Abstract
Respiratory monitoring is crucial during monitored anaesthesia care (MAC) to ensure patient safety. Patients undergoing procedures like gastrointestinal endoscopy and dental interventions under MAC have a heightened risk of aspiration. Despite the risks, no current system or device can evaluate aspiration risk. This study presents a novel acoustic monitoring system designed to detect fluid retention in the upper airway during MAC. We conducted a prospective observational study with 60 participants undergoing dental treatment under MAC. We utilized a prototype acoustic monitoring system to assess fluid retention in the upper airway by analysing inspiratory sounds. Water was introduced intraorally in participants to simulate fluid retention; artificial intelligence (AI) analysed respiratory sounds pre and post-injection. We also compared respiratory sounds pre-treatment and during coughing events. Coughing was observed in 14 patients during MAC, and 31 instances of apnoea were detected by capnography. However, 27 of these cases had breath sounds. Notably, with intraoral water injection, the Stridor Quantitative Value (STQV) significantly increased; furthermore, the STQV was substantially higher immediately post-coughing in patients who coughed during MAC. In summary, the innovative acoustic monitoring system using AI provides accurate evaluations of fluid retention in the upper airway, offering potential to mitigate aspiration risks during MAC.
Clinical trial number: jRCTs 062220054.
Subject terms: Medical research, Translational research
Introduction
Monitored anaesthesia care (MAC) requires various respiratory monitoring devices to ensure patient safety and optimize anaesthesia delivery. However, certain procedures under anaesthesia, such as gastrointestinal endoscopy and dental procedures, pose a high risk of aspiration due to the overlap between the airway and the operative field1–3. Guidelines for MAC recommend respiratory monitoring using capnography, which measures the concentration of carbon dioxide in exhaled breath and provides continuous monitoring of the patient's ventilation4,5. Capnography can also detect changes in respiratory rate and depth, as well as the presence of airway obstruction. However, capnography cannot detect upper airway water retention, and measurements using nasal gas collection can be affected by various factors, including exhaled gas dilution from nasal cannulas used for oxygen administration, high nasal flow, and breathing pattern6–10. During MAC, anaesthetics such as propofol and benzodiazepine agonists, can reduce the salivary swallow reflex by decreasing peripheral muscle activity. Additionally, mechanical stimulation during the procedure can result in excessive salivary retention in the upper airway, necessitating the anaesthesiologist’s constant vigilance to prevent aspiration11–13.
To prevent aspiration during MAC, it is crucial to detect salivary retention early and take appropriate measures, such as adjusting head position, suctioning saliva, and reducing the dose of anaesthetics. However, diagnosing aspiration through classical auscultation is subjective and requires skill, making it impractical for anaesthesiologist’s to constantly use a stethoscope intraoperatively14–16. To address this issue, the present study developed a novel acoustic monitoring system that objectively and visually evaluates breath sounds. The system displays respiratory sounds as a spectrogram in real time17, and uses an algorithm to analyse irregular breath sounds. Furthermore, artificial intelligence (AI) analysis of inspiratory inflow sounds acquired at the neck enables quantification of the level of water retention in the upper airway18.
This study aimed to confirm the clinical feasibility of an AI acoustic analysis system for detecting fluid retention in the upper airway, and to determine whether respiratory monitoring combined with capnography can quantify aspiration risk.
Methods
Ethical approval
This was a prospective observational study conducted at Hiroshima University Hospital, which was approved by the Certified Review Board of Hiroshima University (approval number: CRB2022-0002). The trial was registered prior to patient enrolment at the Japan Registry of clinical trials (jRCTs062220054, Principal investigator: Yoshitaka Shimizu, link to trial registry, Date of registration: August 31, 2022; https://jrct.niph.go.jp/en-latest-detail/jRCTs062220054). All methods were performed in accordance with the relevant guideline and regulations, including Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines19. Written informed consent was obtained from all participants, who could withdraw at any time.
