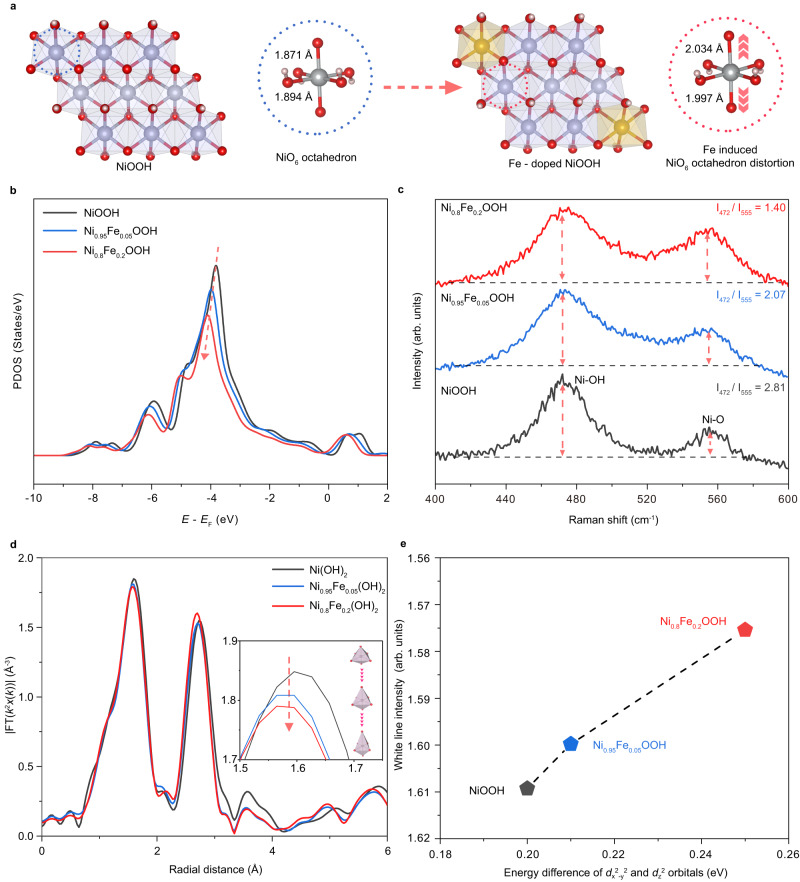
Different eg* band broadening extent in Fe-doped NiOOH system
The extent of eg* band broadening in Ni1-xFexOOH (x = 0, 0.05, 0,2) is discussed utilizing a suite of density functional theory (DFT) calculations, Raman spectroscopy, and extended X-ray absorption fine structure (FT-EXAFS) results. Firstly, the DFT calculation is conducted using Ni1-xFexOOH (x = 0, 0.05, 0,2) as computational models (Supplementary Fig. 7) to systematically investigate the effect of Fe dopants on tuning NiO6 distortion and eg* band broadening. Upon introducing Fe into NiOOH (extract from Ni0.8Fe0.2OOH model, Supplementary Fig. 7), noticeable extensions of Ni-O bonds along the z-axis are observed, with values increasing from 1.871 Å to 2.034 Å and from 1.894 Å to 1.997 Å, respectively, as compared to NiOOH (Fig. 3a). Then, the eg* band partial density of states (PDOS) of Ni1-xFexOOH (x = 0, 0.05, 0,2) is calculated. As shown in the Fig. 3b, the eg* band PDOS peak becomes broader with lower intensity from NiOOH, Ni0.95Fe0.05OOH, to Ni0.8Fe0.2OOH. This trend indicates a stronger eg* band broadening associated with higher concentrations of Fe dopants. Raman spectroscopy of Ni1-xFexOOH (x = 0, 0.05, 0,2) shows two typical peaks at 472 cm−1 and 555 cm−1 (Fig. 3c). Notably, the intensity (I) ratio between these two peaks can be used to identify the crystal structure of NiOOH23. The measured I472/I550 value here is 2.81 for NiOOH, 2.07 for Ni0.95Fe0.05OOH, and 1.40 for Ni0.8Fe0.2OOH, respectively, indicating a significantly increased level of disorder in the crystal structure of NiOOH with higher Fe dopants concentration. Furthermore, FT-EXAFS analysis demonstrates a reduction in the intensity of the Ni-O bond peak from Ni(OH)2, Ni0.95Fe0.05(OH)2, to Ni0.8Fe0.2(OH)2 (Fig. 3d). The lower Ni-O bond peak intensity is corresponded to the increased extent of NiO6 octahedron distortion. Neither the peak intensity around 6 Å nor the Ni-Ni bond peak intensity show great difference, indicating that there is no significant particle size difference among these samples20. Hence, the structural difference among these Ni1-xFex(OH)2 (x = 0, 0.05, 0.2) should be ascribed to the doping effect instead of strain effect. Moreover, the energy difference between dx2-y2 and dz2, calculated based on the band center difference between dx2-y2 and dz2, is 0.21 eV, 0.22 eV, and 0.25 eV for NiOOH, Ni0.95Fe0.05OOH, and Ni0.8Fe0.2OOH, respectively (Supplementary Figs. 8–10). This provides strong evidence for the greater eg* band broadening with higher concentration of Fe dopants, which agrees well with our prior reported X-ray absorption spectroscopy (XAS) analysis (Fig. 3e)20. Hence, both experimental and theoretical results demonstrate that the introduction of Fe dopants into NiOOH induces varying degrees of NiO6 octahedron distortion, resulting in distinct levels of eg* band broadening around the Fermi level, with the order being Ni0.8Fe0.2OOH > Ni0.95Fe0.05OOH > NiOOH.
