Abstract
Background
Many people with atopic eczema are reluctant to use the most commonly recommended treatments because they fear the long‐term health effects. As a result, many turn to dietary supplements as a possible treatment approach, often with the belief that some essential ingredient is 'missing' in their diet. Various supplements have been proposed, but it is unclear whether any of these interventions are effective.
Objectives
To evaluate dietary supplements for treating established atopic eczema/dermatitis.
Evening primrose oil, borage oil, and probiotics are covered in other Cochrane reviews.
Search methods
We searched the following databases up to July 2010: the Cochrane Skin Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE (from 2005), EMBASE (from 2007), PsycINFO (from 1806), AMED (from 1985), LILACS (from 1982), ISI Web of Science, GREAT (Global Resource of EczemA Trials) database, and reference lists of articles. We searched ongoing trials registers up to April 2011.
Selection criteria
Randomised controlled trials (RCTs) of dietary supplements for the treatment of those with established atopic eczema/dermatitis.
Data collection and analysis
Two authors independently screened the titles and abstracts, read the full text of the publications, extracted data, and assessed the risk of bias.
Main results
We included 11 studies with a total of 596 participants. Two studies assessed fish oil versus olive oil or corn oil placebo. The following were all looked at in single studies: oral zinc sulphate compared to placebo, selenium versus selenium plus vitamin E versus placebo, vitamin D versus placebo, vitamin D versus vitamin E versus vitamins D plus vitamin E together versus placebo, pyridoxine versus placebo, sea buckthorn seed oil versus sea buckthorn pulp oil versus placebo, hempseed oil versus placebo, sunflower oil (linoleic acid) versus fish oil versus placebo, and DHA versus control (saturated fatty acids of the same energy value). Two small studies on fish oil suggest a possible modest benefit, but many outcomes were explored. A convincingly positive result from a much larger study with a publicly‐registered protocol is needed before clinical practice can be influenced.
Authors' conclusions
There is no convincing evidence of the benefit of dietary supplements in eczema, and they cannot be recommended for the public or for clinical practice at present. Whilst some may argue that at least supplements do not do any harm, high doses of vitamin D may give rise to serious medical problems, and the cost of long‐term supplements may also mount up.
Keywords: Humans; Dietary Supplements; Dermatitis, Atopic; Dermatitis, Atopic/diet therapy; Fish Oils; Fish Oils/therapeutic use; Plant Oils; Plant Oils/therapeutic use; Pyridoxine; Pyridoxine/therapeutic use; Randomized Controlled Trials as Topic; Selenium; Selenium/therapeutic use; Vitamin D; Vitamin D/therapeutic use; Vitamin E; Vitamin E/therapeutic use; Vitamins; Vitamins/therapeutic use; Zinc; Zinc/therapeutic use
Plain language summary
Dietary supplements for established atopic eczema in adults and children
Eczema is a skin condition characterised by an itchy, red rash, which affects 5% to 20% of people worldwide. There is no cure, but many treatments can help improve the skin's condition, making life easier. In those for whom these treatments do not work well or who fear their long‐term effects, there is often a belief that either something in their diet, or something missing in their diet, is making their eczema worse.
This review looked at the following dietary supplements (products which add ingredients to a diet): fish oil, zinc, selenium, vitamin D, vitamin E, pyridoxine (vitamin B6), sea buckthorn oil, hempseed oil, and sunflower oil.
Three commonly used dietary supplements (evening primrose oil, borage oil, and probiotics) are currently the subject of other Cochrane reviews (Boehm 2003; Boyle 2008).
We looked for trials comparing supplements with placebo (dummy). We included 11 randomised controlled trials (596 participants) when it was clear that the children or adults taking part had atopic eczema. In reviewing the trials, the main outcomes we looked for were evidence of improvement in the symptoms of eczema, such as itching or loss of sleep, in the short‐term (i.e. six weeks). In the longer term, we wanted to see evidence of a reduced need for treatment for the eczema or a reduction in the number of flares. We also looked for evidence of any general improvement in the eczema and in individual symptoms.
Overall, we found no convincing evidence that taking supplements improved the eczema of those involved. In general, studies were small with low numbers of participants and of poor quality in terms of the way they were run. Two trials of fish oil did find slight improvement for the participants in terms of the degree of itchiness and quality of life. However, these trials had small numbers, which means they had little chance of finding real differences if they did exist. That is why larger trials are needed before any recommendations can be made. We found no evidence of adverse (harmful) effects in those who took part in the trials. People sometimes think that supplements can at least do no harm; however, high doses of vitamin D, for example, can cause serious medical problems, and the safety of dietary supplements should not be assumed. The cost of supplements can also mount up.
Background
Description of the condition
Disease definition
Atopic eczema (AE) is a non‐infective, chronic inflammatory skin disease characterised by an itchy, red rash. The terms 'atopic eczema' and 'atopic dermatitis' have been used synonymously throughout this review.
The European Academy of Allergy and Clinical Immunology (EAACI) nomenclature task force has previously defined the term 'atopy' as a personal or familial tendency, or both, usually in childhood and adolescence, to become sensitised to an allergen and to produce IgE antibodies (Johansson 2001).
Up to 40% of children with atopic eczema are not atopic when defined according to allergy tests, such as skin prick tests (Bohme 2001). Others have found that up to two thirds of people with atopic dermatitis are not atopic (Flohr 2004), implying that the continued use of the term 'atopic dermatitis' is problematic. A revised nomenclature for allergy (Johansson 2001) has been updated by the World Allergy Organisation (Johansson 2004). The new nomenclature is based on the mechanisms that initiate and mediate allergic reactions. The term 'eczema' is proposed to replace the provisional term 'atopic eczema/dermatitis syndrome' (AEDS). What is generally known as 'atopic eczema/dermatitis' is probably not one single disease, but rather an aggregation of several diseases with certain characteristics in common. The term 'atopy' cannot be used until an IgE sensitisation has been documented by IgE antibodies in the blood of a person or by a positive skin prick test to common environmental allergens, such as pollen or house dust mite. The term 'eczema' can, therefore, be split into 'atopic eczema' and 'non‐atopic eczema'.
Because most readers of this review will be used to the terms 'atopic eczema' or 'atopic dermatitis' when referring to these conditions, we will use the term 'atopic eczema' in a similar loose way throughout this review on the understanding that the use of the prefix 'atopic' does not imply IgE sensitivity. Where IgE sensitivity has been determined in the studies, we will highlight them accordingly.
Epidemiology and causes
Atopic eczema is the most common inflammatory skin disease of childhood, affecting 15% to 20% of children in the UK at any one time (Hoare 2000). The cumulative prevalence of atopic dermatitis varies from up to 20% in Northern Europe and the USA, to 5% in the South Eastern Mediterranean (Shaw 2011; Thestrup 2002). Prevalence data for the symptoms of atopic eczema were collected in the global ISAAC study (International Study of Asthma and Allergies in Childhood). The results of this study suggest that atopic eczema is a worldwide problem affecting 5% to 20% of children (Odhiambo 2009; Williams 1999). Only 2% of children under the age of 5 years have severe disease, and 84% have mild disease (Emerson 1998). Around 2% of adults have atopic eczema, and many of these have a more chronic and severe form (Charman 2002). Atopic eczema is often associated with other atopic diseases (Beck 2000), e.g. asthma and rhinitis, and sufferers often have a family history of the condition. The incidence of common allergic disease has increased over the last 30 years (Asher 2006). The increase in prevalence of atopic disease in the past three decades appears to be a real phenomenon and has been observed in countries as far apart as Japan, USA, Finland, and Africa (Williams 2008).
The cause of eczema is not well‐understood, but it is probably due to a combination of genetic and environmental factors (Cookson 2002), such as pollution (Polosa 2001), and prenatal or early exposure to infections (Kalliomaki 2002). Research into filaggrin gene mutations has also highlighted the importance of a defective skin barrier as well as altered immune responses (van den Oord 2009).
Clinical features
Atopic eczema may be acute (short and severe) with redness, scaling, oozing, and vesicles; or it may be chronic (long‐term) with skin thickening, altered pigmentation, and exaggerated surface markings. The condition affects mainly the creases of the elbows and knees, and the face and neck, although it can affect any part of the body. The severity of eczema is variable, ranging from localised mild scaling to generalised involvement of the whole body, with redness, oozing, and secondary infection. Itching is the predominant symptom, which can induce a vicious cycle of scratching and, thus, skin damage that in turn leads to more itching ‐ the so‐called "itch scratch itch" cycle. There is a tendency to a dry, sensitive skin, even in those who have 'grown out' of the disease. This is thought to be due to a defect in the barrier of the epidermis (van den Oord 2009). In adulthood, the skin (especially of the hands) may be prone to inflammation in the presence of environmental irritants, such as soaps (Archer 2000).
Natural history
Atopic eczema usually starts within the first 6 months of life, and by 1 year, 60% of those likely to develop it will have done so. Remission occurs by the age of 15 years in 60% to 70% of cases, although some relapse later (Williams 2000). In the more severely affected child, development and puberty may be delayed (Baum 2002).
Impact
Atopic eczema varies in severity, often from one hour to the next. Severity can be measured in a number of ways. The itching and scratching can adversely affect quality of life through chronic sleep disturbance (Meltzer 2008). This may have an impact on family life. The disease can be associated with complications, such as bacterial and viral infections (McHenry 1995). The unsightly appearance of the skin and the need to apply greasy ointments can limit a child's inclination to participate in social and sporting activities and, thus, affect their confidence. Adults with atopic eczema often have low self‐esteem, and relationships can be difficult to initiate and sustain. Everyday tasks, such as housework, gardening, childcare, and food preparation, present problems when the skin on the hands is cracked. Promotion at work may be blocked for people who do not 'look good'.
There is a substantial economic cost not only to the family of the person with atopic eczema (Kemp 2003), but also to the health services of the country as a whole (Herd 1996; Mancini 2008; Verboom 2002). Direct costs to the family are encountered when purchasing treatments, special clothing, and bedding; indirect costs are experienced from lost working days when parents are looking after a sick child. The wider economic implications lie in the costs of healthcare professionals, the lost opportunities of parents of sick children who do not have the option of seeking employment, and the child who, as a result of missing schooling, has limited employment prospects (Su 1997).
Description of the intervention
There is currently no cure for atopic eczema. However, a wide range of treatments are employed that aim to control the symptoms (Fennessy 2000; Hoare 2000; Lamb 2002). Healthcare professionals assist people in the management of their disease using a variety of treatment methods; these include emollients, topical steroids, topical tars, topical tacrolimus, and pimecrolimus (NCC‐WCH & NICE 2007; SIGN 2011). Other treatment methods, such as wet wrap dressings, phototherapy, and complementary therapies (Ernst 2002), are also tried. Many of the treatments are of unknown effectiveness (Hoare 2000). Emollients and topical corticosteroids are universally recommended (Smethurst 2002). In addition, textile (soft clothes) and ambient (dust) measures of control for atopic eczema have been used.
The National Institute for Health Research (NIHR) Health Technology Assessment (HTA) systematic review of treatments for atopic eczema (Hoare 2000) looked at trials that had been conducted on dietary interventions including borage oil, evening primrose oil, pyridoxine, vitamin, and zinc supplementation. This review suggested that there was no clear benefit over placebo with any of these interventions, although no formal meta‐analyses were performed, and the review is now out of date. We could not find any other systematic reviews on this topic.
Diet and atopic eczema
Many people with atopic eczema are reluctant to use the most commonly recommended treatments because they fear the long‐term health effects (Charman 2002). As a result, many turn to dietary supplementation as a possible treatment approach, often with the belief that some essential ingredient is 'missing' in the diet of the affected individual. In other cases, the rationale for use of the supplements may have a sound scientific basis as illustrated below.
A number of dietary supplements have been tried, and these can be broadly classified into the following.
(1) Vitamins, such as pyridoxine (vitamin B6)
Pyridoxine (vitamin B6) is an essential water‐soluble vitamin and a co‐factor in many of the body's chemical pathways. It has been used to treat atopic eczema (Mabin 1995).
(2) Essential fatty acids, such as evening primrose oil (EPO)
Polyunsaturated fatty acids are essential components of all cell membranes. There are 2 families of such essential fatty acids: n‐6 (e.g. linoleic acid and arachidonic acid) and n‐3 (e.g. eicosapentaenoic acid). Some of these substances are precursors of a group of substances called eicosanoids, which may play an important part in the inflammatory and immunological processes of atopic eczema. Fish oils are particularly rich in n‐3 fatty acids, and it has been suggested that these may compete with n‐6 fatty acids in a way that reduces the inflammatory components of atopic eczema. EPO and borage oil are being covered in another Cochrane review currently in preparation (Boehm 2003).
(3) Minerals, such as zinc
Oral supplements of zinc salts have become popular remedies for a range of unrelated medical disorders (Pfeiffer 1978). They have been specifically recommended for the treatment of atopic eczema (Bland 1983; Davies 1987; Franklin 1988), although the scientific basis for this is unclear.
(4) 'Others', such as trace elements (e.g. selenium)
Reduced concentrations of selenium in whole blood, plasma, and white cells; and reduced activity of the selenium‐dependent enzyme, glutathione peroxidase, in red cells have been found in atopic eczema. This has lead to the use of supplements, such as selenium and vitamin E (Fairris 1989a).
(5) Probiotics (friendly gut bacteria)
Probiotics are a treatment that may alter the intestinal microbiota of people with eczema. Probiotics are live micro‐organisms (e.g. Lactobacillus species) that when administered in adequate amounts confer a health benefit on the host. Their precise mode of action is not well‐established, but they have been shown to reduce markers of intestinal inflammation and intestinal permeability in disease states. This might change the way in which antigens that are present in the intestine are recognised by the immune system. Probiotics are dealt with in another Cochrane review (Boyle 2008).
Why it is important to do this review
The rationale for a review on dietary supplements for established atopic eczema is as follows:
(1) previous reviews, such as the UK Health Technology Assessment systematic reviews, are now at least 10 years out of date;
(2) there is a need to cover those dietary supplements that are not mentioned in the other Cochrane reviews of evening primrose oil or probiotics; and
(3) lots of dietary supplements are commonly tried in eczema.
Objectives
To evaluate dietary supplements for treating established atopic eczema/dermatitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of dietary supplements for the treatment of established atopic eczema/dermatitis.
We excluded provocation studies, such as double‐blind food challenges that focused only on the diagnosis of food allergy. However, RCTs of therapeutic interventions that have used food challenges to identify suitable study participants were included.
Types of participants
We included those people who had atopic eczema as diagnosed by a doctor.
In the HTA systematic review of treatments for atopic eczema (Hoare 2000), specific terms were used to identify trial participants as listed in Table 1. The list classifies conditions into 'definite', 'possible', and 'not' atopic eczema ‐ we used this list as a guide.
1. Terms used to categorise trial participants with atopic eczema (AE).
| Definite AE | Possible AE | Not AE |
| Atopic eczema | Periorbital eczema | Seborrhoeic eczema |
| Atopic dermatitis | Childhood eczema | Contact eczema |
| Besnier's prurigo | Infantile eczema | Allergic contact eczema |
| Neurodermatitis atopica (German) | 'Eczema' unspecified | Irritant contact eczema |
| Flexural eczema/dermatitis | Constitutional eczema | Discoid/ nummular eczema |
| ‐ | Endogenous eczema | Asteatotic eczema |
| ‐ | Chronic eczema | Varicose/stasis eczema |
| ‐ | Neurodermatitis | Photo‐/light‐sensitive eczema |
| ‐ | Neurodermatitis (German) | Chronic actinic dermatitis |
| ‐ | ‐ | Dishydrotic eczema |
| ‐ | ‐ | Pompholyx eczema |
| ‐ | ‐ | Hand eczema |
| ‐ | ‐ | Frictional lichenoid dermatitis |
| ‐ | ‐ | Lichen simplex |
| ‐ | ‐ | Occupational dermatitis |
| ‐ | ‐ | Prurigo |
Those studies using terms in the 'not atopic eczema' category, such as 'allergic contact eczema', were excluded. Those studies using terms in the 'possible atopic eczema' category, such as 'childhood eczema', were scrutinised by one or more authors and only included if the description of the participants clearly indicated atopic eczema (i.e. itching and flexural involvement).
