Abstract
Background
Preterm birth represents the single largest cause of mortality and morbidity for newborns and a major cause of morbidity for pregnant women. Tocolytic agents include a wide range of drugs that can inhibit labour to prolong pregnancy. This may gain time to allow the fetus to mature further before being born, permit antenatal corticosteroid administration for lung maturation, and allow time for intra‐uterine transfer to a hospital with neonatal intensive care facilities. However, some tocolytic drugs are associated with severe side effects. Combinations of tocolytic drugs may be more effective over single tocolytic agents or no intervention, without adversely affecting the mother or neonate.
Objectives
To assess the effects on maternal, fetal and neonatal outcomes of any combination of tocolytic drugs for the treatment of preterm labour when compared with any other treatment, no treatment or placebo.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2014) and reference lists of retrieved studies.
Selection criteria
We included randomised controlled trials comparing a combination of tocolytic agents, administered by any route or any dose, for inhibiting preterm labour versus any other treatment (including other combinations of tocolytics or single tocolytics), no intervention or placebo.
Data collection and analysis
Two review authors independently assessed study reports for eligibility, carried out data extraction and assessed risk of bias.
Main results
Eleven studies met our inclusion criteria. Two studies did not report any outcome data relevant to the review, so the results of the review are based on nine trials that contributed data. Primary outcomes were perinatal mortality, serious maternal or infant outcomes, adverse drug reactions, birth before 48 hours of trial entry, birth before 34 weeks' gestation and preterm neonates delivered without a full course of antenatal steroids completed 24 hours before birth. The quality of evidence in included trials was mixed; only three of the trials were placebo controlled.
The included trials examined seven different comparisons: intravenous (IV) ritodrine plus oral or IV magnesium (sulphate or gluconate) versus IV ritodrine alone (three trials, 231 women); IV ritodrine plus indomethacin suppositories versus IV ritodrine alone (one trial, 208 women); IV ritodrine plus vaginal progesterone versus IV ritodrine alone (one trial, 83 women); IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone (one trial, 24 women); IV fenoterol plus oral naproxen versus IV fenoterol alone (one trial, 72 women); oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol (one trial, 125 women); and, IV terbutaline plus oral metoprolol versus IV terbutaline alone (one trial, 17 women). Few studies with small numbers of women were available for each comparison, hence very little data were pooled in meta‐analysis. In all trials, not many of the primary outcomes were reported.
Three trials examined intravenous (IV) ritodrine plus IV or oral magnesium (sulphate or gluconate) compared with IV ritodrine alone. One study, with 41 women, reported more adverse drug reactions in the group receiving the combined tocolytics (risk ratio (RR) 7.79, 95% confidence interval (CI) 1.11 to 54.80). Two trials reported discontinuation of therapy due to severe side effects (results were not combined due to high statistical heterogeneity, I² = 83%); one trial reported increased severe side effects in the group receiving IV ritodrine alone (RR 7.79, 95% CI 1.11 to 54.80, 41 women); in the other trial there was no clear difference between groups (RR 0.23, 95% CI 0.03 to 1.97, 107 women). Other primary outcomes were not reported.
One trial assessed IV ritodrine plus indomethacin suppositories versus IV ritodrine alone. There were no significant differences between groups for perinatal mortality or serious neonatal morbidity. Results for other primary outcomes were not reported.
There were no significant differences between groups receiving IV ritodrine plus vaginal progesterone compared with IV ritodrine alone for most outcomes reported, although the latency period (time from recruitment to delivery) was increased in the group receiving the combination of tocolytics.
For other combinations of tocolytic agents, primary outcomes were rarely reported and for secondary outcomes results did not demonstrate differences between groups.
Authors' conclusions
It is unclear whether a combination of tocolytic drugs for preterm labour is more advantageous for women and/or newborns due to a lack of large, well‐designed trials including the outcomes of interest. There are no trials of combination regimens using widely used tocolytic agents, such as calcium channel blockers (nifedipine) and/or oxytocin receptor antagonists (atosiban). Further trials are needed before specific conclusions on use of combination tocolytic therapy for preterm labour can be made.
Plain language summary
Combinations of tocolytic drugs for inhibiting preterm labour
Preterm birth (birth before 37 weeks) is the single largest cause of deaths and ill health for newborn babies and a major cause of complications for pregnant women. Tocolytic agents include a wide range of drugs that can slow or stop labour contractions so as to prolong pregnancy and potentially improve the health outcomes for the baby. Using a combination of two or more tocolytic drugs may improve the length of time the pregnancy is prolonged over using a single tocolytic drug or no intervention, without adversely affecting the mother or baby or worsening drug side effects.
This review examined the effects of any combination of tocolytic drugs for the treatment of preterm labour, compared with any other treatment, no treatment or placebo. The results of the review are based on data from nine randomised controlled trials that examined seven different drug comparisons.
Three trials examined the betamimetic drug ritodrine plus magnesium compared with ritodrine alone. The trials reported on adverse side effects, with inconsistency between the trials as to which treatment gave fewer severe side effects. Other outcomes were either not reported or not clearly different between treatment groups.
One trial looked at ritodrine plus indomethacin versus ritodrine alone. There were no clear differences between groups for serious newborn ill health. Results for other outcomes were not clearly different. There were no clear differences between groups receiving ritodrine plus progesterone compared with ritodrine alone for most outcomes reported, although the time between giving the drugs and the birth was increased in the group receiving the combination of tocolytics. For other combinations of tocolytic agents, results did not demonstrate differences between groups. There were no trials of combination regimens using widely used tocolytic agents, such as calcium channel blockers (nifedipine) and oxytocin receptor antagonists (atosiban).
Due to insufficient evidence, it is unclear if combination tocolytic regimens are more or less effective than using a single tocolytic drug, or if they have more adverse effects. Some widely used tocolytic drugs have not been examined in trials as part of combination regimens, so further research is needed.
Background
Description of the condition
Preterm birth is defined as birth before 37 completed weeks of gestation (Bryce 2005). It occurs in 11.1% of births globally, affecting an estimated 14.9 million babies every year (Blencowe 2012; Born Too Soon Report 2012). In an analysis of 65 countries with reliable trend data on preterm births (1990 to 2010), 62 countries had stable or rising preterm birth rates (Blencowe 2012). This has been attributed at least in part to rising rates of provider‐initiated preterm birth and improved registration of extremely preterm births (Blencowe 2012; Davidoff 2006). It is generally accepted that approximately 30% to 35% of preterm births are provider‐initiated (by induction or caesarean section) for maternal and/or fetal indications, while 65% to 70% are spontaneous preterm births. Spontaneous preterm births includes those due to spontaneous preterm labour (40% to 45%) and those following preterm rupture of membranes (25% to 30%) (Goldenberg 2008). The precise cause of spontaneous preterm birth is often unknown, however several maternal factors are known to increase risk, including maternal age (adolescence and advanced age), maternal history of preterm birth, race, multiple pregnancy, short inter‐pregnancy interval, infections, medical conditions, poor nutrition, lifestyle factors, psychological factors and genetic predisposition (Goldenberg 2008; Plunkett 2008).
Preterm birth is the single largest cause of newborn mortality and is estimated to account for 29% of deaths in the first four weeks of life (Lawn 2009). Preterm newborns have higher rates of a range of neonatal morbidities, including respiratory (respiratory distress syndrome, persistent pulmonary hypertension) infectious (pneumonia, meningitis, sepsis, necrotizing enterocolitis), central nervous system (intraventricular haemorrhage, seizures) and metabolic (hypoglycaemia, jaundice and hyperbilirubinaemia) morbidities (Teune 2011). Administering antenatal corticosteroids to women at risk of preterm birth has been shown to reduce the overall risk of neonatal death, respiratory distress syndrome, cerebroventricular haemorrhage, necrotising enterocolitis, the need for respiratory support or intensive care admissions and systemic infections in the first 48 hours of life (Roberts 2006).
In the post‐neonatal period, preterm infants are at continued risk of mortality and morbidity (Katz 2013; Teune 2011). In the longer term, they experience more illnesses, hospital admissions and educational and behavioral problems in childhood and early adulthood, (Boyle 2011; Ekeus 2010; Talge 2010; van Baar 2009) as well as higher rates of visual and hearing impairments, cerebral palsy and neuro‐developmental and behavioural impairments (Hagberg 2001; Marlow 2005; O'Connor 2007).
Description of the intervention
Tocolytic therapy
Tocolytic agents include a wide range of drugs that can slow or suppress uterine contractions. Tocolytics are considered advantageous in spontaneous preterm labour to (a) allow time for the fetus to mature, potentially avoiding the deleterious effects of prematurity; (b) allow time for antenatal corticosteroids (ACS) to be administered and have a clinical effect; and (c) allow time for intrauterine transfer to a higher‐care centre, where neonatal intensive care facilities (or other services) are available (Anotayanonth 2004).
The ideal tocolytic agent should be effective, easy to administer, without significant maternal, fetal or neonatal side effects and permit time for antenatal corticosteroids to be administered and take effect (Crowther 2002). A variety of tocolytic treatments have been used to inhibit uterine activity in women in spontaneous preterm labour, including beta‐adrenergic receptor agonists (betamimetics), prostaglandin inhibitors, calcium channel blockers, oxytocin receptor antagonists and magnesium sulphate (Anotayanonth 2004; Crowther 2002; Duckitt 2002; Flenady 2014; Flenady 2014a; Gaunekar 2004; King 2005). However, there is considerable global variation in types, doses and regimens of tocolytic agents used to manage preterm labour.
How the intervention might work
Uterine contractility is stimulated by increases in intracellular calcium ion concentrations in myometrial cells (Gáspár 2013). Intracellular calcium ion concentration is mediated by both voltage‐gated calcium channels (for influx of calcium ions) and release of calcium ions from sarcoplasmic reticulum. Calcium ions bind to calmodulin and activate myosin light chain kinase (MLCK) in myometrial cells, leading to the phosphorylation of myosin light chains, initiating cross‐bridging in contractile proteins and ultimately myometrial contraction (Gáspár 2013; Noble 2009; Wray 2003).
Tocolytic agents inhibit uterine contractions through one of two pathways: Firstly, by affecting the contractile proteins in the myometrium (by blocking myosin phosphorylation through intracellular messengers). Betamimetics, nitric oxide donors, magnesium sulphate and calcium channel blockers affect contractile proteins. Betamimetics bind to beta‐adrenergic receptors (increasing intracellular cyclic adenosine monophosphate, that inhibits myosin light chain kinase) preventing myosin phosphorylation. Nitric oxide donors produce a similar response through cyclic guanosine monophosphate. Conversely, magnesium sulphate and calcium channel blockers prevent intracellular calcium ion influx, also reducing myosin light chain kinase activity (Caritis 2005; Gáspár 2013).
The second pathway to preventing uterine contractility is through inhibiting synthesis or blocking the activity of myometrial stimulants (such as oxytocin and prostaglandins). By binding oxytocin receptors, oxytocin receptor antagonists (such as atosiban) prevent the oxytocin‐mediated intracellular increase of inositol triphosphate, which normally increases intracellular calcium levels. Comparatively, cyclo‐oxygenase (COX) enzymes that catalyse prostaglandin production can be inhibited by general (COX) and selective (COX‐2) enzyme inhibitors (Caritis 2005; Gáspár 2013).