Fig. 3. Analysis of eg* band broadening extent of Ni1-xFexOOH (x = 0, 0.05, 0.2).
a Models of NiOOH with and without Fe dopant to show the Ni-O bond extension along z axis. b The calculated eg* band Ni partial density of states (PDOS) of Ni1-xFexOOH (x = 0, 0.05, 0,2). c Raman spectra of the Ni1-xFexOOH (x = 0, 0.05, 0,2) to compare the intensity ratio between peak at 472 cm−1and 555 cm−1 (I472/I555). d FT-EXAFS spectra of Ni K edges of Ni1-xFex(OH)2 (x = 0, 0.05, 0.2), inset showing the enlarged FT results within the range of 1.5 to 1.75 Å. e The correlation between the Ni K-edge white line intensity with the energy difference of dx2-y2 and dz2 orbitals for Ni1-xFexOOH (x = 0, 0.05, 0.2). It should be noted that the white line intensity of Ni1-xFex(OH)2 (x = 0, 0.05, 0.2) extracted from Fig. 5a in our previous work20 is used to evaluate the eg* band broadening for Ni1-xFexOOH (x = 0, 0.05, 0.2) to avoid the possible effects on the XAS results caused by the self-discharge of Ni3+31.
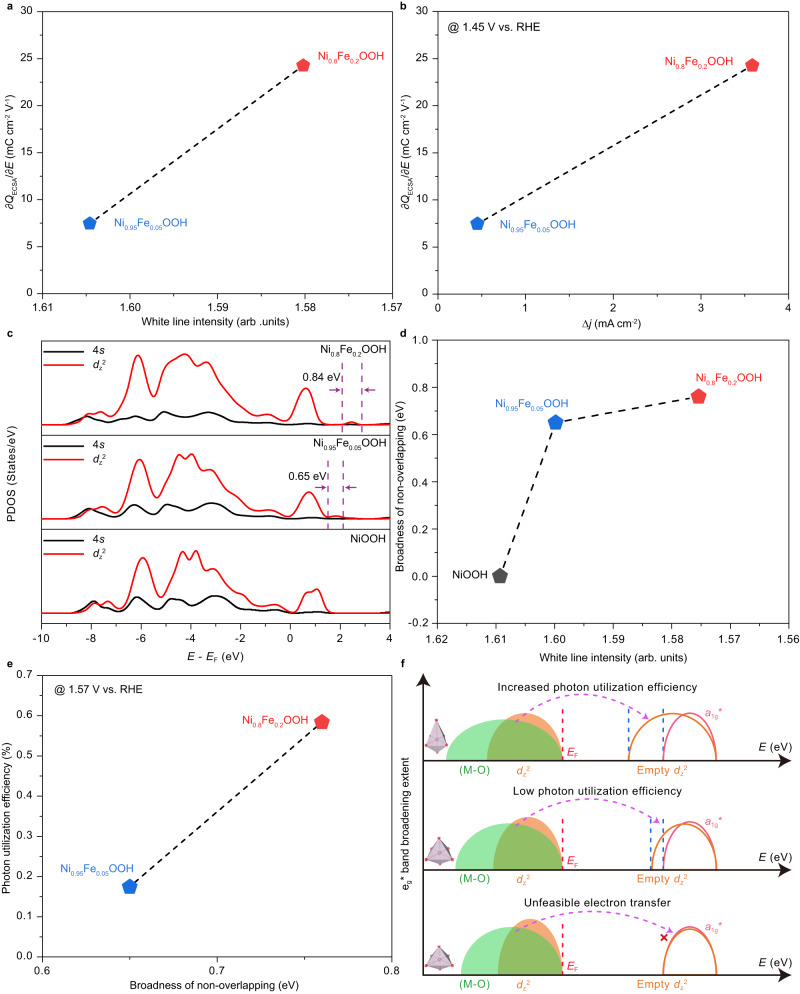
Effects of eg* band broadening on COM
As demonstrated, the main structural distinction among Ni1-xFexOOH (x = 0.05, 0.2) lies in the degree of NiO6 octahedron distortion, leading to varying degrees of eg* band broadening. Moreover, both Ni K-edge and L-edge XAS measurements show that there is negligible structural difference for the samples after OER under dark or light conditions, confirming the reversibility of the electrode surface during the COM-based OER process (Supplementary Fig. 11). In this section, the effect of eg* band broadening on the *OH deprotonation in Ni1-xFexOOH (x = 0.05, 0.2) is investigated. Here, the slope obtained from pulse-voltammetry measurement serves as a representative metric for *OH deprotonation ability, while the white line intensity extracted from Ni K-edge spectra is employed to indicate the degree of eg* band broadening20. As shown in Fig. 4a, the Ni0.8Fe0.2OOH with stronger eg* band broadening shows enhanced *OH deprotonation ability compared to the Ni0.95Fe0.05OOH. Meanwhile, the calculated reaction free energies of the *OH deprotonation process for Ni0.8Fe0.2OOH following the COM route is also lower than that of the Ni0.95Fe0.05OOH, which is consistent with the pulse-voltammetry results (Supplementary Fig. 12). The enhanced proton transfer ability ultimately results in a substantial improvement in the OER activity under light irradiation within the low potential region, where the *OH deprotonation serves as the RDS (Fig. 4b).
Fig. 4. Effects of eg* band broadening on COM.
a The correlation between the Ni K-edge white line intensity with the *OH deprotonation ability of Ni1-xFexOOH (x = 0.05, 0.2). b The correlation between the *OH deprotonation ability with the COM-contributed current density improvement of Ni1-xFexOOH (x = 0.05, 0.2) under light condition at 1.45 V vs. RHE. c The calculated Ni PDOS of 4 s and dz2 orbitals of Ni1-xFexOOH (x = 0, 0.05, 0.2). d The correlation between the broadness of non-overlapping region and the Ni K-edge white line intensity of Ni1-xFexOOH (x = 0, 0.05, 0.2). e The correlation between the broadness of non-overlapping region and the photon utilization efficiency of Ni1-xFexOOH (x = 0.05, 0.2). f Schematic illustration of the effects of eg* band broadening on facilitating the electron transfer from (M-O) to empty dz2 to increase the photon utilization efficiency under COM route.