Types of interventions
Dietary supplements, such as vegetable and fish oils, vitamins, selenium, and zinc. The comparators are no treatment, placebo, or any other active intervention.
Evening primrose oil (EPO) and borage oil are excluded in this review as they are being covered in a separate Cochrane Review (Boehm 2003), and the protocol has been published in The Cochrane Library. Probiotics have been covered in a separate Cochrane review (Boyle 2008).
Types of outcome measures
Primary outcomes
(a) Short‐term (within six weeks). Changes in participant‐rated or parent‐rated symptoms of atopic eczema, such as pruritus (itching) or sleep loss. (b) Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments.
Secondary outcomes
(a) Global severity as rated by the participants or their physician. If this outcome was not available then the following was used: (b) Global changes in composite rating scales using a published named scale. Where this was not possible, we used the following: (c) The author's modification of existing scales or new scales. Also: (d) Quality of life (Chren 1997; Finlay 1996). (e) Palatability. (f) Adverse events including long‐term consequences on growth.
Tertiary outcome measures
Changes in individual signs of atopic eczema as assessed by a physician, e.g. erythema (redness), purulence (pus formation), excoriation (scratch marks), xerosis (skin dryness), lichenification (thickening of the skin), fissuring (cracks), exudation (weeping serum from the skin surface), pustules (pus spots), papules (spots that protrude from the skin surface), vesicles (clear fluid or 'water blisters' in the skin), crusts (dried serum on skin surface), infiltration/oedema (swelling of the skin), and induration (a thickened feel to the skin).
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 8th July 2010:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library using the search strategy in Appendix 2;
MEDLINE (from 2005) using the search strategy in Appendix 3;
EMBASE (from 2007) using the search strategy in Appendix 4;
PsycInfo (from 1806) using the search strategy in Appendix 3;
AMED (Allied and Complementary Medicine, from 1985) using the search strategy in Appendix 3;
LILACS (Latin American and Caribbean Health Science Literature Information database, from 1982) using the search strategy in Appendix 5;
ISI Web of Science (which contains the Science Citation Index); and
The GREAT (Global Resource for EczemA Trials) database.
The UK and US Cochrane Centres (CCs) have ongoing projects to systematically search MEDLINE and EMBASE for reports of trials, which are then included in the Cochrane Central Register of Controlled Trials. Searching has currently been completed in MEDLINE to 2004 and in EMBASE to 2006. Further searching has been undertaken for this review by the Cochrane Skin Group to cover the years that have not been searched by the UK and US CCs.
A final prepublication search for this review was undertaken on 14th September 2011. Although it has not been possible to incorporate RCTs identified through this search within this review, relevant references are listed under Studies awaiting classification. They will be incorporated into the next update of the review.
Ongoing Trials
We searched the following ongoing trials registries on 14th April 2011, using the following search terms: 'atopic eczema', 'atopic dermatitis', 'supplements', 'vitamins', 'zinc', 'essential fatty acids', and 'selenium'.
The metaRegister of Controlled Trials (www.controlled‐trials.com).
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch).
The Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Searching other resources
Reference from published studies
The bibliographies of included and excluded RCTs were checked for possible references to further RCTs.
Language
No language restrictions were imposed.
Data collection and analysis
Selection of studies
Two authors (FB‐H and CJ) identified titles and abstracts from the searches. If it was clear that the study did not refer to a randomised controlled trial of a supplement for the treatment of established atopic eczema then it was excluded. The same two authors independently assessed each study to determine whether it met the pre‐defined selection criteria. Any differences were resolved through discussion with a third author (HW).
Data extraction and management
Two authors (FB‐H and CJ) independently performed data extraction using a specially designed data extraction form. Any differences in opinion were resolved by a third author (HW). One author (FB‐H) checked and entered the data into RevMan 5.
Assessment of risk of bias in included studies
The 'Risk of bias' assessment included an evaluation of the following components for each included study, since there is some evidence that these are associated with biased estimates of treatment effect (Juni 2001):
(a) the method of generation of the randomisation sequence; (b) the method of allocation concealment ‐ it was considered 'adequate' if the assignment could not be foreseen; (c) who was blinded/not blinded (participants, clinicians, outcome assessors) if this was appropriate; and (d) how many participants were lost to follow up in each treatment group, whether reasons for losses were adequately reported, and whether all participants were analysed in the groups to which they were originally randomised.
In addition, we have reported on the following:
(e) the degree of certainty that the participants had atopic eczema; (f) the baseline comparability of the participants for age, sex, and eczema severity; and (g) assessment of compliance with treatment.
To avoid investigator bias or error, two authors (FB‐H and CJ) independently examined all papers.
Measures of treatment effect
The results are expressed as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes, and difference in means (MD) with 95% CI for continuous outcomes. Where it was not possible to perform a meta‐analysis, the data has been summarised narratively.
Unit of analysis issues
We found one cross‐over study. We would have analysed data from the first phase of this cross‐over study separately before pooling, especially since cross‐over studies may not be appropriate for dietary supplements. However, this study gave no figures, and, therefore, it is described narratively.
Dealing with missing data
If participant dropout led to missing data, we conducted an intention‐to‐treat analysis. For dichotomous outcomes, participants with missing outcome data were regarded as treatment failures and included in the analysis. For continuous outcomes, we would have considered using the last recorded value carried forward of participants with missing outcome data; however, these circumstances did not occur.
Assessment of heterogeneity
Statistical heterogeneity was assessed using the I² statistic. Data were synthesised using meta‐analysis techniques if the I² statistic was less than 80%.
Assessment of reporting biases
We had planned to test publication bias by the use of a funnel plot if adequate data had been available for similar types of intervention.
Data synthesis
For studies with a similar type of active intervention, a meta‐analysis was performed to calculate a weighted treatment effect across trials using a fixed‐effect (DerSimonian and Laird) model. Where it was not possible to perform a meta‐analysis, the data were summarised for each trial.
Subgroup analysis and investigation of heterogeneity
Only two studies were pooled statistically.
If substantial heterogeneity (I² statistic > 50%) had existed between studies for the primary outcome, we would have investigated reasons for heterogeneity.
Had there been adequate information, we would have performed a subgroup analysis for infants (under 1 year old) and children (aged 1 to 16 years) versus adults.
Where participant‐rated symptoms were reported on categorical Likert scales (e.g. no improvement, mild improvement, good improvement, excellent), we dichotomised the data by defining a cut‐off at 'good to excellent improvement'.
Had enough studies used SCORAD (SCORing Atopic Dermatitis), we would have split eczema severity into mild, moderate, and severe (where mild is 0 to 15, moderate is 15 to 40, and severe is > 40).
Other
Where there was uncertainty, we planned to contact trial authors for clarification. However, no clarification was needed from the trial authors. A consumer was part of the review team to ensure the relevance and readability of the final review.
Results
Description of studies
Results of the search
The electronic searches identified a total of 120 studies, of which the full text was sought for 18 studies. After reading the full text, 11 studies were included, 6 were excluded, and 1 was found to be an ongoing study.
Included studies
Eleven RCTs were eligible for inclusion in the systematic review (Bjorneboe 1989; Callaway 2005; Ewing 1991; Fairris 1989; Gimenez‐Arnau 1997; Javanbakht 2011; Koch 2008; Mabin 1995; Sidbury 2008; Soyland 1994; Yang 1999). A total of 596 participants were included in these studies, details of which are listed in the 'Characteristics of included studies' tables. The studies, which allowed participants to continue with what they were prescribed to mimic what would normally happen in practice, fell into the following main categories:
1. Fish oil 2. Zinc 3. Selenium 4. Vitamin D and vitamin E 5. Pyridoxine hydrochloride 6. Sea buckthorn oil 7. Hempseed oil 8. Sunflower oil 9. Docosahexaenoic acid (DHA) supplementation
Design
1. Fish oil We found three RCTs ‐ two were a two‐arm parallel group design (Bjorneboe 1989; Soyland 1994), and one was a three‐arm parallel group design (Gimenez‐Arnau 1997):
fish oil versus placebo (olive oil)
fish oil versus placebo (corn oil)
linoleic acid (from sunflower oil) versus fish oil versus placebo
2. Zinc We found one RCT, and it was a two‐arm parallel group design (Ewing 1991):
zinc versus placebo capsules
3. Selenium We found one RCT, and it was a three‐arm parallel group design (Fairris 1989):
selenium versus selenium plus vitamin E versus placebo
4. Vitamin D and E We found two RCTs ‐ one was a two‐arm parallel group design (Sidbury 2008), and the other was a four‐arm parallel group design (Javanbakht 2011):
vitamin D versus placebo
vitamin D plus vitamin E placebo versus vitamin E plus vitamin D placebo versus vitamins D plus vitamin E versus vitamin D and E placebos
5. Pyridoxine hydrochloride We found one RCT, and it was a two‐arm parallel group design (Mabin 1995):
pyridoxine hydrochloride versus placebo
6. Sea buckthorn oil We found one RCT, and it was a three‐arm parallel group design (Yang 1999):
sea buckthorn seed oil versus sea buckthorn pulp oil versus placebo
7. Hempseed oil We found one RCT, and it was a cross‐over study (Callaway 2005):
hempseed oil versus placebo (olive oil)
8. Sunflower oil We found one RCT, and it was a three‐arm parallel group design (Gimenez‐Arnau 1997):
linoleic acid (from sunflower oil) versus fish oil versus placebo
9. DHA (docosahexaenoic acid) We found one RCT, and it was a two‐arm parallel group design (Koch 2008):
DHA versus isoenergetic (equal energy content) control of saturated fatty acids
Sample Sizes
The number of participants in the studies ranged from 20 (Sidbury 2008) to 145 (Soyland 1994).
Setting
The studies were conducted in Norway (Bjorneboe 1989; Soyland 1994), Finland (Callaway 2005; Yang 1999), the UK (Ewing 1991; Fairris 1989; Mabin 1995), Spain (Gimenez‐Arnau 1997), Iran (Javanbakht 2011), Germany (Koch 2008), and the USA (Sidbury 2008).
Excluded studies
Six studies were excluded after reading the full text; the reasons for exclusion are listed in the 'Characteristics of excluded studies' tables.
Studies awaiting classification
One study (Bautista 2010) was identified when the prepublication search was conducted. Details of this are listed in a 'Characteristics of studies awaiting classification' table.
Ongoing studies
One ongoing study was identified (NCT00879424); details of this study are listed in a 'Characteristics of ongoing studies' table.
Risk of bias in included studies
The methodological quality graph, Figure 1, shows the review authors' judgements about each methodological quality item presented as percentages across all of the included studies.
1.
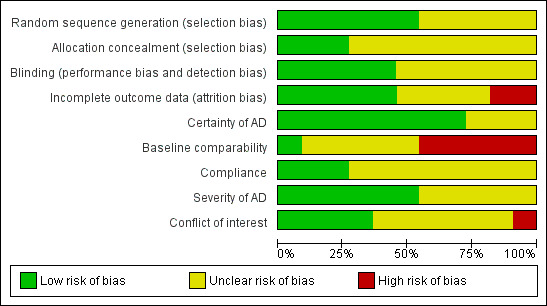
Methodological quality graph: Review authors' judgements about each methodological quality item presented as percentages across all included studies.
The methodological quality summary, Figure 2, shows the review authors' judgements about each methodological quality item for each included study.
2.

Methodological quality summary: Review authors' judgements about each methodological quality item for each included study.
Allocation
In five studies the method of randomisation was not described or was unclear. Six studies clearly described the method of randomisation (Callaway 2005; Fairris 1989; Javanbakht 2011; Koch 2008; Mabin 1995; Sidbury 2008).
Only three studies clearly demonstrated adequate concealment of allocation (Javanbakht 2011; Mabin 1995; Sidbury 2008).
Blinding
One study blinded participants and the outcome assessor (Sidbury 2008). One study blinded participants and clinicians (Javanbakht 2011). One study blinded the participants only (Fairris 1989). Two studies blinded the outcome assessor only (Gimenez‐Arnau 1997; Mabin 1995). These studies were judged at low risk of bias for this domain.
Blinding was unclear in six studies.
Incomplete outcome data
Analysis should be performed according to the intention‐to‐treat (ITT) principle, thus, avoiding bias (Altman 1991; Sackett 1979). However, in many of the studies, analysis of outcomes was carried out only in those participants who completed the study.
Only one small study (n = 11) had no loss to follow up and analysed by intention‐to‐treat (Sidbury 2008). This study was judged at low risk of bias, as were four other studies that stated numbers and reasons for participants lost to follow up (Ewing 1991; Javanbakht 2011; Koch 2008; Mabin 1995).
One study (Fairris 1989) only gave the total number of participants, and it was not clear how many were evaluable in each arm. There was also no mention of ITT. One study (Gimenez‐Arnau 1997) did not mention loss to follow up, possibly due to a highly‐selected population. It was not clear if the analysis was by ITT. These two studies were judged as an unclear risk of bias, as were two other studies that mentioned dropouts, but carried out a per‐protocol (PP) analysis (Bjorneboe 1989; Callaway 2005).
Two studies were judged at high risk of bias for this domain. For the first study (Soyland 1994), 25 participants did not complete the study with no reasons given. In the second study (Yang 1999), 37% of the participants were lost to follow up and a PP analysis was undertaken.
Other potential sources of bias
Degree of certainty that participants had atopic eczema (AE)
The certainty of AE was clear for eight studies that used criteria by Hanifin and/or Rajka. Three studies did not state how they diagnosed atopic eczema (Fairris 1989; Sidbury 2008; Yang 2000).
Baseline comparability of the participants for age, sex, and eczema severity
Only one study (Koch 2008) clearly described baseline comparability and was judged at low risk of bias for this domain.
One study only gave baseline comparability for disease severity (Fairris 1989), so it was judged as unclear.
Four studies (Bjorneboe 1989; Ewing 1991; Javanbakht 2011; Soyland 1994) only gave baseline comparability for participants completing the supplementation, so they were judged as unclear.
Three studies gave no baseline comparability of participants (Callaway 2005; Gimenez‐Arnau 1997; Yang 1999), so they were judged at high risk of bias. The study by Sidbury 2008 gave no details of baseline comparability, and Mabin 1995 mentioned that it had large differences in sex distribution at baseline, so these two studies were judged at high risk of bias.
Assessment of compliance
Compliance was clear in only three studies (Bjorneboe 1989; Soyland 1994; Yang 1999).
Severity of atopic eczema
Severity of atopic eczema was clear for six studies (Fairris 1989; Gimenez‐Arnau 1997; Javanbakht 2011; Mabin 1995; Sidbury 2008; Soyland 1994).
Scales
The rationale for scale development, such as that used in the Fairris study in eczema, is very subjective, despite sounding very quantitative. It is probably not appropriate to use such ordinal data in a quantitative way as several of the studies have done. Furthermore, few of the scales used have been tested adequately for validity, repeatability, and responsiveness to change (Schmitt 2007).
Conflict of interest
Conflict of interest was high for only one study (Callaway 2005), low for four studies (Bjorneboe 1989; Fairris 1989; Javanbakht 2011; Sidbury 2008), and unclear for six studies (Ewing 1991; Gimenez‐Arnau 1997; Koch 2008; Mabin 1995; Soyland 1994; Yang 1999).