A combination of two or more tocolytic agents could be used to reduce uterine contractions by targeting different pathways in myometrial contraction, potentially improving the overall tocolytic effect (either additively or synergistically). Furthermore, combination therapy could permit reductions in dosage and/or frequency of individual tocolytic agents, potentially decreasing the rate of adverse effects while maintaining efficacy.
Why it is important to do this review
There is evidence that some tocolytic agents decrease the number of women in preterm labour giving birth within 48 hours when compared with placebo. This can increase the likelihood of receiving a full course of antenatal corticosteroids, as well as increase the time for antenatal corticosteroids to have a clinical effect. However, due to the lack of systematic reviews examining maternal and newborn morbidity and mortality outcomes following combination tocolytic use, inhibiting preterm labour with more than one tocolytic agent is currently based on an incomplete understanding of risks and benefits. Furthermore, combinations of tocolytic agents have the potential for greater efficacy compared to single agents, as well as the potential for reduced dosage and/or frequency of administration, that could reduce the rate of adverse effects.
Objectives
To assess the effects on maternal, fetal and neonatal outcomes of any combination of tocolytic drugs for the treatment of preterm labour compared with any other treatment, no treatment or placebo.
Methods
Criteria for considering studies for this review
Types of studies
Any adequate published, unpublished or ongoing randomised controlled trial that compares a combination of tocolytic agents, administered by any route or any dose, for inhibiting preterm labour versus any other treatment (including other combinations of tocolytics, or single tocolytic), no intervention or placebo. We excluded quasi‐randomised controlled trials.
Types of participants
Pregnant women assessed as being in spontaneous preterm labour (see definition below) and considered suitable for tocolytic agents.
Types of interventions
The following groups of comparisons were assessed for inclusion.
Combination of tocolytic drugs versus any other combination of tocolytic agents.
Combination of tocolytic drugs versus any other tocolytic agent alone.
Combination of tocolytic drugs versus any other intervention.
Combination of tocolytic drugs versus no intervention.
Combination of tocolytic drugs versus placebo.
Types of outcome measures
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews.
Consensus was reached on a set of six ‘core’ outcomes, which are highlighted below. These will be included in all tocolysis reviews. In addition to these core outcomes, individual teams may include other outcomes as necessary.
Primary outcomes
Seven primary outcomes were chosen as being most representative of the clinically important measures of ineffectiveness and complications.
Serious maternal outcomes (see definition below).
Short‐term and long‐term serious infant outcome (see definition below).
Birth before 48 hours of trial entry.
Preterm neonate delivered without full course of antenatal steroids (see definition below) completed at least 24 hours before birth.
Perinatal death after trial entry (see definition below).
Birth before 34 completed weeks.
Adverse drug reactions (including discontinuation of therapy because of adverse effects).
Definitions
Preterm labour
The presence of regular uterine contractions on a preterm pregnancy (with intact or ruptured membranes) with or without cervical dilatation.
Serious maternal outcomes
Death, cardiac arrest, respiratory arrest, admission to intensive care unit.
Short‐term and long‐term serious infant outcome
Determined by the presence of any of the following: death; chronic lung disease (use of supplemental oxygen therapy at 36 weeks' postmenstrual age or at 28 days of life or later); grade three or four intraventricular haemorrhage or periventricular leukomalacia; major sensorineural disability at two years of age defined as any one or more of the following: severe or profound vision impairment, sensorineural deafness requiring hearing aids, moderate or severe cerebral palsy or developmental delay/intellectual impairment (defined as developmental quotient or intelligence quotient less than two standard deviations below the mean).
Perinatal death after trial entry
Fetal death or neonatal death up to 28 days of life.
Full course of corticosteroids
Betamethasone (24 mg) intramuscularly (IM) divided in two to four doses, given in 24 hours, 20 to 24 mg of dexamethasone IM divided in four to six doses given in 24 hours, or 2 g of hydrocortisone intravenously divided in four doses given in 24 hours.
Secondary outcomes
Maternal
Need for additional tocolytics.
Recurrence of labour.
Caesarean section birth.
Antepartum haemorrhage.
Postpartum haemorrhage.
Length of hospital stay.
Breastfeeding.
Satisfaction with treatment.
Quality of life at 12 to 24 months after the birth (measured by validated instruments).
Psychological aspects of mother and family.
Maternal oedema (non‐prespecified outcome).
Infant/child
Preterm neonate delivered without full course of antenatal steroids (see definition below) completed at least 24 hours before birth.
Birth before seven days of trial entry.
Birth before 28 completed weeks.
Birth before 37 completed weeks.
Pregnancy prolongation (latency: interval between randomisation and birth).
Gestational age at birth.
Birthweight.
Apgar score less than seven at five minutes.
Respiratory distress syndrome.
Use of mechanical ventilation.
Duration of mechanical ventilation.
Persistent pulmonary hypertension of the neonate.
Intraventricular haemorrhage.
Intraventricular haemorrhage ‐ grade three or four.
Periventricular leukomalacia.
Chronic lung disease.
Necrotising enterocolitis.
Retinopathy of prematurity.
Neonatal jaundice.
Neonatal sepsis.
Fetal death.
Neonatal death.
Infant death.
Arterial pH < 7.1 (non pre‐specified).
Health service use
Admission to neonatal intensive care unit.
Neonatal length of hospital stay.
Treatment associated costs.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identify as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, review authors (J Nardin (JN), H West (HW), T Dowswell (TD)) extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (HW, TD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome using different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. If we identify cluster‐randomised trials for inclusion in a subsequent update of this review, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation on the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
We planned to use methods used for the analysis of cluster‐randomised trials to analyse data from trials which included more than 10% multiple pregnancies provided information was available (in the trial reports or from authors) on outcomes for twins or higher multiples. In this version of the review no such trials were included; trials either explicitly excluded women with multiple pregnancies or there was insufficient information to carry out planned analysis.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial is the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. If the average treatment effect was not clinically meaningful, we did not combine trials.
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses.
We planned to carry out the following subgroup analyses.
Gestational age (less than 28 weeks of gestation versus 28 weeks and above).
Intact versus ruptured membranes.
Single versus multiple pregnancy.
We planned to restrict subgroup analyses to the primary outcomes.
However, only one trial reported data that could be analysed based on the status of the membranes and in view of heterogeneity, we have presented results for these subgroups separately (Gamissans 1982). Planned subgroup analyses will be carried out in future updates of this review if more data become available.
We planned to assess differences between subgroups by interaction tests available in RevMan 2012. We would have reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analysis to explore the effect of trial quality. This would involve analysis based on the rating of selection bias and attrition bias. Studies of poor quality (those rated as 'unclear' or 'high risk of bias') would be excluded in the analysis in order to assess for any substantive difference to the overall result. However, all of the included trials were rated as unclear or high risk of bias for selection and/or attrition bias, therefore, no sensitivity analyses were performed
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search strategy identified 31 citations corresponding to 27 studies that were assessed for inclusion. While 11 studies (14 citations) met our inclusion criteria, two of these did not report any outcome data relevant to the review (Castillo 1988; Schauf 2005). Further information about both of these trials is set out in the Characteristics of included studies tables, but these studies do not contribute any data to the analyses, and are not otherwise mentioned in the results below. Sixteen studies (17 citations) were excluded (see: Figure 1).
1.
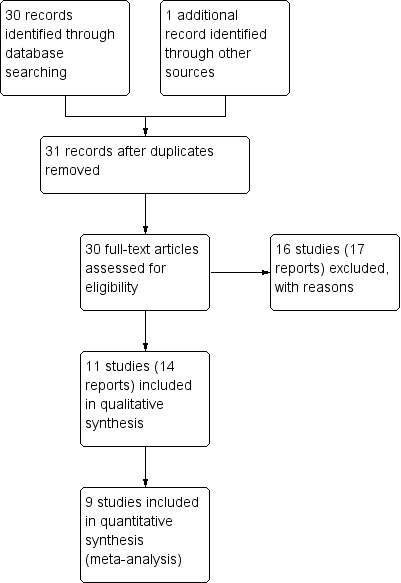
Study flow diagram.
Results are based on nine included trials that contributed data. Some of these studies reported on very few of our pre‐specified primary and secondary outcomes, and for most outcomes only one study contributed data so, overall, very few data were pooled in meta‐analysis.
Included studies
The included studies were categorised according to the type of drugs being compared.
Intravenous (IV) ritodrine plus oral or IV magnesium (gluconate or sulphate) versus IV ritodrine alone: three trials, including a total of 231 women (Ally 1992; Ferguson 1984; Hatjis 1987).
IV ritodrine plus indomethacin suppositories versus IV ritodrine alone: one trial, including 208 women (Gamissans 1982).
IV ritodrine plus vaginal progesterone versus IV ritodrine alone: one trial with 83 women (Arikan 2011).
IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone: one trial, including a total of 24 women (Francioli 1988).
IV fenoterol plus oral naproxen versus IV fenoterol alone: one trial, including 72 women (Rios‐Anez 2001).
Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol: one trial with 125 women (Lauterbach 2012).
IV terbutaline plus oral metoprolol versus IV terbutaline alone: one trial with data for 17 women (Ross 1983).
Settings
The studies were reported between 1982 and 2012 in six countries (USA (Ferguson 1984; Hatjis 1987; Ross 1983); France (Ally 1992; Francioli 1988); Turkey (Arikan 2011); Spain (Gamissans 1982); Poland (Lauterbach 2012); and Venezuela (Rios‐Anez 2001).
Participants
All of the studies recruited women in preterm labour. All studies recruited women with a gestational age less than 37 weeks, but the criteria relating to gestation varied particularly with regard to the lower gestational age (22 to 35 weeks (Ally 1992); 24 to 34 weeks (Arikan 2011); up to 36 weeks (Ferguson 1984); 28 to 36 weeks (Francioli 1988); 20 to 36 weeks (Gamissans 1982); 20 to 35 weeks (Hatjis 1987); 23 to 34 weeks (Lauterbach 2012); 31 to 36 weeks (Rios‐Anez 2001) and 26 to 36 weeks (Ross 1983).
In one trial it was specifically stated that women with both intact and ruptured amniotic membranes were included (Gamissans 1982); however, four studies specifically mentioned excluding women with preterm prelabour rupture of membranes (Ally 1992; Arikan 2011; Francioli 1988; Ross 1983) and in the remaining studies, it was not clear whether inclusion was confined to women with intact membranes. Five studies specifically mentioned exclusion of women with multiple pregnancies (Arikan 2011; Francioli 1988; Gamissans 1982; Lauterbach 2012; Rios‐Anez 2001). Women with serious complications or disease (including infection and renal problems) were specifically excluded by Ally 1992; Arikan 2011; Gamissans 1982; Lauterbach 2012; and Ross 1983. Other exclusion criteria included previous uterine or cervical surgery, fetal anomaly and deteriorating condition of the mother or fetus.
Interventions
All of the trials included therapy through intravenous infusion in at least one of the groups, and in most trials there was maintenance therapy with oral drugs following successful tocolysis. Dosage and duration of initial tocolysis varied, and several trials mentioned adjusting infusion rates depending on side effects and the effect of tocolysis on uterine contractions. Co‐interventions were not always well described. Use of corticosteroids (betamethasone or dexamethasone) for fetal lung maturation were mentioned by Arikan 2011; Ferguson 1984; Gamissans 1982; Lauterbach 2012; and Ross 1983. In the remaining studies it was not clear whether or not women received corticosteroids during the first 24 hours of tocolytic therapy.
