Abstract
Despite the high prevalence of peripheral neuropathic pain (NP) conditions and significant progress in understanding its underlying mechanisms, the management of peripheral NP remains inadequate. Existing pharmacotherapies for NP act primarily on the central nervous system (CNS) and are often associated with CNS-related adverse effects, limiting their clinical effectiveness. Mounting preclinical evidence indicates that reducing the heightened activity in primary sensory neurons by targeting G-protein coupled receptors (GPCRs), without activating these receptors in the CNS, relieves pain without central adverse effects. This review focuses on recent advancements in GPCR-mediated peripheral pain relief and discusses strategies to advance the development of more effective and safer therapies for peripheral NP by shifting from traditional CNS modulatory approaches towards selective targeting of GPCRs on primary sensory neurons.
Keywords: G-protein coupled receptors, Neuropathic pain, Peripheral nervous system, Primary Sensory Neurons, Spontaneous discharge, Drug discovery
The quest for new therapies for peripheral neuropathic pain with less adverse effects
Neuropathic pain (NP) that results from injury or disease of the peripheral somatosensory nervous system, is associated with abnormal spontaneous and ectopic activity in primary sensory neurons (PSNs) and sensitization in the pain signaling pathways. These neuroplastic changes in both the peripheral and central nervous systems (PNS, CNS) result in pain even in the absence of external stimuli (spontaneous pain), and an amplified response to both noxious and innocuous stimuli (hyperalgesia and allodynia, respectively) (Box 1). Despite significant progress in understanding the cellular and molecular maladaptive changes in the nervous system that play critical roles in the mechanisms of NP, the translation of this knowledge to improved therapies has been disappointing. Current therapies for peripheral NP are limited in effectiveness and have significant CNS adverse effects, such as sedation, dizziness, fatigue, cognitive dysfunction, dependence and addiction. Additionally, there has not been a single new drug approval for any NP condition by the Food and Drug Administration (FDA) in the past decade. This growing unmet health-care need for improved, non-addictive drugs for the management of peripheral NP and the meager success rate in translating drugs with proven efficacy in pre-clinical models to new clinically effective drugs underscores the need for a paradigm shift in the quest for novel, effective and safe therapies for NP (https://www.bio.org/ia-reports) [1–3].
Box 1. Neuropathic Pain: Clinical presentation, mechanisms and pharmacological treatment.
Neuropathic pain (NP) results from injury or disease of the peripheral or central somatosensory nervous system. The prevalence of NP in the general population is estimated as 7–10% [85]. NP is traditionally classified based on the affected injury or disease location as peripheral or central NP, and further described by its etiology. Common causes of peripheral NP are diabetic neuropathy, lumbar or cervical nerve root compressions, nerve injuries, amputation and surgical procedures, cancer, chemotherapy, HIV, and vitamin deficiency. NP is characterized by ongoing pain, hyperalgesia, allodynia, and hyperpathia. Diagnosing NP and differentiating from nociceptive pain are critical as the treatment strategies vary. Although laboratory investigations and clinical neurophysiological testing may help identify the underlying etiology, NP remains primarily a clinical diagnosis. The pharmacological management of NP remains challenging as present therapies are only moderately effective in about 50% of patients, and are associated with several CNS-related adverse effects [86]. To maximize treatment efficacy and provide personalized therapies for patients with peripheral NP, attempts have been made to stratify patients according to their specific pain mechanisms [87,88]. Using quantitative sensory testing protocols in large cohorts of subjects with peripheral NP of varying etiologies, three broad phenotypes with characteristic sensory profiles have been identified [87,89,90]. These include: 1) Sensory loss cluster characterized by a loss of small and large fiber function (heat and pain, touch and pressure, respectively; deafferentation), 2) Thermal hyperalgesia cluster characterized by a preserved sensory function with thermal hyperalgesia indicating peripheral sensitization (irritable nociceptor), and 3) Mechanical hyperalgesia cluster exemplified by pinprick hyperalgesia and dynamic mechanical allodynia combined with a loss of small fiber function (central sensitization). Patient stratification and individualized therapeutic approaches, based on the sensory profiles and other characteristics, may help bridge the translation gap between bench and bedside [88]. The detailed battery of sensory tests performed in human phenotyping studies are not typically employed presently in preclinical studies. Thus, establishing a correlation between disease phenotypes in humans and those seen in preclinical models is challenging [91,92]. In addition, the determination of the pathophysiologic mechanism of pain for each patient is yet to be fully adopted in routine clinical practice. Furthermore, the response of various patient phenotypes to specific drug treatments remains underresearched. While preliminary evidence suggests that drugs targeting the PSNs are more effective in the irritable nociceptor phenotype of peripheral NP, further studies are needed to confirm these observations.
Traditionally, NP treatments have focused on targeting CNS sites. However, growing clinical evidence and studies using human sensory neurons indicate the critical involvement of PSNs in the maintenance of peripheral NP [4–6]. Importantly, the PSN has gained attention in selective analgesic drug discovery as various molecular targets that participate in pain generation (pro-nociceptive) and relief (anti-nociceptive) have been identified in recent years [7–10]. Targeting the PSNs offers several advantages over the conventional CNS-focused approach (Box 2). Peripheral targets modulate pain signals at their source, thereby potentially minimizing the diverse neurochemical, anatomical, and functional changes across the PNS and CNS. G-protein coupled receptors (GPCRs) present a range of therapeutic opportunities, given their role in modulating pain signals by facilitating the transduction and neural activity in PSNs and in regulating endogenous mechanisms of pain control (Figure 1, Box 3 [10–12]. Thus, it is important and timely to review GPCRs in PSNs that have been of recent interest as potential targets for drugs in the management of peripheral NP. The increased recognition that targeting GPCRs in PSNs may achieve pain relief with minimal adverse effects has also inspired innovations in drug development and delivery. Here, we highlight current novel neurophysiologic and gene expression studies that provide evidence for a critical role of the PSNs in peripheral NP. We discuss select emerging GPCRs with genes expressed in mouse and human sensory neurons and promising pre-clinical evidence for efficacy of drugs that target these receptors in PSNs. We also highlight innovations in peripheral analgesic drug development and delivery and present potential directions for future research.
Box 2. Targeting GPCRs in PSNs for NP treatment: Benefits.
GPCRs are among the most extensively researched drug targets due to their modulatory functions, roles in disease pathophysiology, and druggability. The success rate of GPCR-based therapeutics in clinical trials is also noteworthy (78%, 39%, and 29% for phase I, II, and III trials, respectively). Furthermore, nearly a third of FDA-approved drugs target GPCRs [93]. The activity of PSNs is regulated by a variety of molecular switches including ion channels, transducers, GPCRs, enzymes, and biomolecules. While ion channels and transducers are indispensable for action potential generation and neuronal excitability, drugs selectively targeting specific ion or transducer channels (e.g, NaV1.7, TRPV1) have failed in clinical pain trials due to lack of selectivity or efficacy, or because of CNS and cardiovascular adverse effects [94,95].
Targeting GPCRs on PSNs may offer several advantages for NP treatment. Firstly, unlike common ion channel blockers that primarily target one down-stream effector (e.g., sodium channels), activation of Gi-coupled GPCRs can modulate multiple ion channels (e.g., calcium channel inhibition, potassium channel opening) and can also interact with other GPCRs (e.g, hetero-dimerization, intracellular signaling cross-talk) [40,96,97]. Secondly, GPCRs may be more desirable targets for chronic pain treatment as their activation may have long-lasting effects, unlike the brief action of conventional ion channel blockers. Thirdly, activation of GPCRs can regulate a wide range of down-stream cell signaling cascades which may lead to changes in transcriptional, translational and post-translational changes (e.g., phosphorylation) of these ion channels and induce broad changes that may reduce neuronal excitability [98–100]. Fourthly, GPCR are also suitable for developing several new strategies for pain inhibition owing to their cellular distribution (cell surface, endosome), compartmentalized signaling, and diverse drug discovery pipelines (e.g., orthosteric agonist or allosteric modulator) [3,93,97,98]. For example, small molecule positive allosteric modulators can enhance the GPCR activation and fine-tune actions of endogenous ligands for neuronal inhibition [38,99]. Moreover, some GPCRs have preferential anatomical distribution in PSNs (eg, MrgprX1) for potential pain-selective treatment.
However, targeting GPCRs on PSNs is not without potential adverse effects and limitations. These receptors may be present in non-neuronal tissues, and also develop tolerance from desensitization. These challenges in analgesic drug discovery can be mitigated by adopting alternate innovative approaches (see discussion on “Innovations in drug design and delivery”) [37,71,93,101].
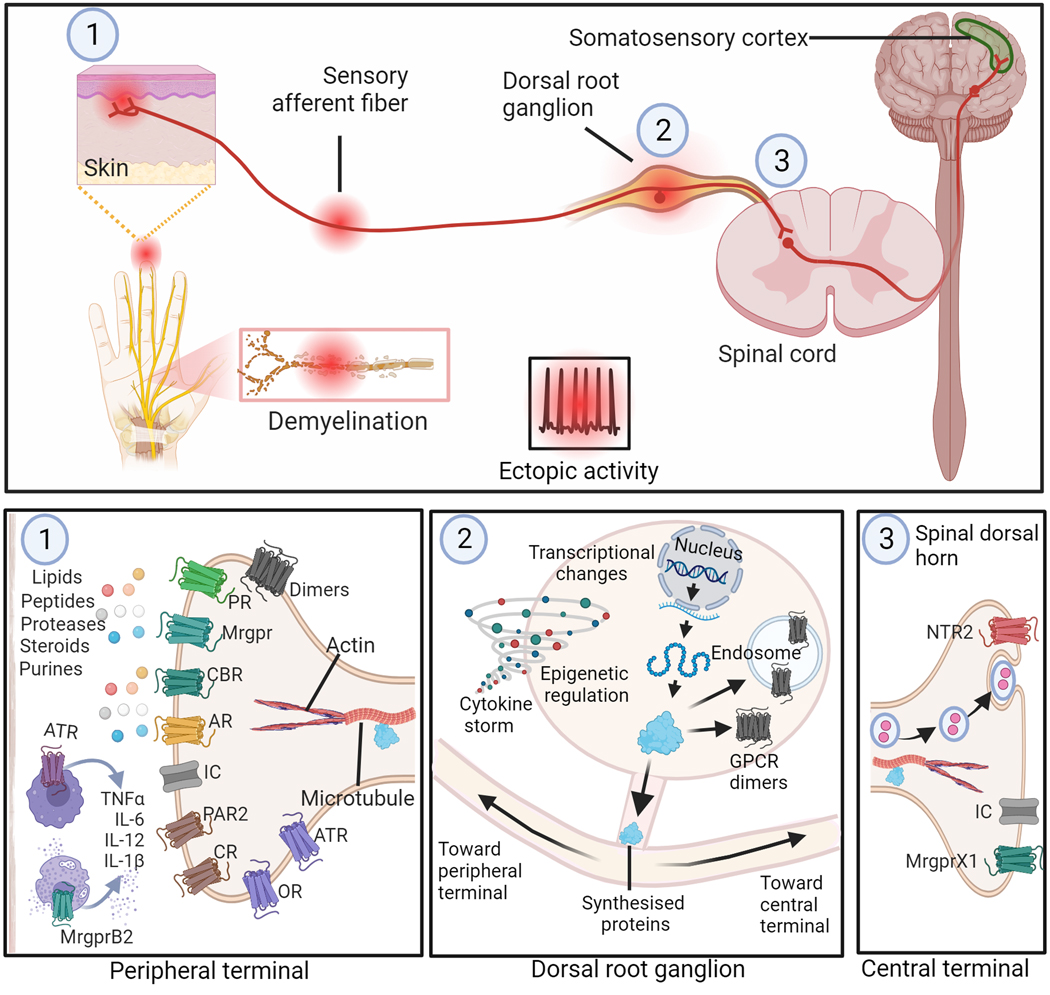
Figure 1: Possible sites on primary sensory neurons for GPCR-mediated inhibition of peripheral neuropathic pain.