With the applied potential increased, the COM-based OER activity would be limited by the photon utilization efficiency. Our prior research demonstrated that the non-overlapping region between dz2 and a1g* orbitals played a key role in the photon absorption process, which was greatly related to the eg* band broadening14. To unveil the underlying science of the extent of eg* band broadening and enhanced OER activities in the high potential region, the relationship between the eg* band broadening and the photon utilization efficiency is investigated. In theory, if the non-overlapping region between dz2 and a1g* orbitals is broader, it would make the electron transfer from (M-O) to dz2 easier under light irradiation. This, in turn, would result in a higher photon utilization efficiency. As shown in Fig. 4c, the dz2 orbital for NiOOH is completely overlapped with the a1g* orbital. Hence, electrons would be unfeasible to transfer from (M-O) to dz2. After doping Fe into NiOOH, a distinctive non-overlapping region emerges between dz2 and a1g* for both Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH, indicating the possible electron transfer under light irradiation. More importantly, the broadness of the non-overlapping region increases from 0.65 eV for Ni0.95Fe0.05OOH to 0.84 eV for Ni0.8Fe0.2OOH. The broadening of the non-overlapping region could be ascribed to the stronger extent of eg* band broadening induced by Fe dopants (Fig. 4d). Subsequently, the relationship between the broadness of nonoverlapping region and the corresponding photon utilization efficiency is analyzed. As shown in Fig. 4e, with the increase of broadness of the non-overlapping region, the photon utilization efficiency is significantly promoted from 0.175% to 0.584%. To further confirm such effect, LSV measurements of Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH under light with wavelengths of 575 nm, 475 nm, and 365 nm are performed. The Ni0.8Fe0.2OOH with stronger eg* band broadening exhibits higher current density promotion at 1.57 V vs. RHE between dark and light conditions at each wavelength compared to Ni0.95Fe0.05OOH (Supplementary Fig. 13). From a calculated perspective, the reaction free energy of the light-dominated step for Ni0.8Fe0.2OOH is 0.89 eV, much lower than that of the Ni0.95Fe0.05OOH (0.94 eV) (Supplementary Fig. 12). As such, these results prove that increasing the non-overlapping region via tuning eg* band broadening would facilitate the electron transfer from (M-O) to dz2 under light irradiation, leading to higher photon utilization efficiency (Fig. 4f).