Participants
Inclusion criteria were: ≧18 years of age, capability to undergo dental procedures on an outpatient basis, dental phobia, abnormal (excessive) gag reflex, and undergoing invasive dental procedures. Exclusion criteria were: allergy and a history of serious adverse reactions to midazolam or propofol, dysphagia, poor oral hygiene, and mental disability. Sixty-seven patients, who underwent supervised anaesthesia management at Hiroshima University Hospital between September and November 2022, were initially enrolled in this study. However, five participants who did not consent to the study and two who had anaesthesiologist protocol errors were excluded.
Anaesthesia and data collection
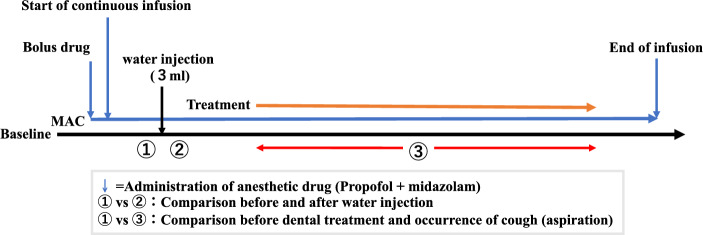
The CARESCAPETM B850 bedside monitor (GE Healthcare, Chicago, IL, USA) was used to measure blood pressure, SPO2, electrocardiogram, and capnogram. Subsequently, the venous route was secured, and propofol (10 or 20 mg bolus, followed by 0.8–4 mg/kg/h as continuous dosage) and midazolam (1–2.5 mg bolus) were administered. Sedation levels were managed within the range of 3–4 on the Observer's Assessment of Alertness/Sedation (OAA/S) scale20. An acoustic respiratory rate (RRa) sensor of the Radical 7 monitor (Masimo Corp., Irvine, CA, USA) was attached to the skin of the left paralarynx using adhesive tape, whereas the sensor of the continuous monitoring system for respiratory sounds was attached to the skin of the right paralarynx. (Fig. 1). Oxygen was administered at 3 L/min through a nasal cannula. Subsequently, in accordance with the recommendations for the swallowing evaluation drinking test, 3 cc of water was injected into the oral cavity using a syringe, and the stridor quantitative value (STQV) was compared before and after the injection21. Dental treatment was then initiated, and STQV was noted when coughing (aspiration) occurred during dental treatment. The quantitative waveforms in each respiratory monitoring device were confirmed using the respiratory sound continuous monitoring system when no respiration was detected for > 20 s, according to the capnogram and RRa. (Fig. 2). Sampling of all subjects was performed in the supine position. The capnogram in the capnography, the audiogram in the RRa, and the spectrogram in the new respiratory sound monitoring system were observed. Electronic data used in this study was from an electronic data capture system (REDCap™).
Figure 1.

The cervical sensor is placed just lateral to the thyroid cartilage and medial to the sternocleidomastoid muscle (on the right side, indicated by a red arrow). The RRa sensor is attached contralaterally (on the left side).
Figure 2.
Anaesthesia and data collection protocol diagram. STQV assessments were performed in a quiet environment to avoid noise effects. MAC monitored anaesthesia care, STQV stridor quantitative value.
Respiratory sound monitoring system and calculation of the STQV
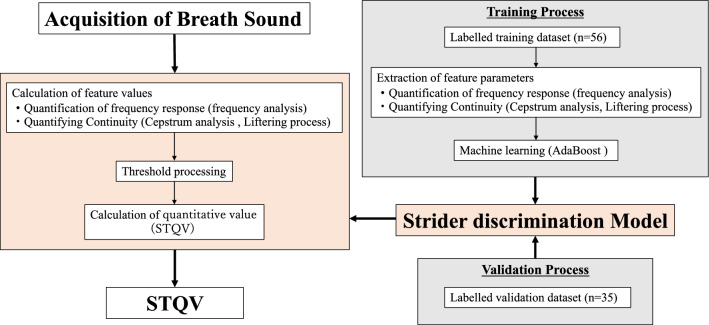
The respiratory sound monitoring system consists of a sensor for breath sound detection, a control unit (prototype) and a laptop computer (DESKTOP-H94L8BJ, Dell Technoligies Inc, Round Rock, TX, USA), and the AI acoustic analysis algorithm for calculating STQV is embedded in a dedicated installed application. (Fig. 3). The STQV was calculated in this study as a quantitative measure of mucus retention using cervical auscultation. An AI analysis algorithm was developed to calculate the STQV, using 56 labelled sounds as the training dataset for machine learning and 35 labelled sounds as the validation dataset. Feature parameters were extracted from snoring, caused by upper airway obstruction because of subsidence of the tongue root, and from stridor sounds, generated by the resonance of water-soluble reservoirs between inspiration and expiration. This extraction utilised frequency analysis, cepstrum analysis, the Liftering process, and other methods.