Outcomes
All of the studies included some measure of pregnancy prolongation, although this was not measured consistently in different studies. Other pre‐specified outcomes were not consistently reported across studies. Side effects were reported in a minority of trials, and fewer than half of the included studies reported infant mortality and serious morbidity.
Excluded studies
We excluded 16 studies. The main reason for exclusion was that the method of allocation was not random; this applied to 11 studies (Caballero 1979; How 2006; Ieda 1991; Illia 1993; Katz 1983; Ogburn 1985; Reynolds 1978; Richter 1975; Richter 1979; Spearing 1979; Wischnik 1989). In three studies the intervention was not relevant to the review; either the women did not receive a combination of tocolytic drugs (Bedoya 1972; Dunlop 1986; Morales 2013 ) or women in both arms received the same drugs in different sequences (Kawagoe 2011). Finally, in the study by Herzog 1999, women were randomised to receive the same combination of tocolytic drugs but the way drugs were administered varied.
Risk of bias in included studies
Assessment of the methodological quality of the included studies was based on risk of bias in relation to selection bias (method of randomisation and allocation concealment), performance bias, detection bias, attrition bias (loss of participants from the analyses) and reporting bias. A summary of 'Risk of bias' assessments for each study, and for included trials overall, are set out in Figure 2 and Figure 3.
2.
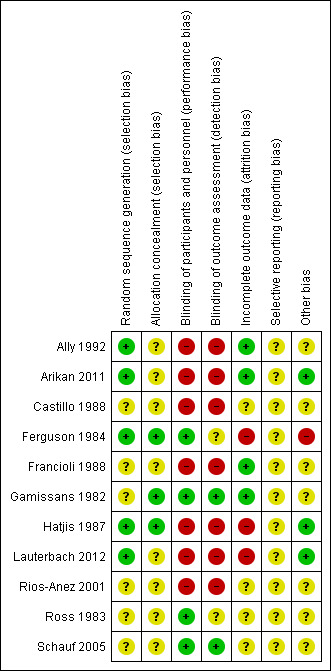
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
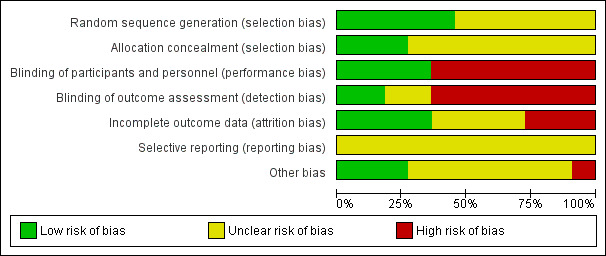
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Generation of the randomisation sequence
Five studies described methods for generating the randomisation sequence, which we judged were at low risk of bias; Ally 1992; Ferguson 1984; Hatjis 1987; and Lauterbach 2012 described using random number tables or pre‐prepared random lists, while Arikan 2011 reported using a computerised random‐number generator. In the remaining studies, the method for determining the randomisation sequence was either not clear, or not mentioned at all (Castillo 1988; Francioli 1988; Gamissans 1982; Rios‐Anez 2001; Ross 1983; Schauf 2005).
Allocation concealment
In eight of the studies, the method for concealing group allocation at the point of randomisation was not clear (Ally 1992; Arikan 2011; Castillo 1988; Francioli 1988; Lauterbach 2012; Rios‐Anez 2001; Ross 1983; Schauf 2005). Gamissans 1982 described a double‐blind design and Ferguson 1984 and Hatjis 1987 concealed allocations in sealed envelopes.
Blinding
Four of the studies were placebo‐controlled and were assessed as being at low risk of bias for blinding (Ferguson 1984; Gamissans 1982; Ross 1983; Schauf 2005). In two of these studies, outcome assessors were also blinded (Gamissans 1982; Schauf 2005), while this was not mentioned in Ferguson 1984 or Ross 1983. The remaining studies either did not attempt, or did not mention blinding. As the regimens in different arms of the trials were different, it is likely that staff providing care and evaluating outcomes would be aware of treatment allocation, and these studies have been assessed as high risk of bias due to lack of blinding (Ally 1992; Arikan 2011; Castillo 1988; Francioli 1988; Hatjis 1987; Lauterbach 2012; Rios‐Anez 2001).
Incomplete outcome data
There was no serious sample attrition in the trials by Ally 1992, Arikan 2011, Francioli 1988 and Gamissans 1982, and loss to follow‐up was not clear for Castillo 1988, Rios‐Anez 2001, Ross 1983 and Schauf 2005. In three trials, sample attrition was a serious source of bias. In these studies a significant proportion of the women were excluded from the study after randomisation or were lost to follow‐up for reasons that may have related to treatment, and this may have affected outcomes (e.g. delivered early or withdrew due to side effects) (Ferguson 1984; Hatjis 1987; Lauterbach 2012).
Selective reporting
Although the assumption that if something was not reported it was not done could be true for most of the trials that are currently published, the inadequate reporting for most of the trials included in this review could be also related to the period during which the majority of them were published. The lack of information for those published during the 1980s made methodological quality evaluation difficult (Castillo 1988; Ferguson 1984, Francioli 1988; Gamissans 1982; Hatjis 1987; Ross 1983). In addition, two studies were assessed using translated notes (Ally 1992; Francioli 1988) and, again, this made assessment of selective reporting more difficult.
Other potential sources of bias
Most studies described the characteristics of women in each of the study groups, and there was no clear evidence of baseline imbalance. The gestational age was slightly different between groups in the Ally 1992 trial, and in the Ferguson 1984 trial, recruitment was stopped early due to the high incidence of side effects, and this may have introduced bias.
Effects of interventions
This systematic review included 11 studies with a total of 895 women. Data from nine randomised controlled trials with a total of 776 women reported outcome data relevant to this review.
The sample sizes of all the trials that fulfilled the inclusion criteria were small, most comparisons used data from single studies only and none of them had sufficient clinical power to identify differences between groups for many outcomes. The biggest trial included 208 women and was further subgrouped by state of membranes to deal with clinical heterogeneity (Gamissans 1982).
It was not feasible to compare all combinations together versus single drugs, as the mechanisms of action among different drug families are not the same and combining data in meta‐analysis might have led to heterogeneous and spurious results. Therefore, we examined different combinations of drugs separately. For most of the comparisons and outcomes analysed, only one or few trials reported findings.
Comparison 1: IV ritodrine plus IV or oral magnesium (gluconate or sulphate) versus IV ritodrine alone
Three trials compared IV ritodrine plus IV or oral magnesium (gluconate or sulphate) versus IV ritodrine alone.
Primary outcomes
Only one trial, including 41 women, evaluated differences in 'adverse drug reactions', showing a higher incidence in the combination group (risk ratio (RR) 7.79, 95% confidence interval (CI) 1.11 to 54.80) (Ferguson 1984) (Analysis 1.1).
1.1. Analysis.
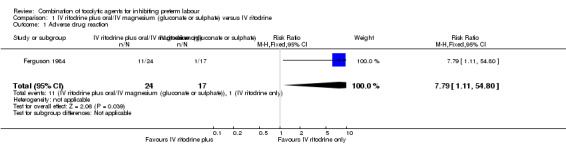
Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 1 Adverse drug reaction.
'Discontinuation of therapy' was reported in two trials (Ally 1992; Ferguson 1984); we have not pooled results due to the high heterogeneity observed between the included trials (I² = 83%); one trial reported increased side effects in the group receiving ritodrine alone (RR 7.79, 95% CI 1.11 to 54.80, 41 women); in the other trial there were no clear differences between groups (RR 0.23, 95% CI 0.03 to 1.97, 107 women), (Analysis 1.2).
1.2. Analysis.
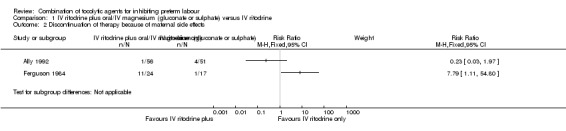
Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 2 Discontinuation of therapy because of maternal side effects.
None of the studies included in this comparison reported on our other primary outcomes.
Secondary outcomes
Only one trial (Hatjis 1987), including 64 women, reported the outcome 'birth before seven days after trial entry'; the difference between groups did not reach statistical significance (RR 0.62, 95% CI 0.38 to 1.01), (Analysis 1.3). One trial of 107 women (Ally 1992) assessed birthweight, showing no clear difference between treatment groups (mean difference (MD) 77 g, 95% CI ‐87.86 to 241.86) (Analysis 1.4).
1.3. Analysis.
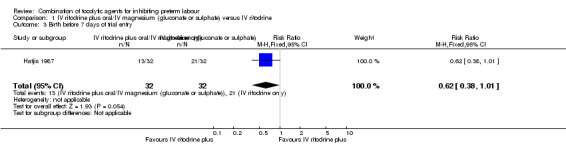
Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 3 Birth before 7 days of trial entry.
1.4. Analysis.
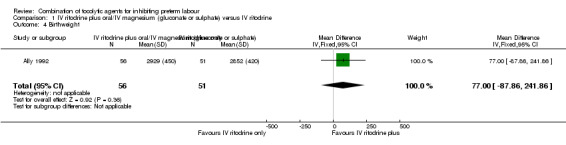
Comparison 1 IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine, Outcome 4 Birthweight.
Other secondary outcomes were not reported.
Comparison 2: IV ritodrine plus indomethacin suppositories versus IV ritodrine alone
For the second comparison, only one trial fulfilled the inclusion criteria (Gamissans 1982). The trial involved 208 women, and the results were reported separately according to the state of the membranes (intact versus ruptured), to handle an evident clinical cause of heterogeneity. Due to significant heterogeneity for the results of some outcomes, we are presenting the results separately for women with intact membranes (153 women) or ruptured membranes (55 women) reflecting the approach used in the trial report.
Primary outcomes
There was no clear difference between groups for perinatal mortality in women with intact membranes (RR 0.46, 95% CI 0.17 to 1.26), or ruptured membranes (RR 0.96, 95% CI 0.15 to 6.37) (Analysis 2.1).
Data on respiratory distress syndrome (RDS) were reported for the women with intact amniotic membranes; there were very few events and no strong evidence of difference between groups (RR 0.51, 95% CI 0.05 to 5.47) (Analysis 2.2).
2.2. Analysis.
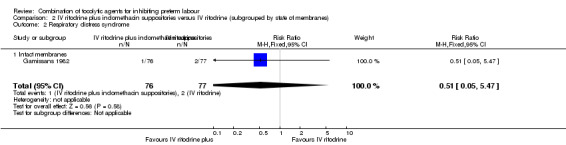
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 2 Respiratory distress syndrome.
For maternal adverse drug reactions there was no clear difference between groups receiving the combined therapy versus ritodrine alone in either the intact or ruptured membranes groups (RR 1.11, 95% CI 0.67 to 1.87; RR 0.77, 95% CI 0.36 to 1.66, respectively) (Analysis 2.3).