Top panel depicts sites on peripheral sensory neurons (PSNs) that may develop spontaneous or ectopic activity following injury or disease affecting peripheral nerves. These sites include (1) peripheral nerve terminals near skin and site of nerve injury or demyelination, and (2) dorsal root ganglion (DRG). Activity originating at these sites are transmitted to the dorsal horn of the spinal cord where the (3) central terminals of afferent nerve fibers terminate. The bottom panel depicts potential GPCR targets at these three sites for modulation of the neuronal activity. In the periphery (1), the somatosensory insult could result in release of various biological mediators (e.g., lipids, purines, steroids, proteases, peptides lipids and cytokines) that sensitize the nociceptors. Promising GPCR targets at this site are shown. At the level of the DRG (2) transcriptomic changes may contribute to altered GPCR and ion channel expression in neurons that may result in suppressing anti-nociceptive GPCR activity and/or promoting\ pro-nociceptive GPCR functions, contributing to the pathophysiology of NP. The central terminals of PSNs (3) also offer potential targets for modulating the signals transmitted to the neurons in the spinal cord. Targeting these GPCRs at various sites along the PSNs offers opportunities for drug development for the treatment of peripheral NP. Abbreviations: OR, Opioid Receptors; AR, Adenosine Receptors; IC, Ion Channels; CBR, Cannabinoid Receptors; MrgprB2, Mas-related G-protein-coupled receptor B2; MrgprX1, Mas-related G-protein-coupled receptor X1; CR, Chemokine receptors; ATR, Angiotensin II receptors; PAR2, Protease Activated Receptor 2; PR, purinergic receptors other than ARs; NTR2, Neurotensin Receptor 2.
Box 3. GPCR Targets on PSNs: Mechanisms of pain relief.
Targeting GPCRs could potentially inhibit neuronal activity at multiple sites in the PNS and through various mechanisms, resulting in pain relief (figure 1). Peripherally-acting drugs may modulate the transduction of pain signals at peripheral nerve terminals, and/or target GPCRs on the axons and soma of PSNs to modulate neuronal excitability and pain transmission. The intricate mechanisms behind analgesia resulting from activation of Gi-coupled GPCRs in PSNs have been previously discussed [3,99,102]. The modulation of ten different ion channel families by 35 different GPCRs, which are targeted by more than 20 endogenous modulators, activation of which can result in pro- and anti-nociceptive effects has been reviewed by Salzer et al. [103]. Activation of Gi-coupled GPCRs such as MOR decreases intracellular cAMP level and also interacts with multiple ion channels, resulting in neuronal inhibition and pain relief. For example, activation of MOR results in a signaling cascade that involves the Gβγ subunit that forms a complex with and inhibits the TRPM3 channels expressed in DRG neurons [96,104], which have been reported to be involved in the heat hypersensitivity and spontaneous pain after nerve injury [105]. The activation of down-stream Protein kinase A could result in the phosphorylation of voltage-gated sodium channels and modulation of cAMP-gated ion channels (e.g., HCN, ASIC, GIRK). In addition, Gq/G11 signaling in nociceptors is also considered to play a role in sensitization mechanisms in pathological pain states, possibly by modulation of TREK, Na+ and TRPV1 channels [106]. Furthermore, pain transmission and conduction in PNS may also be modulated through GPCR-ion channel interplay (e.g. MOR-TRPV1) and interaction (e.g. MOR-NMDAR), which could impact activity of various ion channels [3,49,96,98,103]. Other significant mechanisms of GPCRs on neuronal modulation include receptor dimerization (heteromers) which could result in distinct cellular signaling, inhibition of neurotransmitter release, and suppression of pro-inflammatory pathways [3,21,76]. The multi-site and complex cellular mechanisms of GPCRs provide opportunities to develop effective and potentially safer pain therapies.
Decoding and interrupting the pain signals arising in PSNs: Bench to bedside
Single-cell transcriptomics of primate and mouse sensory neurons have shown strong cross-species parallels in sensory neuron types, indicating that the fundamental cellular basis for somato-sensation is preserved across species [7]. Recent single-nucleus RNA sequencing studies across various peripheral nerve injury models in mice reveal that nerve injury initiates a profound transcriptional reprogramming in PSNs [7–9]. Changes in the expression of genes encoding for various GPCRs, ion channels and multiple neuropeptides that could potentially lead to ectopic activity across PSNs have been detected. Additionally, transcriptomic profiling of DRG from NP patients suggests an increase in inflammation-associated transcripts [13,14]. Nerve injury also results in the release of inflammatory mediators by immune cells and of microRNAs by DRG neurons that results in the sensitization of PSNs [13]. These findings suggest that both direct changes in PSN excitability and indirect mechanisms may play important roles in the onset and maintenance of NP. Moreover, these studies highlight that change in gene expression in PSNs could serve as important guides for validating potential drug targets and developing new therapies for relief of NP.
Numerous pre-clinical studies in rodent models of NP involving traumatic, metabolic, or toxic injuries to one or more peripheral nerves demonstrate that nociceptive PSNs are sensitized and exhibit abnormal ectopic and spontaneous activity [15]. The hyperactivity in PSNs leads to central sensitization, and these plastic changes play a significant role in the mechanisms of NP. Microneurography studies in human subjects with diabetic NP further confirm these earlier reports of spontaneous activity in C-nociceptive afferents in animal models [16,17]. In addition, patch-clamp recordings from dissociated dorsal root ganglion (DRG) neurons from patients with radicular NP exhibit depolarizing spontaneous fluctuations, a decrease in action potential threshold, and spontaneous activity [17–19]. Similar observations were made in patch-clamp recordings from fibroblasts obtained from small fiber neuropathy (SFN) patients, which were reprogrammed into pluripotent stem cells (iPSC) and differentiated into nociceptive PSNs. SFN-derived nociceptors revealed spontaneous activity in nearly 20% of cells compared to <2% in nociceptors from healthy subjects [4]. Pharmacological studies with iPSC-derived nociceptors from a patient with SFN demonstrated that lacosamide, which suppressed ectopic activity in nociceptive C-fibers, markedly attenuated NP [6]. These neurophysiologic studies in human cells indicate that heightened activity in PSNs plays a critical role in ongoing pain and the development of evoked hypersensitivity in peripheral NP. Moreover, these innovative approaches of recordings from surgical or post-mortem DRG neurons and insights from iPSCs-derived nociceptors from patients with NP provide model systems for screening of molecules and opportunities for the development of novel analgesics [6,19–22].
GPCRs in primary sensory neurons: Promising therapeutic targets
Several studies, using animal models of chronic pain, highlight the role of GPCRs in NP, underscore the potential benefits of targeting GPCRs in PSNs for the treatment of NP (Box 2), and elucidate the underlying mechanisms (Box 3). Here, we discuss the evidence for GPCRs in PSNs in various animal models of NP and efficacy of drugs targeting these receptors, with emphasis on drugs that have limited CNS penetration.
Opioid receptors
Opioids are widely used for pain management but their utility is limited due to abuse potential and addiction risks, and other CNS side effects. The opioid epidemic and opioid-related overdoses and fatalities in the U.S. has raised significant public health concerns [20]. Targeting peripheral μ, δ, and κ-opioid receptors on PSNs offers a potentially safer and more selective approach to pain management.
μ-opioid receptors (MORs):
MORs are predominantly expressed in peptidergic PSNs and also in immune cells, where their activation can modulate pain signals at the site of injury. In addition to their classical GPCR Gi/o signaling, MORs regulate various cellular processes such as the inhibition of voltage-sensitive calcium channels, induction of hyperpolarization through modulation of potassium efflux, inhibition of neurotransmitter release, and suppression of neuroinflammatory cytokine signaling. The peripherally restricted MOR-preferring agonist, dermorphin [D-Arg2, Lys4](1–4) amide (DALDA) inhibited both evoked and ongoing pain in a spared nerve injury model (SNI) and also suppressed ectopic discharges in sensory neurons [21][12]. A novel strategy involving a pH-sensitive MOR agonist, (±)-N-(3-fluoro-1-phenethylpiperidine-4-yl)-N-phenyl propionamide (NFEPP), resulted in a peripheral OR-mediated analgesia in rats with chronic constriction injury (CCI), without the typical opioid-associated central side effects [22].
δ-opioid receptors (DORs):
Oprd1, the gene for DORs, is expressed in mouse and human nociceptive DRG neurons and their expression is decreased after SNI, CCI and sciatic nerve transection (ScNT) in mice [8,9,23]. DOR conditional knock-out mice (DOR-cKO−/−) lacking DORs selectively in PSNs exhibit heightened mechanical and heat hypersensitivity following nerve injury, suggesting that DORs in PSNs attenuate NP [24]. Studies using the DOR-cKO−/− mice also indicated that DORs in PSNs were essential for analgesia mediated by the DOR agonists, SNC-80 and Tan-67 [24]. However, agonists of DORs (ADL5859 and ADL5747), which showed robust analgesia in pre-clinical studies, failed in clinical trials [25]. Further research is needed to determine if the failure of the DOR agonists may be related, in part, to the decrease in expression of DORs after nerve injury.
