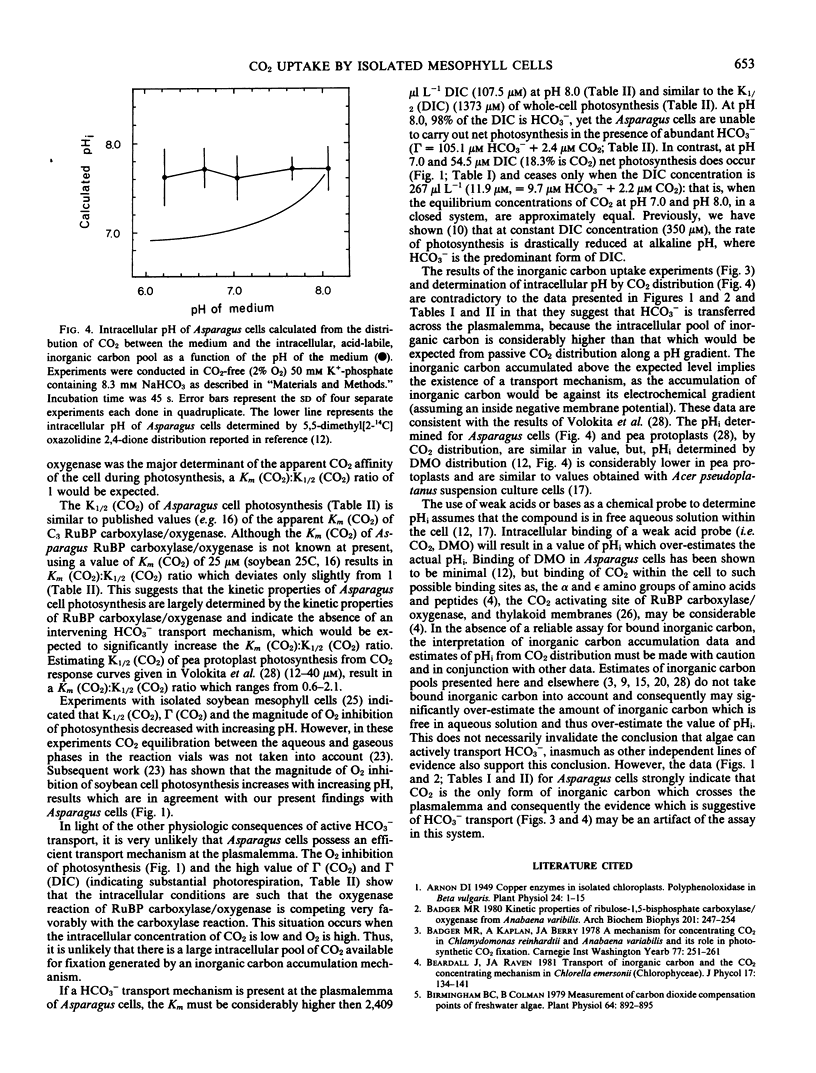
Abstract
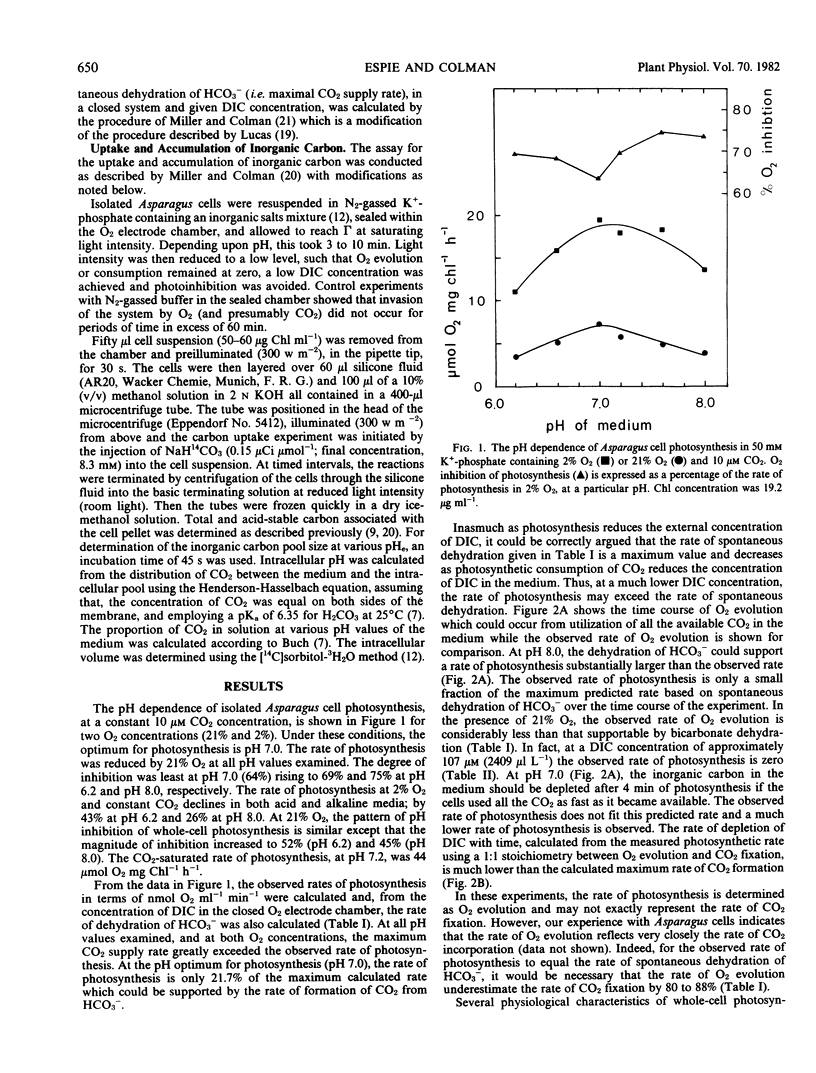
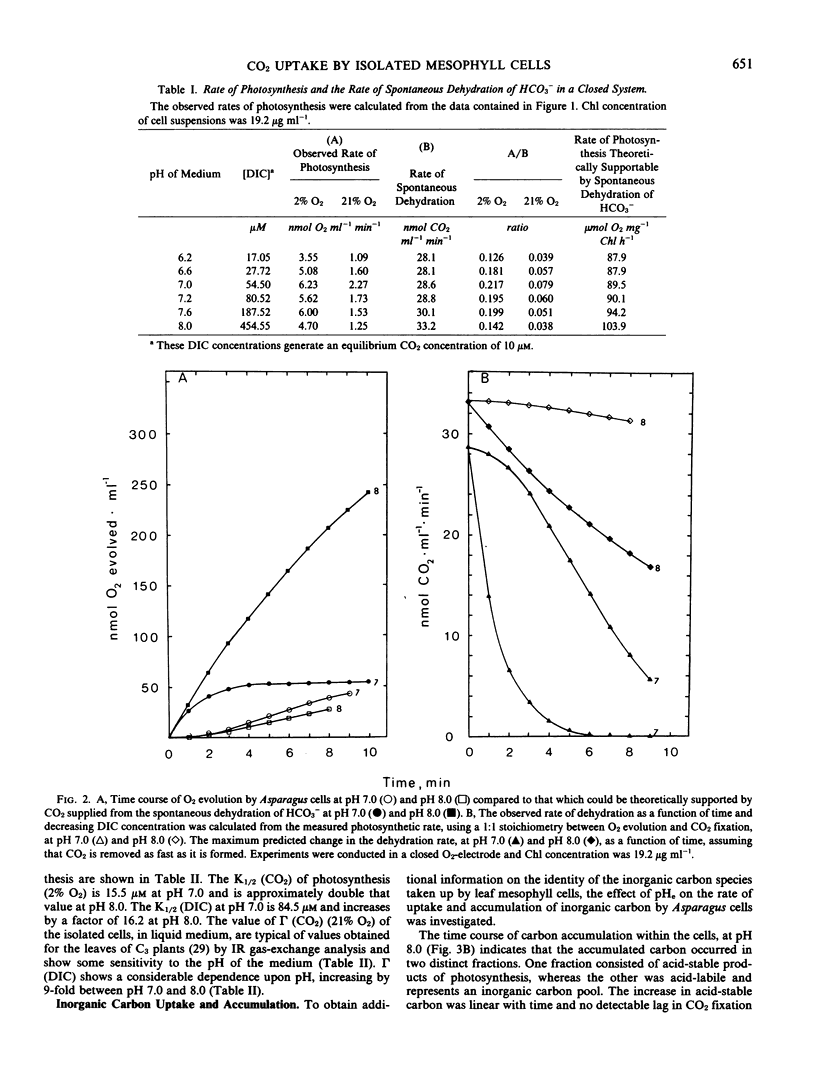
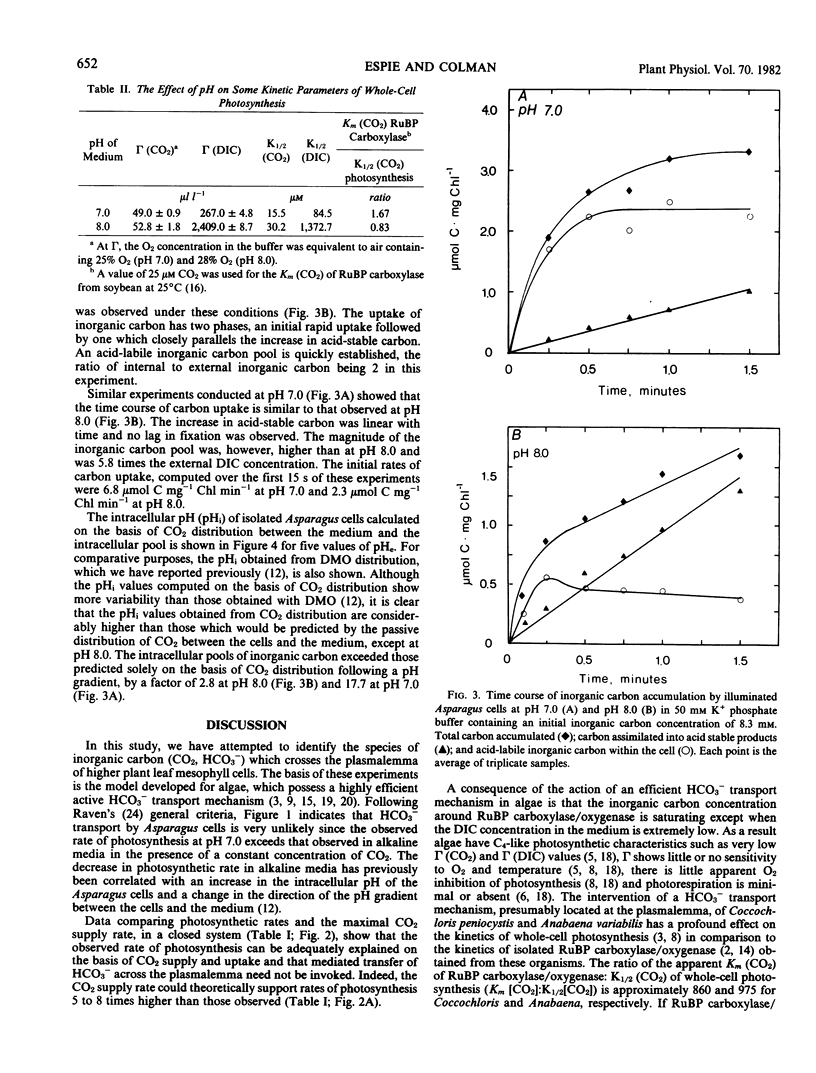
The possibility of HCO3− transport into isolated leaf mesophyll cells of Asparagus sprengeri Regel has been investigated. Measurement of the inorganic carbon pool in these cells over an external pH range 6.2 to 8.0, using the silicone-fluid filtration technique, indicated that the pool was larger than predicted by passive 14CO2 distribution, suggesting that HCO3− as well as CO2 crosses the plasmalemma. Intracellular pH values, calculated from the distribution of 14CO2 between the cells and the medium, were found to be higher (except at pH 8.0) than those previously determined by 5,5-dimethyl[2-14C]oxazolidine-2,4-dione distribution. It is suggested that the inorganic carbon accumulated above predicted concentrations may be bound to proteins and membranes and thus may not represent inorganic carbon actively accumulated by the cells, inasmuch as in a closed system at constant CO2 concentration, the photosynthetic rates at pH 7.0 and 8.0 were 5 to 8 times lower than the maximum rate which could be supported by CO2 arising from the spontaneous dehydration of HCO3−. Furthermore, CO2 compensation points of the cells in liquid media at 21% O2 at pH 7.0 and 8.0, and the K½ CO2 (CO2 concentration supporting the half maximal rate of O2 evolution) at 2% O2 at pH 7.0 and 8.0 are not consistent with HCO3− transport. These results indicate that the principal inorganic carbon species crossing the plasmalemma in these cells is CO2.