Figure 3.
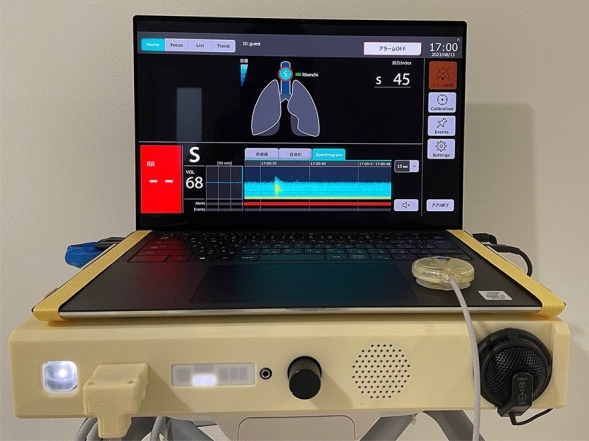
The respiratory sound monitoring system comprises sensors for detecting respiratory sounds and the PC for visualization.
Features are calculated after every extremely short time period (< 100 ms, fixed). If a feature exceeds the threshold determined by machine learning, the period is identified as an "abnormal sound period," and if it does not exceed the threshold, it is identified as a "non-abnormal sound period." The larger the feature, especially when the abnormal sound is loud, the higher is the probability of it being in the "abnormal sound period". A quantitative value (range, 0–1) of abnormal respiratory sounds can be obtained by calculating the ratio between the number of "abnormal sound periods" and the total number of sections (Fig. 4).
Figure 4.
Machine learning algorithms for the values of the strider component. Based on a training dataset of strider sounds recorded from patients and labelled by experts, features were extracted using frequency and cepstrum analyses, and machine learning algorithms were applied.
Sample size and statistical analysis
In this pilot study, it was not possible to obtain abnormal detection data based on STQV in cervical auscultation, so the sample size estimation was based on a previous study by the same research group that used fine crackles sound AI analysis for interstitial lung disease detection. This previous study showed that the fine crackles sound AI index was 0.032 ± 0.023 and 0.121 ± 0.090 in the control and interstitial lung disease group, respectively17, from which an effect size of 0.95 was calculated using the G*Power software (version 3.1.9.6). The final target sample size was estimated as 17 participants using an alpha level of 0.05 and a power of 0.95. However, assuming a 30% incidence of aspiration in MAC, the target number of participants needed was 51. Assuming a participation rate of 0.8, the final target number of patients was set at 60.
SPSS (version 25 for Mac, IBM, Inc, Chicago, IL, USA) was used for statistical analyses, and statistical significance was set at P < 0.05. The STQVs were analysed using a paired t-test.
Results
Sixty participants (35 women and 25 men) were included in this study, with a mean age of 47.9 ± 15 [min–max: 20–84] years, a mean height of 160.2 ± 9.5 [min–max: 140–183] cm, and a mean weight of 60.6 ± 16.3 [min–max: 38–118] kg. Patients with American Society of Anesthesiologists Physical Status Classification 1 or 2 were included. The mean anaesthetic doses of propofol and midazolam used for MAC were 100 ± 44 [min–max: 37–240] mg and 2.2 ± 0.6 [min–max: 1–5] mg, respectively, and the mean anaesthetic duration was 50.4 ± 16.5 [min–max: 21–97] min.
During the MAC procedure, 14 participants developed cough. Apnoea was detected by capnogram during expiratory nasal sampling in 31 cases (52%), of which 27 cases had breath sounds (Fig. 5).
Figure 5.