κ-opioid receptors (KORs):
KORs are largely expressed in peptidergic sensory neurons and peripheral nerve injury (CCI, SNI, ScNT) reduced its gene (Oprk1) expression in mouse DRG [8,9,23]. Peripheral KORs inhibit neurogenic inflammation and nociceptor sensitization by inflammatory mediators. Peripherally restricted KOR agonists reduce pain and itch behaviors, and mechanical hypersensitivity in a surgical incision model by mechanisms similar to traditional MOR agonists [26]. A peripherally restricted KOR agonist, CR845 (Difelikefalin), demonstrated high efficacy in CCI model without CNS adverse effects and is being tested intravenously in Phase 3 trials for post-operative pain (NCT00877799, NCT01789476 NCT02542384). Other promising peripherally restricted KOR agonists effective in animal models include FE200041, peripherally acting derivatives of TRK-820 (17-hydroxy-cyclopropylmethyl and 10a-hydroxy), ICI204,488, FE200665, JT09 and SHRO687 [26–28].
Cannabinoid receptors (CBRs)
CB1Rs are expressed in peripheral and central terminals of small nociceptive as well as large neurons. In mouse and human DRG, the Cnr1, gene for CB1R is expressed in peptidergic and non-peptidergic neurons [8,29]. The CB1 receptor expression at the mRNA level was decreased in DRG after CCi in mice [9] . Studies using conditional knockout mice lacking CB1Rs in PSNs and the peripherally restricted dual cannabinoid receptor agonist, CB13, revealed the role of peripheral CB1 receptors in attenuating inflammatory hypersensitivity [30]. CB13 inhibited high voltage-activated calcium currents in cultured DRG neurons [30]. Peripherally restricted CB1R-selective agonists (LBP1, AZ11713908, PRNMI) exhibit significant analgesic effects in animal models of spinal nerve ligation (SNL), bone-cancer pain, and chemotherapy-induced polyneuropathy (CIPN) without CNS side effects [31].
Although CB2 receptors are considered to be localized mainly in non-neuronal cells, studies with human DRG demonstrate the colocalization of CB2 receptor proteins (CB2R) with TRPV1 in small/medium neurons [32]. In addition, CB2 receptor protein levels are increased in human DRG and peripheral nerves after injury. Similarly, CB2 receptors and mRNA expression levels in the DRG are increased after SNL injury in rodents [33]. Peripheral nerve injury enhanced CB2 expression in PSNs via epigenetic bivalent histone modifications and activation of CB2 receptors reduced NP [33]. A peripheral CB2 agonist, AM1710, inhibited CIPN pain in mice and this effect was blocked by co-administration of a peripherally acting CB2R antagonist [34]. Furthermore, in an animal model of neuropathy induced by the antiretroviral agent Zalcitabine, AM1710 decreased mechanical and cold allodynia. These effects were not observed in advillinCre/+;CB2f/f conditional knockout mice, suggesting that the anti-allodynic effects were mediated via CB2 receptors on PSNs [35].
Mas-related G-protein coupled receptors (Mrgprs)
Mrgprs include a family of about 40 GPCRs. Some Mrgpr subtypes are exclusively expressed in PSNs and play an important role in modulating pain transmission and itch. For example, mouse MrgprC11 and its human homologue, MrgprX1, are expressed specifically in nociceptive DRG neurons (predominantly non-peptidergic) and their terminals, but not in the brain. Peripheral nerve injury decrease Mrgprc11 gene expression in injured DRG neurons of mouse [9,36]. Activation of MrgprX1 at central terminals of PSNs in humanized MrgprX1 mice by a full agonist, BAM8–22, attenuates NP, but activation of MrgprX1 at the peripheral cutaneous terminals produces itch [37]. In NP models, the endogenous agonist of MrgprX1, BAM22, was elevated in the spinal cord around the central terminals of DRG neurons but not in the periphery. Positive allosteric modulators (PAM) of MrgprX1, unlike BAM8–22, are inactive by themselves but in the presence of BAM8–22, enhance its effects. MrgprX1 PAM attenuated both evoked and ongoing pain in nerve-injured mice [38,39]. Thus, MrgprX1 PAM has the advantage of promoting spatial and temporal selectivity, potentiating the actions of endogenous MrgprX1 agonist without causing itch. MrgprX1 agonists also enhance opioid-induced analgesia potentially leading to lower opioid dose [40].
Other Mrgpr receptors have also been suggested to play a role in NP and analgesia. For example, ionotropic stimulation of MrgprA3+ C-fibers through native ATP gated P2X3 channels induced pain behavior [41]. Additionally, activation of MrgprD at peripheral terminals of PSNs leads to DRG hyperexcitability and mechanical allodynia whereas silencing of MrgprD+ neurons attenuates mechanical hypersensitivity in a high-fat diet-induced painful diabetic neuropathy model [42]. Mrgpr may also indirectly contribute to pain mechanisms. MrgprB2, a Gi-coupled GPCR that is specifically expressed in mast cells and not in sensory neurons, serves as a receptor for the neuropeptide substance P and plays a crucial role in neurogenic inflammation and pain modulation [43]. Mrgpr receptor ligands and PAMs are thus promising non-opioid analgesics for NP.
Chemokine receptors
Chemokine receptors are hepta-helical surface GPCRs that play an important role in regulating immune response, specifically cell migration. Several chemokine receptors have been implicated to play a role in NP. For instance, C-X-C chemokine receptor type 5 (CXCR5) is expressed in peptidergic and non-peptidetgic PSNs in mouse [44], and CXCL13/CXCR5 axis in DRG was found to contribute in initiation and maintenance of NP whereas its blockade reversed the SNL-induced hypersensitivity in mice [45]. Likewise, chronic compression of DRG induces CXCL12 dependent hyperexcitability of PSNs and inhibition of CXCR4, the receptor for CXCL13, attenuates NP behaviors [46]. Development and validation of peripherally restricted drugs or site specific delivery of compounds targeting chemokine signaling will help further determine the potential therapeutic value of this target.
Purinergic receptors
Two classes of purinergic receptors, P1 and P2Y receptors belong to GPCRs. Activation of P2Y receptors by endogenous nucleotides such as ATP, released during nerve or tissue damage, modulates pain transmission and nociceptive signaling in DRG. A subset of this receptor system, P2Y Purinoceptor 14 (P2Y14R), is expressed in both the peptidergic and non-peptidergic PSNs [29]. CCI and ScNT increases the expression of the gene encoding for P2Y14R in mouse DRG neurons [8,9]. The upregulation of P2Y14R in DRG sensitizes PSNs and promotes pain hypersensitivity in a model of infraorbital nerve injury-induced NP in mice [47]. Trigeminal ganglionic administration of P2Y14R agonist (UDP-glucose) evoked orofacial pain in mice and stimulated the release of inflammatory cytokines, whereas its antagonist 4-[4-(4-Piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid hydrochloride (PPTN) inhibited infraorbital nerve injury-induced hypersensitivity [47]. The peripheral site of action in these studies with local microinjections needs further validation with other approaches, such as optogenetic or chemogenetic techniques and conditional knockout strategies.
Among P1 receptors, adenosine A1 and A3 receptors are Gi coupled receptors (A1Rs, A3Rs) whereas A2 receptors (A2R) are associated with Gs signaling. Adora1, gene for A1R is expressed in both peptidergic and non-peptidergic neurons [29]. Activation of the A1Rs by N6-cyclopentyladenosine in DRG neurons attenuates α,β-meATP induced inward currents and inhibits pain in rats [48]. A1R-mediated peripheral analgesia is possibly mediated through the inhibition of acid-sensing ion channels in PSNs [49]. However, the translation of A1R ligands for pain therapy has been hampered by side effects, such as bradycardia and vasodilation [50]. A3Rs are expressed in both human and rodent immune cells, particularly CD4+ and CD8+ T cells which play a crucial role in pain by modulating cytokine release and DRG neuronal activity. A3R agonists lose their antiallodynic activity in mice lacking T and B cells [51]. The analgesic mechanisms of A3R could involve inhibition of neurotransmitter release mediated by N-type calcium channels in DRG neurons [52]. Extrapolation of potential therapeutic use of A1R and A3R agonists requires dose optimization to eliminate the hypothermic effects associated with these receptor systems [50].
Oxytocin receptor (OXTR)
The role of oxytocin in central analgesia has been well documented, but their involvement in peripheral analgesia was only recently revealed. Single-cell mRNA expression studies of DRG neurons indicate that both small and large cells express OXTR [53], and the OXTR-expressing neurons also contain TRPV1 and Piezo2. Using qRT-PCR, in situ hybridization, and immunohistochemical techniques an increased expression of OXTR mRNA and receptors in small and medium DRG neurons was observed in a model of chemotherapy-induced polyneuropathy (CIPN) [53]. Systemic oxytocin, which has limited ability to cross the blood-brain barrier, reduced the mechanical and thermal hypersensitivity in CIPN. Treatment with a chemotherapeutic agent, paclitaxel, increased OXTR expression in DRG neurons and activation of the receptor relieved NP by inhibiting pPKC-mediated upregulation of Nav 1.7 currents [54]. Additionally, whole-cell patch-clamp recordings of cultured small-diameter DRG neurons revealed that oxytocin decreased the spontaneous activity in neurons from paclitaxel treated mice [54]. Similarly, in a model of orofacial NP, the microinjection of oxytocin at the trigeminal ganglion relieved mechanical hypersensitivity [55]. The translational investigations on oxytocin use for NP are under investigations (NCT02100956, NCT04903002), and a careful evaluation of potential adverse events is needed, considering gender specific side-effects of oxytocin (e.g., uterine contraction) as well as other adverse effects such as headaches, blurred vision, and drowsiness.
Calcitonin gene-related peptide (CGRP) receptor
Calca, the gene for CGRP, labels the peptidergic neurons in mouse DRG [29]. Early after SNI Calca expression decreases in mouse DRG, while at later timepoints there is a trend toward increased expression [23]. Physical or chemical insult to the somatosensory nervous system releases CGRP in PSNs and facilitates the development of NP. Consistent with the clinical success of monoclonal CGRP antibodies for migraine, clinical reports also suggest their efficacy in managing peripheral NP [56]. Further research is required in this area to validate the role of PSN targeting with peripherally restricted CGRP antagonists or site-specific monoclonal antibodies.