Full text
PDF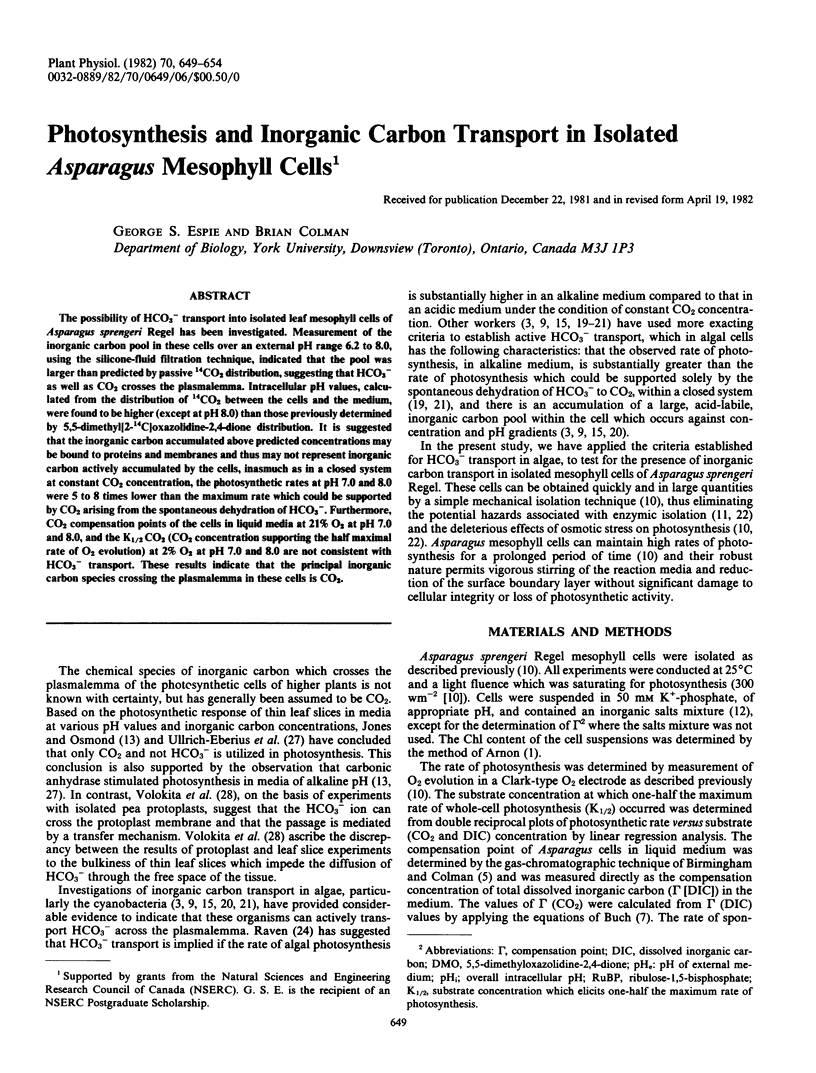

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch Biochem Biophys. 1980 Apr 15;201(1):247–254. doi: 10.1016/0003-9861(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Birmingham B. C., Coleman J. R., Colman B. Measurement of photorespiration in algae. Plant Physiol. 1982 Jan;69(1):259–262. doi: 10.1104/pp.69.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham B. C., Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979 Nov;64(5):892–895. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Effect of oxygen and temperature on the efficiency of photosynthetic carbon assimilation in two microscopic algae. Plant Physiol. 1980 May;65(5):980–983. doi: 10.1104/pp.65.5.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Inorganic Carbon Accumulation and Photosynthesis in a Blue-green Alga as a Function of External pH. Plant Physiol. 1981 May;67(5):917–921. doi: 10.1104/pp.67.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguay J. J. The 5,5-dimethyloxazolidine-2[14C],4-dione distribution technique and the measurement of intracellular pH in Acer pseudoplatanus cells. Biochim Biophys Acta. 1977 Mar 29;497(1):329–333. doi: 10.1016/0304-4165(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Hunt L. D. Comparative biochemistry of ribulose bisphosphate carboxylase in higher plants. Basic Life Sci. 1978;11:127–128. doi: 10.1007/978-1-4684-8106-8_9. [DOI] [PubMed] [Google Scholar]
- Servaites J. C. pH Dependence of Photosynthesis and Photorespiration in Soybean Leaf Cells. Plant Physiol. 1977 Nov;60(5):693–696. doi: 10.1104/pp.60.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler A. The binding of bicarbonate ions to washed chloroplast grana. Biochim Biophys Acta. 1977 Jun 9;460(3):511–522. doi: 10.1016/0005-2728(77)90089-5. [DOI] [PubMed] [Google Scholar]
- Volokita M., Kaplan A., Reinhold L. Evidence for Mediated HCO(3) Transport in Isolated Pea Mesophyll Protoplasts. Plant Physiol. 1981 Jun;67(6):1119–1123. doi: 10.1104/pp.67.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]