Effects of interventions
In this section we have addressed our prespecified outcome measures (Types of outcome measures) in relation to the intervention types we listed in the Included studies section.
1. Fish oil
Two studies were in adults with moderate/severe atopic eczema (Bjorneboe 1989; Soyland 1994). A third study was in adults with severe atopic eczema (Gimenez‐Arnau 1997). Two of these studies were considered sufficiently similar to pool for individual signs of AE (Bjorneboe 1989; Soyland 1994).
The first study (Bjorneboe 1989) was a parallel study of 31 adults (16 to 56 years) over a 12‐week period, which compared extract of fish oil (each capsule also contained an antioxidant, vitamin A, and vitamin D) to an isoenergetic (same energy value) placebo supplement containing olive oil. The main outcomes, which were symptom scores evaluated by the physician, were erythema, scale, visibility, severity, excoriation, weeping, lichenification, and the area affected. Symptom scores evaluated by participants were erythema, visibility, itch, scale, and effect on daily living.
The second study (Soyland 1994) was a parallel study of 145 adults (18 to 64 years) comparing fish oil (each capsule also contained an antioxidant) to a placebo (corn oil). The main outcomes, which were total symptom scores as rated by physicians and participants at 16 weeks, were effects of daily life as rated by the participants, and changes in individual signs of AE as assessed by a physician.
With regard to our primary outcome (a) 'Short‐term (within six weeks). Changes in participant‐rated or parent‐rated symptoms of atopic eczema, such as itching (pruritus) or sleep loss', no data were available for either study.
With regard to our primary outcome (b) 'Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments', there was no significant difference in topical steroid use for the Bjorneboe 1989 study. Only the mean and ranges were given: fish group, 95.8 g (40 to 250 g); and placebo, 77.7 g (10 to 200 g). In the Soyland 1994 study, there was no significant difference in antihistamine or mild topical steroid use ‐ this was only mentioned in the text.
With regard to our secondary outcome (a) 'Global severity as rated by the participants or their physician', analysis of total symptom score as rated by the participant in the first study (Bjorneboe 1989) showed significantly higher overall improvement in the fish oil group, compared to the control group (one study; MD 10.00, 95% CI 0.14 to 19.86) (see Analysis 1.1). Analysis of total symptom score as rated by the physician showed no significant difference in overall improvement when fish oil was compared to placebo. No figures were given for this.
1.1. Analysis.
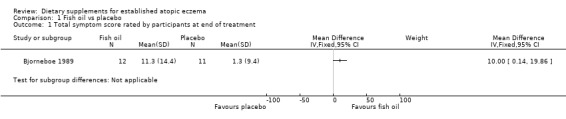
Comparison 1 Fish oil vs placebo, Outcome 1 Total symptom score rated by participants at end of treatment.
In the Soyland 1994 study, the total symptom scores rated by the physician and the participant after treatment significantly improved in both groups; no figures were given for the difference between groups ‐ only mean values were given per group with no standard deviations (fish group, 3.1; and placebo, 3.2).
With regard to our secondary outcome (d) 'Quality of life', there was a significant difference in effect of daily living as evaluated by participants at the end of treatment in favour of fish oil (treatment group), compared to placebo (pooled analysis; 2 studies; MD ‐0.84, 95% CI ‐1.52 to ‐0.15) (see Analysis 1.11).
1.11. Analysis.
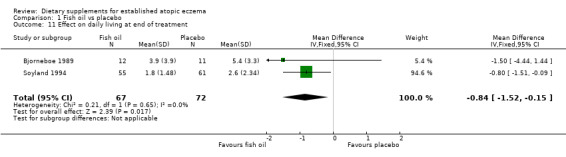
Comparison 1 Fish oil vs placebo, Outcome 11 Effect on daily living at end of treatment.
With regard to our tertiary outcome measure 'Changes in individual signs of atopic eczema as assessed by a physician', no significant difference was found for erythema (pooled analysis; 2 studies; MD 0.38, 95% CI ‐0.36 to 1.13) (see Analysis 1.2), excoriation (1 study; MD 0.40, 95% CI ‐1.24 to 2.04) (see Analysis 1.3), lichenification (pooled analysis; 2 studies; MD 0.04, 95% CI ‐0.70 to 0.78) (see Analysis 1.4), severity (1 study; MD ‐0.30, 95% CI ‐2.23 to 1.63) (see Analysis 1.5), scale (2 studies; MD ‐0.2, 95% CI ‐0.84 to 0.45) (see Analysis 1.6), and visibility (1 study; MD ‐0.40, 95% CI ‐2.49 to 1.69) (see Analysis 1.7).
1.2. Analysis.
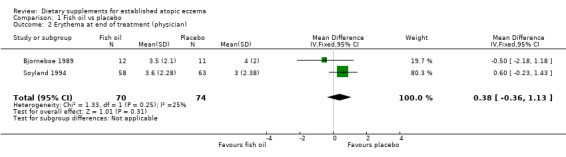
Comparison 1 Fish oil vs placebo, Outcome 2 Erythema at end of treatment (physician).
1.3. Analysis.
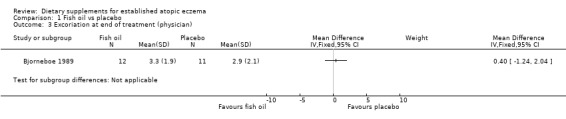
Comparison 1 Fish oil vs placebo, Outcome 3 Excoriation at end of treatment (physician).
1.4. Analysis.
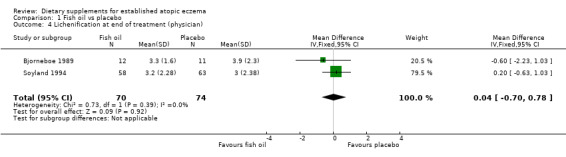
Comparison 1 Fish oil vs placebo, Outcome 4 Lichenification at end of treatment (physician).
1.5. Analysis.
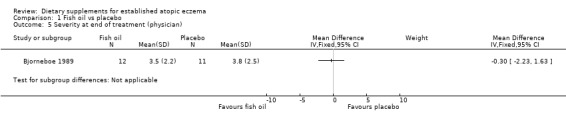
Comparison 1 Fish oil vs placebo, Outcome 5 Severity at end of treatment (physician).
1.6. Analysis.
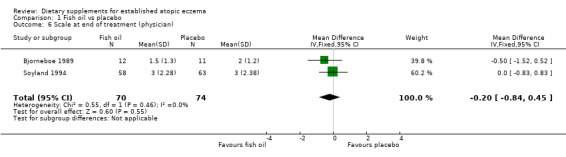
Comparison 1 Fish oil vs placebo, Outcome 6 Scale at end of treatment (physician).
1.7. Analysis.
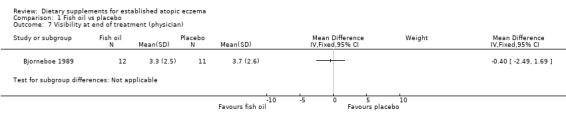
Comparison 1 Fish oil vs placebo, Outcome 7 Visibility at end of treatment (physician).
There was a significant difference in the area affected (pooled analysis; 2 studies; MD ‐0.59, 95% CI ‐1.13 to ‐0.06) (see Analysis 1.8) in favour of fish oil. No significant difference was found for induration (1 study; MD 0.8, 95% CI ‐0.03 to 1.63) (see Analysis 1.9) or pruritus (1 study; MD ‐0.2, 95% CI ‐1.03 to 0.63) (see Analysis 1.10). Results for itch at the end of treatment, as rated by the participant, significantly favoured the fish oil group (1 study; MD ‐2.50, 95% CI ‐4.46 to ‐0.54) (see Analysis 1.12).
1.8. Analysis.
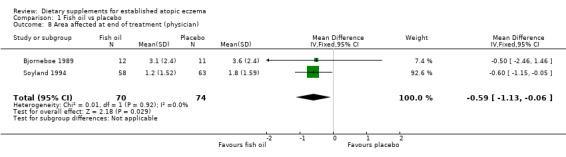
Comparison 1 Fish oil vs placebo, Outcome 8 Area affected at end of treatment (physician).
1.9. Analysis.
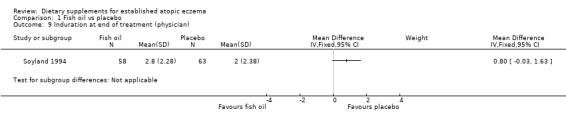
Comparison 1 Fish oil vs placebo, Outcome 9 Induration at end of treatment (physician).
1.10. Analysis.
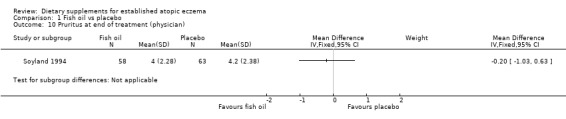
Comparison 1 Fish oil vs placebo, Outcome 10 Pruritus at end of treatment (physician).
1.12. Analysis.
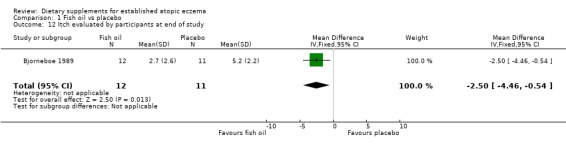
Comparison 1 Fish oil vs placebo, Outcome 12 Itch evaluated by participants at end of study.
Our secondary outcomes (b), (c), (e), and (f) were not addressed by Bjorneboe 1989 or Soyland 1994.
The third study (Gimenez‐Arnau 1997) was a three‐armed parallel study of 48 adults, which compared linoleic acid (from sunflower) versus fish oil versus placebo (oleic acid). The main outcomes were disease severity assessed by the Rajka score and eczema extension assessed by the Rule of Nines. Only six‐week data were given for all three groups due to a high dropout rate in the vegetable oil and placebo group. No numbers were given per arm.
With regard to our primary outcome (a) 'Short‐term (within six weeks). Changes in participant‐rated or parent‐rated symptoms of atopic eczema', such as itching (pruritus) or sleep loss', no data were given for short‐term changes in participant‐rated or parent‐rated symptoms.
With regard to our secondary outcome (b) 'Global changes in composite rating scales using a published named scale', significant differences were reported in the text of Gimenez‐Arnau 1997 in the percentage of Rajka score and Rule of Nine reduction between participants treated with linoleic acid and fish oil, and also between linoleic acid and placebo (oleic acid).
There were no data given for our prespecified tertiary outcome measures in the study by Gimenez‐Arnau 1997.
Neither our primary outcome (b), nor our secondary outcomes (a), (c), (d), (e), and (f), were addressed by Gimenez‐Arnau 1997.
2. Zinc
One parallel study (Ewing 1991) of 50 children (1 to 16 years) compared zinc sulphate versus placebo over 8 weeks. The main outcomes were extent of eczema estimated from percentage surface area of body affected by eczema, severity graded by degree of erythema on an arbitrary scale from one to five, and the surface area affected when multiplied by severity score, which was expressed as a combined disease severity score.
With regard to our primary outcome (a) 'Short‐term (within six weeks). Changes in participant‐rated or parent‐rated symptoms of atopic eczema, such as itching (pruritus) or sleep loss', there was no significant difference in mean itch score at four weeks (no figures given). However, the mean itch score was significantly higher in the zinc group at 8 weeks (MD 1.20, 95% CI 0.02 to 2.38) (see Analysis 2.5).
2.5. Analysis.
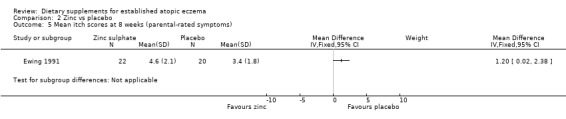
Comparison 2 Zinc vs placebo, Outcome 5 Mean itch scores at 8 weeks (parental‐rated symptoms).
With regard to our secondary outcome (b) 'Global changes in composite rating scales using a published named scale', there was no significant difference in surface area of body affected by eczema at 4 weeks (MD 4.20, 95% CI ‐6.19 to 14.59) (see Analysis 2.6) or 8 weeks (MD 2.90, 95% CI ‐6.08 to 11.88) (see Analysis 2.7).
2.6. Analysis.
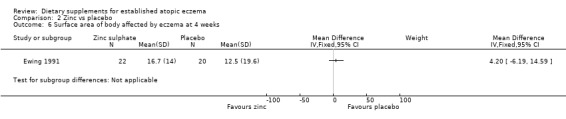
Comparison 2 Zinc vs placebo, Outcome 6 Surface area of body affected by eczema at 4 weeks.
2.7. Analysis.
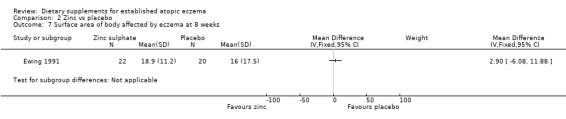
Comparison 2 Zinc vs placebo, Outcome 7 Surface area of body affected by eczema at 8 weeks.
With regard to our secondary outcome (c) 'The author's modification of existing scales or new scales', there was no significant difference in combined disease severity score at 4 weeks (MD 4.00, 95% CI ‐43.07 to 51.07) (see Analysis 2.1) or 8 weeks (MD 9.40, 95% CI ‐25.87 to 44.67) (see Analysis 2.2).
2.1. Analysis.
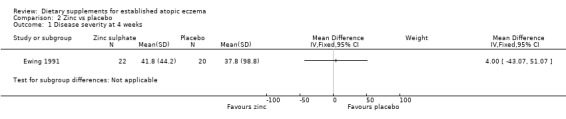
Comparison 2 Zinc vs placebo, Outcome 1 Disease severity at 4 weeks.
2.2. Analysis.
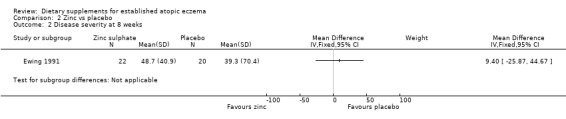
Comparison 2 Zinc vs placebo, Outcome 2 Disease severity at 8 weeks.
With regard to our tertiary outcome measure 'Changes in individual signs of atopic eczema as assessed by a physician, e.g. erythema (redness)', there was no significant difference in erythema as assessed by the physician at 4 weeks (MD 0.00, 95% CI ‐0.53 to 0.53) (see Analysis 2.3) or 8 weeks (MD 0.50, 95% CI ‐0.04 to 1.04) (see Analysis 2.4).
2.3. Analysis.
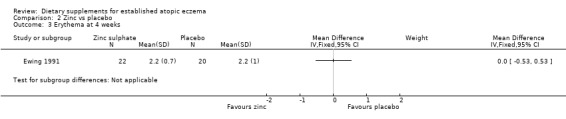
Comparison 2 Zinc vs placebo, Outcome 3 Erythema at 4 weeks.
2.4. Analysis.
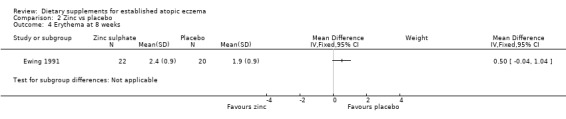
Comparison 2 Zinc vs placebo, Outcome 4 Erythema at 8 weeks.
Our primary outcomes (b) and (c); and our secondary outcomes (a), (b), (d), (e), and (f) were not addressed by Ewing 1991.
3. Selenium
One 3‐arm parallel group design study (Fairris 1989) of 60 adults compared selenium versus selenium plus vitamin E versus placebo over 12 weeks. The main outcomes were disease severity by separate assessment of the head plus neck, arms, trunk plus legs; and the degree of inflammation, lichenification, and scaliness on a scale of zero to four, adding the three values and multiplying them to give a weighted total score.