Protease activated receptor 2 (PAR2)
PAR2 is expressed in approximately 3–4% of total PSNs, mostly in small-diameter neurons that co-express the Nppb and IL31ra genes [57]. The expression of F2rl1, the gene encoding for PAR2, increases in mouse DRG neurons after CCI and SNI [9,23]. and PAR2 has been suggested to play a role in the development and maintenance of pain-like behavior. Studies with PAR-conditional knockout mice with reduced expression of PAR2 in PSNs further highlight the role of PAR2 in the induction of mechanical hyperalgesia in CIPN [57]. Additionally, intraperitoneal administration of a β-arrestin signaling biased PAR2 antagonist, C781, transiently reversed mechanical hypersensitivity in paclitaxel-treated mice [58].
Angiotensin II type 1 and 2 receptors (AT1Rs, AT2Rs)
In rats, treatment with the AT1R antagonist Losartan both delayed the development and ameliorated paclitaxel-induced CIPN [59]. Although prior reports suggested that AT2Rs were expressed in DRG neurons and its activation induced peripheral sensitization, transcriptome analysis revealed that AT2R gene (Agtr2) was not expressed in DRG cells. Agtr2 was found in macrophages infiltrating injury sites [60]. EMA401, a selective, orally active and peripherally restricted AT2R antagonist, showed promising analgesic effects in pre-clinical models of NP and a Phase 2 trial for postherpetic neuralgia [61]. However, recent multi-center clinical trials were terminated prematurely based on results from preclinical toxicity studies in primates demonstrating unexpected drug-related hepatotoxicity [61,62]. It is unclear if the toxicity is specific to the molecule or a target-related adverse effect. Another ATR2 ligand, CFTX1554, is presently in Phase 1 trial (clinicaltrials.gov NCT05260658). While AT2 receptor agonists remain of interest as potential therapeutic agents for managing NP, the toxicology profile of lead compounds should be cautiously examined.
Neurotensin receptors (NTSR)
Two seven transmembrane proteins of this class (NTSR1 and NTSR2) are expressed in PSNs and superficial dorsal horn. Sc-RNAseq data suggest a decrease in Nrsr2 at the injured DRGs in mice with sciatic nerve CCI [9]. The conotoxin, contulakin-G (CGX), which binds to both NTSR1 and NTSR2, has analgesic effects in NP models by activating presynaptic NTRS2 in the spinal dorsal horn [63]. In-vitro calcium imaging studies demonstrated that CGX inhibits the high-voltage-activated calcium channels in PSNs. DRG voltage clamp recordings suggest that the decrease in calcium currents primarily resulted from inhibition of R-type (Cav2.3) and to some extent N-type (Cav2.2) calcium currents. Intrathecal administration of the ntsr2-specific guide RNA suggests that the likely site of action of the drug was the central terminals of PSNs.
Orphan receptors
Certain orphan receptors whose ligands and functions are being explored have emerged as potential mediators of NP in the PNS. Whole genome sequencing in patients with peripheral neuropathy following taxane and platinum-based chemotherapies revealed that GPR68 is highly expressed in mechano-thermo-sensitive neurons in DRG [64]. Similarly, GPR183, primarily expressed in immune cells, was found to play a role in peripheral sensitization and mechanical and thermal hypersensitivity in rats [65]. A partial infraorbital nerve transection in mice led to the increased expression of GPR151, GPR153, GPR85, GPR119, GPR65 and GPR27 in the trigeminal ganglion (TG) [66]. Among these GPR151 was found to be most significantly increased and associated with heightened neuronal activity and neuroinflammation in TG. Likewise, GPR177 expression in large-diameter A-fibers was found to be associated with the development of diabetic NP in mice via WNT-mediated TRPV1 activation [18]. Endogenous ligands of GPR132 are concentrated in the sciatic nerve and DRG, but not in spinal cord tissues following oxaliplatin treatment in mice. Notably, the activation of GPR132 by these endogenous molecules is driven by the TRPV1 dependent effects in PSNs [67]. These studies highlight the need for further investigation of the role of novel orphan receptors in the modulation of NP mechanisms.
Innovations in peripheral analgesic drug development and delivery
The need to develop new therapeutic strategies to overcome barriers to novel drug development for pain have been highlighted in recent reviews [2,3]. We discuss pharmaceutical and targeted strategies that may provide innovative approaches to drug development for NP. One strategy for specifically targeting peripheral GPCRs involves the synthesis of drugs with restricted permeability across the blood-brain barrier (BBB) (Figure 2). For example, peripherally-restricted KOR agonists have progressed from pre-clinical research to clinical trials for pain indications [27]. Strategies employed in developing these compounds include altering the polarity by the incorporation of amphiphilic moieties exhibiting hydrophilic or hydrophobic properties, use of zwitterionic compounds, and compounds with hydrophilic substituents. The success of the peripherally restricted KOR agonist, CR845 (Difelikefalin) for itch treatment has further fueled interest in developing other peripherally-restricted compounds (Table 1) that target GPCRs. While these compounds are not entirely restricted to the PNS, they exert their biological effects within dose ranges that are largely independent of CNS mechanisms.
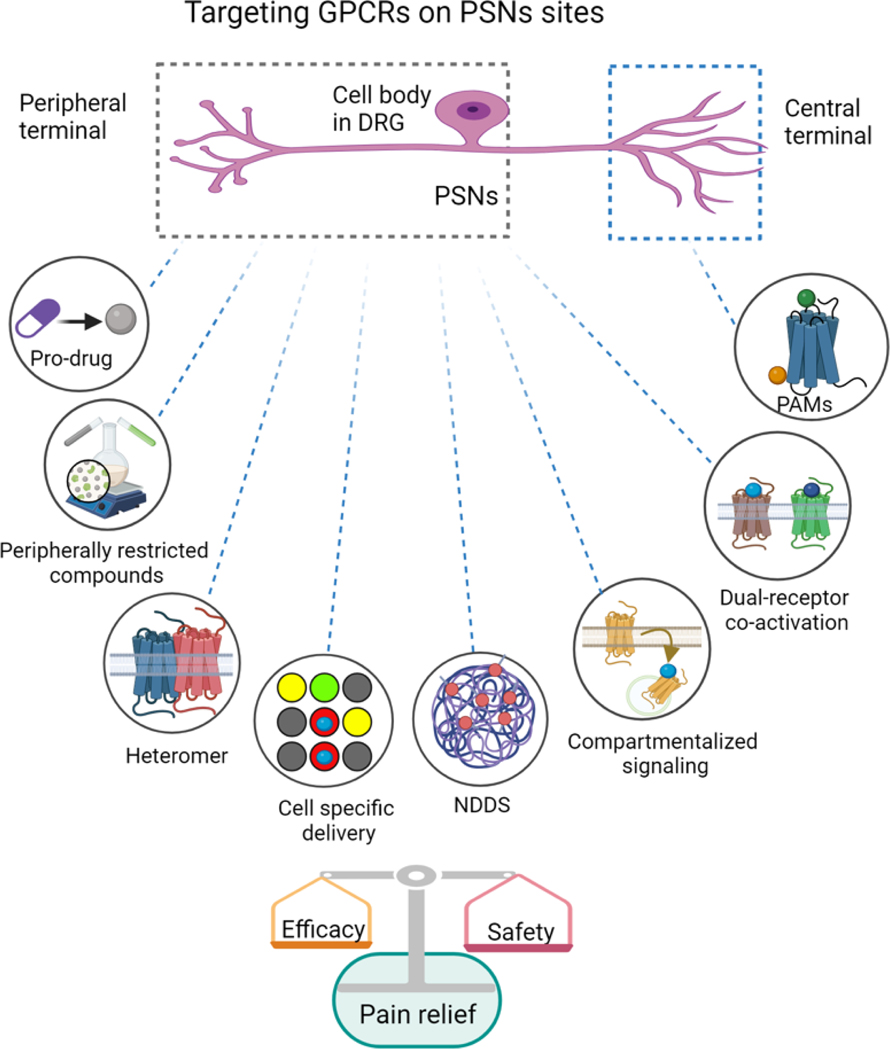
Figure 2. Innovative strategies to target GPCRs on primary sensory neurons for achieving site-specific analgesia.
Approaches aimed at targeting GPCRs on the peripheral terminals and the cell body of primary sensory neurons (PSNs) to develop analgesics with increased safety and efficacy are depicted. These include development of peripheral restricted compounds, prodrugs, targeting GPCR heteromers, and co-activation of dual GPCR receptors. Other strategies such as NDDS (novel drug delivery systems), cell specific discovery, and compartmentalized signaling are also shown. In addition, the approach of enhancing the activity of endogenous agonists at the central terminals of PSNs by positive allosteric modulators (PAMs), such as MrgX1 PAMs, is also shown.
Table 1.