2.3. Analysis.
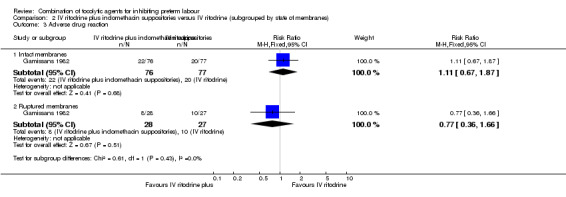
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 3 Adverse drug reaction.
Other primary outcomes were not reported.
Secondary outcomes
Results showed a statistically significant difference in the number of women giving birth before 37 weeks of gestation and recurrence of labour favouring the combination group in those women with intact membranes (RR 0.70, 95% CI 0.50 to 0.97; RR 0.59, 95% CI 0.36 to 0.99, respectively) (Analysis 2.4; Analysis 2.5).
2.4. Analysis.
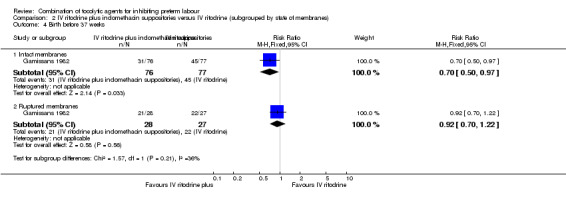
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 4 Birth before 37 weeks.
2.5. Analysis.
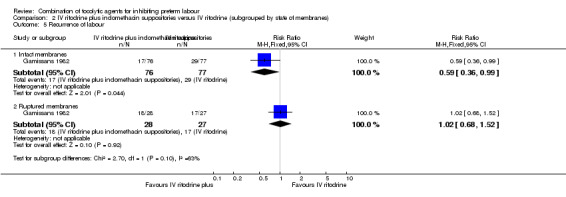
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 5 Recurrence of labour.
For women with ruptured membranes the evidence did not demonstrate a difference between groups for birth before 37 weeks (RR 0.92, 95% CI 0.70 to 1.22) (Analysis 2.4), and frequency of labour recurrence was very similar in the two treatment groups (RR 1.02, 95% CI 0.68 to 1.52) (Analysis 2.5).
No differences were found for infant Apgar score less than seven at five minutes irrespective of membrane status (intact: RR 1.01, 95% CI 0.31 to 3.36; ruptured: RR 1.29, 95% CI 0.32 to 5.22) (Analysis 2.6).
2.6. Analysis.
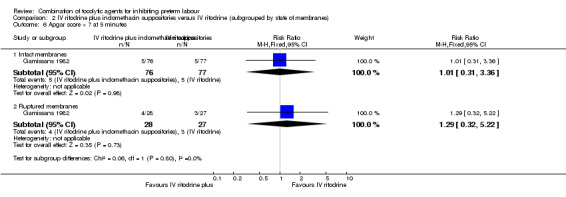
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 6 Apgar score < 7 at 5 minutes.
Other outcomes were not reported.
Comparison 3: IV ritodrine plus vaginal progesterone versus IV ritodrine alone
One study with data for 83 women is included in this comparison (Arikan 2011). The study did not have sufficient statistical power to identify differences between groups for most of the outcomes reported.
Primary outcomes
There was only one infant death, no significant differences between groups, and very wide 95% CIs around the effect estimate for this outcome (RR 0.31, 95% CI 0.01 to 7.41) (Analysis 3.1).
3.1. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 1 Infant mortality.
Event rates were low for serious morbidity in newborns, and there were no significant differences between groups for infant sepsis (RR 0.93, 95% CI 0.14 to 6.30) (Analysis 3.2), RDS (RR 0.93, 95% CI 0.06 to 14.38) (Analysis 3.3), or for need for mechanical ventilation (RR 1.86, 95% CI 0.18 to 19.73) (Analysis 3.4). There were no estimable data for infant necrotising enterocolitis or intraventricular haemorrhage.
3.2. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 2 Infant sepsis.
3.3. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 3 RDS.
3.4. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 4 Use of mechanical ventilation.
Secondary outcomes
There were low event rates for poor infant outcomes at birth and no apparent differences between groups for low infant Apgar score at five minutes (RR 0.23, 95% CI 0.03 to 1.99) (Analysis 3.7), arterial blood pH less than 7.1 (non pre‐specified outcome) (RR 0.47, 95% CI 0.09 to 2.40) (Analysis 3.8), infant admission to neonatal intensive care unit (NICU) (RR 0.93, 95% CI 0.20 to 4.34) (Analysis 3.9), or mean length of NICU stay (days) (MD ‐7.90, 95% CI ‐48.95 to 33.15) (the denominator for this last non‐prespecified outcome was not clear, and in the analysis we have used the number of babies admitted to NICU rather than the whole sample) (Analysis 3.10).
3.7. Analysis.
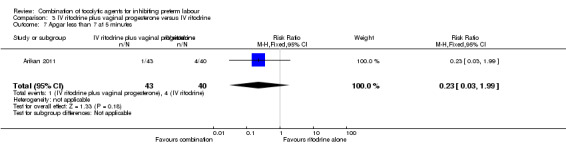
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 7 Apgar less than 7 at 5 minutes.
3.8. Analysis.
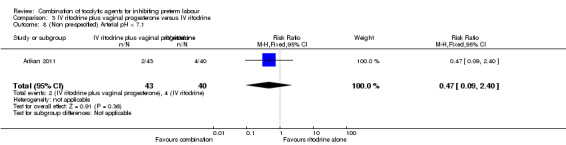
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 8 (Non pre‐specified) Arterial pH < 7.1.
3.9. Analysis.
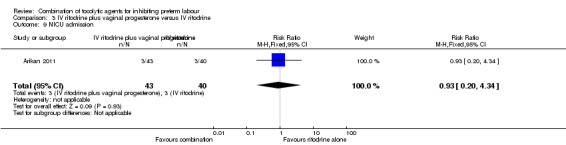
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 9 NICU admission.
3.10. Analysis.
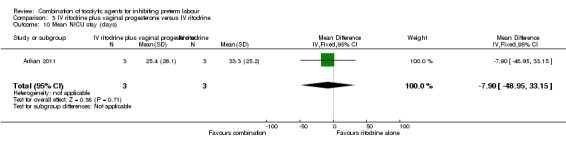
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 10 Mean NICU stay (days).
Infants whose mothers were randomised to receive the combination of tocolytics were less likely to have a low birthweight (less than 2500 g) (RR 0.41, 95% CI 0.20 to 0.84) (Analysis 3.12), and a higher mean birthweight (MD 398.0 g, 95% CI 86.37 to 709.63) (Analysis 3.11).
3.12. Analysis.
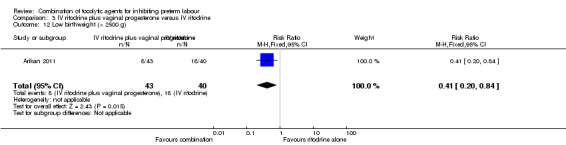
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 12 Low birthweight (< 2500 g).
3.11. Analysis.
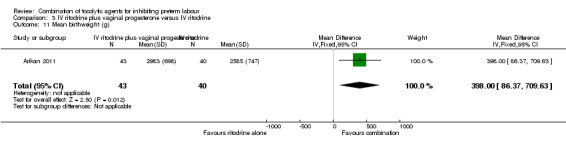
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 11 Mean birthweight (g).
The period between randomisation and birth (latency) was increased for women in the combined group by a mean of almost 11 days (MD 10.90, 95% CI 3.56 to 18.24) (Analysis 3.14). This finding was reflected in fewer women giving birth before 37 weeks in the combined tocolytic group (RR 0.66, 95% CI 0.45 to 0.97) (Analysis 3.13), and an increase in the mean gestational age at delivery (MD 1.20 weeks, 95% CI 0.08 to 2.32) (Analysis 3.15).
3.14. Analysis.
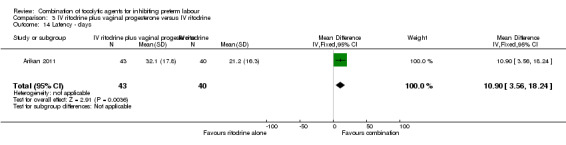
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 14 Latency ‐ days.
3.13. Analysis.
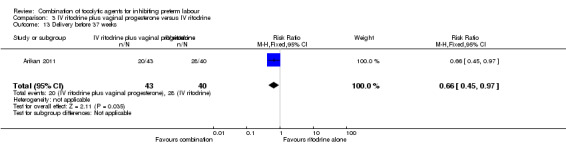
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 13 Delivery before 37 weeks.
3.15. Analysis.
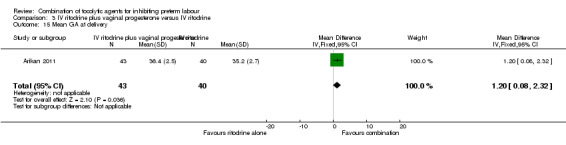
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 15 Mean GA at delivery.
There was no significant difference between groups for caesarean section (RR 0.75, 95% CI 0.51 to 1.10) (Analysis 3.16). Other outcomes were not reported.
3.16. Analysis.
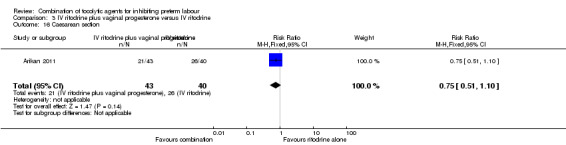
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 16 Caesarean section.
Comparison 4: IV hexoprenaline sulphate plus IV magnesium hydrochloride versus IV hexoprenaline sulphate alone
Only 24 women were recruited in the only trial included in this comparison (Francioli 1988), with no difference observed in the only outcome analysed by the authors: birth before seven days of trial entry (RR 0.85, 95% CI 0.14 to 5.06) (Analysis 4.1).
4.1. Analysis.
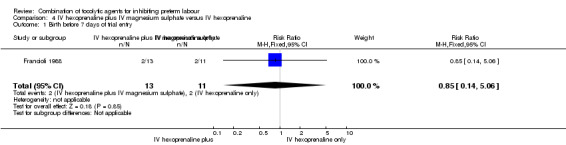
Comparison 4 IV hexoprenaline plus IV magnesium sulphate versus IV hexoprenaline, Outcome 1 Birth before 7 days of trial entry.
Comparison 5: IV fenoterol plus oral naproxen versus IV fenoterol alone
For the comparison IV fenoterol plus oral naproxen versus IV fenoterol alone, only one trial was included (Rios‐Anez 2001). In this trial only 72 women were recruited and results did not show any difference in the only outcome relevant to the review that was reported: 'birth before 37 weeks of gestation' (RR 0.50, 95% CI 0.23 to 1.09) (Analysis 5.1).
5.1. Analysis.
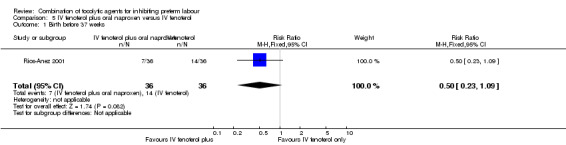
Comparison 5 IV fenoterol plus oral naproxen versus IV fenoterol, Outcome 1 Birth before 37 weeks.
Comparison 6: Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol
One trial examined this comparison (Lauterbach 2012). One‐hundred and forty‐eight women were randomised; however, there was considerable loss to follow‐up following randomisation.