Increasing eg* band broadening can promote participation of COM – a universal concept
Based on the above discussion, it is anticipated that increasing the extent of eg* band broadening in NiOOH-based materials could promote the participation of COM during OER, realizing higher OER activities. Our previous work revealed that the eg* band broadening could be induced by both cation dopants and strain effect20. Hence, to validate the proposed hypothesis, it is necessary to verify this in systems influenced by strain effects. Specifically, the reconstruction derived X-NiOOH (X = NiS2, NiSe2, and Ni5P4) samples are prepared through the electro-oxidation of NiS2/NiSe2/Ni5P4 at a current density of 10 mA cm−2 for 10 h, which would exhibit different extent of eg* band broadening due to the strain effect, with the following order: NiS2-NiOOH>NiSe2-NiOOH >Ni5P4-NiOOH >NiOOH20. Here, the OER activities of X-NiOOH (X = NiS2, NiSe2, Ni5P4) are investigated under both light and dark conditions. As shown in Fig. 5a, discernible discrepancies could be observed in the degree of activity enhancement among X-NiOOH (X = NiS2, NiSe2, Ni5P4) between light and dark conditions. NiS2-NiOOH displays the most substantial improvement in OER activity under light irradiation, with an overpotential drop of 23 mV at 10 mA cm−2. In comparison, NiSe2-NiOOH exhibits a lower degree of OER activity promotion, with a 15 mV reduction in overpotential, but a higher promotion compared to Ni5P4-NiOOH, which shows an 11 mV overpotential reduction. As such, a positive correlation between the extent of eg* band broadening and the enhanced OER activities is revealed (Fig. 5b). At the same time, both the *OH deprotonation ability and photon utilization efficiency of X-NiOOH (X = NiS2, NiSe2, Ni5P4) follow the same order: NiS2-NiOOH>NiSe2-NiOOH >Ni5P4-NiOOH (Fig. 5c, d, Supplementary Fig. 14). These results demonstrate the universal concept that increased eg* band broadening could simultaneously facilitate *OH deprotonation and promote photon utilization, resulting in highly efficient COM-based OER activity.
Fig. 5. Effects of increasing eg* band broadening extent on COM-based OER activity of X-NiOOH (X = NiS2, NiSe2, and Ni5P4).
a LSV polarization curves of X-NiOOH (X = NiS2, NiSe2, and Ni5P4) based on a backward scan conducted at a scan rate of 0.1 mV s−1.(without iR-correction) b The correlation between the COM contributed overpotential drop at 10 mA cm−2 and the Ni K-edge white line intensity of X-NiOOH (X = NiS2, NiSe2, and Ni5P4). c The correlation between the Ni K-edge white line intensity with the *OH deprotonation ability of NiOOH (X = NiS2, NiSe2, and Ni5P4). d The correlation between the Ni K-edge white line intensity and the photon utilization efficiency of X-NiOOH (X = NiS2, NiSe2, and Ni5P4). It should be noted that the white line intensity of X-Ni(OH)2 (X = NiS2, NiSe2, Ni5P4) is extracted from Fig. 3a in our previous work20 to evaluate the eg* band broadening for X-NiOOH to avoid the possible effects on the XAS results caused by the self-discharge of Ni3+31.