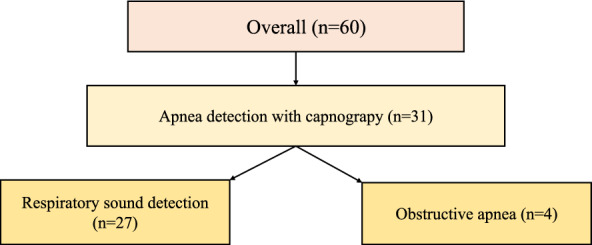
Breakdown of individuals with apnoea detected by capnogram. Respiratory sounds were confirmed using audiograms and spectrograms.
The results of the stridor quantitative value (STQV) analysis based on AI were as follows. The STQV was significantly higher after intraoral water injection than that before (0.136 ± 0.103 vs. 0.05 ± 0.045, P < 0.001 [95% confidence interval (CI); –0.11 to –0.061]; Fig. 6). The STQV pre-treatment and immediately after the onset of coughing in patients who developed cough during MAC (n = 14) was 0.042 ± 0.04 and 0.342 ± 0.038, respectively, indicating that the STQV was significantly higher immediately after the onset of coughing (P < 0.002 [95% CI − 0.47 to − 0.13]; Fig. 7).
Figure 6.
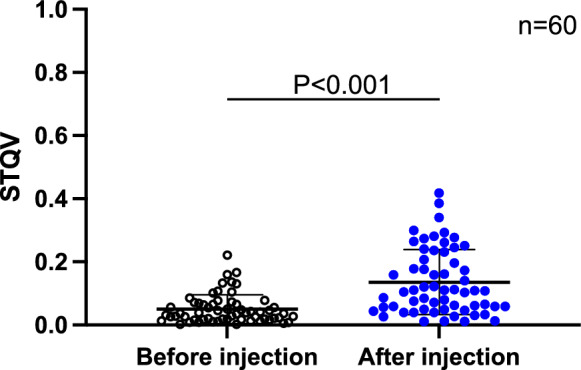
Comparison of the values of STQV before and after intraoral injection of 3 cc of water. The STQV was significantly higher after intraoral water injection than that before (0.136 ± 0.103 vs. 0.05 ± 0.045, P < 0.001 [95% confidence interval (CI) − 0.11 to − 0.061]. The horizontal line indicates the mean, and error bar indicates the standard deviation; analysis was performed using the student’s t-test. STQV stridor quantitative value.
Figure 7.
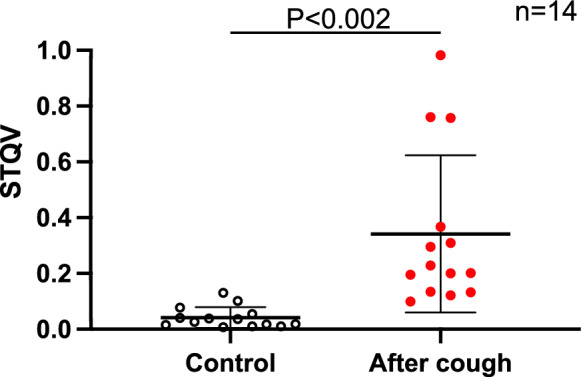
Comparison of the STQV in patients with cough. The STQV pre-treatment and immediately after the onset of coughing in patients who developed cough during MAC (n = 14) was 0.042 ± 0.04 and 0.342 ± 0.038, respectively, indicating that the STQV was significantly higher immediately after the onset of coughing (P < 0.002 [95% CI − 0.47 to − 0.13]. The horizontal line indicates the mean, and error bar indicate standard deviation; analysis was performed using the student’s t-test. STQV stridor quantitative value.
Discussion
In this study, capnography with nasal expiratory gas sampling was effective in detecting apnoea. Notably, of the 31 patients (52%) who had apnoea detected using capnography during MAC, 27 (87%) had breath sounds detected, and obstructive apnoea was present in < 13%. Our results suggest that capnography can detect mouth breathing under MAC.
The STQV, as analysed by AI, increased during the dental procedure due to fluid retention in the upper airway, caused by water injection and bleeding. It was particularly increased following the coughing event.