Data for perinatal death and delivery within seven days were available for 125 women. There was no significant difference between groups for perinatal death (RR 0.19, 95% CI 0.02 to 1.55) (Analysis 6.1), or delivery within seven days (RR 0.77, 95% CI 0.29 to 2.03) (Analysis 6.2).
6.1. Analysis.
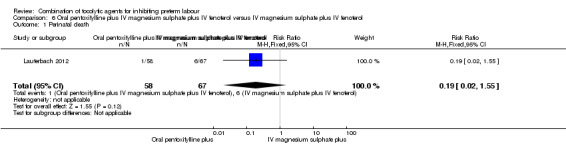
Comparison 6 Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol, Outcome 1 Perinatal death.
6.2. Analysis.
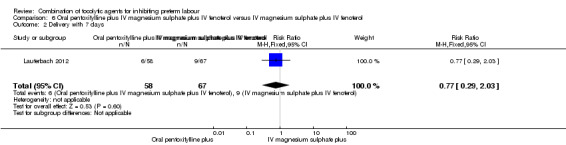
Comparison 6 Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol, Outcome 2 Delivery with 7 days.
Other outcomes were reported in this trial but analyses excluded women who either delivered within the first week of tocolysis, or withdrew from the trial, or changed the place of birth, or were intolerant to pentoxifylline. Of the 148 women randomised, data for pregnancy outcomes and serious infant morbidity were reported for only 96 of the original sample: 39% of the intervention group and 31% of the control group were not included in the analysis of these outcomes. In view of the high risk of bias for such outcomes, we have not, therefore, reported them in this review.
Comparison 7: IV terbutaline plus oral metoprolol versus IV terbutaline alone
One trial with data for 17 women is included in this comparison (Ross 1983). Only two outcomes relevant to the review were reported. There was no clear difference between groups for mean time to delivery and the standard deviations for this outcome suggest considerable variation within groups (MD ‐6.80 days, 95% CI ‐14.92 to 1.32) (Analysis 7.2). No women in either arm of this trial were reported to have oedema (non pre‐specified outcome).
7.2. Analysis.
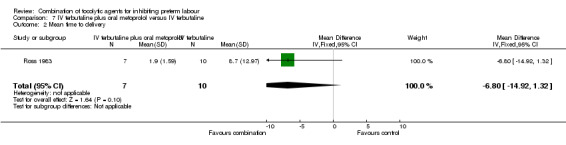
Comparison 7 IV terbutaline plus oral metoprolol versus IV terbutaline, Outcome 2 Mean time to delivery.
Discussion
This systematic review included 11 studies with a total of 895 women. Data from nine randomised controlled trials with a total of 776 women reported outcome data relevant to this review. Overall, seven different combinations of tocolytic drugs were examined with only one or few small trials per comparison, so very few data were pooled in meta‐analysis. While most of the included studies reported some measure of pregnancy prolongation, other outcomes were infrequently reported.
Summary of main results
Three trials examined intravenous (IV) ritodrine plus oral or IV magnesium (sulphate or gluconate) compared with IV ritodrine alone. One study with 41 women reported more adverse drug reactions in the group receiving the combination of tocolytic drugs. Two trials reported discontinuation of therapy due to severe side effects, with one trial reporting increased side effects in the group receiving ritodrine alone, while in the other trial there was no clear difference between groups. Other outcomes were either not reported, or not statistically significant.
One trial compared IV ritodrine plus indomethacin suppositories versus IV ritodrine alone. There were no significant differences between groups for perinatal death serious neonatal morbidity. Results for other primary outcomes were not reported..
There were no significant differences between groups receiving IV ritodrine plus vaginal progesterone compared with IV ritodrine alone for most outcomes reported, although the latency period (time from randomisation to delivery) was increased in the group receiving the combination of tocolytics.
For other combinations of tocolytic agents, results did not demonstrate differences between groups.
Overall completeness and applicability of evidence
Although the aim of this systematic review was to evaluate combinations of tocolytic agents compared with any other treatment on maternal and neonatal outcomes, we pre‐specified outcomes chosen as the most representative clinical measures of effectiveness and complications. Unfortunately, some of these outcomes, particularly those to which the tocolytic treatment would be most relevant (e.g. 'birth before 48 hours of trial entry' to allow the administration of a complete course of corticosteroids), were not assessed by any of the included studies.
The samples sizes of the individual trials were generally small. Moreover, the analysis of the largest study (which included only 208 women) was stratified into two groups because of a clinical source of heterogeneity (state of membranes). This and the poor quality of reporting in the majority of the included trials made conclusions difficult, and in most cases studies were underpowered to identify differences between groups. Antenatal corticosteroid use has an important effect on neonatal outcomes, however, its use was reported in only five of the included studies. Thus review findings must to be interpreted with considerable caution.
The most serious practical limitation of this systematic review is that none of the identified studies (included and excluded) evaluated two of the most commonly used tocolytic agents in high‐resource settings, atosiban and nifedipine, as part of a combination or single tocolytic therapy. Many settings have abandoned the use of betamimetics for tocolysis due to their unfavourable side‐effect profile in comparison with other agents (Flenady 2014). These results are therefore not clinically relevant in settings where betamimetics are no longer used.
A theoretically effective combination of tocolytics would include agents that act through different mechanisms, affecting contractile proteins in the myometrial cell and also blocking the action of myometrial stimulants. The latter action is only known to be performed by cyclo‐oxygenase (COX) inhibitors (indomethacin) and oxytocin antagonists (atosiban). Therefore, future trials of combinations of tocolytic agents should, where appropriate, include at least one of these two agents, as well as considering the need to evaluate calcium channel blockers in combination regimens.
Quality of the evidence
It was difficult to assess the methodological quality of most of the trials included in the review because of poor reporting. In most of the trials, methods for generating the randomisation sequence and concealing allocation at the point of randomisation were not clear or presented high risk of bias. Only four of the trials reported that blinding had been attempted. Lack of blinding may mean findings are at high risk of bias, as care would have been provided, and many of the outcomes assessed, by staff who would have been aware of what treatment a woman had received. In three trials, sample attrition was a serious source of bias because women were excluded from the study after randomisation or were lost to follow‐up for reasons that may have related to treatment and this may have affected outcomes.
Potential biases in the review process
We are aware that the review process itself is subject to bias, and we took steps to minimise bias. At least two review authors carried out data extraction and assessed risk of bias independently; however, a different review team may not have made identical decisions.
Agreements and disagreements with other studies or reviews
As with previous Cochrane reviews of tocolytic drugs, this review showed that betamimetics are the most commonly studied tocolytic agent. However, one review has demonstrated that (when used alone) calcium channel blockers are preferred to other single tocolytic drugs, particularly betamimetics (mostly ritodrine), due to comparable efficacy and fewer side effects (Flenady 2014). Despite this, we found no trial that included calcium channel blockers in a combination tocolytic regimen. A review of the use of COX inhibitors alone (including indomethacin) for treating preterm labour could not recommend COX inhibitor use due to insufficient data (King 2005). However, in a small number of participants COX inhibitors reduced the proportion of women delivering preterm. The risks associated with magnesium sulphate alone in threatened preterm labour (Crowther 2002) should be carefully considered in tocolytic combination regimens.
Authors' conclusions
Implications for practice.
It remains unclear whether using a combination of tocolytic drugs for inhibiting preterm labour is more advantageous for women and/or newborns given the lack of studies. There is no direct trial evidence to demonstrate that combination tocolytic therapies are equivalent or superior (in either efficacy or side‐effect profile) to commonly used single tocolytic agents, such as calcium channel blockers or oxytocin receptor antagonists. Further trials are needed before specific conclusions on use of combination tocolytic therapy for preterm labour can be made.
Implications for research.
Additional studies are urgently needed to explore the relationships between combinations of tocolytics versus any other treatment for preterm labour. These studies should prioritise (where appropriate) the inclusion of commonly used tocolytic agents, such as calcium channel blockers and/or oxytocin antagonists. Reporting of a range of maternal and newborn mortality and morbidity outcomes, as well as reporting the use of antenatal corticosteroids, is needed in order to obtain meaningful data for clinical decision‐making.
History
Protocol first published: Issue 4, 2006 Review first published: Issue 7, 2014
| Date | Event | Description |
|---|---|---|
| 20 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Guillermo Carroli and Zarko Alfirevic for their contributions to the protocol (Nardin 2006).
This work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
The World Health Organization and Juan Manuel Nardin, Therese Dowswell, and Helen M West retain copyright and all other rights in their respective contributions to the manuscript of this Review as submitted for publication.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. IV ritodrine plus oral/IV magnesium (gluconate or sulphate) versus IV ritodrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse drug reaction | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.79 [1.11, 54.80] |
| 2 Discontinuation of therapy because of maternal side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Birth before 7 days of trial entry | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 4 Birthweight | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 77.0 [‐87.86, 241.86] |
Comparison 2. IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.17, 1.26] |
| 1.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.37] |
| 2 Respiratory distress syndrome | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 2.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 3 Adverse drug reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.67, 1.87] |
| 3.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.66] |
| 4 Birth before 37 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.97] |
| 4.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 5 Recurrence of labour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.36, 0.99] |
| 5.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.52] |
| 6 Apgar score < 7 at 5 minutes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Intact membranes | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.36] |
| 6.2 Ruptured membranes | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.32, 5.22] |
2.1. Analysis.
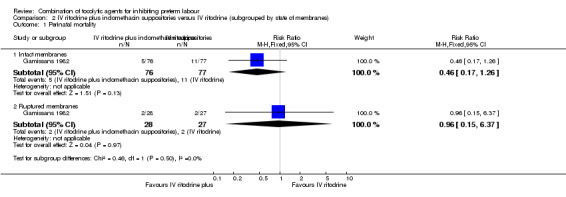
Comparison 2 IV ritodrine plus indomethacin suppositories versus IV ritodrine (subgrouped by state of membranes), Outcome 1 Perinatal mortality.
Comparison 3. IV ritodrine plus vaginal progesterone versus IV ritodrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infant mortality | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.41] |
| 2 Infant sepsis | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.14, 6.30] |
| 3 RDS | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.06, 14.38] |
| 4 Use of mechanical ventilation | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.18, 19.73] |
| 5 Necrotising enterocolitis | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Intraventricular haemorrhage | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Apgar less than 7 at 5 minutes | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.99] |
| 8 (Non pre‐specified) Arterial pH < 7.1 | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.09, 2.40] |
| 9 NICU admission | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.20, 4.34] |
| 10 Mean NICU stay (days) | 1 | 6 | Mean Difference (IV, Fixed, 95% CI) | ‐7.90 [‐48.95, 33.15] |
| 11 Mean birthweight (g) | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 398.00 [86.37, 709.63] |
| 12 Low birthweight (< 2500 g) | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.84] |
| 13 Delivery before 37 weeks | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.97] |
| 14 Latency ‐ days | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 10.90 [3.56, 18.24] |
| 15 Mean GA at delivery | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.08, 2.32] |
| 16 Caesarean section | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.51, 1.10] |
3.5. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 5 Necrotising enterocolitis.
3.6. Analysis.
Comparison 3 IV ritodrine plus vaginal progesterone versus IV ritodrine, Outcome 6 Intraventricular haemorrhage.