Discussion
In this work, Ni1-xFexOOH (x = 0, 0.05, 0.2) samples are prepared to investigate the role of eg* band broadening on enhancing the COM-based OER activity. The results demonstrate that a stronger eg* band broadening would not only facilitate *OH deprotonation, but also promote photon utilization efficiency by generating a broader non-overlapping region between dz2 and a1g*. As such, both of the two RDS in the COM route could be effectively alleviated by realizing stronger eg* band broadening, resulting in higher OER activity. Furthermore, the universality of this concept is demonstrated by revealing the same effect on promoting the COM-based OER activity in X-NiOOH (X = NiS2, NiSe2, Ni5P4) with different eg* band broadening extent. Previously, it was also unveiled that the stronger eg* band broadening would trigger the emergence of more electronic states around the Fermi level. This effect notably facilitated the electron transfer from electrocatalysts to external circuit via *OH deprotonation, resulting in higher OER activities (AEM-pathway)20. As for the LOM route, the deprotonation step is usually the RDS. The conceptual framework encompassing NiO6 distortion, eg* band broadening, and *OH deprotonation also holds the potential for application in the context of the LOM process, thereby enabling the realization of enhanced catalytic performance. Therefore, it is believed that increasing the degree of eg* band broadening could be a crucial factor on promoting the participation of COM under light irradiation and even provide valuable guidelines for future design of highly efficient OER catalysts following various reaction pathways.
Methods
Materials
Nickel nitrate hexahydrate (Ni(NO)3·6H2O), iron nitrate nonahydrate (Fe(NO3)3·9H2O), Urea, Sulfur, Selenium, Sodium phosphate monobasic were purchased from the Sigma-Aldrich. These chemicals were reagent grade and used as received without further purification.
Synthesis of the Ni1-xFex(OH)2 (x = 0, 0.05, 0,2)
The Ni1-xFex(OH)2 (x = 0, 0.05, 0,2) were grown on the carbon cloth by a facile hydrothermal treatment20. Before hydrothermal treatment, the carbon cloth was pre-treated under 500 °C for 1 h in the air condition and then dealt with Ultra-Violet Ozone device for 30 min to make it fully hydrophilic. Next, 2 mmol total amount of Ni(NO)3·6H2O and Fe(NO3)3·9H2O, 10 mmol urea were added into 35 mL deionized (DI) water and stirred for 15 min to form uniform solution. One piece of carbon cloth with size of 2 cm × 3 cm was immersed into the solution. Then, they were transferred into a 50 mL Teflon-lined stainless-steel autoclave and kept in oven at 120 °C for 10 h. The obtained Ni1-xFex(OH)2 (x = 0, 0.05, 0,2) was then washed by DI water and ethanol for at least three times, and further dried under 70 °C in air for 4 h.
Synthesis of the X-NiOOH (X = NiS2, NiSe2, Ni5P4)
The X-NiOOH (X = NiS2, NiSe2, Ni5P4) was obtained from the reconstruction of NiS2, NiSe2, and Ni5P4 via a 10 h chronopotentiometry treatment20. First, one piece of hydrothermal-derived Ni(OH)2 loaded carbon cloth was placed at the downstream position in crucible, while 400 mg sulfur/selenium/sodium phosphate monobasic power was placed at the upstream position. Then, the crucible was kept at 400 °C for 2 h under N2 atmosphere and then cooled down to temperature. After that, the synthesized NiS2/NiSe2/Ni5P4 was washed using DI water and ethanol for at least three times, and dried at 70 °C for 1 h. Next, the chronopotentiometry treatment was conducted under 10 mA cm−2 for 10 h to fully transfer the NiS2/NiSe2/Ni5P4 to X-NiOOH (X = NiS2, NiSe2, and Ni5P4).
Removal of Fe impurity
The 1 M KOH solution was purified to remove Fe impurity before electrochemical measurement. To be specific, 0.5 g Ni(NO)3·6H2O powder was added into 30 mL 1 M KOH solution, forming Ni(OH)2 precipitation. Next, the suspension was centrifuged at 7826 × g for 10 minutes. Then, the obtained Ni(OH)2 powder was added into 50 mL KOH solution and mechanically agitated for 10 min. After standing still for 24 h, the suspension was centrifuged at 7826 × g for 10 minutes, and the KOH supernatant was kept in a clean electrochemical cell for use. The pH value of the purified 1 M KOH is detected to be 13.65 and the error bars represent mean ± standard error (Supplementary Fig. 2).