While the risk of aspiration during MAC can be minimized through preoperative fasting, parasympathetic nerve blockers, and other techniques, such as head elevation and proper patient positioning, it cannot be completely eliminated in all cases. Therefore, patients receiving MAC should be closely monitored for signs of aspiration throughout the perioperative period1–4.
Capnography is a device that can continuously monitor exhaled carbon dioxide concentration and is useful in detecting changes in respiratory rate, respiratory depth, and the presence of airway obstruction. However, it cannot detect aspiration. Moreover, if the sampling tube for capnography is implanted only the nose, measuring capnogram is impossible in mouth breathing6–10. There is a correlation between mouth breathing and aspiration in patients with sleep apnoea22,23. Therefore, this information may be useful to identify patients at risk of aspiration.
The respiratory centre increases alveolar ventilation to regulate breathing during sedation, when there is an acute partial obstruction of the upper airway resulting in symptoms, such as snoring. Therefore, when a partial obstruction causes a decrease in alveolar ventilation, the respiratory centre senses it and prolongs the inspiratory duty cycle (the time spent inhaling relative to the entire respiratory cycle) to stabilize ventilation24–26. However, the prolonged inspiratory duty cycle in the mouth-breathing state further increases the risk of aspiration.
In such a mouth-breathing state, if the level of fluid retention in the upper airway of patients receiving MAC could be accurately determined, the risk of aspiration could be reduced using capnography. The present study’s research group has developed smart stethoscopes that utilize machine learning algorithms to analyse tones and provide real-time analysis results. One of the valuation scales developed, called the STQV, was designed to identify excessive mucus accumulation in the upper airway using its acoustic technology. In the study, the STQV was found to increase in patients who received MAC due to the infusion of water into the upper airway, which is known to increase with salivation, suppression of swallowing function by anaesthetics, and mechanical stimulation by devices inserted through the mouth such as bite blocks. In the present study, intentional intervention (intraoral water injection) increased water retention in the upper airway and STQV, suggesting that the effect was triggered by a fluid retention in the upper airway.
The STQV may not be the most effective method for evaluating aspiration risks because liquid in the larynx may not necessarily produce distinctive sounds that can be detected using acoustic sound monitoring. Nonetheless, the acoustic sound analysis technology using AI analysis can detect increased water retention in the upper airway.
The RRa of an existing respiratory sound monitoring device can detect breath depression (a decrease in breathing rate) before it becomes severe, helping to prevent respiratory failure and the need for invasive care. However, there is little advantage in using the existing device in combination with capnograms, as their measurement points overlap27–31.
Acoustic monitoring using AI analysis technology has the potential to diagnose various pathological conditions, such as wheezing and blistering sounds. The present study confirmed its usefulness in assessing upper airway presence of fluid. In the future, the diagnostic capabilities of this system could be expanded by confirming the location of airway reservoirs and analysing voice patterns17,18,32,33.
This study had some limitations that should be considered when interpreting the results. Firstly, the criteria for sedation management, such as the anaesthetics dosage and sedation level, were not clearly defined in advance and were left to each anaesthesiologist’s discretion. This may have led to variability in the level of sedation and thus influenced the study's findings. Secondly, the STQV values calculated in this study were absolute values and were not calibrated for each patient, which may have contributed to variations in the measurements. Thirdly, this study only included patients undergoing dental treatment who are exposed to a high risk of aspiration33, which therefore, limits the generalizability of the findings to other types of procedures with different aspiration risks. Fourth, the condition of the upper airway fluid reservoir, caused by intraoral water injection performed as an intervention, has different properties from those of saliva or blood; the noises generated may also exhibit different tones. Fifth, the STQV readings can be affected by the noise generated by dental instruments, it was not possible to confirm the exact STQV immediately before this event. It is plausible that it was even higher prior to the coughing than after it. Finally, future research should expand the application of the proposed technique to other medical procedures and examine its validity in a randomized controlled trial.
In summary, the results of this study confirm that an AI-powered respiratory sound diagnostic system can assess upper airway fluid retention in the non-intubated state, suggesting its usefulness for evaluating the risk of aspiration during MAC.