Comparison 4. IV hexoprenaline plus IV magnesium sulphate versus IV hexoprenaline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth before 7 days of trial entry | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.14, 5.06] |
Comparison 5. IV fenoterol plus oral naproxen versus IV fenoterol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth before 37 weeks | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.23, 1.09] |
Comparison 6. Oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol versus IV magnesium sulphate plus IV fenoterol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.55] |
| 2 Delivery with 7 days | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.03] |
Comparison 7. IV terbutaline plus oral metoprolol versus IV terbutaline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 (Non‐prespecified) Maternal oedema | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Mean time to delivery | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐14.92, 1.32] |
7.1. Analysis.
Comparison 7 IV terbutaline plus oral metoprolol versus IV terbutaline, Outcome 1 (Non‐prespecified) Maternal oedema.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ally 1992.
| Methods | 2‐arm RCT of IV ritodrine plus oral magnesium gluconate vs IV ritodrine alone. Randomised by a pre‐established list. Allocation concealment: not stated. |
|
| Participants | 107 women randomised ‐ 51 women in control group and 56 in experimental group. Study conducted between April 1988‐March 1990. Inclusion criteria: preterm labour between 22‐35 weeks. Exclusion criteria: PPROM, infection in evolution, nephropathy. |
|
| Interventions | Experimental group: IV ritodrine plus oral magnesium gluconate. Control: IV ritodrine. In both groups treatment continued for 24 hrs after the arrest of contractions. Then, oral treatment was continued until 36‐37 weeks. | |
| Outcomes | Pregnancy prolongation index, duration of perfusion (hrs), total dose of IV ritodrine (mg). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pre‐established randomisation list was used to allocate patients to the treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatment regimens. Staff would be aware. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not stated, but assessors would be aware. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 107 participants total: 51 in the control group, 56 in the treatment group. There were 3 patients excluded from the study (groups not stated) for medical reasons. It was not clear if an ITT analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from translated notes. |
| Other bias | Unclear risk | The control group was at 31.1 ± 0.4 weeks; the treatment group was at 29.7 ± 0.4 weeks. |
Arikan 2011.
| Methods | 2‐arm RCT of IV ritodrine plus vaginal progesterone vs IV ritodrine alone. | |
| Participants | 83 women randomised in a hospital in Turkey ‐ 40 women to the control group and 43 to the experimental group. Inclusion criteria: singleton pregnancy between 24 and 34 weeks; admitted with threatened preterm labour (persistent contractions (> 6 in a 30‐minute period) with cervical dilation or effacement); intact membranes, cervical dilatation 2 cm or less, with no previous cervical cerclage. Exclusion criteria: chorioamnionitis, evidence of deteriorating maternal or fetal condition, history of cervical surgery, uterine anomaly, fetal anomaly. |
|
| Interventions | All women were hydrated with 500 mL of Ringers lactate over 30 minutes and then all received a ritodrine infusion adjusted every 20 minutes from 10 to 70 mL/hr (maximum 0.35 mg/min) until the cessation of uterine contractions, the failure of therapy (not clear within what time period) or the occurrence of unacceptable side effects including tachycardia, hypotension or chest pain. The therapy was continued for 6 hrs after the cessation of contractions. All women also received a single course of betamethasone (2 x 12 mg injections during the first 24 hrs after admission) Experimental intervention: together with the ritodrine infusion, women in the experimental group also received vaginal micronized natural progesterone 200 mg. After ritodrine was discontinued women continues to receive micronized progesterone until 37 weeks’ gestation (frequency of 200 mg doses not clear) (43 women) (i.e. progesterone used for both tocolysis and maintenance therapy). The control group received the ritodrine therapy only (40 women). |
|
| Outcomes | Time to delivery (latency period), gestational age at delivery, delivery before 37 weeks, infant birthweight, umbilical cord pH, Apgar score and perinatal mortality and morbidity, admission to NICU, RDS, confirmed sepsis. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Stated that a convincing placebo was not feasible and blinding was not attempted. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 women were randomised and it was reported that none were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Reported that groups were comparable at baseline. |
Castillo 1988.
| Methods | 4‐arm clinical trial, comparing ritodrine only, indomethacin only, ritodrine plus indomethacin vs control. | |
| Participants | 35 women participated. Inclusion criteria: pregnant women being treated for premature labour. Exclusion criteria: no information given. |
|
| Interventions | Ritodrine only n = 10. Indometacine only n = 10. Ritodrine plus indomethacin n = 10. Control/comparison intervention: bedrest with IV hydration, n = 5. |
|
| Outcomes | Biophysical and biochemical markers. | |
| Notes | Conference abstract with no useable data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “at random”, not described. Equal numbers in groups suggests that it was not truly random. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, and groups received different treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Little information given. |
| Selective reporting (reporting bias) | Unclear risk | Assessed from conference abstract, with lack of detail about the study. |
| Other bias | Unclear risk | Little information on study methods. |
Ferguson 1984.
| Methods | Randomised, blinded clinical trial of IV ritodrine plus IV magnesium sulphate vs IV ritodrine alone. Allocation concealment by sealed envelopes. IV solutions prepared by a nurse not otherwise involved in providing care. | |
| Participants | 50 women originally randomised ‐ 22 women to the control group and 28 women to the experimental group. Data from only 41 were analysed. Study conducted between August 1982 to January 1983. Inclusion criteria: preterm labour prior to 36 weeks. Exclusion criteria: absolute contraindication to ritodrine or magnesium sulphate |
|
| Interventions | Experimental: IV ritodrine plus IV magnesium sulphate. Control: IV ritodrine. | |
| Outcomes | Side effects, successful suppression of contractions > 48 hrs. | |
| Notes | 18% (n = 9) excluded from the analysis for several reasons. Trial stopped early due to high incidence of side effects (sample size sought = 60). Groups were also randomised (randomisation not achieved due to early end of recruitment to trial) into 3 groups of IV solutions: 5% dextrose in water, 5% dextrose in half normal saline, lactated Ringer's solution. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "From a previously randomized file.” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a placebo‐ controlled trial, the staff preparing the medications (which were described as indistinguishable from each other) did not have any involvement administering treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study stopped after 50 women recruited. Data available for 40 women “who had completed at least 6 hours of treatment”. Data were available for 17 women in the ritodrine only group and for 24 in the ritodrine plus magnesium sulphate group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published reports. |
| Other bias | High risk | Study stopped early because of the high number of women having side effects. |
Francioli 1988.
| Methods | 2‐arm randomised trial of IV hexoprenaline sulphate alone vs IV hexoprenaline sulphate plus IV magnesium hydrochloride. Allocation concealment not stated. | |
| Participants | 24 women randomised ‐ 11 women to control group and 13 to experimental group. Inclusion criteria: preterm labour between 28‐36 weeks. Exclusion criteria: PROM, complete cervical effacement or cervical dilatation > 2 cm dilatation, multiple pregnancy, history of cervical incompetence or cerclage. |
|
| Interventions | Experimental: IV hexoprenaline sulphate plus IV magnesium hydrochloride. Control: IV hexoprenaline sulphate. | |
| Outcomes | Prolongation of delivery > 7 days, birthweight (average), days of prolongation of pregnancy (average), magnesaemia, kalaemia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned but different rates of infusion and treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described, but likely that outcome assessors knew treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appeared to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Not clear (assessment from translated notes). |
| Other bias | Unclear risk | Groups appeared comparable at baseline. |
Gamissans 1982.
| Methods | 2‐arm, randomised, double‐ blinded trial of IV ritodrine plus placebo suppositories vs IV ritodrine plus indomethacin suppositories. Allocation concealment: authors said "the key code was not available to the investigators". | |
| Participants | 208 women in total were randomised ‐ 104 women were in the control group and 104 women were in the experimental group Women were divided according to inclusion criteria into 2 subgroups for the analysis. The first group with 'intact membranes' included 153 women ‐ 77 women were in the control group and 76 women were in the experimental group The second group with 'ruptured membranes' included 55 women ‐ 27 women were in the control group and 28 women were in the experimental group. Inclusion criteria: preterm labour between 20‐36 weeks and between 24‐34 weeks respectively for first and second subgroups, single fetus. Exclusion criteria: pre‐eclampsia, renal or hypertensive disease, Rh immunisation, history suggestive of peptic ulcers. |
|
| Interventions | Experimental group: IV ritodrine plus indomethacin suppositories.
Control: IV ritodrine plus placebo suppositories. Suppositories were given every 8 hrs for 2 weeks unless labour occurred, rectal intolerance or gastric pain. Ritodrine infusion was administered for 24 hrs, then IM or orally up to 38 weeks unless labour occurred. |
|
| Outcomes | For both subgroups: gain in days (mean, median, < 11, 11‐30, 31‐60, > 60), gestational age at delivery < 37 weeks, recurrences, side effects, birthweight, Apgar score < 7 (at 1 and 5 min), fetal and postnatal death, umbilical blood ph (vein and artery). First subgroup only: RDS. | |
| Notes | This study was performed in 2 phases: during the first 2 years of the study, 85 women were recruited. None received corticosteroid therapy for lung maturation. During the second phase, 58 women out of the total 68 recruited, received treatment with betamethasone. In total 28/76 and 30/77 women received betamethasone in the R + I and R + P groups respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Placebo‐controlled trial, the code key was not available to the investigators. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo preparation was described as identical in this double‐ blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code key was not available to the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Women who developed side effects were reported to have been included in the main analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study reports. |
| Other bias | Unclear risk | Baseline characteristics described as similar. |
Hatjis 1987.
| Methods | 2‐arm, RCT of IV ritodrine plus IV magnesium sulphate vs IV ritodrine. Randomised by table of random numbers. Allocation concealment: sealed envelopes. |
|
| Participants | 64 women were randomised ‐ 36 women were in the control group and 38 women were in the experimental group. Study conducted in a tertiary care centre from October 1982 to July 1984. Inclusion criteria: preterm labour between 20‐35 weeks. Exclusion criteria: contraindications to IV ritodrine. |
|
| Interventions | Experimental: IV ritodrine plus IV magnesium sulphate. Control: IV ritodrine. In both groups ritodrine infusion was maintained for approximately 10 hrs after reaching the effective dose, then decreased gradually and oral ritodrine or terbutaline was administered until 37 weeks or recurrence. Magnesium sulphate was administered for variable periods with the aim of maintaining a magnesaemia between 5 to 7 mg%, and continued for approximately 10 hrs after contractions stopped. | |
| Outcomes | Prolongation of at least 7 days, days from treatment to delivery (mean), recurrence. | |
| Notes | 10 women excluded for different unjustified reasons (intention‐to‐treat analysis not performed). Side effects, birthweight and neonatal outcomes impossible to assess due to the use of rescue therapy in only 1 of the groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | “sealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Likely to be high risk, different treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described, but likely that outcome assessors knew treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 74 women were randomised, 10 were subsequently excluded because they did not complete treatment. Reasons for not completing treatment may have related to treatment allocation and outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published paper. |
| Other bias | Low risk | Baseline characteristics were described as similar. No other obvious bias. |
Lauterbach 2012.