Material characterizations
The Raman spectra were recorded by a Raman Spectrophotometer with an excitation wavelength of 514.4 nm. Nickel K-edge X-ray absorption fine structure (XAFS) spectra were recorded at the XAFCA beamline at the Singapore Synchrotron Light Source (SSLS) under transmission mode where the storage ring is running at 0.7 GeV with current nearly 200 mA24. The energy calibrations were finished by using standard Nickel foil. The k2-weighted Fourier transforms were conducted using the Hanning window function for the EXAFS results, with the k-range of 2.5–10.5 Å−1.
Electrochemical measurements
All the electrochemical measurements were performed using an electrochemical workstation (VPM3, BiO-logic Inc) in a three-electrode setup in 1 M KOH. The working electrode was the Ni1-xFexOOH (x = 0, 0.05, 0,2) and X-NiOOH (X = NiS2, NiSe2, Ni5P4). The Hg/HgO was chosen as the reference electrode. The Pt was used as the counter electrode. The linear scan voltammetry (LSV) was measured at scan rate 0.1 mV s−1. In the dark situation, all electrochemical measurements are conducted in a dark box. Under light condition, a solar simulator (NBeT Solar-500, 300 W) was used as the light source to match AM 1.5 G. The intensity of the light irradiated on the electrocatalyst was 100 mW cm−2 as calibrated by a light power meter (Newport 843-R). The experimental set-up is provided in Supplementary Fig. 15. Before evaluating the OER activity, all samples were subjected to chronopotentiometry measurements at a current density of 10 mA cm−2 under dark for 24 h to ensure the complete oxidation of Ni2+ to Ni3+.
Pulse-voltammetry measurement
One small piece of electrocatalysts on carbon cloth (0.2 cm2) after electrochemical oxidation was used for the pulse-voltammetry measurement. The potential was set firstly at a low potential (El = 1.40 V) for 80 s to stabilize the electrode surface. Then the potential was turned to a higher potential (Eh = 1.42 V) for 12 s and back to El for 12 s as one cycle. This cycle was repeated while increasing Eh from 1.42 V to 1.48 V versus RHE in 20 mV/step with constant El = 1.40 V. The transferred charge normalized to ECSA during each cycle was evaluated by integrating the current pulse/ECSA over time.
Electrochemically surface area
The electrochemically surface area (ECSA) data was evaluated from recording the electrochemical double-layer capacitance of the catalyst via cyclic voltammograms (CVs). Here, the potential range was set at 0.02–0.12 V (versus Hg/HgO) to avoid the Faradaic process. The CVs were conducted in the quiescent electrolyte with the potential swept across the set potential range with at 6 scan rates 5, 10, 20, 30, 40, 50 mV s−1. The charging current was plotted versus scan rate and a straight line could be derived with the slope value equalled to the double-layer capacitance (Cdl). The ECSA was obtained by dividing the Cdl to the specific capacitance Cs = 0.04 mF cm−2 25.
Photon utilization calculation
In COM route, two photons participated into one oxygen molecule evolution, which corresponded to four electrons. Hence, the utilized photon number was calculated by the equation:
| 1 |
in which J was the current density difference between light and dark conditions at 1.57 V vs. RHE, t was reaction time, S was surface area, e was elementary charge. The number of photons per second and surface unit for certain wavelength was calculated by
| 2 |
where I [W m−2 nm−1] was the irradiance for certain wavelength, which could be known from Supplementary Fig. 5a; EP was the photon energy, h was Planck constant, c was speed of light, λ was wavelength. The NP-λ spectra could therefore be obtained, as shown in Supplementary Fig. 5b. Integration of the NP-λ spectra yields a theoretical maximum photon flux N of 4.936 × 1022 photons m−2 s−1. Hence, the photon utilization efficiency was calculated as
| 3 |
Computational method
All calculations were conducted via the DFT with the generalized Perdew-Burke-Ernzerhof (PBE)26, and the projector augmented-wave (PAW)27 pseudopotential planewave method as implemented in the Vienna ab initio Simulation Package (VASP) code28. For the PAW pseudopotential, 3d84s2, 3d74s1, 2s22p4 and 1s1 were treated as valence electrons for Ni, Fe, O and H atoms, respectively. The bulk β-NiOOH structure was optimized within the local-spin-polarized density approximations (LSDA + U, Ueff = 5.3 eV for Ni and 5 eV for Fe, respectively) .The plane wave cutoff energy of 500 eV was set for all calculations and the energy and force convergence were set to 10−5 eV and 0.02 eV Å−1, respectively. Grimme’s DFT-D3(BJ) dispersion correction29 were used in all calculations. A 12 × 12 × 10 Monkhorst-Pack (MP) K-point grid was used for β-NiOOH unit cells geometry optimization calculations. The layered NiOOH structure was optimized using the same basic settings of the bulk β-NiOOH structure optimization except the k-point sampling (12 × 6 × 1). The optimized unit cell of layered NiOOH was expanded to 6 × 3 supercells (a = 17.556 Å and b = 17.739 Å) containing 72 Ni, 72 H and 144 O atoms with a vacuum thickness of 18 Å for Fe embedding systems. Based on our experimental results, two different Fe doping concentrations were considered in this study, they are Ni0.95Fe0.05OOH and Ni0.8Fe0.2OOH corresponding to computational models of Fe4Ni68 and Fe14Ni58, respectively (Supplementary Fig. 7, the CONTCAR of the optimized Ni1-xFexOOH (x = 0, 0.05, 0.2) models is provided in Supplementary Data 1). It should be noted that we constrained lattice constants and relaxed all atoms in Fe embedding systems simulations. For supercell case, only γ K-point was considered.