There were no data available for any of our prespecified primary and tertiary outcomes. None of our secondary outcomes were addressed apart from (a).
For our secondary outcome (a) 'Global severity as rated by the participants or their physician', there were no significant differences between mean severity score in the 3 groups at 12 weeks ‐ only means given with no standard deviations or number of participants per arm (selenium, 13.7; selenium plus vitamin E, 15.3; and placebo, 14.5).
4. Vitamin D and vitamin E
Two RCTs studied vitamin D and vitamin E: one four‐arm parallel group design (Javanbakht 2011) and one two‐arm parallel group design (Sidbury 2008).
The first study (Javanbakht 2011) was a 4‐arm parallel group design of 52 adults (13 to 45 years), which compared vitamin D plus vitamin E placebo versus vitamin E plus vitamin D placebo versus vitamins D plus vitamin E versus vitamin D and E placebos over 60 days. The main outcomes were severity of eczema using SCORAD.
With regard to our primary outcome (b) 'Degree of long‐term (over six months) control, such as reduction in number of flares or reduced need for other treatments', there was no significant difference in the use of topical steroids for vitamin D (MD ‐0.31, 95% CI ‐0.97 to 0.35) (see Analysis 3.11), vitamin E (MD ‐0.34, 95% CI ‐0.97 to 0.29) (see Analysis 4.9), or vitamin D plus E (MD ‐0.42, 95% CI ‐1.05 to 0.21) (see Analysis 5.9) when compared to placebo.
3.11. Analysis.
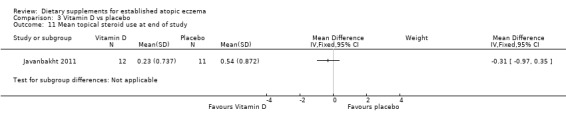
Comparison 3 Vitamin D vs placebo, Outcome 11 Mean topical steroid use at end of study.
4.9. Analysis.
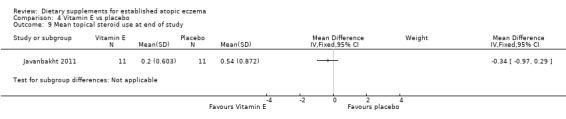
Comparison 4 Vitamin E vs placebo, Outcome 9 Mean topical steroid use at end of study.
5.9. Analysis.
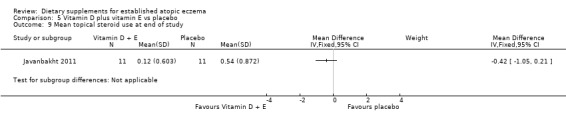
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 9 Mean topical steroid use at end of study.
With regard to our secondary outcome (b) 'Global changes in composite rating scales using a published named scale', there was no significant difference in SCORAD at the end of treatment when vitamin D was compared to placebo (MD 1.00, 95% CI ‐7.04 to 9.04) (see Analysis 3.1), or when vitamin E was compared to placebo (MD ‐1.90, 95% CI ‐9.43 to 5.63) (see Analysis 4.1). There was no significant difference in objective SCORAD at the end of treatment when vitamin D was compared to placebo (MD 1.90, 95% CI ‐3.23 to 7.03) (see Analysis 3.2), or when vitamin E was compared to placebo (MD 0.20, 95% CI ‐4.79 to 5.19) (see Analysis 4.2).
3.1. Analysis.
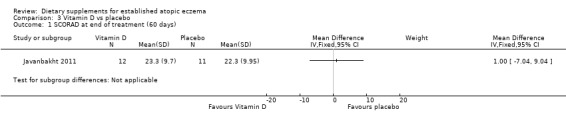
Comparison 3 Vitamin D vs placebo, Outcome 1 SCORAD at end of treatment (60 days).
4.1. Analysis.
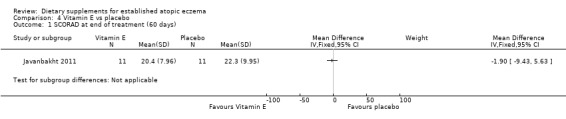
Comparison 4 Vitamin E vs placebo, Outcome 1 SCORAD at end of treatment (60 days).
3.2. Analysis.
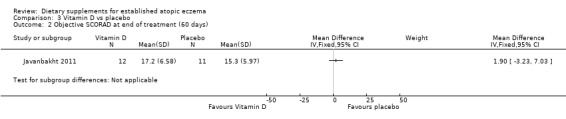
Comparison 3 Vitamin D vs placebo, Outcome 2 Objective SCORAD at end of treatment (60 days).
4.2. Analysis.
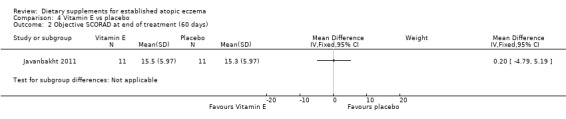
Comparison 4 Vitamin E vs placebo, Outcome 2 Objective SCORAD at end of treatment (60 days).
However, there was a significant difference in favour of vitamin D plus E compared to placebo for SCORAD at the end of treatment (MD ‐9.8, 95% CI ‐17.21 to ‐2.39) (see Analysis 5.1) and objective SCORAD at the end of treatment (MD ‐14.10, 95% CI ‐18.95 to ‐9.25) (see Analysis 5.2).
5.1. Analysis.
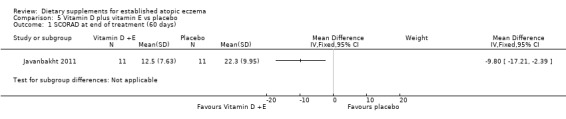
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 1 SCORAD at end of treatment (60 days).
5.2. Analysis.
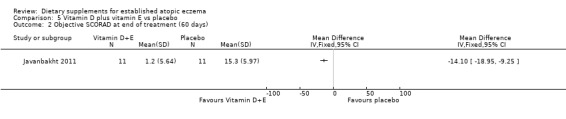
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 2 Objective SCORAD at end of treatment (60 days).
With regard to our tertiary outcome measure 'Changes in individual signs of atopic eczema as assessed by a physician', there were no significant differences in erythema as assessed by the clinician at the end of treatment for vitamin D versus placebo (MD ‐0.20, 95% CI ‐0.47 to 0.07) (see Analysis 3.3), or vitamin E versus placebo (MD 0.00, 95% CI ‐2.16 to 2.16) (see Analysis 4.3).
3.3. Analysis.
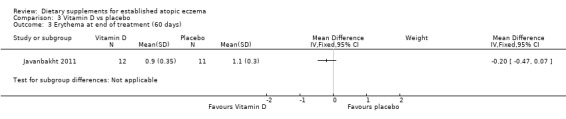
Comparison 3 Vitamin D vs placebo, Outcome 3 Erythema at end of treatment (60 days).
4.3. Analysis.
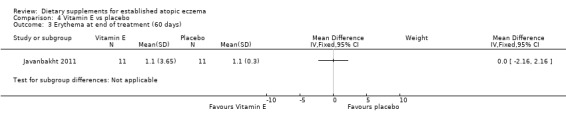
Comparison 4 Vitamin E vs placebo, Outcome 3 Erythema at end of treatment (60 days).
There was a significant difference in erythema for vitamin D plus E versus placebo (MD ‐0.5, 95% CI ‐0.76 to ‐0.24) (see Analysis 5.3).
5.3. Analysis.
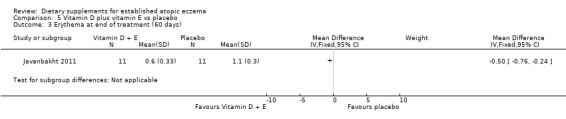
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 3 Erythema at end of treatment (60 days).
There was no significant difference in oedema as assessed by the clinician at the end of treatment for vitamin D versus placebo (MD ‐0.10, 95% CI ‐0.33 to 0.13) (see Analysis 3.4), vitamin E versus placebo (MD ‐0.10, 95% CI ‐0.54 to 0.34) (see Analysis 4.4), or vitamin D plus E versus placebo (MD ‐0.40, 95% CI ‐0.84 to 0.04) (see Analysis 5.4).
3.4. Analysis.
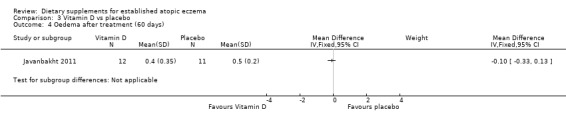
Comparison 3 Vitamin D vs placebo, Outcome 4 Oedema after treatment (60 days).
4.4. Analysis.
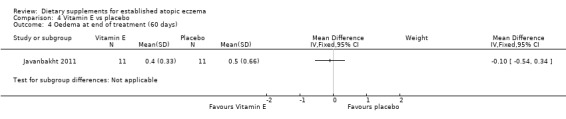
Comparison 4 Vitamin E vs placebo, Outcome 4 Oedema at end of treatment (60 days).
5.4. Analysis.
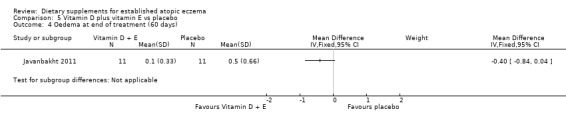
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 4 Oedema at end of treatment (60 days).
There was no significant difference in oozing as assessed by the clinician at the end of treatment for vitamin D versus placebo (MD 0.02, 95% CI ‐0.26 to 0.30) (see Analysis 3.5).
3.5. Analysis.
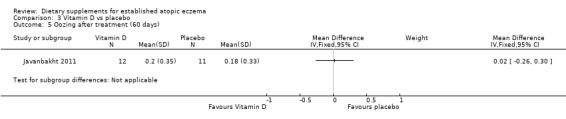
Comparison 3 Vitamin D vs placebo, Outcome 5 Oozing after treatment (60 days).
There was no significant difference in excoriation as assessed by the clinician at the end of treatment for vitamin D versus placebo (MD 0.10, 95% CI ‐0.34 to 0.54) (see Analysis 3.6), vitamin E versus placebo (MD 0.10, 95% CI ‐0.34 to 0.54) (see Analysis 4.5), or vitamin D plus E versus placebo (MD ‐0.3, 95% CI ‐0.74 to 0.14) (see Analysis 5.5).
3.6. Analysis.
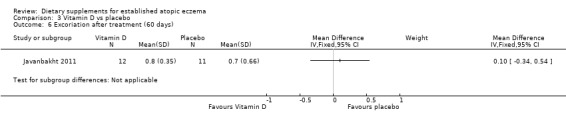
Comparison 3 Vitamin D vs placebo, Outcome 6 Excoriation after treatment (60 days).
4.5. Analysis.
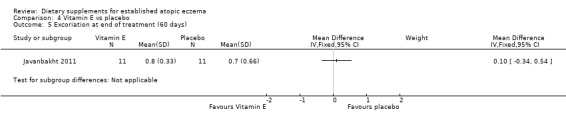
Comparison 4 Vitamin E vs placebo, Outcome 5 Excoriation at end of treatment (60 days).
5.5. Analysis.
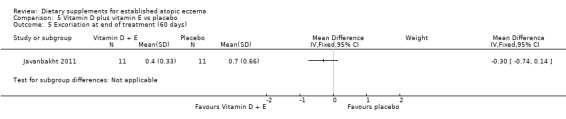
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 5 Excoriation at end of treatment (60 days).
There was no significant difference in lichenification for vitamin D versus placebo (MD 0.10, 95% CI ‐0.34 to 0.54) (see Analysis 3.7), or vitamin D plus E versus placebo (MD ‐0.40, 95% CI ‐0.95 to 0.15) (see Analysis 5.6). However, there was a significant difference in lichenification when vitamin E was compared to placebo (MD ‐0.60, 95% CI ‐1.04 to ‐0.16) (see Analysis 4.6).
3.7. Analysis.
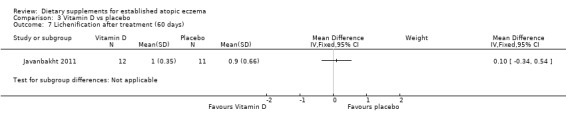
Comparison 3 Vitamin D vs placebo, Outcome 7 Lichenification after treatment (60 days).
5.6. Analysis.
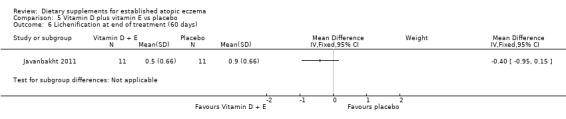
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 6 Lichenification at end of treatment (60 days).
4.6. Analysis.
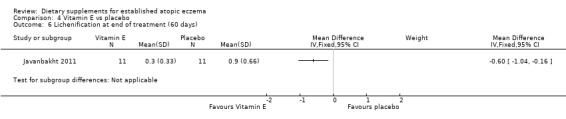
Comparison 4 Vitamin E vs placebo, Outcome 6 Lichenification at end of treatment (60 days).
There was no significant difference in dryness for vitamin D versus placebo (MD ‐0.20, 95% CI ‐0.45 to 0.05) (see Analysis 3.8), but dryness was significantly better in the vitamin E group compared to placebo (MD ‐1.10, 95% CI ‐1.38 to ‐0.82) (see Analysis 4.7), and significantly better in the vitamin D plus E group when compared to placebo (MD ‐0.30, 95% CI ‐0.58 to ‐0.02) (see Analysis 5.7).
3.8. Analysis.
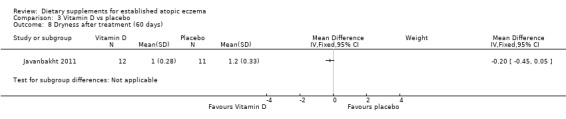
Comparison 3 Vitamin D vs placebo, Outcome 8 Dryness after treatment (60 days).
4.7. Analysis.
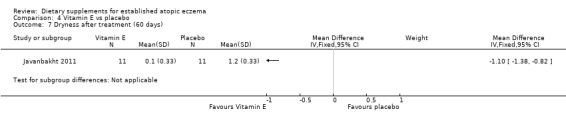
Comparison 4 Vitamin E vs placebo, Outcome 7 Dryness after treatment (60 days).
5.7. Analysis.
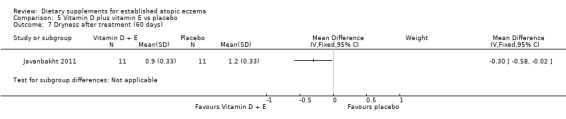
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 7 Dryness after treatment (60 days).
There was no significant difference in pruritis for vitamin D versus placebo (MD 1.00, 95% CI ‐0.96 to 2.96) (see Analysis 3.9), or vitamin E versus placebo for pruritus (MD ‐0.60, 95% CI ‐2.13 to 0.93) (see Analysis 4.8). Pruritis was significantly better in the vitamin D and vitamin E group compared with placebo (MD ‐2.0, 95% CI ‐3.53 to ‐0.47) (see Analysis 5.8).
3.9. Analysis.
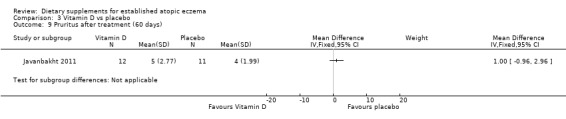
Comparison 3 Vitamin D vs placebo, Outcome 9 Pruritus after treatment (60 days).
4.8. Analysis.
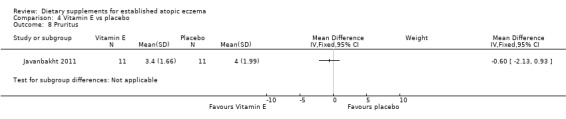
Comparison 4 Vitamin E vs placebo, Outcome 8 Pruritus.