Drugs acting on GPCR targets on primary sensory neurons (PSNs) investigated in pre-clinical models of inflammatory and peripheral neuropathic pain (Evidence from 2018 to 2023)
| GPCR class | Receptor subtype | Expression/localization in different subtypes of PSNs in mouse and human DRG | Drug candidate | Route of administration | Animal model | Outcomes/mechanism of action |
|---|---|---|---|---|---|---|
| Opioid receptors | MOR | IHC [107]: Mouse: Peptidergic neuron (CGRP+) and myelinated neuron (NF200+). Sc-RNAseq: [36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8). Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8). |
DALDA (Agonist) (#) | Systemic (subcutaneous) | L5-SNL (rats) | Inhibits spontaneous and ongoing pain [21] |
| Systemic (subcutaneous) | Tibial SNI (mice) | • Inhibits mechanical and heat hypersensitivity • Inhibits high voltage activated calcium currents in DRG neurons [12] |
||||
| Systemic (subcutaneous) | CCI (rats) | • Inhibition of mechanical and thermal hypersensitivity [108] | ||||
| (±)-N-(3-fluoro-1-phenethylpiperidine-4-yl)-N-phenyl propionamide (NFEPP) *Low Pka mediated pH dependent effect at injury site only |
Systemic (intravenous) | CCI (rats) | • Inhibition of mechanical and thermal hypersensitivity • Naloxone methiodide, a peripheral opioid antagonist, completely reversed the pain relief caused by NFEPP |
|||
| KOR | IHC [26]: Mouse: Peptidergic neuron (CGRP+) and myelinated neuron (NF200+) Sc-RNAseq: [36] Mouse: NP1 (Gfra1, Gfra2), NP2 (Gfra1), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1) Human: PEP1 (Adcyap1), PEP2 (Ntrk1) |
HSK21542 (Agonist) (#) | Systemic (intravenous) |
Paw incision; Sciatic CCI; (rats) |
• Inhibitions mechanical allodynia • Minimal central side effects in comparison to morphine [109] |
|
| SHR0687 (Agonist) (#) | Systemic (intravenous) | Carrageenan-induced pain | • Inhibits mechanical hypersensitivity [28] | |||
| FE200041 (Agonist) (#) | Systemic (Intravenous, subcutaneous) | Tail flick test Formalin test |
• Antinociceptive effect [28] | |||
| JT09 (Agonist) (#) | Systemic (oral) | Hot plate test Acetic acid induced writhing | • Antinociceptive effect | |||
| Opioid/neuropeptide FF receptors | Sc-RNAseq: [36] NPFFR1: Mouse: NP2 (Gfra1), PEP1 (Adcyap1), Cold (Trpm8) Human: PEP1 (Adcyap1), PEP2 (Ntrk1) NPFFR2: Mouse:NP1 (Gfra1, Gfra2), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1) Human: NP1 (Gfra1, Gfra2), PEP1 (Adcyap1), PEP2 (Fam19a1), Cold (Trpm8) |
Analogues of DN-9 (Agonist) (#) |
Systemic (oral) | Sciatic CCI; (mice) | • Inhibition of mechanical and thermal hypersensitivity • No tolerance, dependence, constipation, or respiratory depression at effective analgesic doses [75] |
|
| DOR/KOR | DOR: IHC [110]: Non peptidergic unmyelinated neuron (IB4+) and myelinated neuron (NF200+) Sc-RNAseq:[36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), PEP2 (Ntrk1) and PEP2 (Fam19a1) Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8) |
CAV1001 (Agonist) (#) | Systemic (intraperitoneal) | L5-L6 SNL (rats) | • Inhibition of mechanical hypersensitivity [74] | |
| Cannabinoid receptors | CB1 | ISH [111]: Mouse: Non peptidergic unmyelinated neuron (IB4+), Peptidergic neuron (Trpv1+) and myelinated neuron (NF200+) Sc-RNAseq:[36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8) Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8) |
4-{2-[-(1E)-1[(4-propylnaphthalen-1-yl)methylidene]-1H-inden-3-yl]ethyl}morpholine or PRNMI (Agonist) (#) |
Systemic (intraperitoneal) Local (intraplantar) | CIPN (rats) | • Inhibition of mechanical and cold hypersensitivity • No tolerance and CNS associated side effects [112] |
| CB2 | RNAscope ISH [33]: Small and medium diameter sensory neurons after nerve injury. Single-cell-RNAseq: [36] Human: PEP2 (Fam19a1) |
AM1710 (Agonist) | Local (intraplantar) | CIPN (rats) | • Anti-allodynic effect [34] | |
| LY2828360 (Biased agonist) | Local (intraplantar) | Carrageenan | • Anti-allodynic effect [79] | |||
| CB1/CB2 | CB13 (Agonist) (#) | Systemic (oral) | Sciatic CCI (rats) | • Anti-allodynic effect [113] | ||
| Local (bath application) | CFA induced inflammatory pain (mice) | • Inhibits high voltage activated calcium currents in DRG neurons [30] | ||||
| Purinergic receptors | P2Y14R | IHC [114]: Mouse: Peptidergic neuron and myelinated neuron (NFH+) Single-cell-RNAseq: [36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8) Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (Trpm8) |
4-[4-(4-Piperidinyl)phenyl]-7-[4-(trifluoromethyl)phenyl]-2-naphthalenecarboxylic acid hydrochloride (Antagonist) |
Injection to trigeminal ganglion | CCI of the infraorbital nerve (mice) | • Inhibition of mechanical hypersensitivity [47] |
| Adenosine A1 receptor | IHC [115]: Mouse: Non peptidergic unmyelinated neuron (IB4+), Peptidergic neuron (CGRP+) and myelinated neuron (NF200+) Single-cell-RNAseq: [36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1) Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1) |
N6-cyclopentyladenosine (Agonist) | Local (Intraplantar) | Intraplantar glutamate injection (rats) | • Reduced spontaneous pain behavior [48] | |
| Adenosine A3 receptor | ICC [116]: Rat cultured DRG neurons Single-cell-RNAseq: [36] Human: NP3 (Sst), PEP2 (Ntrk1) *Debatable inter-species differences |
MRS5980 (Agonist) | Systemic (intraperitoneal) | Sciatic CCI (mice) | • PSNs A2 dependent anti-allodynia [51] | |
| Oxytocin receptor | IHC [117] Rat: Non peptidergic unmyelinated neuron (IB4+) and Peptidergic neuron (CGRP+) Single-cell-RNAseq: [36] Mouse: Cold (trpm8) Human: PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1) |
Oxytocin (agonist) | Injection to trigeminal ganglion | Alveolar nerve transection | • Inhibits mechanical hypersensitivity [55] | |
| Local (intraplantar) | SNL (rats) | • Inhibition of mechanical allodynia [118] | ||||
| Mrgpr | MrgprX1 | IHC [36]: Non peptidergic unmyelinated neuron (IB4+) and Peptidergic neuron (CGRP+) Single-cell-RNAseq: [36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Fam19a1), Cold (Trpm8) Human [10]: NP1 (Gfra2) |
6-(tert-butyl)-5-(3,4-dichlorophenyl)-4-(2-(trifluoromethoxy)phenoxy)t hieno[2,3-d]pyrimidine (Positive allosteric modulator) | Systemic (Oral) | Sciatic CCI (MrgprX1 humanized mice) | • Attenuates heat hypersensitivity [38] |
| Chemokine receptors | CXCR5 | IHC [44]: Mouse: Non peptidergic unmyelinated neuron (IB4+), Peptidergic neuron (CGRP+) and myelinated neuron (NF200+) Single-cell-RNAseq: [36] Mouse: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst) Human [10]:TRPA1+, PENK+, Cold, Aδ high-threshold nociceptors |
Cxcl13 siRNA | DRG microinjection | SNL (mice) | • Inhibition of mechanical and heat hypersensitivity [45] |
| CGRP receptor | Peptidergic neuron (CGRP+) Single-cell-RNAseq:[36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (trpm8) Human: NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (trpm8) |
Olcegepant (Antagonist) | Temporo-mandibular joint injection | CFA injection in temporomandi bular joint (mice) | • Inhibition of Temporomandibular disorder associated pain [119] |
|
| Angiotensin II type 1 and 2 receptors | AT1Rs | IHC [120]: Rat: Non peptidergic unmyelinated neuron (IB4+) Single-cell-RNAseq: [36] Mouse:NP1 (Gfra1, Gfra2), NP2 (Gfra1), NP3 (Sst), PEP1 (Adcyap1), PEP2 (Ntrk1) and PEP2 (Fam19a1), Cold (trpm8) Human: NP1 (Gfra1, Gfra2), PEP1 (Adcyap1) |
Losartan (Antagonist) | Systemic (intraperitoneal) | CIPN | • Inhibition of cytokine release in DRG • Inhibits mechanical hypersensitivity [59] |
| AT2Rs | IHC: [120]: Rat: Non peptidergic unmyelinated neuron (IB4+) Single-cell-RNAseq: [36] Mouse: NP2 (Gfra1) |
EMA401 (Antagonist) | Systemic (Intraperitoneal, oral) | CIPN, sciatic CCI, SNI (rats and mice) | • Inhibits mechanical, heat and cold hypersensitivities [61] |
Abbreviations: NP, Non-peptidergic; PEP, peptidergic; IHC, Immunohistochemistry; ISH, In-situ hybridization; Sc-RNAseq, Single cell RNA sequencing; CGRP, Calcitonin gene-related peptide; IB4, isolectin B4; NF200, neurofilament-200; gfra1, GDNF Family Receptor Alpha 1; gfra2, GDNF Family Receptor Alpha 2; Sst, Somatostatin; Adcyap1, Adenylate Cyclase Activating Polypeptide 1; Ntrk1, neurotrophic tyrosine kinase receptor; Fam19a1, Family with sequence similarity 19 (chemokine (C-C motif)-like), member A1; Trpm8, Transient Receptor Potential Cation Channel Subfamily M Member 8.
The neuronal clustering approach is adopted from Jung et. al. 2023 [29].
Resources: https://sensoryomics.shinyapps.io/RNA-Data/
denotes peripherally restricted compounds
With technological advancements, novel drug delivery systems (NDDS) have evolved that enhance the activity of pharmaceutical compounds, optimize their pharmacokinetics, and enable site-specific release. A promising strategy involves the development of peripherally restricted nano-formulations of existing CNS acting opioids. A poly-glycol-morphine conjugate has been formulated that hinders BBB permeation due to its large molecular size and hydrophilicity, but facilitates diffusion through leaky blood vessels in inflamed tissues [68]. A peripherally-restricted transthyretin-based opioid drug delivery system has also been reported to mitigate undesired CNS side effects [69]. The approach involved conjugating active pharmaceutical ingredients (e.g., morphine) with AG-10, a hydrophilic derivative that binds to serum protein transthyretin, thereby impeding the drug complex’s ability to penetrate the BBB. By optimizing drug delivery to selective sites, NDDS unfolds new avenues for improving the treatment efficacy and safety of conventional drugs.
Targeting compartmentalized GPCR signaling is an emerging approach to develop advanced cell-specific delivery system for desired drug molecules [70]. Compartmentalized signaling refers to the intricate organization of GPCR signaling pathways within specific cellular compartments that enables precise control and coordination of cellular responses. Precision cell-specific delivery could aid in customization of therapeutic interventions and help target the spatial GPCR regulation, allowing cells to respond efficiently to diverse external stimuli. A recent study reported that DOR endocytosis and endosomal signaling by Protein kinase C (PKC) mediated the inhibitory effects of endogenous opioids and DOR agonists [71]. Using a nanoparticle cell-specific delivery strategy, the DOR agonist DADLE was coupled to a liposome shell and incorporated into a mesoporous silica core. This resulted in the release of the drug in the endosomal microenvironment of DOR-expressing neurons.
Importantly, cell-specific delivery could potentially minimize off-target effects and enhance the efficacy of GPCR-targeted therapies. Other important approaches for endosomal targeting include the development of endosomal biased agonists and antagonists, pH-responsive and lipid-anchored nanoformulation [70,72].
Other strategies include developing prodrugs that are activated specifically at the injury site in the PNS to achieve targeted analgesia. For example a peripherally restricted prodrug PL265, a dual enkephalinase inhibitor, attenuated NP in a murine model by activation of opioid receptors on nociceptors without the development of tolerance [73]. Additionally, several drugs originally designed for other clinical conditions, such as tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and anticonvulsants, have been repurposed for NP management. Drug repurposing circumvents various stages of conventional drug discovery, resulting in reduced time and cost for drug development.