For COM route, the thermodynamic correction of Gibbs free energy was implemented by VASPKIT30 and the relative energy was calculated by:
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Research Foundation, Singapore, under its Competitive Research Programme (Award No.: NRF-CRP26-2021-0003) and Ministry of Education, Singapore, under its Academic Research Fund (AcRF) Tier 2 (Award No: MOE-T2EP501220010). The computational resource was provided by A*STAR Computational Resource Centre, Singapore (A*CRC) and the National Supercomputing Centre, Singapore (https://www.nscc.sg). Y-W. Z acknowledges the support from A*STAR-SERC-CRF Award.
Author contributions
H.Y.Z., X.P.W., S.B.X., J.Z., and J.M.X. conceived the idea. H.Y.Z. and X.P.W. performed synthesis and electrochemical measurement of the samples. H.Y.Z., X.P.W., J.C.Y, X.Z, H.A., Q.Z., Y.F.M., H.W., were responsible for the analysis of electrochemical results. S.B.X, C.W., C.Z.D., were responsible for the XAFS characterization. Y-W. Z., Z.G.Y., and Q.Z. carried out DFT simulations. J.M.X. is in charge of the overall project and preparation of the manuscript.
Peer review
Peer review information
Nature Communications thanks Junfeng Gao, Chuan Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All the data supporting of the finding of this study are included within the paper and its supporting files and are available from the corresponding authors on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhi Gen Yu, Email: yuzg@ihpc.a-star.edu.sg.
Shibo Xi, Email: xi_shibo@isce2.a-star.edu.sg.
Xiaopeng Wang, Email: msewxia@nus.edu.sg.
Junmin Xue, Email: msexuejm@nus.edu.sg.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43302-2.
References
- 1.Ren X, et al. Spin-polarized oxygen evolution reaction under magnetic field. Nat. Commun. 2021;12:2608. doi: 10.1038/s41467-021-22865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bak J, Yun TG, An J-S, Bae HB, Chung S-Y. Comparison of Fe-enhanced oxygen evolution electrocatalysis in amorphous and crystalline nickel oxides to evaluate the structural contribution. Energy Environ. Sci. 2022;15:610–620. doi: 10.1039/D1EE01826D. [DOI] [Google Scholar]
- 3.Gao L, Cui X, Sewell CD, Li J, Lin Z. Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction. Chem. Soc. Rev. 2021;50:8428–8469. doi: 10.1039/D0CS00962H. [DOI] [PubMed] [Google Scholar]
- 4.Song J, et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020;49:2196–2214. doi: 10.1039/C9CS00607A. [DOI] [PubMed] [Google Scholar]
- 5.Suntivich J, May KJ, Gasteiger HA, Goodenough JB, Shao-Horn Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science. 2011;334:1383–1385. doi: 10.1126/science.1212858. [DOI] [PubMed] [Google Scholar]
- 6.Zhao S, et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy. 2016;1:1–10. doi: 10.1038/nenergy.2016.184. [DOI] [Google Scholar]
- 7.Zhao J-W, et al. Key roles of surface Fe sites and Sr vacancies in the perovskite for an efficient oxygen evolution reaction via lattice oxygen oxidation. Energy Environ. Sci. 2022;15:3912–3922. doi: 10.1039/D2EE00264G. [DOI] [Google Scholar]
- 8.Peng X, et al. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy. 2020;78:105234. doi: 10.1016/j.nanoen.2020.105234. [DOI] [Google Scholar]
- 9.Wang X, Zhong H, Xi S, Lee WSV, Xue J. Understanding of Oxygen Redox in the Oxygen Evolution Reaction. Adv. Mater. 2022;34:2107956. doi: 10.1002/adma.202107956. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z-F, et al. Chemical and structural origin of lattice oxygen oxidation in Co–Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy. 2019;4:329–338. doi: 10.1038/s41560-019-0355-9. [DOI] [Google Scholar]