5.8. Analysis.
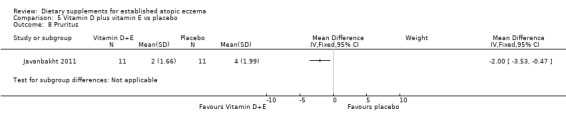
Comparison 5 Vitamin D plus vitamin E vs placebo, Outcome 8 Pruritus.
Javanbakht 2011 did not address our primary outcomes (a) or (c); or our secondary outcomes (a), (c), (d), (e), and (f).
The second study (Sidbury 2008) compared vitamin D versus placebo in 11 children (aged 2 to 13 years) with mild AE over 1 month. The main outcome was the Investigator's Global Assessment (IGA) after one month.
There was no data available for any of our prespecified primary and tertiary outcomes or any of our secondary outcomes except (a).
With regard to our secondary outcome (a) 'Global severity as rated by the participants or their physician', there was no significant difference in Investigator's Global Assessment (IGA) for the number of participants who stayed the same or got worse after 1 month of treatment (RR 0.24, 95% CI 0.04 to 1.44) (see Analysis 3.10).
3.10. Analysis.
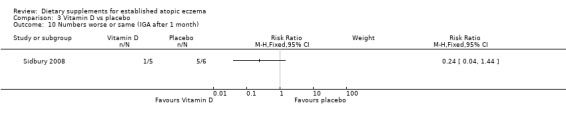
Comparison 3 Vitamin D vs placebo, Outcome 10 Numbers worse or same (IGA after 1 month).
There was no significant difference in the change of mean Eczema Area and Severity Index (EASI) scores between the 2 groups (2.4, 95% CI, ‐8.4 to 3.7) (P = 0.40).
5. Pyridoxine hydrochloride (vitamin B6)
One study (Mabin 1995) of 48 children (older than 12 months) compared pyridoxine hydrochloride with placebo over 4 weeks. The main outcomes were global severity and parental‐rated symptoms of eczema after 4 weeks.
For our primary outcome (a)'Changes in participant‐rated or parent‐rated symptoms of atopic eczema', there was no significant difference in parental‐rated symptoms of eczema between the 2 groups at the end of treatment (RR 1.05, 95% CI 0.83 to 1.33) (see Analysis 6.1).
6.1. Analysis.
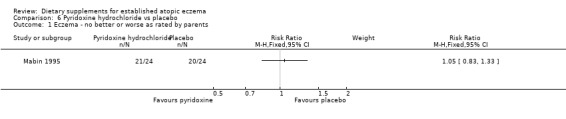
Comparison 6 Pyridoxine hydrochloride vs placebo, Outcome 1 Eczema ‐ no better or worse as rated by parents.
For our secondary outcome (a) 'Global severity as rated by the participants or their physician', there was no significant difference between the groups in global severity after 4 weeks ‐ only medians and ranges were given in the text: vitamin B6 = 109 (16 to 334) and placebo = 77 (13.3 to 346).
There were no data available for our prespecified primary outcomes (b) and (c), all our secondary outcomes except (a), and for our tertiary outcome measures.
6. Sea buckthorn oil
One study (Yang 1999) of 78 adults compared sea buckthorn seed oil versus sea buckthorn pulp oil versus placebo over 4 months. The main outcome was severity of atopic eczema measured by SCORAD.
There were no data available for any of our prespecified primary and tertiary outcomes, or any of our secondary outcomes except (b).
For our secondary outcome (b)'Global changes in composite rating scales using a published named scale', there was no significant difference in SCORAD at 4 weeks for sea buckthorn oil versus placebo (MD ‐5.70, 95% CI ‐17.75 to 6.35) (see Analysis 7.1), or for buckthorn pulp versus placebo (MD 0.80, 95% CI ‐11.32 to 12.92) (see Analysis 8.1).
7.1. Analysis.
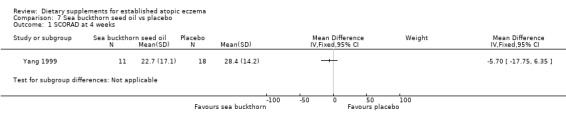
Comparison 7 Sea buckthorn seed oil vs placebo, Outcome 1 SCORAD at 4 weeks.
8.1. Analysis.
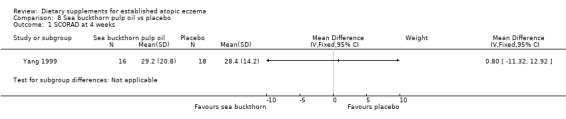
Comparison 8 Sea buckthorn pulp oil vs placebo, Outcome 1 SCORAD at 4 weeks.
There was no significant difference in SCORAD at 4 months for sea buckthorn oil versus placebo (MD 1.40, 95% CI ‐11.31 to 14.11) (see Analysis 7.2), or for buckthorn pulp versus placebo (MD 6.2, 95% CI ‐6.2 to 18.6) (see Analysis 8.2).
7.2. Analysis.
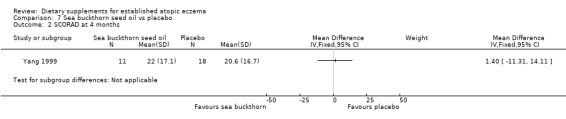
Comparison 7 Sea buckthorn seed oil vs placebo, Outcome 2 SCORAD at 4 months.
8.2. Analysis.
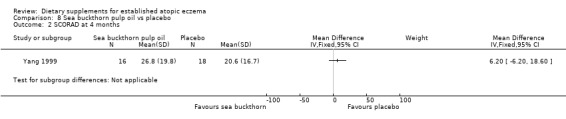
Comparison 8 Sea buckthorn pulp oil vs placebo, Outcome 2 SCORAD at 4 months.
7. Hempseed oil
One cross‐over study (Callaway 2005) of 20 adults (aged 25 to 60 years) compared hempseed oil versus placebo (olive oil), with 2 8‐week intervention periods and a 4‐week washout. The main outcome was severity of atopic dermatitis measured by participants' perceptions of changes in skin dryness and itchiness.
Subjective decreases in both skin dryness and itchiness were statistically significant after hempseed oil intervention (P = 0.027 and 0.023, respectively, as intra‐group comparisons). No such improvements were observed after olive oil intervention.
None of our prespecified primary, secondary, or tertiary outcomes were addressed by this study.
8. Sunflower oil
One study (Gimenez‐Arnau 1997) of 48 adults with chronic or severe atopic eczema, compared linoleic acid from sunflower oil versus fish oil versus placebo over 12 weeks. The main outcome was disease severity assessed by Rajka and eczema extension assessed by the Rule of Nines.
There were no data available for any of our prespecified primary and tertiary outcomes, or for our secondary outcomes except for (b).
For our secondary outcome (b) 'Global changes in composite rating scales using a published named scale', the text quoted a significant difference in the per cent reduction of the Rajka score and the Rule of Nines between sunflower oil and fish oil and between sunflower oil and placebo; however, no numbers were given for the number of participants in each arm.
9. Docosahexaenoic acid (DHA) supplementation
One study (Koch 2008) of 53 adults with atopic eczema compared DHA versus an isoenergetic control over 8 weeks. Global reduction in SCORAD was measured at eight weeks.
There were no data available for our prespecified primary outcomes or for our secondary outcomes except (b).
For our secondary outcome (b), the paper reported a significant reduction in SCORAD from baseline to eight weeks for the DHA group, but not for the isoenergetic control group.
For our tertiary outcomes, the paper reported that the diminished SCORAD in the DHA group was mainly attributed to the significant reduction of the affected areas from 12 (2 to 41 SCORAD points (median, range) at baseline to 7 (2 to 25) at week 8 (P = 0.02 calculated using Wilcoxon test for nonparametrical, paired data).
Discussion
Summary of main results
This systematic review identified 11 randomised controlled trials of dietary supplements for the treatment of established atopic eczema. Two studies assessed fish oil versus olive oil or corn oil placebo. Oral zinc sulphate was compared to placebo in one study, and other single RCTs compared selenium versus selenium plus vitamin E versus placebo, vitamin D versus placebo, vitamin D versus vitamin E versus vitamins D and E together versus placebo, pyridoxine versus placebo, sea buckthorn seed oil versus sea buckthorn pulp oil versus placebo, hempseed oil versus placebo, sunflower oil (linoleic acid) versus fish oil versus placebo, and DHA versus control (saturated fatty acids of the same energy value).
Most of the studies evaluated were far too small in size to exclude even large treatment differences, so it is not surprising that most have failed to show any treatment differences. Such small studies are similarly unable to conclude that the treatments were ineffective due to the lack of precision of effects estimates inherent in such small studies.
Some of the interventions tested were quite complex in terms of combination of various products that might have had opposing beneficial or harmful effects. For example, the study by Bjorneboe included a combination of n‐3 and n‐6 fatty acids along with vitamins A, D, and E. At such an early stage of research, it is probably better to explore the effects of single compounds in placebo‐controlled studies so that each can be retained or eliminated as appropriate.
The three studies (Bjorneboe 1989; Gimenez‐Arnau 1997; Soyland 1994) looking at fish oil versus placebo found no significant differences for any of the primary outcomes. However, pooled analysis of two of the studies (Bjorneboe 1989; Soyland 1994) found that fish oil significantly improved the effect on daily living compared to placebo (our secondary outcome looking at quality of life). This analysis also found a significant difference in area affected at the end of treatment as assessed by the physician (one of our tertiary outcomes). One study evaluated itch at the end of the study and found that fish oil significantly improved itch compared to the placebo group (Bjorneboe 1989). The largest of the fish oil supplementation studies did not show any benefit over placebo (Soyland 1994).
One small study (Ewing 1991) of poor methodological quality failed to show the benefit of zinc supplementation in atopic eczema. The study found no significant difference between zinc sulphate and placebo in the severity of eczema at eight weeks in children. In addition, parental‐rated mean itch score was significantly better in the placebo group compared to the zinc group.
One small study (Fairris 1989) of poor methodological quality failed to show any benefit of selenium supplementation or selenium plus vitamin E supplementation over placebo.
Two studies (Javanbakht 2011; Sidbury 2008) found no benefit of vitamin D over placebo. One study (Javanbakht 2011) found no benefit of vitamin E over placebo, but found a significant difference in SCORAD at the end of treatment in favour of vitamin D plus E over placebo. A more recent study comparing vitamin E to placebo and the combination of vitamin E and D to placebo found no benefit of either vitamin E or D compared to placebo.
One study (Mabin 1995) of pyridoxine (vitamin B6) provided insufficient evidence to assess pyridoxine in people with atopic eczema. The study found no significant difference between pyridoxine and placebo in the severity of eczema at four weeks.
One study (Yang 1999) found no significant difference in SCORAD at four weeks in adults when sea buckthorn seed oil was compared to placebo or sea buckthorn pulp oil was compared to placebo.
One study (Callaway 2005) found no benefit of hempseed oil over placebo in adults.
One study (Gimenez‐Arnau 1997) found no benefit of sunflower oil over placebo in adults with chronic or severe atopic eczema. This study quoted a significant difference in the per cent reduction of Rajka score and Rule of Nines when sunflower oil was compared to fish oil and when sunflower oil was compared to placebo; however, we were unable to substantiate this since the number of participants in each arm was not given.
Quality of the evidence
Eleven poor quality studies were included in this review involving 596 participants: 3 fish oil supplement diets, 1 zinc supplement diet, 1 selenium supplement diet, 2 vitamin D supplement diets, 1 vitamin E supplement diet, 1 pyridoxine hydrochloride (vitamin B6) supplement diet, 1 sea buckthorn oil supplement diet, 1 hempseed oil supplement diet, and 1 sunflower oil supplement diet.
All three of the fish oil studies in adults were of poor methodological quality; two of the studies were in adults with moderate to severe atopic eczema and the third study was in adults with severe atopic eczema. Concealment of allocation was unclear in all three studies. None of the studies looked at short‐term (within six weeks) changes in participant‐rated or parent‐rated symptoms of atopic eczema, such as itching or sleep loss. None of the studies looked at reduction in the number of flares; however, one study reported a reduction in the use of topical steroids, and one study reported no difference in the use of topical steroids or antihistamine use.
Only three studies clearly demonstrated adequate concealment of allocation (Javanbakht 2011; Mabin 1995; Sidbury 2008). Blinding was unclear in over half of the studies. Only one small study (Sidbury 2008) had no losses to follow up and analysed by intention‐to‐treat. For two studies, the number of participants in each arm was not given. Only one study (Koch 2008) clearly described baseline comparability, whereas the other studies just gave comparability for participants completing the supplementation. Compliance was only clear in three studies. The severity of atopic eczema was only clear in seven of the studies; however, for most of the studies (73%) the certainly of atopic eczema was clear.
Whilst the promotion of various products claiming various skin benefits not supported by good evidence might be commonplace in the world of dietary supplements, we did not find any major concerns regarding conflicts of interest in the trials identified in this review. The inclusion of those studies where conflicts of interest with commercial organisations were declared did not alter our overall conclusions. The field of dietary supplements for health benefit is a large and complex one, which some have tried to summarise using "balloon race" depictions: http://www.informationisbeautiful.net/visualizations/snake‐oil‐supplements/
Potential biases in the review process
It is possible that we could have missed some published studies in our searches or that we are unaware of other unpublished studies. No potential biases were identified in relation to the review process.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with a previous HTA review (Hoare 2000).
Authors' conclusions
Implications for practice.
Disappointingly, we have not found any convincing evidence of the benefit of dietary supplements in eczema. It is not possible for us to claim that all of the supplements that were examined in this review are not effective, because all of the studies were of low quality and most were much too small (underpowered) to exclude even relatively moderate treatment benefits.
Whilst this review cannot exclude the possibility that dietary supplements may play a role in improving eczema, the absence of positive evidence means that they cannot be recommended to the public or for clinical practice at present.
Two small studies on fish oil suggest a possible modest benefit, but many outcomes were explored, and a convincingly positive result from a much larger study with a publicly registered protocol is needed before clinical practice can be influenced. In addition, one study comparing vitamin D plus E to placebo found a significant improvement of SCORAD at the end of treatment where vitamin D or vitamin E alone had not. This study was small, and a larger study is needed to confirm these results. There is also a real need to look closely at adverse events. It is unclear if these small but statistically significant differences are clinically worthwhile in the absence of better information on the scales used.
Whilst it is possible that some may argue that at least supplements do not do any harm, high doses of vitamin D may give rise to serious medical problems, and the cost of long‐term supplements may also mount up.
Implications for research.
The grounds for recommending further clinical trial research in this field are limited. Whilst it is true that public interest in supplements of various sorts for chronic diseases is quite high, the main lack of anything positive to date may reflect poorly‐designed, poorly‐reported, and underpowered studies. There needs to be a stronger theoretical, biological, epidemiological, and experimental rationale before recommending further research in this area. Much attention has already been focused on evening primrose oil with no clear sign of benefit (Williams 2003), although another Cochrane review will address this specific topic (Boehm 2003). Given the presence of at least some positive secondary/tertiary outcomes in two of the studies that have explored fish oil, and that there is at least some good theoretical basis why they might play a role in dampening down the inflammatory pathway, a much larger placebo‐controlled and well‐designed study might be worthwhile. Although recent evidence has suggested that increased vitamin D intake may play a role in altering immune responses (Schwalfenberg 2011) and in protecting atopic diseases, such as asthma, we are not aware of any convincing role in eczema prevention or treatment (Miyake 2010; Miyake 2011). There is, however, some emerging data on the role of vitamin D in possibly modifying immune responses in eczema (the sunshine hypothesis, Searing 2010), and one recent study has shown a possible link between eczema severity and low vitamin D levels.
Another study has shown that vitamin D supplementation could reverse some of the defects in the innate immune system associated with atopic eczema, such as the capacity to increase the production of broad spectrum antimicrobial peptides like cathelicidin, which may in turn decrease the risk of secondary infections (Hata 2008).