| Methods | 2‐arm RCT individual randomisation, comparing oral pentoxifylline plus IV magnesium sulphate plus IV fenoterol vs IV magnesium sulphate plus IV fenoterol. | |
| Participants | 148 women randomised in a hospital in Cracow, Poland, between 2005 and 2008 ‐ 77 women were in the control group and 71 women were in the experimental group. Inclusion criteria: women with gestational age 23 to 34 weeks with more than 6 contractions in a 60‐minute period, progressive cervical changes, Bishop score < 5, intact membranes, Exclusion criteria: multiple pregnancy, fetal malformation, uterine malformation, pre‐eclampsia, underlying prolonged illness treated by immunosuppressants, hypotension, thrombocytopenia or clotting disorder, coronary heart disease, renal disease, diabetes, need for immediate delivery. |
|
| Interventions | Experimental intervention: (71 women) combined tocolytic therapy: IV magnesium sulphate 4 g loading dose followed by 2 g/hr to maximum dose of 24 g/day with fenoterol as a continuous infusion of 0.5 mcg/min plus pentoxifylline 50 mg per hr for 6 hrs then oral doses of 400 mg for 12 hrs with total daily dose of 800 mg/day (pentoxifylline for up to 3 weeks). Control/comparison intervention: (77 women) combined tocolytic therapy: IV magnesium sulphate 4 g loading dose followed by 2 g/hr to maximum dose of 24 g/day with fenoterol as a continuous infusion of 0.5 mcg/min. No pentoxifylline. Both groups received a single course of betamethasone in 2 doses of 12 mg for every 24 hrs for fetal lung maturation, In both groups doses of tocolytic drugs were adjusted depending on the strength and frequency of contractions. |
|
| Outcomes | Fetal‐placental blood flow, birthweight, Apgar score at 5 minutes, clinical index risk score for babies, sepsis and need for persistent ductus arteriosus closure, IVH, periventricular leukomalacia, death, need for respiratory support at 36 weeks. | |
| Notes | Considerable loss to follow‐up following randomisation. Analysis excludes women who were randomised but refused treatment, delivered within the first week of tocolysis, withdrew from the trial, changed the place of birth, or were intolerant to pentoxifylline. 148 randomised. Some data available for 125. Follow‐up of 96. 39% of the intervention group and 31% of the control group were therefore not included in the analysis of some outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The allocation was performed according to the sequence generated by a random number calculator.” |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The intervention was blinded neither for patients nor for doctors and nurses.” “placebo was not used in the control arm.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Likely that outcome assessors knew treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Considerable loss to follow‐up following randomisation. (Analysis excludes women who were randomised but refused treatment, delivered within the first week of tocolysis, withdrew from the trial, changed the place of birth, or were intolerant to pentoxifylline.) 148 randomised. Some data available for 125. Follow‐up of 96. 39% of the intervention group and 31% of the control group were therefore not included in the analysis of some outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Low risk | Groups described as comparable at baseline. |
Rios‐Anez 2001.
| Methods | 2‐arm randomised trial comparing IV fenoterol plus oral naproxen versus IV fenoterol alone. Allocation concealment: not stated. | |
| Participants | 72 women were randomised ‐ 36 women were in the control group and 36 in the experimental group. Inclusion criteria: preterm labour between 31‐36 weeks, normal singleton pregnancy. | |
| Interventions | Experimental: IV fenoterol plus oral naproxen. Control: IV fenoterol. Fenoterol infusion was maintained for 24 hrs, then, oral fenoterol was administered. Naproxen was given for 24 hrs. | |
| Outcomes | Birth < 37 weeks, birthweight. | |
| Notes | Reported only as abstract with very poor presentation. No other data to be extracted. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Equal numbers in groups suggests that it was not truly random. |
| Allocation concealment (selection bias) | Unclear risk | “divided into two 36 groups of each one, selected at random.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned. Different regimens and no mention of placebo, so likely to be at high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not described, but likely that outcome assessors knew treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. All women appeared to be accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Very brief conference abstract. |
| Other bias | Unclear risk | Brief abstract with little description of study methods. |
Ross 1983.
| Methods | 2‐armed RCT with individual randomisation, comparing IV terbutaline plus oral metoprolol vs IV terbutaline plus placebo. | |
| Participants | 20 women entered into the study (6 women had previous treatment before entry to the study; 3 had received either IV ritodrine or isoxsuprine, and 3 had received sc terbutaline). 3 women were excluded due to fetal death (1) concurrent varicella (1) and woman refused after start of protocol (1) ‐ 10 women were in the control group and 7 women in the experimental group. Recruitment in a Boston hospital (USA), 1981‐1982. Inclusion criteria: women in advanced premature labour, described as: gestational age 26‐34 weeks; cervical dilatation 3 or greater cm and effacement 50% or greater; regular uterine contractions lasting 30‐60 seconds at least every 10 minutes; intact membranes. (Cervical effacement was 100% in 13/17 women.) Exclusion criteria: maternal cardiovascular, renal, hepatic or respiratory disease; pre‐eclampsia; diabetes; signs of infection or sepsis; fetal compromise; vaginal bleeding suggesting placenta previa or abruption. |
|
| Interventions | Both groups: terbutaline 0.25 mg as a slow IV bolus followed by a continuous infusion of terbutaline at 10 mcg/min with infusion doses increased by 10 mcg/min every 15 minutes until contractions ceased or until maximum dose of 80 mcg with a reduction in doses if maternal pulse exceeded 150 per minute and therapy was discontinued if the women had palpitations, chest pain or oedema. If contractions ceased IV therapy continued for 6 hrs, and 30 minutes before the end of IV therapy oral terbutaline was administered (5 mg every 4 hrs). During oral therapy women had oral diet and fluids and IV hydration was discontinued. Women in both groups received dexamethasone 4 mg every 8 hrs IM up to 6 doses unless the attending doctor refused. All women were on bed rest during the treatment and contractions and fetal heart were monitored throughout. IV hydration was prescribed (5% glucose in Ringers lactate at 100 mL
/hr) with no additional oral fluid. Experimental intervention: (Results for 7 women (1 twin pregnancy)). In addition to terbutaline women in the intervention group received oral metoprolol 100 mg followed by 50 mg every 12 hrs. Control/comparison intervention: (Results for 10 women (1 twin pregnancy)). Terbutaline plus placebo. |
|
| Outcomes | Maternal pulse and blood pressure; mean and median time to delivery. Delay for 48 hrs and neonatal outcomes were not reported by randomisation group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was described as a double‐blind study. The control group received placebo (not described). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not clear when the randomisation code was broken. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 women entered into the study. Exclusions due to fetal death (1) concurrent varicella (1) and 1 woman refused after start of protocol. Not clear how many randomised to each group. Data for 7 women in the metoprolol group and 10 in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes were not reported by randomisation group (delay in delivery for 48 hrs and neonatal outcomes). |
| Other bias | Unclear risk | Groups appeared comparable at baseline. |
Schauf 2005.
| Methods | 3‐armed RCT with individual randomisation, comparing IV magnesium (group 1) vs IV magnesium plus IV fenoterol plus IV verapamil (group 2) vs placebo (group 3). | |
| Participants | 84 women randomised in a hospital in Germany ‐ 23 women to group 1, 32 women to group 2 and 29 women to the control group (group 3). Inclusion criteria: women with gestational age 25‐35 weeks with premature labour (at least 5 contractions in a 30 minute period). Exclusion criteria: multiple pregnancy, placenta previa, pre‐eclampsia, vaginal bleeding, PROM, cervical dilatation greater than 2 cm, contraindications to study medications. |
|
| Interventions | 3 groups. Group 1. (23 women) IV magnesium (50 mL bolus dose containing 5 g magnesium followed by 2 g/hr magnesium). Group 2 (32 women) fenoterol plus verapamil. Bolus of sodium chloride 0.9% followed by fenoterol 270 mcg/hr and verapamil 0.2 mg/hr. Control placebo: (29 women) sodium chloride (bolus and then IV infusion). |
|
| Outcomes | Red blood cell deformability after treatment. | |
| Notes | No relevant outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as placebo‐controlled double‐blind trial (no relevant outcomes, laboratory analysis only). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory analysis only. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. Not clear if there were any missing data. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. |
| Other bias | Unclear risk | Groups appeared comparable at baseline. |
hr(s): hour(s) IM: intramuscular ITT: intention‐to‐treat IV: intravenous IVH: intravenous hydration mcg: microgram mg: milligram NICU: neonatal intensive care unit PPROM: preterm prelabour rupture of membranes PROM: preterm rupture of membranes RCT: randomised controlled trial RDS: respiratory distress syndrome Rh: rhesus vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bedoya 1972 | It was not clear that any group received a combination of tocolytic drugs. It appeared that the intervention drug was compared with 1 of various other drugs. |
| Caballero 1979 | It was not clear that this was an RCT. “We have treated two groups of 100 patients each.” No mention of random allocation in this brief abstract. It was not clear that women were in preterm labour. |
| Dunlop 1986 | Authors evaluated a combination of 2 drugs only 1 being a tocolytic agent and the other an antibiotic. |
| Herzog 1999 | Authors used the same combination of tocolytic agents in the 3 groups being compared. Groups differ only in ways of administration, which was not the aim of this review. |
| How 2006 | Second tocolytic agent (nifedipine) was not randomly assigned. Nifedipine was only given if women remain without contractions after 24 hours of magnesium sulphate, otherwise, magnesium sulphate tocolysis was recommenced. |
| Ieda 1991 | It was not clear that this was an RCT, and although 1 group received a combination of tocolytic agents it was not clear what the control group received. |
| Illia 1993 | Inadequate method of randomisation. Women with even number on their case notes were assigned to the intervention group, odd numbers were assigned to the control group. |
| Katz 1983 | Not random allocation (alternate allocation, it was also stated that groups were "matched"). |
| Kawagoe 2011 | This study used a design which meant that both groups received the same combination of tocolytic agents but in a different order, and allocation at some stages in the study depended on clinician discretion. |
| Morales 2013 | This is an ongoing study comparing women randomised to receive nifedipine versus progesterone for maintenance therapy after tocolysis with atosiban. The study does not examine the use of a combination of agents for tocolysis. |
| Ogburn 1985 | This was not an experimental study (there was no control group); treatment with a combination of tocolytics was initiated in 23 women in preterm labour after failure of initial tocolysis. |
| Reynolds 1978 | Alternate allocation. Not an RCT. Not a combination of tocolytics. |
| Richter 1975 | Not clear that this was an RCT. Historical controls. |
| Richter 1979 | Not clear that this was an RCT. Not all the women recruited were randomly allocated (some historical controls). |
| Spearing 1979 | Not clear that this was an RCT. Alternate allocation. |
| Wischnik 1989 | Not an RCT. Alternate allocation. |
RCT: randomised controlled trial
Differences between protocol and review
Clinically relevant outcomes for trials of tocolysis for inhibiting preterm labour have been prespecified following consultation with the editors and authors of the individual reviews.
Consensus was reached on six ‘core’ outcomes. These have now been added to methods section of the review, March 2014.
Two outcomes, not pre‐specified in the protocol, have been included in this review: Arterial pH < 7.1; and maternal oedema.
Contributions of authors
JM Nardin (JMN) drafted the first version of this review. H West and T Dowswell carried out data extraction, assessed risk of bias and contributed to the analysis and the text. JP Vogel (JPV) prepared the second version of the review and finalised it in response to comments from O Oladapo and JMN. All authors approved the version submitted for editorial approval by JPV.