Simultaneously targeting dual receptors in the PNS has been proposed as a strategy to achieve synergistic effects and enhance treatment efficacy. Co-activation of DORs and KORs in the periphery by a dual agonist resulted in potent analgesic effects in a mouse model of inflammatory pain, with reduced tolerance compared to morphine [74]. Similarly, orally active opioid/neuropeptide FF receptor agonist administration led to a greater reduction in CCI-induced mechanical and heat pain in mice without exhibiting central side effects [75].
While receptor selectivity and its functional manipulation with exogenous ligands has been the standard approach for drug development, receptors also work in complex organizations forming homomers or heteromers that are coupled to distinct downstream mechanisms and may have effects unlike that of the primary receptors. MOR-DOR heteromers are expressed in DRGs and their pharmacological modulation could alleviate NP [76]. Other GPCR heteromers are also identified in PSNs and their respective exogenous ligands, such as DOR-KOR, CB1-DOR and MOR-DOR agonists, alleviate NP [77,78].
The use of biased agonists to target GPCRs in PSNs could be another innovative strategy to develop new drugs for NP with fewer undesirable effects. Biased GPCR ligands, unlike traditional orthosteric agonists, selectively activate a subset of the receptor’s downstream signaling cascade. Intra-plantar administration of LY2828360, a biased agonist of CB2R, attenuates carrageenan induced chronic pain in rats, whereas this effect was absent in mice with conditional deletion of CB2R in PSNs [79]. The potential utility of biased GPCR agonists is worth examining in animal models of peripheral NP. In addition, as described earlier for MrgprX1 [38], exploring PAMs presents an appealing strategy for selectively targeting GPCRs expressed in small neurons when endogenous ligands are selectively upregulated under pain conditions. The PAM agonists for CB1 and CB2 receptors are also being developed and tested in preclinical models to minimize the adverse effects associated with orthosteric agonists [80,81].
Epigenetic changes in PSNs following nerve injury appear to play a crucial role in the altered expression of various GPCR genes responsible for endogenous analgesia and exogenous ligand-mediated responses. Peripheral nerve injury led to downregulation of zinc finger protein 382 (ZFN382) which formed complexes with HDAC1 and SETDB1 and regulated the CXCL13 expression. Importantly, rescuing the ZFN382 downregulation by DRG microinjection attenuated NP-like behaviors in nerveinjured mice [45]. G9a, a histone methyltransferase, was enriched at the oprm1 promoter in nerve-injured mice DRG, leading to decreased MOR expression [82]. Silencing G9a with siRNA fully restored MOR expression and enhanced morphine’s analgesic effect. Another study revealed G9a’s role in reducing CB1R expression after nerve injury and genetic suppression of G9 restored CB1R agonist efficacy by inhibiting glutamate release in primary afferents [83]. Thus, targeting epigenetic regulators in PSNs, such as G9a and ZFN382, to modulate gene expression can be a potential novel strategy for NP relief.
Concluding remarks and future perspectives
An extensive body of research has shed light on the role of PSNs in the pathophysiology of peripheral NP and has resulted in the search for targets on these neurons. While targeting GPCR on PSNs to achieve analgesia has shown remarkable success in pre-clinical studies, several outstanding questions remain that hinder the translation to clinical studies (see outstanding questions). Improved animal models and outcomes that more accurately reflect human pain conditions and patient symptoms, combined with genetic and mechanistic studies in human sensory neurons, could pave the way for efficient and personalized therapies for NP [2]. Development of more effective and safer peripherally restricted drugs and allosteric modulators of GPCRs would be an essential step prior to clinical trials. Additionally, detailed studies of the long-term safety and efficacy of peripherally restricted compounds and nanoformulations are essential to determine their translational potential. A better understanding of the protein structure, the ligand binding sites, and the precise mechanisms of action of various peripheral analgesics could accelerate drug development. In this context, the AlphaFold Protein Structure Database and other artificial intelligence (AI) approaches may provide opportunities to re-engineer drug discovery and development, select and prioritize combinations of drug targets, enhance clinical trial design, and provide insights on personalized treatment strategies [84]. Utilizing more rigorous tools such as human DRG cultures, stem cell-based sensory neuron investigations, and developing human organoid chips to study the effect of novel compounds would significantly advance precision medicine for NP management. In conclusion, drugs targeting GPCRs on peripheral sensory neurons could lead to significant advancement in the management of peripheral NP as complementary or alternative approaches to centrally acting analgesics.
Outstanding questions
What cellular and molecular changes occur after disease or injury to peripheral nerves that lead to enhanced excitability of sensory neurons and results in the onset and maintenance of NP?
What are the precise molecular interactions between GPCRs, neuronal, and non-neuronal cells that lead to the modulation of excitability in PSNs?
How does activity in PSNs contribute to the transition from acute to chronic pain, and could targeting these pathophysiologic mechanisms provide opportunities for preventive strategies?
What cellular, physiologic, or behavioral biomarkers are likely to be most helpful in understanding clinical phenotypes of NP and serve as models to develop and test effective treatment options for patients?
How can the reproducibility and predictability of animal models and outcome measures be improved and validated for translating pre-clinical observations to human pain conditions?
What is the role of crosstalk between GPCRs and metabotropic receptors in the initiation and maintenance of transcriptional changes that leads to sensitization of PSNs in neuropathic pain?
What new approaches would help predict and help minimize off-site target engagement of novel GPCR ligands in other tissues (cardiac, hepatic, etc.)?
Can site-specific biased agonists produce selective analgesia and minimize tolerance to chronic treatment with GPCR agonists?
What are the key challenges that need to be overcome to improve the success of translating the multiple potential GPCR targets in PSNs to safe and effective novel therapies for NP?
Highlights
Pharmacological management of peripheral neuropathic pain (NP) remains inadequate due to limited efficacy and dose-limiting central side effects of drugs presently approved for clinical use.
NP is associated with abnormal spontaneous discharge and ectopic activity in the primary sensory neurons (PSNs) that leads to heightened sensitivity of central pain processing neurons (central sensitization).
The majority of existing treatments for peripheral NP target the central nervous system (CNS). Growing clinical evidence that indicates a crucial role of the PSNs in the maintenance of NP has led to the search for novel safer therapeutic strategies and targets.
Selective targeting of GPCRs expressed on PSNs blocks pain signals at its source by suppressing spontaneous and ectopic activity in nociceptive neurons and results in the attenuation of ongoing and evoked pain in preclinical models of peripheral NP.
Analgesia aimed at GPCR targets on PSNs represents a promising strategy for developing new therapies for peripheral NP management, offering a complementary or alternative approach to alleviating pain with minimal central adverse effects.
Acknowledgements
This work was conducted at the Johns Hopkins University, Baltimore, U.S.A and Indian Institute of Technology (B.H.U), Varanasi, India. The authors were supported by National Institute of Health (Bethesda, Maryland, USA) grants NS026363 (S.R.), NS110598 (Y.G.), NS117761 (Y.G.), NS115718 (T.T.), and core research grant (CRG/2020/002621/BHS) and ICMR-ECD grant (P-16/ECD/Adhoc/49/2022-23) awarded to Dr. Vinod Tiwari by Science and Engineering Research Board and Indian Council of Medical Research, India respectively. Funders had no role in study conceptualization and data interpretation, and in the decision to submit the work for publication.
Glossary
- *Allodynia
Pain due to a stimulus that does not normally provoke pain
- *Central sensitization
Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input
- Chemotherapy-induced neuropathy (CIPN)
An animal model that mimics the neuropathy and pain experienced by patients undergoing treatment with chemotherapeutic agents, such as paclitaxel and oxaliplatin. In this model, animals (typically rodents) are exposed to chemotherapeutic agents for a few days to induce a chemotoxic neuropathy, e.g., paclitaxel induced peripheral neuropathy
- Chronic constriction Injury (CCI)
A commonly employed model in pain research that involves placing a series of loose ligatures around the sciatic nerve in rodents that results in a constriction injury of the nerve and hypersensitivity in the nerve-injured paw
- Gi/o signaling
A Gi/o protein-coupled intracellular signaling pathway when triggered by activation of the GPCR results in decrease in cAMP levels through inhibition of adenylyl cyclase activity. The cAMP pathway connects to multiple cellular machines, including ion channels, transcription factors, and metabolic enzymes. In neurons, activation of this pathway usually results in inhibition of neuronal activity
- Gs signaling
A Gs protein-coupled intracellular signaling pathway when triggered by activation of the GPCR results in stimulation of adenylyl cyclase, increase in cAMP levels, and activation of multiple downstream cascades
- High-fat diet-induced painful diabetic neuropathy model
An experimental model of neuropathy in which rodents are fed a high-fat diet for an extended period of time to induce diabetes and mimic the metabolic and neuropathic changes associated with the disease
- *Hyperalgesia
Increased pain from a stimulus that normally provokes pain
- *Hyperpathia
A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold
- Infraorbital nerve injury-induced neuropathic pain model
A chronic pain model, usually in rodents, that involves injury to the infraorbital nerve, a sensory nerve that innervates a region of the face above the lip
- *Pain
An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage
- *Peripheral sensitization
Increased responsiveness and reduced threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields
- *Neuropathic Pain
Pain caused by a lesion or disease of the somatosensory nervous system
- *Nociceptor
A high-threshold sensory receptor of the peripheral somatosensory nervous system that is capable of transducing and encoding noxious stimuli
- *Nociceptive Pain
Pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors. Note: This term is designed to contrast with neuropathic pain. The term is used to describe pain occurring with a normally functioning somatosensory nervous system to contrast with the abnormal function seen in neuropathic pain
- *Sensitization
Increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs
- Spared nerve injury (SNI)
It involves the transection of two of the three terminal branches of the sciatic nerve in rodents while preserving the integrity of the third branch (commonly the sural or tibial nerve) that results in chronic pain and hypersensitivity
- Spinal nerve ligation (SNL)
A surgical procedure that involves tight ligation and/or sectioning of the lumbar spinal nerve at one or more levels that results in chronic pain behaviors including hypersensitivity in the affected extremity
- Spontaneous activity
Firing of action potentials by a nerve fiber in the absence of an external stimulus
Footnotes
Commonly used pain terms as defined by The International Association for the Study of Pain (IASP) (https://www.iasp-pain.org/resources/terminology/).