It is also worth pointing out that many of the "placebos" used substances, such as olive oil, which might not be inert, thereby, reducing the chances of finding a true biological effect in the tested interventions. Future researchers should think carefully about the nature of placebos that are used in such studies.
The clinical data is not sufficiently strong to warrant a large trial in this area yet, although it is an area worth following up. As for the other dietary supplements, we are unconvinced that there is sufficient biological or clinical data to suggest that further clinical trials are needed, although we would be happy to revise such a statement in the future if a new and convincing critical mass of evidence emerges.
Future studies should use outcome measures that are more readily clinically interpretable and should be informed by the outcome of the Harmonizing Outcomes for Eczema (HOME) project (Schmitt 2010; Schmitt 2011).
The measurement of surface area involvement is just one example of lack of consistency that could be addressed by harmonisation of outcome measures. Some have used terms such as 'eczema extent', 'eczema extension', 'area affected', and 'affected areas' in unclear ways; and it is equally unclear how and whether extent should be combined with other signs of eczema, such as oedema, redness, and scaliness.
Because little is known about how these supplements could work at a biologic level in allergic disease, little is known about their duration of action. This means that they are probably unsuitable for testing in cross‐over designs where each participant is given both treatments to try, because the effect of taking the first treatment may hang over into the next treatment phase.
What's new
| Date | Event | Description |
|---|---|---|
| 18 May 2015 | Amended | Two erroneous links in the plain language summary have been fixed. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 16 May 2014 | Review declared as stable | A search of the GREAT (The Global Resource of Eczema Trials) database in February 2013 found just 1 study on Vitamin D supplementation, and another search of the GREAT database in May 2014 found only a few papers. Thus, this review has been marked stable because most of the papers were on probiotics and prebiotics, which are dealt with in another Cochrane review, and the paper on vitamin D is unlikely to move the existing vitamin D data in the review on in any meaningful way. An update has not been considered necessary for two successive years. Our Trials Search Co‐ordinator will run a new search in 2015 to re‐assess whether an update is needed. |
| 15 October 2008 | Amended | Converted to new review format. |
Notes
A search of the GREAT (The Global Resource of Eczema Trials) database in February 2013 found just 1 study on Vitamin D supplementation, and another search of the GREAT database in May 2014 found only a few papers. Thus, this review has been marked stable because most of the papers were on probiotics and prebiotics, which are dealt with in another Cochrane review, and the paper on vitamin D is unlikely to move the existing vitamin D data in the review on in any meaningful way. An update has not been considered necessary for two successive years. Our Trials Search Co‐ordinator will run a new search in 2015 to re‐assess whether an update is needed.
Acknowledgements
The authors would like to thank Finola Delamere and Weija Zhang for their contribution to the published protocol.
The editorial base would like to thank the Key Editor, Urba Gonzalez, Jo Leonardi‐Bee and Sarah Garner who were our Statistics and Methods Editors respectively, Eric Simpson who was a clinical referee, and another clinical referee who wishes to remain anonymous. We also wish to thank Kim Thomas who was our consumer referee.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register search strategy
((atopic AND eczema) OR (atopic AND dermatitis) OR (besnier* AND prurigo) OR (neurodermatitis) OR (infant* AND eczema) OR (childhood AND eczema) OR eczema) AND ((fat* and acid*) or (linoleic and acid*) or (arachidonic and acid*) or (eicosapentanoic and acid*) or eicosanoid* or (fish and oil*) or pyridoxine or (vitamin* and B6) or (vitamin* and E) or (zinc and salt*) or (zinc and compound*) or selenium or (diet* and supplement*))
Appendix 2. Cochrane Library search strategy
#1(atopic dermatitis) or (atopic eczema) or (eczema*) or (neurodermatitis) or (infantile eczema) #2(child* eczema*) or (Besnier's prurigo) #3MeSH descriptor Neurodermatitis explode all trees #4MeSH descriptor Eczema explode all trees #5MeSH descriptor Dermatitis, Atopic explode all trees #6(#1 OR #2 OR #3 OR #4 OR #5) #7(fat* acid*) or (fish oil*) or (diet* near supplement*) or (pyridoxine) or (vitamin B6) #8(zinc compound*) or (zinc and salt*) or(selenium) or (vitamin E) #9(linoleic acid*) or (arachidonic acid*) or (eicosapentanoic acid *) or (eicosanoid*) #10MeSH descriptor Fish Oils explode all trees #11MeSH descriptor Fatty Acids explode all trees #12MeSH descriptor Linoleic Acids explode all trees #13MeSH descriptor Arachidonic Acid explode all trees #14MeSH descriptor Vitamin B 6 explode all trees #15MeSH descriptor Vitamin E explode all trees #16MeSH descriptor Selenium explode all trees #17MeSH descriptor Zinc, this term only #18(#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19(#6 AND #18) #20SR‐SKIN #21(#19 AND NOT #20)
Appendix 3. MEDLINE (OVID) search strategy
(This search strategy also used for AMED and PsycInfo).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. (animals not (human and animals)).sh. 10. 8 not 9 11. exp Dermatitis, Atopic/ 12. atopic dermatitis.mp. 13. atopic eczema.mp. 14. exp NEURODERMATITIS/ 15. neurodermatitis.mp. 16. infantile eczema.mp. 17. childhood eczema.mp. 18. Besniers' Prurigo.mp. 19. exp Eczema/ or eczema.mp. 20. 16 or 11 or 19 or 14 or 13 or 17 or 12 or 15 or 18 21. 10 and 20 22. fatty acids.mp. or exp Fatty Acids/ 23. linoleic acid.mp. or exp Linoleic Acid/ 24. arachidonic acid.mp. or exp Arachidonic Acid/ 25. exp Eicosapentaenoic Acid/ or eicosapentanoic acid.mp. 26. eicosanoid*.mp. or exp Eicosanoids/ 27. fish oils.mp. or exp Fish Oils/ 28. dietary supplements.mp. or exp Dietary Supplements/ 29. pyridoxine.mp. or exp Pyridoxine/ 30. vitamin B6.mp. or exp Vitamin B 6/ 31. zinc salts.mp. 32. zinc compounds.mp. or exp Zinc Compounds/ 33. selenium.mp. or exp Selenium/ 34. exp Vitamin E/ or vitamin E.mp. 35. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 36. 21 and 35
Appendix 4. EMBASE (OVID) search strategy
1. random$.mp. 2. factorial$.mp. 3. (crossover$ or cross‐over$).mp. 4. placebo$.mp. or PLACEBO/ 5. (doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. (singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. (assign$ or allocat$).mp. 8. volunteer$.mp. or VOLUNTEER/ 9. Crossover Procedure/ 10. Double Blind Procedure/ 11. Randomized Controlled Trial/ 12. Single Blind Procedure/ 13. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14. exp Dermatitis, Atopic/ 15. atopic dermatitis.mp. 16. atopic eczema.mp. 17. exp NEURODERMATITIS/ 18. neurodermatitis.mp. 19. infantile eczema.mp. 20. childhood eczema.mp. 21. Besniers' Prurigo.mp. 22. exp Eczema/ or eczema.mp. 23. 19 or 14 or 22 or 17 or 16 or 20 or 15 or 18 or 21 24. fatty acids.mp. or exp Fatty Acids/ 25. linoleic acid.mp. or exp Linoleic Acid/ 26. arachidonic acid.mp. or exp Arachidonic Acid/ 27. exp Eicosapentaenoic Acid/ or eicosapentanoic acid.mp. 28. eicosanoid*.mp. or exp Eicosanoids/ 29. fish oils.mp. or exp Fish Oils/ 30. dietary supplements.mp. or exp Dietary Supplements/ 31. pyridoxine.mp. or exp Pyridoxine/ 32. vitamin B6.mp. or exp Vitamin B 6/ 33. zinc salts.mp. 34. zinc compounds.mp. or exp Zinc Compounds/ 35. selenium.mp. or exp Selenium/ 36. exp Vitamin E/ or vitamin E.mp. 37. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 38. 13 and 23 and 37
Appendix 5. LILACS search strategy
((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) [Words] and eczema or eccema [Words]
Data and analyses
Comparison 1. Fish oil vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total symptom score rated by participants at end of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Erythema at end of treatment (physician) | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.36, 1.13] |
| 3 Excoriation at end of treatment (physician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Lichenification at end of treatment (physician) | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.70, 0.78] |
| 5 Severity at end of treatment (physician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Scale at end of treatment (physician) | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.84, 0.45] |
| 7 Visibility at end of treatment (physician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Area affected at end of treatment (physician) | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.13, ‐0.06] |
| 9 Induration at end of treatment (physician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Pruritus at end of treatment (physician) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 Effect on daily living at end of treatment | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.52, ‐0.15] |
| 12 Itch evaluated by participants at end of study | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.46, ‐0.54] |
Comparison 2. Zinc vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Disease severity at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Erythema at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Erythema at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Mean itch scores at 8 weeks (parental‐rated symptoms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Surface area of body affected by eczema at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Surface area of body affected by eczema at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 3. Vitamin D vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Objective SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Erythema at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Oedema after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Oozing after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Excoriation after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Lichenification after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Dryness after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Pruritus after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10 Numbers worse or same (IGA after 1 month) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Mean topical steroid use at end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 4. Vitamin E vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Objective SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Erythema at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Oedema at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Excoriation at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Lichenification at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Dryness after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Pruritus | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Mean topical steroid use at end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 5. Vitamin D plus vitamin E vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Objective SCORAD at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Erythema at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Oedema at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Excoriation at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Lichenification at end of treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Dryness after treatment (60 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Pruritus | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Mean topical steroid use at end of study | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 6. Pyridoxine hydrochloride vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eczema ‐ no better or worse as rated by parents | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 7. Sea buckthorn seed oil vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCORAD at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 SCORAD at 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Comparison 8. Sea buckthorn pulp oil vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SCORAD at 4 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 SCORAD at 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bjorneboe 1989.
| Methods | This was a randomised controlled trial (single centre, Norway). D = parallel AC =unclear RS = block randomisation B = unclear |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: fish oil (18% eicosapentaenoic acid (EPA), 12% docosahexaenoic acid (DHA), 30% total n‐3 fatty acids, and 3% total n‐6 fatty acids). Each capsule contained 1 IU alpha‐tocopherol as antioxidant, 100 IU vitamin A, and 10 IU vitamin D (n = 16) T2: olive oil placebo (n = 15) T1 vs T2 ‐ 10 capsules daily for 12 weeks |
|
| Outcomes | 1) The objective evaluation of erythema, scale, visibility, severity, excoriation, weeping, lichenification, and area affected were separately assessed in a 10‐point score system 2) Participants, in addition, noted the degree of erythema, visibility, itch, scale, and effect on daily living in a similar score system Each final symptom score (after 12 weeks of intervention) in the objective and subjective evaluation was subtracted from the initial symptom score for each participant. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The blinding was not clear (double‐blind was stated in the Methods). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 4 dropouts in each group due to inability to swallow capsules. No ITT was carried out. |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin and Rajka. |
| Baseline comparability | Unclear risk | Baseline characteristics (age and sex, severity and intensity of AD) were described only for those completing supplementation. |
| Compliance | Low risk | This was evaluated by measuring the fatty acid composition of serum phospholipids at baseline and at the end of the study by gas chromatography. |
| Severity of AD | Unclear risk | This was not stated. |
| Conflict of interest | Low risk | This was a university study. |
Callaway 2005.
| Methods | This was a randomised controlled trial (single centre, Finland). D = cross‐over AC = unclear RS = clear B = unclear |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: hempseed oil T2: olive oil placebo 2 8‐week intervention periods with a 4‐week wash‐out period in between periods. Participants were instructed to consume 30 mls (2 Tbsp) of oil each day during the intervention period. |
|
| Outcomes | 1) FU at baseline, 4 weeks, and at the end of the intervention period (total of 6 visits) 2) Severity of AD measured by participants' perceptions of changes in skin dryness and itchiness throughout study period using a rating scale from 0 (no dryness or itching) to 5 (severe dryness or itching, sleep disturbance). Use of dermal medication rated on a similar scale: 0 (no medication) and 5 (regular usage) 3) Permeability barrier function of skin determined by measuring transepidermal water loss (TEWL). Plasma lipid profiles and dermal medication usage were also looked at |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronically‐generated random digits were used. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Single‐blind was stated. Oils were bottled in unlabelled brown glass. It was not clear who was blinded, but the appearance of the oils differed in colour, and the olive oil tasted of olives, so participants possibly knew what they were taking. It's stated that members of the study team were blinded with the exception of 1 (no further details). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants were lost to FU, and 3 dropped out due to personal reasons (it was unclear whether this was from the olive or hempseed oil group). 1 dropped out at week 13 due to the taste of the hempseed oil. |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin. |
| Baseline comparability | High risk | There was a large sex imbalance (1 man, 15 women). There was no baseline characteristics table or description. |
| Compliance | Unclear risk | No details were given. |
| Severity of AD | Unclear risk | No details were given. |
| Conflict of interest | High risk | The first author had financial interest in the production of hempseed oil. |
Ewing 1991.
| Methods | This was a randomised controlled trial (single centre, Manchester UK). D = parallel AC = unclear RS = unclear B = unclear (states double‐blind in Methods) |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: oral Z Span spansules (sustained release capsules each containing 61.8 mg zinc sulphate, equivalent to 22.5 mg zinc) (n = 22) T2: placebo capsules (n = 20) T1 vs T2, 3 times daily for 8 weeks Children who were being maintained on exclusion diets, which had been initiated previously, were included, but they were instructed not to embark on any food challenges during the study. |
|
| Outcomes | 1) Families recorded daily the degree of redness of skin, day‐time itch, and night‐time sleep disturbance on a 1 to 10 scale, as well as the number of applications of corticosteroid and/or emollient and evening dose of trimeprazine. Children were examined at 4 and 8 weeks. The extent of eczema was estimated from the percentage surface area of the body affected by eczema, the body having been divided into 14 separate areas, each of which was scored. Severity was graded by degree of erythema on an arbitrary scale from 1 to 5. The surface area affected when multiplied by severity score was expressed as a combined disease severity score. Weighed tubes of participants' topical steroids and emollients were returned at each visit 2) Overall efficacy was recorded by an observer and participants/parents by use of a 1 to 5 scale (1 = much better, 5 = much worse) at 4 and 8 weeks |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind was stated in the Methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 people dropped out ‐ 2 (1 zinc, 1 placebo) were withdrawn for non‐compliance (failed to attend or failed to take capsules), and 6 stopped taking capsules: 1 (placebo) due to loose stools, 1 (zinc) due to exacerbation of eczema attributed to capsules by parents, 3 (1 zinc, 2 placebo) developed widespread itchy maculopapular rash 6 to 15 days after starting treatment, and 1 (placebo) developed a recurrence of the herpes simplex virus skin infection. |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin. |
| Baseline comparability | Unclear risk | There was no mention of baseline comparability of the whole group, only those finishing the trial. Also, there was no mention of sex breakdown, only age, wt, and clinical signs of eczema in those completing the trial. |
| Compliance | Unclear risk | 1 of the reasons for dropout was non‐compliance; however, how this was actually measured was not documented. |
| Severity of AD | Unclear risk | No details were given. |
| Conflict of interest | Unclear risk | Smith, Kline, & French Laboratories supplied active and placebo capsules. The lead author was supported by grants from Glaxo Group Research and Glaxo Laboratories. |
Fairris 1989.