Acknowledgements
We thank Dr. Hitoshi Higuchi of Okayama university, Hideyuki Ohkubo, Mr. Yuya Watanabe and Mr. Yuji Shimizu of Air Water Inc., Mr. Yoshihiro Takayanagi of Nihon Kohden Corp, and Dr. Norifumi Shigemoto and Ms. Yuka Abe of the clinical Research Center at the Hiroshima University Hospital for their assistance.
Author contributions
Y.S. made a study design, collected the data, and drafted the paper. N.S. made a study design and discussed the results. K.O., U.S., H.K., S.I. and E.I. collected the data and discussed the results. S.O. and T.S. advised the study design and discussed the results. Y.M.T. and N.S. supervised the study, analysed the data, and revised the paper. The authors read and approved the final manuscript.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) Grant (21he1602002h0004) and JSPS KAKENHI Grant (JP 21K10072).
Data availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Janik LS, Stamper S, Vender JS, Troianos CA. Pro-Con debate: Monitored anesthesia Care versus General endotracheal anesthesia for endoscopic retrograde cholangiopancreatography. Anesth. Analg. 2022;134:1192–1200. doi: 10.1213/ANE.0000000000005851. [DOI] [PubMed] [Google Scholar]
- 2.Smith ZL, et al. A randomized controlled trial evaluating general endotracheal anesthesia versus monitored anesthesia care and the incidence of sedation-related adverse events during ERCP in high-risk patients. Gastrointest. Endosc. 2019;89:855–862. doi: 10.1016/j.gie.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Smith ZL, Nickel KB, Olsen MA, Vargo JJ, Kushnir VM. Type of sedation and the need for unplanned interventions during ERCP: Analysis of the clinical outcomes research initiative national endoscopic database (CORI-NED) Frontline Gastroenterol. 2020;11:104–110. doi: 10.1136/flgastro-2019-101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists Practice guidelines for sedation and analgesia by non- anesthesiologists. Anesthesiology. 2002;96:1004–1017. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 5.Hinkelbein J, et al. European Society of Anaesthesiology and European Board of anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur. J. Anaesthesiol. 2018;35:6–24. doi: 10.1097/EJA.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 6.Siobal MS. Monitoring exhaled carbon dioxide. Respir. Care. 2016;61:1397–1416. doi: 10.4187/respcare.04919. [DOI] [PubMed] [Google Scholar]
- 7.Saunders R, Struys MMRF, Pollock RF, Mestek M, Lightdale JR. Patient safety during procedural sedation using capnography monitoring: A systematic review and meta-analysis. BMJ Open. 2017;7:e013402. doi: 10.1136/bmjopen-2016-013402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soto RG, Fu ES, Vila H, Jr, Miguel RV. Capnography accurately detects apnea during monitored anesthesia care. Anesth. Analg. 2004;99:379–382. doi: 10.1213/01.ANE.0000131964.67524.E7. [DOI] [PubMed] [Google Scholar]
- 9.Wall BF, Magee K, Campbell SG, Zed PJ. Capnography versus standard monitoring for emergency department procedural sedation and analgesia. Cochrane Database Syst. Rev. 2017;3:CD010698. doi: 10.1002/14651858.CD010698.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lightdale JR, et al. Microstream capnography improves patient monitoring during moderate sedation: A randomized, controlled trial. Pediatrics. 2006;117:e1170–e1178. doi: 10.1542/peds.2005-1709. [DOI] [PubMed] [Google Scholar]
- 11.Bohman JK. Aspiration during monitored anesthesia care. Anesthesiology. 2015;122:471–472. doi: 10.1097/ALN.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 12.Pardo E, Camus M, Verdonk F. Anesthesia for digestive tract endoscopy. Curr. Opin. Anaesthesiol. 2022;35:528–535. doi: 10.1097/ACO.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 13.Savilampi J, Ahlstrand R, Magnuson A, Geijer H, Wattwil M. Aspiration induced by remifentanil: A double-blind, randomized, crossover study in healthy volunteers. Anesthesiology. 2014;121:52–58. doi: 10.1097/ALN.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 14.Green SM, Mason KP, Krauss BS. Pulmonary aspiration during procedural sedation: A comprehensive systematic review. Br. J. Anaesth. 2017;118:344–354. doi: 10.1093/bja/aex004. [DOI] [PubMed] [Google Scholar]