- 11.Wu T, et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2019;2:763–772. doi: 10.1038/s41929-019-0325-4. [DOI] [Google Scholar]
- 12.Grimaud A, Hong WT, Shao-Horn Y, Tarascon J-M. Anionic redox processes for electrochemical devices. Nat. Mater. 2016;15:121–126. doi: 10.1038/nmat4551. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Unraveling oxygen vacancy site mechanism of Rh-doped RuO2 catalyst for long-lasting acidic water oxidation. Nat. Commun. 2023;14:1412. doi: 10.1038/s41467-023-37008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, X. et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature, 611, 702–708 (2022). [DOI] [PubMed]
- 15.Grimaud A, et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017;9:457–465. doi: 10.1038/nchem.2695. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z-F, et al. Tuning of lattice oxygen reactivity and scaling relation to construct better oxygen evolution electrocatalyst. Nat. Commun. 2021;12:3992. doi: 10.1038/s41467-021-24182-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Zhang KH, Hofmann JP, Oropeza FE. The electronic structure of transition metal oxides for oxygen evolution reaction. J. Mater. Chem. A. 2021;9:19465–19488. doi: 10.1039/D1TA03732C. [DOI] [Google Scholar]
- 18.Zeng L, et al. Anti-dissolution Pt single site with Pt (OH)(O3)/Co (P) coordination for efficient alkaline water splitting electrolyzer. Nat. Commun. 2022;13:3822. doi: 10.1038/s41467-022-31406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heo, Y., Choi, S., Bak, J., Kim, H. S., Bae, H. B. & Chung, S. Y. Symmetry‐broken atom configurations at grain boundaries and oxygen evolution electrocatalysis in perovskite oxides. Advanced Energy Materials8, 1802481 (2018).
- 20.Zhong H, et al. Optimization of oxygen evolution activity by tuning e* g band broadening in nickel oxyhydroxide. Energy Environ. Sci. 2023;16:641–652. doi: 10.1039/D2EE03413A. [DOI] [Google Scholar]
- 21.Trotochaud L, Young SL, Ranney JK, Boettcher SW. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 2014;136:6744–6753. doi: 10.1021/ja502379c. [DOI] [PubMed] [Google Scholar]
- 22.Nong HN, et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature. 2020;587:408–413. doi: 10.1038/s41586-020-2908-2. [DOI] [PubMed] [Google Scholar]
- 23.Jiao S, et al. Accelerating oxygen evolution electrocatalysis of two-dimensional NiFe layered double hydroxide nanosheets via space-confined amorphization. Nanoscale. 2019;11:18894–18899. doi: 10.1039/C9NR07465A. [DOI] [PubMed] [Google Scholar]
- 24.Du Y, et al. XAFCA: a new XAFS beamline for catalysis research. J. Synchrotron Radiat. 2015;22:839–843. doi: 10.1107/S1600577515002854. [DOI] [PubMed] [Google Scholar]
- 25.McCrory CC, Jung S, Peters JC, Jaramillo TF. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013;135:16977–16987. doi: 10.1021/ja407115p. [DOI] [PubMed] [Google Scholar]
- 26.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 27.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 28.Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 29.Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011;32:1456–1465. doi: 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- 30.Wang V, Xu N, Liu J-C, Tang G, Geng W-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021;267:108033. doi: 10.1016/j.cpc.2021.108033. [DOI] [Google Scholar]
- 31.Wang X, et al. Strain stabilized nickel hydroxide nanoribbons for efficient water splitting. Energy Environ. Sci. 2020;13:229–237. doi: 10.1039/C9EE02565K. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All the data supporting of the finding of this study are included within the paper and its supporting files and are available from the corresponding authors on request.