Declaration of interest
TT, XD, and YG are listed as inventors on a patent application claiming novel MrgprX1 PAMs. X.D. is a co-founder of Escient Pharmaceuticals. Other authors declare no competing interests. SR is a consultant for AbbVie, Vertex, and Lexicon Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Taneja A. et al. (2017) Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm. Eur. J. Clin. Pharmacol 73, 1219–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf CJ (2020) Capturing novel non-opioid pain targets. Biol. Psychiatry 87, 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yekkirala AS et al. (2017) Breaking barriers to novel analgesic drug development. Nat. Rev. Drug Discov. 16, 545–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namer B. et al. (2019) Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine 39, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landmann G. et al. (2022) Local hyperexcitability of C-nociceptors may predict responsiveness to topical lidocaine in neuropathic pain. PLoS One 17, e0271327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dull MM et al. (2019) Methylglyoxal causes pain and hyperalgesia in human through C-fiber activation. Pain 160, 2497–2507 [DOI] [PubMed] [Google Scholar]
- 7.Kupari J. et al. (2021) Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun 12, 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renthal W. et al. (2020) Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal Injury. Neuron 108, 128–144.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C. et al. (2022) scRNA-sequencing reveals subtype-specific transcriptomic perturbation in DRG neurons of PirtEGFPf mice in neuropathic pain condition. Elife 11, e76063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavares-Ferreira D. et al. (2022) Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med 14, eabj8186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell R. et al. (2020) ErbB1-dependent signalling and vesicular trafficking in primary afferent nociceptors associated with hypersensitivity in neuropathic pain. Neurobiol. Dis 142, 104961. [DOI] [PubMed] [Google Scholar]
- 12.Barpujari A. et al. (2020) Role of peripheral sensory neuron mu-opioid receptors in nociceptive, inflammatory, and neuropathic pain. Reg. Anesth. Pain Med. 45, 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray PR et al. (2023) RNA profiling of human dorsal root ganglia reveals sex differences in mechanisms promoting neuropathic pain. Brain 146, 749–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall BE et al. (2022) Transcriptomic analysis of human sensory neurons in painful diabetic neuropathy reveals inflammation and neuronal loss. Sci. Rep 12, 4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raja SN et al. (2020) John J. Bonica Award Lecture: Peripheral neuronal hyperexcitability: the “low-hanging” target for safe therapeutic strategies in neuropathic pain. Pain 161, S14–S26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker AK et al. (2023) Spontaneous activity of specific C-nociceptor subtypes from diabetic patients and mice: Involvement of reactive dicarbonyl compounds and (sensitized) transient receptor potential channel A1. J. Peripher. Nerv. Syst 28, 202–225 [DOI] [PubMed] [Google Scholar]
- 17.North RY et al. (2022) Electrophysiological alterations driving pain-associated spontaneous activity in human sensory neuron somata parallel alterations described in spontaneously active rodent nociceptors. J. Pain 23, 1343–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie YK et al. (2022) GPR177 in A-fiber sensory neurons drives diabetic neuropathic pain via WNT-mediated TRPV1 activation. Sci. Transl. Med 14, eabh2557 [DOI] [PubMed] [Google Scholar]
- 19.North RY et al. (2019) Electrophysiological and transcriptomic correlates of neuropathic pain in human dorsal root ganglion neurons. Brain 142, 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barocas JA et al. (2022) Clinical impact, costs, and cost-effectiveness of hospital-based strategies for addressing the US opioid epidemic: a modelling study. Lancet Public Heal. 7, e56–e64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiwari V. et al. (2018) Peripherally acting μ-opioid receptor agonists attenuate ongoing pain-associated behavior and spontaneous neuronal activity after nerve injury in rats. Anesthesiology 128, 1220–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Gaztelumendi A. et al. (2018) Analgesic effects of a novel pH-dependent m-opioid receptor agonist in models of neuropathic and abdominal pain. Pain 159, 2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry AM et al. (2023) Deep RNA-seq of male and female murine sensory neuron subtypes after nerve injury. Pain 164, 2196–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin D. et al. (2022) δ-Opioid receptors in primary sensory neurons tonically restrain nociceptive input in chronic pain but do not enhance morphine analgesic tolerance. Neuropharmacology 217, 109202. [DOI] [PubMed] [Google Scholar]
- 25.Berthiaume S. et al. (2020) Alleviating pain with delta opioid receptor agonists: evidence from experimental models. J. Neural Transm. 127, 661–672 [DOI] [PubMed] [Google Scholar]
- 26.Snyder LM et al. (2018) Kappa opioid receptor distribution and function in primary afferents. Neuron 99, 1274–1288.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanucci A. et al. (2022) Discovery of κ opioid receptor (KOR)-selective d - tetrapeptides with improved in vivo antinociceptive effect after peripheral administration. ACS Med. Chem. Lett 13, 1707–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan MIH et al. (2022) A systematic review on the kappa opioid receptor and its ligands: New directions for the treatment of pain, anxiety, depression, and drug abuse. Eur. J. Med. Chem 243, 114785. [DOI] [PubMed] [Google Scholar]
- 29.Jung M. et al. (2023) Cross-species transcriptomic atlas of dorsal root ganglia reveals species-specific programs for sensory function. Nat. Commun 14, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford NC et al. (2022) Role of primary sensory neurone cannabinoid type-1 receptors in pain and the analgesic effects of the peripherally acting agonist CB-13 in mice. Br. J. Anaesth 128, 159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finn DP et al. (2021) Cannabinoids, the endocannabinoid system and pain: a review of preclinical studies. Pain 162, S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand U. et al. (2008) Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 138, 667–680 [DOI] [PubMed] [Google Scholar]
- 33.Ghosh K. et al. (2022) Cannabinoid CB2 receptors are upregulated via bivalent histone modifications and control primary afferent input to the spinal cord in neuropathic pain. J. Biol. Chem 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin X. et al. (2022) A peripheral CB2 cannabinoid receptor mechanism suppresses chemotherapy-induced peripheral neuropathy: evidence from a CB2 reporter mouse. Pain 163, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carey LM et al. (2023) Peripheral sensory neuron CB2 cannabinoid receptors are necessary for both CB2-mediated antinociceptive efficacy and sparing of morphine tolerance in a mouse model of anti-retroviral toxic neuropathy. Pharmacol. Res 187, 106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He SQ et al. (2014) Temporal changes in MrgC expression after spinal nerve injury. Neuroscience 261, 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z. et al. (2017) Targeting human Mas-related G protein-coupled receptor X1 to inhibit persistent pain. Proc. Natl. Acad. Sci. U. S. A 114, E1996–E2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berhane I. et al. (2022) Thieno[2,3-d]pyrimidine-based positive allosteric modulators of human mas-related G protein-coupled receptor X1 (MRGPRX1). J. Med. Chem 65, 3218–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S. et al. (2022) Synthesis and biological characterization of a series of 2-sulfonamidebenzamides as allosteric modulators of mrgX1. ACS Med. Chem. Lett 13, 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He SQ et al. (2018) Oligomerization of MrgC11 and μ-opioid receptors in sensory neurons enhances morphine analgesia. Sci. Signal 11, eaao3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharif B. et al. (2020) Differential coding of itch and pain by a subpopulation of primary afferent neurons. Neuron 106, 940–951.e4 [DOI] [PubMed] [Google Scholar]
- 42.George DS et al. (2022) A subpopulation of peripheral sensory neurons expressing the Mas-related G Protein-Coupled Receptor d (Mrgprd) generates pain hypersensitivity in painful diabetic neuropathy. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green DP et al. (2019) A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 101, 412–420.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu XB et al. (2016) CXCL13/CXCR5 enhances sodium channel Nav1.8 current density via p38 MAP kinase in primary sensory neurons following inflammatory pain. Sci. Rep 6, 34836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma L. et al. (2021) Znf382 controls mouse neuropathic pain via silencer-based epigenetic inhibition of cxcl13 in drg neurons. J. Exp. Med 218, e20210920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y. et al. (2017) Effect of CXCL12/CXCR4 signaling on neuropathic pain after chronic compression of dorsal root ganglion. Sci. Rep 7, 5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin J. et al. (2022) P2Y14 receptor in trigeminal ganglion contributes to neuropathic pain in mice. Eur. J. Pharmacol 931, 175211. [DOI] [PubMed] [Google Scholar]
- 48.Hao JW et al. (2022) A1 adenosine receptor activation inhibits P2X3 receptor–mediated ATP currents in rat dorsal Root ganglion neurons. Mol. Neurobiol 59, 7025–7035 [DOI] [PubMed] [Google Scholar]
- 49.Wei S. et al. (2022) Suppression of ASIC activity by the activation of A1 adenosine receptors in rat primary sensory neurons. Neuropharmacology 205, 108924. [DOI] [PubMed] [Google Scholar]
- 50.Coppi E. et al. (2022) Therapeutic potential of highly selective A3 adenosine receptor ligands in the central and peripheral nervous system. Molecules 27, 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durante M. et al. (2021) Adenosine A3 agonists reverse neuropathic pain via T cell-mediated production of IL-10. J. Clin. Invest 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppi E. et al. (2019) Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons. Pain 160, 1103–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguri T. et al. (2022) Profile of dorsal root ganglion neurons: study of oxytocin expression. Mol. Brain 15, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li L. et al. (2023) Up-regulation of oxytocin receptors on peripheral sensory neurons mediates analgesia in chemotherapy-induced neuropathic pain. Br. J. Pharmacol 180, 1730–1747 [DOI] [PubMed] [Google Scholar]
- 55.Huang CL et al. (2021) Activation of oxytocin receptor in the trigeminal ganglion attenuates orofacial ectopic pain attributed to inferior alveolar nerve injury. J. Neurophysiol 125, 223–231 [DOI] [PubMed] [Google Scholar]
- 56.Kang SA and Govindarajan R. (2021) Anti-calcitonin gene–related peptide monoclonal antibodies for neuropathic pain in patients with migraine headache. Muscle and Nerve 63, 563–567 [DOI] [PubMed] [Google Scholar]
- 57.Hassler SN et al. (2020) The cellular basis of protease-activated receptor 2–evoked mechanical and affective pain. JCI Insight 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kume M. et al. (2023) Protease-activated receptor 2 (PAR2) expressed in sensory neurons contributes to signs of pain and neuropathy in paclitaxel treated mice. J. Pain DOI: 10.1016/j.jpain.2023.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim E. et al. (2019) Losartan, an angiotensin II type 1 receptor antagonist, alleviates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain by inhibiting inflammatory cytokines in the dorsal root ganglia. Mol. Neurobiol 56, 7408–7419 [DOI] [PubMed] [Google Scholar]