| Methods | This was a randomised controlled trial (single centre, Southampton, UK). D = parallel AC = unclear (no details were given) RS = set of random numbers B = unclear |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | T1: 600 µg selenium (in the form of selenium‐enriched yeast) T2: 600 µg selenium (in the form of selenium‐enriched yeast) plus 600 IU of vitamin E (d‐alpha‐tocopherol acetate) T3: visually identical placebo tablets T1 vs T2 vs T3 ‐ daily each morning with food for 12 weeks |
|
| Outcomes | 1) Severity of atopic dermatitis was assessed by separately measuring, on the head and neck, arms, and trunk and legs, the degree of inflammation, lichenification, and scaliness on a scale from 0 to 4, adding the 3 values and multiplying them to produce a weighted total score. Measured at baseline (before supplementation), 2, 4, 8, and 12 weeks after supplementation commenced and again at 12 weeks after supplementation ceased 2) Concentration of selenium in whole blood, vitamin E in plasma, and activity of glutathione peroxidase in platelets was measured. Selinium in skin measured at baseline, and at the end of week 12, by 6 mm punch biopsy of uninvolved skin from lateral buttock |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A set of random numbers were used. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants were blinded. It was stated that the placebo was visually identical. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants were lost to FU (but no mention of the group): 2 could not be contacted after week 8, 1 was excluded at week 4 after changing topical therapy, and 1 at week 8 after taking unprescribed vitamins. |
| Certainty of AD | Unclear risk | No details were given. |
| Baseline comparability | Unclear risk | Baseline characteristics (before supplementation) include disease severity only (no mention of age or sex). |
| Compliance | Unclear risk | No details were given. |
| Severity of AD | Low risk | This was rated as moderate to severe. |
| Conflict of interest | Low risk | A grant was received from Wessex Regional Health Authority Research Committee. |
Gimenez‐Arnau 1997.
| Methods | This was a randomised controlled trial (single centre, Spain). D = parallel AC = unclear RS = unclear B = outcome assessor was blinded |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: linoleic acid (LA) (from sunflower oil) (3 g/day) T2: fish oil (eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) (2.0 + 1.3 g/day)) T3: placebo ‐ oleic acid (3 g/day) T1 vs T2 vs T3 ‐ 12 capsules daily for 12 weeks The numbers per arm were not given. |
|
| Outcomes | 1) Disease severity assessed by Rajka score 2) Eczema extension assessed by Rule of Nines Follow‐up was at 6 and 12 weeks. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Nothing was stated. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It was stated that physicians who did the outcome assessment were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no mention of withdrawals or loss to FU. |
| Certainty of AD | Low risk | This was measured using criteria by Rajka. |
| Baseline comparability | High risk | No details were given. |
| Compliance | Unclear risk | This was not stated. |
| Severity of AD | Low risk | This was rated as chronic and severe (Rajka > = 7). |
| Conflict of interest | Unclear risk | This was not stated. |
Javanbakht 2011.
| Methods | This was a randomised controlled trial (single centre, Iran). D = parallel AC = clear RS = clear B = participants and outcome assessor |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | T1: vitamin D₃ (cholecalciferol 1600 IU) plus vitamin E placebo (n = 12) T2: 600 IU synthetic all‐rac‐α‐tocopherol in 2 softgels (400 and 200 IU) and vitamin D placebo (n = 11) T3: vitamin D₃ (cholecalciferol 1600 IU) and 600 IU synthetic all‐rac‐a‐tocopherol in 2 softgels (400 and 200 IU) (n = 11) T4: vitamin D placebo and vitamin E placebo (n = 11) T1 vs T2 vs T3 vs T4 ‐ 1 capsule and 2 softgels per day for 60 days |
|
| Outcomes | 1) Severity of AD measured by SCORAD before and after intervention | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted randomised blocks were used. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by a third person not involved in the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and clinicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants discontinued treatment (1 was excluded due to phototherapy, 2 due to oral or parenteral corticosteroids, 2 due to immunosuppressive drugs, and 2 were not willing to continue). |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin and Rajka. |
| Baseline comparability | Unclear risk | Baseline characteristics were shown only for those completing supplementation. |
| Compliance | Unclear risk | No details were given. |
| Severity of AD | Low risk | This was rated as mild, moderate, and severe. |
| Conflict of interest | Low risk | Authors report no conflict of interest. The authors alone were responsible for the content and writing of the paper. The study was supported by grant No 2842 from the Research Undersecretary of Tehran University of Medical Sciences. |
Koch 2008.
| Methods | This was a randomised controlled trial (single centre, Germany). D = parallel AC = sealed envelope up to completion of data analysis RS = stratified randomised allocation schedule based on block randomisation B = unclear |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | T1: DHA (docosahexaenoic acid) (n = 28) T2: isoenergetic (same energy value) control of saturated fatty acids (n = 25) T1 vs T2 ‐ 7 capsules daily for 8 weeks |
|
| Outcomes | 1) Severity of atopic eczema measured by SCORAD index at 0, 4, and 8 weeks, and 12 weeks after finishing the supplementation (week 20) 2) IgE production and activation of peripheral blood mononuclear cells (PBMC) analysed (week 0 and week 8) 3) Plasma fatty acids measured by gas chromatography (week 0 and week 8) | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This was stratified according to gender, age, and BMI in a ratio of 1:1 using block randomisation. Allocation was carried out by an independent person. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used, but it was not stated whether these were opaque. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not clear, although the discussion related to DHA and control capsules being indistinguishable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 participants were lost to FU ‐ 7 in the DHA group (5 due to non‐compliance, and 2 because of drastic changes in the clinical outcome due to external reasons), and 2 in the control group (1 due to non‐compliance, and 1 due to drastic changes in the clinical outcome due to external reasons). |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin and Rajka. |
| Baseline comparability | Low risk | This was clear. |
| Compliance | Unclear risk | Within the text of the paper, compliance was not obviously measured. However, 3 participants were lost to FU because of non‐compliance. |
| Severity of AD | Unclear risk | This was not mentioned. |
| Conflict of interest | Unclear risk | R Ruhl was supported by funds from the EU‐RTN 'Nutriceptors' programme. The DHA oil was provided by CRODA GmbH, Nettetal, Germany. |
Mabin 1995.
| Methods | This was a randomised controlled trial (single centre, UK). D = parallel AC = clear ‐ randomisation schedule supervised by the Director of Pharmacy RS = random number tables using blocks of 4 B = outcome assessor |
|
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
|
| Interventions | T1: pyridoxine hydrochloride (n = 24) T2: placebo tablet (n = 24) T1 vs T2 ‐ 1 tablet (50 mg) per day for 4 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables using blocks of 4 were used. |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule were supervised by the Director of Pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants were lost to FU ‐ 3 from the treatment group, 2 from the placebo group (3 because of eczema flare up due to bacterial infection requiring antibiotics, and 2 due to exacerbations of coexistent asthma requiring oral prednisolone treatment), 1 stopped taking the tablets suspecting food allergy, and 1 failed to return for the 4‐week assessment. |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin and Rajka. |
| Baseline comparability | High risk | Randomisation led to differences in sex distribution with 79% men in the treatment group versus 32% men in the placebo group. |
| Compliance | Unclear risk | This was not described. |
| Severity of AD | Low risk | This was rated as moderate and severe. |
| Conflict of interest | Unclear risk | No details were given. |
Sidbury 2008.
| Methods | This was a randomised controlled trial (single centre, Boston, USA). D = parallel AC = clear RS = clear B = participant and outcome assessor (clinician) |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: vitamin D (ergocalciferol 1000 IU) (n = 5) T2: placebo (n = 6) T1 vs T2 ‐ once per day for 1 month |
|
| Outcomes | 1) Severity of atopic eczema measured by the Investigator's Global Assessment (IGA) ‐ 6 categories ranging from clear (1) to very severe (6). Change in IGA category from baseline to FU | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital pharmacy randomly assigned participants. |
| Allocation concealment (selection bias) | Low risk | Assignments were concealed from both participants and investigators. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and outcome assessor (clinician) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 11 randomised participants were analysed. |
| Certainty of AD | Unclear risk | This was unclear. |
| Baseline comparability | High risk | No details were given. |
| Compliance | Unclear risk | No details were given. |
| Severity of AD | Low risk | 10 out of 11 participants had mild AD, and none had severe AD according to the Eczema Area and Severity Index score (EASI). |
| Conflict of interest | Low risk | The study was supported by a grant from the Massachusetts General Hospital Centre for D‐receptor Activation Research (Boston, MA, USA). |
Soyland 1994.
| Methods | This was a randomised controlled trial (multicentre, Norway). D = parallel AC = unclear RS = unclear B = unclear |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: fish oil (N = 57) ‐ each capsule contained 1 g of ethyl esters of very long chain n‐3 fatty acids ‐ 51% eicosapentaenoic acid (EPA), 32% docosahexaenoic acid (DHA) ‐ and 3.6 IU/g dl‐alpha‐tocopherol as antioxidant T2: placebo (N = 63) ‐ corn oil (26% oleic acid, 56% linoleic acid as triacylglycerol) ‐ each capsule contained 3.6 IU/g dl‐alpha‐tocopherol as antioxidant T1 vs T2 ‐ 6 capsules daily for 16 weeks (November to April) |
|
| Outcomes | 1) Evaluation, at baseline and every 4 weeks thereafter for 4 months, of erythematic, induration, pruritis, lichenification, scaling/dryness, and size of area involved, scored on a scale of 0 to 10. A selected area, 10 x 10 cm, which was moderately severely affected, was evaluated separately by the same physician for erythema, induration, and scaling according to a 10‐point scale. In addition, participants subjectively scored the degree of erythema, scaling/dryness, itching, area involved, and effect on daily living on a 7‐point scale (7 = severe involvement) | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind was stated in the methods. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25 participants did not complete the trial, and no reasons were given. |
| Certainty of AD | Low risk | This was measured using criteria by Hanifin. |
| Baseline comparability | Unclear risk | There was no difference at the start of the trial in total mean clinical condition; however, age and sex breakdown was described only for those completing supplementation, but figures relate only to the whole group, not per arm. |
| Compliance | Low risk | This was evaluated by counting capsules consumed in each period and by measuring the fatty acid composition of serum phospholipids at the beginning and end of the study. |
| Severity of AD | Low risk | This was rated as moderate to severe. |
| Conflict of interest | Unclear risk | Pronova AS provided K85 and corn oil capsules. The work was supported by a grant from The Norwegian Cancer Society (NCS). E Soyland was/is a research fellow of NCS. |
Yang 1999.
| Methods | This was a randomised controlled trial (single centre, Finland). D = parallel AC = unclear RS = unclear B = unclear |
|
| Participants |
Inclusion criteria of the trial
|
|
| Interventions | T1: sea buckthorn seed oil capsules (n = 12) T2: sea buckthorn pulp oil capsules (n = 16) T3: placebo‐paraffin oil capsules (n = 21) T1 vs T2 vs T3 ‐ 10 oil capsules (500 mg oil) per day for 4 months |
|
| Outcomes | 1) Severity of atopic eczema measured by SCORAD symptom score of 0 (no symptoms) to 3 (extensive severe). Mean values at baseline, 1 month, and 4 months of treatment (end of trial). Pruritis and sleep loss evaluated separately During all visits, plasma and serum samples were taken from the participants for analysis of the fatty acids of plasma phospholipids and neutral lipids and levels of cholesterol, triglycerols, serum total, and specific IgE. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were given. |
| Allocation concealment (selection bias) | Unclear risk | No details were given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details were given, although double‐blind was stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29 participants were lost to FU (37%) due to irregular or incomplete use of capsules, unscheduled plasma sampling, or clinical examination. The remaining 49 participants were included in the analyses. |
| Certainty of AD | Unclear risk | All participants had a history of AD from childhood with persistent symptoms during the last 6 months. |
| Baseline comparability | High risk | Baseline characteristics were not shown. There was a sex imbalance in the 49 participants that were included in the analyses (16 men and 33 women). |
| Compliance | Low risk | Consumption of capsules was controlled using oral communication, and any unused capsules were returned by the participant at the end of the trial. |
| Severity of AD | Unclear risk | No details were given. |
| Conflict of interest | Unclear risk | Aromtech Ltd (Tornio, Finland) provided oil capsules. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Czeizel 1994 | There was postnatal somatic and mental development after periconceptional multivitamin supplementation. This trial looked at various disease rates, of which AE was 1. |
| Eriksen 2006 | This was not a randomised controlled trial. |
| Hayakawa 1989 | We had an English translation of the abstract, and there was not enough detail available for inclusion. |
| Koller DY 1992 | The abstract does not mention that the study was randomised, and very little information was given. A larger randomised controlled trial was based on this study, and it was included in this review (Mabin 1995). |
| Mayser 2002 | There were daily intra‐venous infusions (not a dietary supplement) of either a n‐3 fatty acid‐based lipid emulsion (fish oil) or a conventional n‐6 lipid emulsion (soybean oil). |
| Yang 2000 | There was a biochemical outcome only. |
Characteristics of studies awaiting assessment [ordered by study ID]
Bautista 2010.
| Methods | This was a randomised controlled trial (single centre). D: parallel AC: unclear RS: unclear B: unclear |
| Participants |
Inclusion criteria of the trial
|
| Interventions | T1: daily oral fish supplementation for 2 months (n = 13) T2: no additional treatment (n = 15) |
| Outcomes | 1) Baseline and 2 months 2) Serum levels of IL‐10, total IgE 3) SCORAD 4) Corneometer moisture readings |
| Notes | ‐ |
AC = method of allocation concealment
AE = atopic eczema
B = blinding
Co‐T = co‐treatments
Dig = diagnosis
D = design
FU = follow up
ITT = intention‐to‐treat
PP = per‐protocol
N = number
RS = method of generating randomisation sequence
SAE = severity of atopic eczema
T = treatment
vs = versus
Characteristics of ongoing studies [ordered by study ID]
NCT00879424.
| Trial name or title | Randomized Trial of Vitamin D Supplementation in Winter‐related, Childhood Atopic Dermatitis |
| Methods | This is a randomised controlled trial. |
| Participants |
|
| Interventions | T1: vitamin D T2: placebo |
| Outcomes | 1) EASI score at 1 month 2) Investigator's Global Assessment (IGA) at 1 month |
| Starting date | February 2009 |
| Contact information | Carlos A Camargo Massachusetts General Hospital |
| Notes | ‐ |
Differences between protocol and review
Finola Delamere and Weija Zhang are no longer authors for the full review, and Claire Jenkinson has been added as an author.
We made minor changes to the Background, mainly to include relevant references since the protocol was published. We changed to the effect measure risk ratio (RR) in line with Skin Group policy.
We had not planned to search the GREAT database, but this has been developed since the protocol to this review was published. Therefore, it was searched, but we found no further studies.