- 15.Parker JD. Pulmonary aspiration during procedural sedation for colonoscopy resulting from positional change managed without oral endotracheal intubation. JA Clin. Rep. 2020;6:53. doi: 10.1186/s40981-020-00360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk JK, Chan DK. Preventing aspiration pneumonia in older people: Do we have the “know-how”? Hong Kong Med. J. 2014;20:421–427. doi: 10.12809/hkmj144251. [DOI] [PubMed] [Google Scholar]
- 17.Horimasu Y, et al. A machine-learning based approach to quantify fine crackles in the diagnosis of interstitial pneumonia: A proof-of-concept study. Med. (Baltim.) 2021;100:e24738. doi: 10.1097/MD.0000000000024738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikutani K, et al. Quantification of respiratory sounds by a continuous monitoring system can be used to predict complications after extubation: A pilot study. J. Clin. Monit. Comput. 2023;37:237–248. doi: 10.1007/s10877-022-00884-4. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Chernik DA, et al. Validity and reliability of the observer’s assessment of alertness/sedation scale: Study with intravenous midazolam. J. Clin. Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 21.Tohara H, et al. Three tests for predicting aspiration without videofluorography. Dysphagia. 2003;18:126–134. doi: 10.1007/s00455-002-0095-y. [DOI] [PubMed] [Google Scholar]
- 22.Koo SK, et al. Acoustic analysis of snoring sounds recorded with a smartphone according to obstruction site in OSAS patients. Eur. Arch. Otorhinolaryngol. 2017;274:1735–1740. doi: 10.1007/s00405-016-4335-4. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, et al. Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function. Sci. Rep. 2023;13:778. doi: 10.1038/s41598-023-27784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton JR, et al. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–1164. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Litman RS, et al. Use of dynamic negative airway pressure (DNAP) to assess sedative-induced upper airway obstruction. Anesthesiology. 2002;96:342–345. doi: 10.1097/00000542-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino Y, et al. The compensatory responses to upper airway obstruction in normal subjects under propofol anesthesia. Respir. Physiol. Neurobiol. 2009;166:24–31. doi: 10.1016/j.resp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson DB, et al. The evaluation of a noninvasive respiratory volume monitor in mechanically ventilated neonates and infants. Anesth. Analg. 2022;134:141–148. doi: 10.1213/ANE.0000000000005562. [DOI] [PubMed] [Google Scholar]
- 28.Atkins JH, Mandel JE. Performance of Masimo rainbow acoustic monitoring for tracking changing respiratory rates under laryngeal mask airway general anesthesia for surgical procedures in the operating room: A prospective observational study. Anesth. Analg. 2014;119:1307–1314. doi: 10.1213/ANE.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 29.Yabuki S, et al. Influences of environmental noise level and respiration rate on the accuracy of acoustic respiration rate monitoring. J. Clin. Monit. Comput. 2018;32:127–132. doi: 10.1007/s10877-017-9997-y. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay MA, et al. The accuracy, precision and reliability of measuring ventilatory rate and detecting ventilatory pause by rainbow acoustic monitoring and capnometry. Anesth. Analg. 2013;117:69–75. doi: 10.1213/ANE.0b013e318290c798. [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg ME, Givony D, Levin R. Acoustic respiration rate and pulse oximetry-derived respiration rate: A clinical comparison study. J. Clin. Monit. Comput. 2020;34:139–146. doi: 10.1007/s10877-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng ZR, et al. Assessing the accuracy of artificial intelligence enabled acoustic analytic technology on breath sounds in children. J. Med. Eng. Technol. 2022;46:78–84. doi: 10.1080/03091902.2021.1992520. [DOI] [PubMed] [Google Scholar]
- 33.Sugimura M, et al. Considerations during intravenous sedation in geriatric dental patients with dementia. Clin. Oral. Investig. 2015;19:1107–1114. doi: 10.1007/s00784-014-1334-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.