- 60.Shepherd AJ et al. (2018) Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc. Natl. Acad. Sci. U. S. A 115, E8057–E8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith MT (2022) Nonopioid analgesics discovery and the Valley of Death: EMA401 from concept to clinical trial. Pain 163, S15–S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rice ASC et al. (2021) Efficacy and safety of EMA401 in peripheral neuropathic pain: results of 2 randomised, double-blind, phase 2 studies in patients with postherpetic neuralgia and painful diabetic neuropathy. Pain 162, 2578–2589 [DOI] [PubMed] [Google Scholar]
- 63.Martin L. et al. (2022) Conotoxin contulakin-G engages a neurotensin receptor 2/R-type calcium channel (Cav2.3) pathway to mediate spinal antinociception. Pain 163, 1751–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan Z. et al. (2023) Whole genome sequencing across clinical trials identifies rare coding variants in GPR68 associated with chemotherapy-induced peripheral neuropathy. Genome Med. 15, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oberkrom K. et al. (2023) GPR183 activation in the peripheral nervous system induces behavioral hypersensitivities in ratsASPET, 7 [Google Scholar]
- 66.Jiang BC et al. (2021) G protein-coupled receptor GPR151 is involved in trigeminal neuropathic pain through the induction of Gβγ/extracellular signal-regulated kinase-mediated neuroinflammation in the trigeminal ganglion. Pain 162, 1434–1448 [DOI] [PubMed] [Google Scholar]
- 67.Hohmann SW et al. (2017) The G2A receptor (GPR132) contributes to oxaliplatin-induced mechanical pain hypersensitivity. Sci. Rep 7, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González-Rodríguez S. et al. (2017) Polyglycerol-opioid conjugate produces analgesia devoid of side effects. Elife 6, e27081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tuhin MTH et al. (2022) Peripherally restricted transthyretin-based delivery system for probes and therapeutics avoiding opioid-related side effects. Nat. Commun 13, 3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramirez-Garcia PD et al. (2023) Targeting endosomal receptors, a new direction for polymers in nanomedicine. J. Mater. Chem. B 11, 5390–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jimenez-Vargas NN et al. (2020) Endosomal signaling of delta opioid receptors is an endogenous mechanism and therapeutic target for relief from inflammatory pain. Proc. Natl. Acad. Sci. U. S. A 117, 15281–15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramírez-García PD et al. (2019) A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat. Nanotechnol 14, 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bonnard E. et al. (2016) Preventive and alleviative effects of the dual enkephalinase inhibitor (Denki) PL265 in a murine model of neuropathic pain. Eur. J. Pharmacol 788, 176–182 [DOI] [PubMed] [Google Scholar]
- 74.Hartrick CT et al. (2020) Dual-acting peripherally restricted delta/kappa opioid (Cav1001) produces antinociception in animal models of sub-acute and chronic pain. J. Pain Res. 13, 2461–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang M. et al. (2021) Development of multifunctional and orally active cyclic peptide agonists of opioid/neuropeptide FF receptors that produce potent, long-lasting, and peripherally restricted antinociception with diminished side effects. J. Med. Chem 64, 13394–13409 [DOI] [PubMed] [Google Scholar]
- 76.Tiwari V. et al. (2020) Activation of μ-d Opioid receptor heteromers inhibits neuropathic pain behavior in rodents. Pain 161, 842–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita W. (2020) The possible role of MOPr-DOPr heteromers and its regulatory protein RTP4 at sensory neurons in relation to pain perception. Front. Cell. Neurosci 14, 609362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sierra S. et al. (2019) Targeting cannabinoid 1 and delta opioid receptor heteromers alleviates chemotherapy-induced neuropathic pain. ACS Pharmacol. Transl. Sci 2, 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guenther KG et al. (2023) Conditional deletion of CB2 cannabinoid receptors from peripheral sensory neurons eliminates CB2-mediated antinociceptive efficacy in a mouse model of carrageenan-induced inflammatory pain. Neuropharmacology 237, 109601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slivicki RA et al. (2020) Positive allosteric modulation of CB1 cannabinoid receptor signaling enhances morphine antinociception and attenuates morphine tolerance without enhancing morphine- induced dependence or reward. Front. Mol. Neurosci 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gado F. et al. (2019) Identification of the first synthetic allosteric modulator of the CB 2 receptors and evidence of its efficacy for neuropathic pain relief. J. Med. Chem 62, 276–287 [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y. et al. (2016) Nerve injury diminishes opioid analgesia through lysine methyltransferase-mediated transcriptional repression of μ-opioid receptors in primary sensory neurons. J. Biol. Chem 291, 8475–8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo Y. et al. (2020) Histone methyltransferase G9a diminishes expression of cannabinoid CB1 receptors in primary sensory neurons in neuropathic pain. J. Biol. Chem 295, 3553–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pun FW et al. (2023) AI-powered therapeutic target discovery. Trends Pharmacol. Sci 44, 561–572 [DOI] [PubMed] [Google Scholar]
- 85.Colloca L. et al. (2017) Neuropathic pain. Nat. Rev. Dis. Prim 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finnerup NB et al. (2015) Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 14, 162–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bannister K. et al. (2020) Neuropathic pain: mechanism-based therapeutics. Annu. Rev. Pharmacol. Toxicol 60, 257–274 [DOI] [PubMed] [Google Scholar]
- 88.Baron R. et al. (2023) Maximizing treatment efficacy through patient stratification in neuropathic pain trials. Nat. Rev. Neurol 19, 53–64 [DOI] [PubMed] [Google Scholar]
- 89.Sachau J. and Baron R. (2023) Precision medicine in neuropathic pain. In Handbook of Experimental Pharmacology 280, pp. 187–210, Springer; [DOI] [PubMed] [Google Scholar]
- 90.Baron R. et al. (2017) Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 158, 261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abboud C. et al. (2021) Animal models of pain: Diversity and benefits. J. Neurosci. Methods 348, 108997. [DOI] [PubMed] [Google Scholar]
- 92.Kankowski S. et al. (2021) Neuropathic pain: Spotlighting anatomy, experimental models, mechanisms, and therapeutic aspects. Eur. J. Neurosci 54, 4475–4496 [DOI] [PubMed] [Google Scholar]
- 93.Hauser AS et al. (2017) Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garami A. et al. (2020) Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol. Ther 208, 107474. [DOI] [PubMed] [Google Scholar]
- 95.Eagles DA et al. (2022) Fifteen years of NaV1. 7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br. J. Pharmacol 179, 3592–3611 [DOI] [PubMed] [Google Scholar]
- 96.Quallo T. et al. (2017) G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. Elife 6, e26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franco R. and Navarro G. (2023) Neuroprotection afforded by targeting G protein-coupled receptors in heteromers and by heteromer-selective drugs. Front. Pharmacol 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davies A. and Tomas A. (2023) Appreciating the potential for GPCR crosstalk with ion channels. Prog. Mol. Biol. Transl. Sci 195, 101–120 [DOI] [PubMed] [Google Scholar]
- 99.Che T. (2021) Advances in the treatment of chronic pain by targeting GPCRs. Biochemistry 60, 1401–1412 [DOI] [PubMed] [Google Scholar]
- 100.Sadeghi M. et al. (2017) Analgesic conopeptides targeting G protein-coupled receptors reduce excitability of sensory neurons. Neuropharmacology 127, 116–123 [DOI] [PubMed] [Google Scholar]
- 101.Varga BR et al. (2023) Strategies towards safer opioid analgesics—A review of old and upcoming targets. Br. J. Pharmacol 180, 975–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berta T. et al. (2023) Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain: an update. Expert Opin. Ther. Targets 21, 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salzer I. et al. (2019) Nociceptor signalling through ion channel regulation via GPCRs. Int. J. Mol. Sci 20, 2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dembla S. et al. (2017) Anti-nociceptive action of peripheral mu-opioid receptors by G-beta-gamma protein-mediated inhibition of TRPM3 channels. Elife 6, e26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su S. et al. (2021) TRPM3 channels play roles in heat hypersensitivity and spontaneous pain after nerve injury. J. Neurosci 41, 2457–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gangadharan V. and Kuner R. (2013) Pain hypersensitivity mechanisms at a glance. Dis. Model. Mech 6, 889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bardoni R. et al. (2014) Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron 81, 1312–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaidya S. et al. (2021) Attenuation of ongoing neuropathic pain by peripheral acting opioid involves activation of central dopaminergic neurocircuitry. Neurosci. Lett 754, 135751. [DOI] [PubMed] [Google Scholar]
- 109.Wang X. et al. (2021) Antinociceptive and antipruritic effects of HSK21542, a peripherally-restricted kappa opioid receptor agonist, in animal models of pain and itch. Front. Pharmacol 12, 773204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scherrer G. et al. (2009) Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137, 1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agarwal N. et al. (2007) Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci 10, 870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mulpuri Y. et al. (2018) Synthetic peripherally-restricted cannabinoid suppresses chemotherapy-induced peripheral neuropathy pain symptoms by CB1 receptor activation. Neuropharmacology 139, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berrocoso E. et al. (2017) Single oral dose of cannabinoid derivate loaded PLGA nanocarriers relieves neuropathic pain for eleven days. Nanomedicine Nanotechnology, Biol. Med 13, 2623–2632 [DOI] [PubMed] [Google Scholar]
- 114.Malin SA and Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol. Pain 6, 1744–8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Metzner K. et al. (2021) Lack of efficacy of a partial adenosine A1 receptor agonist in neuropathic pain models in mice. Purinergic Signal. 17, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cherchi F. et al. (2023) Covalently binding adenosine A3 receptor agonist ICBM irreversibly reduces voltage-gated Ca2+ currents in dorsal root ganglion neurons. Purinergic Signal. DOI: 10.1007/s11302-023-09929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreno-López Y. et al. (2013) Identification of oxytocin receptor in the dorsal horn and nociceptive dorsal root ganglion neurons. Neuropeptides 47, 117–123 [DOI] [PubMed] [Google Scholar]
- 118.González-Hernández A. et al. (2019) Recurrent antinociception induced by intrathecal or peripheral oxytocin in a neuropathic pain rat model. Exp. Brain Res. 237, 2995–3010 [DOI] [PubMed] [Google Scholar]
- 119.Suttle A. et al. (2023) Sensory neuron-TRPV4 modulates temporomandibular disorder pain Via CGRP in mice. J. Pain 24, 782–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benitez SG et al. (2020) Cutaneous inflammation differentially regulates the expression and function of Angiotensin-II types 1 and 2 receptors in rat primary sensory neurons. J. Neurochem 152, 675–696 [DOI] [PubMed] [Google Scholar]