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (FB‐H) Draft protocol (FB‐H, FD, and HW) Consumer ‐ input to draft protocol (RH) Run searches (FB‐H and FD) Identify relevant titles and abstracts from searches (FB‐H and CJ) Obtain copies of trials (FB‐H) Selection of trials (FB‐H and CJ) Extract data from trials (FB‐H and CJ) Enter data into RevMan (FB‐H) Carry out analysis (FB‐H) Interpret data (FB‐H and HW) Writing of final review (FB‐H and HW)
Sources of support
Internal sources
University of Nottingham, School of Nursing, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bjorneboe 1989 {published data only}
- Bjorneboe A, Soyland E, Bjorneboe GE, Rajka G, Drevon CA. Effect of n‐3 fatty acid supplement to patients with atopic dermatitis. Journal of Internal Medicine 1989;225(731):233‐6. [DOI] [PubMed] [Google Scholar]
Callaway 2005 {published data only}
- Callaway J, Schwab U, Harvima I, Halonen P, Mykkanen O, Hyvonen P, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. Journal of Dermatological Treatment 2005;16(2):87‐94. [DOI] [PubMed] [Google Scholar]
Ewing 1991 {published data only}
- Ewing CI, Gibbs AC, Ashcroft C, David TJ. Failure of oral zinc supplementation in atopic eczema. European Journal of Clinical Nutrition 1991;45(10):507‐10. [PubMed] [Google Scholar]
Fairris 1989 {published data only}
- Fairris GM, Perkins PJ, Lloyd B, Hinks L, Clayton BE. The effect on atopic dermatitis of supplementation with selenium and vitamin E. Acta Dermato‐Venereologica 1989;69(4):359‐62. [PubMed] [Google Scholar]
Gimenez‐Arnau 1997 {published data only}
- Gimenez‐Arnau A, Barranco C, Alberola M, Wale C, Serrano S, Buchanan MR, et al. Effects of linoleic acid supplements on atopic dermatitis. Advances in Experimental Medicine & Biology 1997;433:285‐9. [DOI] [PubMed] [Google Scholar]
Javanbakht 2011 {published data only}
- Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomised controlled trial using vitamins E and D supplementation in atopic dermatitis. Journal of Dermatological Treatment 2011;22(3):144‐50. [DOI] [PubMed] [Google Scholar]
Koch 2008 {published data only}
- Koch C, Dolle S, Metzger M, Rasche C, Jungclas H, Ruhl R, et al. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double‐blind, controlled trial. British Journal of Dermatology 2008;158(4):786‐792. [DOI] [PubMed] [Google Scholar]
Mabin 1995 {published data only}
- Mabin DC, Hollis S, Lockwood J, David TJ. Pyridoxine in atopic dermatitis. British Journal of Dermatology 1995;133(5):764‐7. [DOI] [PubMed] [Google Scholar]
Sidbury 2008 {published data only}
- Sidbury R, Sullivan AF, Thadhanis RI, Camargo CA Jr. Randomised controlled trial of vitamin D supplementation for winter‐related atopic dermatitis in Boston: a pilot study. British Journal of Dermatology 2008;159(1):245‐247. [DOI] [PubMed] [Google Scholar]
Soyland 1994 {published data only}
- Soyland E, Funk J, Rajka G, Sandberg M, Thune P, Rustad L, et al. Dietary supple‐mentation with very long‐chain n‐3 fatty acids in patients with atopic dermatitis. A double‐blind, multicentre study. British Journal of Dermatology 1994;130(6):757‐64. [DOI] [PubMed] [Google Scholar]
Yang 1999 {published data only}
- Yang B, Kalimo KO, Mattila LM, Kallio SE, Katajisto JK, Peltola OJ, et al. Effects of dietary supplementation with sea buckthorn (Hippophaerhamnoides) seed and pulp oils on atopic eczema. Journal of Nutritional Biochemistry 1999;10(11):622‐630. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Czeizel 1994 {published data only}
- Czeizel AE, Dobo M. Postnatal somatic and mental development after periconceptional multivitamin supplementation. Archives of Disease in Childhood 1994;70(3):229‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksen 2006 {published data only}
- Eriksen BB, Kare DL. Open trial of supplements of omega 3 and 6 fatty acids, vitamins and minerals in atopic dermatitis. Journal of Dermatological Treatment 2006;17(2):82‐5. [DOI] [PubMed] [Google Scholar]
Hayakawa 1989 {published data only}
- Hayakawa R, Ogino Y. Effects of combination therapy with vitamins E and B2 on skin diseases. Double blind controlled clinical trial. Skin Research 1989;31(6):856‐81. [Google Scholar]
Koller DY 1992 {published data only}
- Koller DY, Pirker C, Jarisch R. Pyridoxine HCI improves atopic eczema dermatitis: changes of IL‐1 beta, IL‐2, ACTH and cortisol in plasma. Clinical & Experimental Allergy 1992;22(1):126. [Google Scholar]
Mayser 2002 {published data only}
- Mayser P, Mayer K, Mahloudjian M, Benzing S, Kramer HJ, Schill WB, et al. A double‐blind, randomized, placebo‐controlled trial of n‐3 versus n‐6 fatty acid‐based lipid infusion in atopic dermatitis. Journal of Parenteral & Enteral Nutrition 2002;26(3):151‐8. [DOI] [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang B, Kalimo KO, Tahvonen RL, Mattila LM, Katajisto JK, Kallio HP. Effect of dietary supplementation with sea buckthorn (Hippophae rhamnoides) seed and pulp oils on the fatty acid composition of skin glycerophospholipids of patients with atopic dermatitis. Journal of Nutritional Biochemistry 2000;11(6):338‐340. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bautista 2010 {published data only}
- Bautista LC, Mendoza A, Sumpaico M, Recto M, Castor M, Leon J. The effects of fish oil supplementation on serum levels of interleukin 10 and total immunoglobulin e among pediatric patients with atopic dermatitis: a randomized controlled single blind clinical trial [Poster P364]. 2010 Annual Meeting of the American College of Allergy, Asthma and Immunology, 14‐15 November 2010, Phoenix, Arizona United States. Annals of Allergy, Asthma & Immunology 2010;105(5 Suppl):A125. [Google Scholar]
References to ongoing studies
NCT00879424 {unpublished data only}
- NCT00879424. Vitamin D Supplementation in Childhood Atopic Dermatitis. clinicaltrials.gov/ct2/show/NCT00879424 (accessed 14 April 2011).
Additional references
Altman 1991
- Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
Archer 2000
- Archer CB. The pathophysiology and clinical features of atopic dermatitis. In: Williams H editor(s). Atopic Dermatitis. The epidemiology, causes and prevention of atopic eczema. 1st Edition. Cambridge University Press, 2000:25‐40. [Google Scholar]
Asher 2006
- Asher MI, Montefort S, Bjorksten B, Lai CKW, Strachan DP, et al. the ISAAC Phase Three Study Group. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISSAAC Phase One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368(9537):733‐743. [DOI] [PubMed] [Google Scholar]
Baum 2002
- Baum WF, Schneyer U, Lantzsch AM, Kloditz E. Delay of growth and development in children with bronchial asthma, atopic dermatitis and allergic rhinitis. Experimental & Clinical Endocrinology & Diabetes 2002;110(2):53‐9. [DOI] [PubMed] [Google Scholar]
Beck 2000
- Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. Journal of Allergy & Clinical Immunology 2000;106(5 Suppl):S258‐63. [DOI] [PubMed] [Google Scholar]
Bland 1983
- Bland J. Mineral Analysis. In: Bland J editor(s). Hair tissue mineral analysis. An emergent diagnostic technique. Wellingborough: Thorsons, 1983:25‐6. [Google Scholar]
Boehm 2003
- Boehm K, Pittler MH, Wilson N, Gool C, Humphreys R, Ernst E. Oral evening primrose oil and borage oil for atopic eczema (Protocol).. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004416] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohme 2001
- Bohme M, Svensson A, Kull I, Nordvall SL, Wahlgren CF. Clinical features of atopic dermatitis at two years of age: a prospective, population‐based case‐control study. Acta Dermato‐Venereologica 2001;81(3):193‐7. [DOI] [PubMed] [Google Scholar]
Boyle 2008
- Boyle RJ, Bath‐Hextall FJ, Leonardi‐Bee J, Murrell DF, Tank MLK. Probiotics for treating eczema. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006135.pub2] [DOI] [PubMed] [Google Scholar]
Charman 2002
- Charman C, Williams HC. Epidemiology. In: Bieber T, Leung DYM editor(s). Atopic Dermatitis. 1st Edition. New York: Marcel Dekker Inc, 2002:21‐42. [Google Scholar]
Chren 1997
- Chren MM, Lasek RT, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of SKINDEX, a quality‐of‐life instrument for patients with skin diseases. Archives of Dermatology 1997;133(11):1433‐40. [PubMed] [Google Scholar]
Cookson 2002
- Cookson W. Genetics and genomics of asthma and allergic diseases. Immunological Review 2002;190:195‐206. [DOI] [PubMed] [Google Scholar]
Davies 1987
- Davies S, Steward A. Nutritional Medicine. The drug free guide to better family health. London: Pan, 1987. [Google Scholar]
Emerson 1998
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. British Journal of Dermatology 1998;139(1):73‐6. [DOI] [PubMed] [Google Scholar]
Ernst 2002
- Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence‐assessed efficacy of two diseases and two treatments. American Journal of Clinical Dermatology 2002;3(5):341‐8. [DOI] [PubMed] [Google Scholar]
Fairris 1989a
- Fairris GM, Lloyd B, Hinks L, Perkins PJ, Clayton BE. The effect of supplementation with selenium and vitamin E in psoriasis. Annals of Clinical Biochemistry 1989;26(Pt 1):83‐8. [DOI] [PubMed] [Google Scholar]
Fennessy 2000
- Fennessy M, Coupland S, Popay J, Naysmith K. The epidemiology and experience of atopic eczema during childhood: a discussion paper on the implications of current knowledge for health care, public health policy and research. Journal of Epidemiology & Community Health 2000;54(8):581‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 1996
- Finlay AY. Measurement of disease activity and outcome in atopic dermatitis. The British Journal of Dermatology 1996;135(4):509‐15. [PubMed] [Google Scholar]
Flohr 2004
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis?. Journal of Allergy & Clinical Immunology 2004;114(1):150‐8. [DOI] [PubMed] [Google Scholar]
Franklin 1988
- Franklin AJ, Blair C. Atopic eczema and other allergic disorders of the skin. In: Franklin AJ editor(s). The recognition and management of food allergy in children. Carnforth: Parthenon, 1988:39‐42. [Google Scholar]
Hata 2008
- Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. Journal of Allergy & Clinical Immunology 2008;122(8):829‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herd 1996
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. The cost of atopic eczema. British Journal of Dermatology 1996;135(1):20‐3. [PubMed] [Google Scholar]
Hoare 2000
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment (Winchester, England) 2000; Vol. 4, issue 37:1‐191. [PMC free article] [PubMed]
Johansson 2001
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel‐Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy: an EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56(9):813‐24. [DOI] [PubMed] [Google Scholar]
Johansson 2004
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. Journal of Allergy & Clinical Immunology 2004;113(5):832‐6. [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalliomaki 2002
- Kalliomaki M, Isolauri E. Pandemic of atopic disease ‐ a lack of microbial exposure in early infancy?. Current Drug Targets ‐ Infectious Disorders 2002;2(3):193‐9. [DOI] [PubMed] [Google Scholar]
Kemp 2003
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21(2):105‐13. [DOI] [PubMed] [Google Scholar]
Lamb 2002
- Lamb SR, Rademaker M. Pharmacoeconomics of drug therapy for atopic dermatitis. Expert Opinion on Pharmacotherapy 2002;3(3):249‐55. [DOI] [PubMed] [Google Scholar]
Mancini 2008
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatric Dermatology 2008;25(1):1‐6. [DOI] [PubMed] [Google Scholar]
McHenry 1995
- McHenry PM, Williams HC, Bingham EA. Management of atopic eczema. Joint Workshop of the British Association of Dermatologists and the Research Unit of the Royal College of Physicians of London. BMJ 1995;310(6983):843‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 2008
- Meltzer LJ, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: prevalence, causes and consequences. Journal of Pediatric Psychology 2008;33(3):279‐91. [DOI] [PubMed] [Google Scholar]
Miyake 2010
- Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. European Respiratory Journal 2010;35(6):1228‐34. [DOI] [PubMed] [Google Scholar]
Miyake 2011
- Miyake Y, Sasaki S, Tanaka K, Hirota Y. Maternal B vitamin intake during pregnancy and wheeze and eczema in Japanese infants aged 16‐24 months: the Osaka Maternal and Child Health Study. Pediatric Allergy & Immunology 2011;22(1 Pt 1):69‐74. [DOI] [PubMed] [Google Scholar]
NCC‐WCH & NICE 2007
- National Collaborating Centre for Women’s and Children’s Health (Commissioned by the National Institute for Health and Clinical Excellence (NICE)). Atopic eczema in children. Management of atopic eczema in children from birth up to the age of 12 years. http://www.nice.org.uk/nicemedia/live/11901/38559/38559.pdf 2007.
Odhiambo 2009
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase III Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. Journal of Allergy & Clinical Immunology 2009;124(6):1251‐8. e23. [DOI] [PubMed] [Google Scholar]
Pfeiffer 1978
- Pfeiffer CC. Zinc and other micronutrients. Keats Publishing Inc, 1978. [Google Scholar]
Polosa 2001
- Polosa R. The interaction between particulate air pollution and allergens in enhancing allergic and airway responses. Current Allergy & Asthma Reports 2001;1(2):102‐7. [DOI] [PubMed] [Google Scholar]
Sackett 1979
- Sackett DL, Gent M. Controversy in counting and attributing events in clinical trials. New England Journal of Medicine 1979;301(26):1410‐2. [DOI] [PubMed] [Google Scholar]
Schmitt 2007
- Schmitt J, Langan S, Williams HC, European Dermato‐Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. Journal of Allergy & Clinical Immunology 2007;120(6):1389‐98. [DOI] [PubMed] [Google Scholar]
Schmitt 2010
- Schmitt J, Williams H, HOME Development Group. Harmonising Outcomes Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. British Journal of Dermatology 2010;163(6):1166‐8. [DOI] [PubMed] [Google Scholar]
Schmitt 2011
- Schmitt J, Langan S, Stamm T, Williams H, Harmonising Outcome Measurments in Eczema (HOME) Delphi panel. Core outcome domains for controlled trials and clinical recordkeeping in eczema: international multi‐perspective Delphi consensus process. Journal of Investigative Dermatology 2011;131(3):623‐30. [DOI] [PubMed] [Google Scholar]
Schwalfenberg 2011
- Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Molecular Nutrition & Food Research 2011;55(1):96‐108. [DOI] [PubMed] [Google Scholar]
Searing 2010
- Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunology & Allergy Clinics of North America 2010;30(3):397‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2011
- Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. Journal of Investigative Dermatology 2011;131(1):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2011
- Scottish Intercollegiate Guidelines Network (SIGN). Management of atopic eczema in primary care (SIGN publication no. 125). http://www.sign.ac.uk/pdf/sign125.pdf 2011.
Smethurst 2002
- Smethurst D. Atopic eczema. Clinical Evidence. Vol. 8, BMJ, 2002:1664‐82. [PubMed] [Google Scholar]
Su 1997
- Su JC, Kemp AS, Varigos GA, Nolan TM. Atopic eczema: its impact on the family and financial cost. Archives of Diseases of Childhood 1997;76(2):159‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thestrup 2002
- Thestrup‐Pedersen K. Treatment principles of atopic dermatitis. Journal of the European Academy of Dermatology & Venereology 2002;16(1):1‐9. [DOI] [PubMed] [Google Scholar]
van den Oord 2009
- Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta‐analysis. BMJ 2009;339(b):2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verboom 2002
- Verboom P, Hakkaart‐Van L, Sturkenboom M, Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. British Journal of Dermatology 2002;147(4):716‐24. [DOI] [PubMed] [Google Scholar]
Williams 1999
- Williams H, Robertson C, Stewart A, Ait‐Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. Journal of Allergy & Clinical Immunology 1999;103(1 Pt 1):125‐38. [DOI] [PubMed] [Google Scholar]
Williams 2000
- Williams HC, Wuthrich B. The natural history of atopic dermatitis. In: Williams HC editor(s). Atopic Dermatitis: the epidemiology, causes and prevention of atopic eczema. Cambridge: Cambridge University Press, 2000:41‐59. [Google Scholar]
Williams 2003
- Williams HC. Evening primrose oil for atopic dermatitis. Time to say goodnight. BMJ 2003;327(7428):1358‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2008
- Williams H, Steward A, Mutius E, Cookson B, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide?. Journal Allergy & Clinical Immunology 2008;121(4):947‐54. [DOI] [PubMed] [Google Scholar]