Sources of support
Internal sources
Centro Rosarino de Estudios Perinatales (CREP), Rosario, Argentina.
The University of Liverpool, UK.
External sources
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Therese Dowswell and Helen West were paid for work on this review from a grant to their institution (University of Liverpool) from the World Health Organization.
New
References
References to studies included in this review
Ally 1992 {published data only}
- Ally K, Nicolas A, Thoumsin H, Lambotte R. Magnesium gluconate and intravenous tocolysis with ritodrine. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1992;21:370‐4. [PubMed] [Google Scholar]
Arikan 2011 {published data only}
- Arikan I, Barut A, Harma M, Harma IM. Effect of progesterone as a tocolytic and in maintenance therapy during preterm labor. Gynecologic and Obstetric Investigation 2011;72(4):269‐73. [DOI] [PubMed] [Google Scholar]
Castillo 1988 {published data only}
- Castillo JM, Alonso J, Hernandez‐Garcia JM, Sancho B, Martinez V. Study of biochemical and biophysical modifications produced on pregnant women treated in threatened of premature labor with ritodrine and indometacine. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988:20.
Ferguson 1984 {published data only}
- Ferguson JE, Hensleigh PA, Kredenster D. Adjunctive use of magnesium sulfate with ritodrine for preterm labor tocolysis. American Journal of Obstetrics and Gynecology 1984;148(2):166‐71. [DOI] [PubMed] [Google Scholar]
- Ferguson JE, Holbrook H, Stevenson DK, Hensleigh PA, Kredentser D. Adjunctive magnesium sulphate infusion does not alter metabolic changes associated with ritodrine tocolysis. American Journal of Obstetrics and Gynecology 1987;156:103‐7. [DOI] [PubMed] [Google Scholar]
Francioli 1988 {published data only}
- Francioli M, Meuron A. Usefulness of the addition of aspartate magnesium hydrochloride via intravenous route to beta mimetics in the treatment of threatened preterm labor. Revue Medicale de la Suisse Romande 1988;108:283‐9. [PubMed] [Google Scholar]
Gamissans 1982 {published data only}
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. European Journal of Obstetrics and Gynecology and Reproductive Biology 1978;8:123‐8. [DOI] [PubMed] [Google Scholar]
- Gamissans O, Canas E, Cararach V, Ribas J, Puerto B, Edo A. A study of indomethacin combined with ritodrine in threatened preterm labor. Proceedings of the 6th European Congress of Perinatal Medicine, August 29th‐September 1st. Vienna, Austria, 1978. [DOI] [PubMed]
- Gamissans O, Cararach V, Serra J. The role of prostaglandin‐inhibitors, beta‐adrenergic drugs and glucocorticoids in the management of threatened preterm labor. Beta‐mimetic Drugs in Obstetrics and Perinatology. 3rd Symposium on Beta‐mimetic Drugs, Aagen, November 1980. Thieme‐Stratton Inc, 1982:71‐84.
Hatjis 1987 {published data only}
- Hatjis CG, Swain M, Nelson LH, Meis PJ, Ernest JM. Efficacy of combined administration of magnesium sulfate and ritodrine in the treatment of premature labor. Obstetrics and Gynecology 1987;69:317‐22. [PubMed] [Google Scholar]
Lauterbach 2012 {published data only}
- Lauterbach R, Rytlewski K, Pawlik D, Hurkala J, Wojtowicz A, Breborowicz G, et al. Effect of Pentoxifylline, administered in preterm labour, on the foetal‐placental circulation and neonatal outcome: a randomized, prospective pilot study. Basic and Clinical Pharmacology and Toxicology 2012;110(4):342‐6. [DOI] [PubMed] [Google Scholar]
Rios‐Anez 2001 {published data only}
- Rios‐Anez R, Santos‐Luque M, Noguera ME. Use of an antagonist of the prostaglandins associated to a b‐sympathomimetic agent in the treatment of pre‐term labour [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):165. [Google Scholar]
Ross 1983 {published data only}
- Ross MG, Nicolls E, Stubblefield PG, Kitzmiller JL. Intravenous terbutaline and simultaneous beta‐blockade for advanced premature labor. American Journal of Obstetrics and Gynecology 1983;147:897‐902. [DOI] [PubMed] [Google Scholar]
Schauf 2005 {published data only}
- Schauf B, Becker S, Abele H, Klever T, Wallwiener D, Aydeniz B. Effect of magnesium on red blood cell deformability in pregnancy. Hypertension in Pregnancy 2005;24(1):17‐27. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bedoya 1972 {published data only}
- Bedoya JM. Use of orciprenaline in the treatment of threatened premature labour. Proceedings of International Symposium on the Treatment of Fetal Risks; 1972; Baden, Austria. 1972:27‐9.
Caballero 1979 {published data only}
- Caballero A, Tejerina A, Dominguez A, Nava JM, Caballero A Jr. Indomethacine alone or associated to ritodrine in the prevention of premature labour [abstract]. 9th World Congress of Gynecology and Obstetrics; 1979 Oct 26‐31;Tokyo, Japan. 1979:300.
Dunlop 1986 {published data only}
- Dunlop PDM, Crowley PA, Lamont RF, Hawkins DF. Preterm ruptured membranes, no contractions. Journal of Obstetrics and Gynaecology 1986;7:92‐6. [Google Scholar]
Herzog 1999 {published data only}
- Herzog S, Cunze T, Martin M, Osmers R, Gleiter C, Kuhn W. Pulsatile vs continuous parenteral tocolysis: comparison of side effects. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;85:199‐204. [DOI] [PubMed] [Google Scholar]
- Herzog S, Cunze T, Osmers R, Kuhn W. Comparative study of maternal side effects of various forms of intravenous therapy with fenoterol in premature labor. Gynakologisch Geburtshilfliche Rundschau 1995;35(1):73‐9. [DOI] [PubMed] [Google Scholar]
How 2006 {published data only}
- How HY, Zafaranchi L, Stella CL, Recht K, Maxwell RA, Sibai BM, et al. Tocolysis in women with preterm labor between 32 0/7 and 34 6/7 weeks of gestation: a randomized controlled pilot study. American Journal of Obstetrics and Gynecology 2006;194(4):976‐81. [DOI] [PubMed] [Google Scholar]
Ieda 1991 {published data only}
- Ieda K, Sasaki J, Mizuno S, Mimura S, Suzuki C, Miyazaki T, et al. The clinical effects of maternally administered magnesium sulfate on the neonate. Journal of Perinatal Medicine 1991;Suppl 2:225. [Google Scholar]
Illia 1993 {published data only}
- Illia R, Diego I, Solana C. Threatened preterm labour. Evaluation of perinatals results in patients treated with betamimetics only and associated with indometacin [Amenaza de parto pretérmino. Evaluación de resultados perinatales de pacientes tratadas con betamiméticos solos y asociados a indometacina]. Tokoginecologia Practica 1993;52:383‐7. [Google Scholar]
Katz 1983 {published data only}
- Katz Z, Lancet M, Yemini M, Mogilner BM, Feigl A, Ben‐Hur H. Treatment of premature labor contractions with combined ritodrine and indomethacine. International Journal of Gynecology & Obstetrics 1983;21:337‐42. [DOI] [PubMed] [Google Scholar]
Kawagoe 2011 {published data only}
- Kawagoe Y, Sameshima H, Ikenoue T, Yasuhi I, Kawarabayashi T. Magnesium sulfate as a second‐line tocolytic agent for preterm labor: a randomized controlled trial in Kyushu Island. Journal of Pregnancy 2011;2011:965060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morales 2013 {published data only}
- Morales CV. Utility of tocolytic therapy for maintenance tocolysis in the management of threatened preterm delivery (UTM/2012). ClinicalTrials.gov (accessed 21 May 2013) 2013.
Ogburn 1985 {published data only}
- Ogburn PL Jr, Hansen CA, Williams PP, Butler JC Jr, Joseph MS, Julian TM. Magnesium sulfate and beta‐mimetic dual‐agent tocolysis in preterm labor after single‐agent failure. Journal of Reproductive Medicine 1985;30(8):583‐7. [PubMed] [Google Scholar]
Reynolds 1978 {published data only}
- Reynolds JW. A comparison of salbutamol and ethanol in the treatment of premature labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1978;18:107‐9. [Google Scholar]
Richter 1975 {published data only}
- Richter R, Hammacher K, Hinselmann M. Prophylaxis of threatened premature labour. Clinical results in a prospective comparative study with ritodrine and buphenine. Gynakologische Rundschau 1975;15:58‐9. [PubMed] [Google Scholar]
Richter 1979 {published data only}
- Richter R, Hinselmann MJ. The treatment of threatened premature labor by betamimetic drugs: a comparison of fenoterol and ritodrine. Obstetrics & Gynecology 1979;53:81‐7. [PubMed] [Google Scholar]
Spearing 1979 {published data only}
- Spearing G. Alcohol, indomethacin and salbutamol. A comparative trial of their use in preterm labour. Obstetrics & Gynecology 1979;53:171‐4. [PubMed] [Google Scholar]
Wischnik 1989 {published data only}
- Wischnik A, Hettenbach A, Schmidt R, Zieger W, Hug G, Melchert F. The effect of magnesium sulfate on volume balance in tocolysis with the beta mimetic agent fenoterol. Gynakologische Rundschau 1989;29 Suppl 2:188‐91. [PubMed] [Google Scholar]
Additional references
Anotayanonth 2004
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since1990 for selected countries: a systematic analysis and implications. Lancet 2012;9(379):2162‐72. [DOI] [PubMed] [Google Scholar]
Born Too Soon Report 2012
- March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon Report: The Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization, 2012. [Google Scholar]
Boyle 2011
- Boyle EM, Poulsen G, Field DJ, Kurinczuk JJ, Wolke D, Alfirevic Z, et al. Effects of gestational age at birth on health outcomes at 3 and 5 years of age: population based cohort study. BMJ 2011;344:e896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryce 2005
- Bryce J, Boschi‐Pinto C, Shibuya K, Black RE, for the WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet 2005;365(9465):1147‐52. [DOI] [PubMed] [Google Scholar]
Caritis 2005
- Caritis S. Adverse effects of tocolytic therapy. BJOG: an international journal of obstetrics and gynaecology 2005;112(Suppl 1):74‐8. [DOI] [PubMed] [Google Scholar]
Crowther 2002
- Crowther CA, Hiller JE, Doyle LW. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001060] [DOI] [PubMed] [Google Scholar]
Davidoff 2006
- Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Seminars in Perinatology 2006;30(5):313. [DOI] [PubMed] [Google Scholar]
Duckitt 2002
- Duckitt K, Thornton S. Nitric oxide donors for the treatment of preterm labour. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002860] [DOI] [PubMed] [Google Scholar]
Ekeus 2010
- Ekeus C, Lindström K, Lindblad F, Rasmussen F, Hjern A. Preterm birth, social disadvantage, and cognitive competence in Swedish 18‐ to 19‐year‐old men. Paediatrics 2010;125:e67‐e73. [DOI] [PubMed] [Google Scholar]
Flenady 2014
- Flenady V, Wojcieszek AM, Papatsonis DNM, Stock OM, Murray L, Jardine LA, Carbonne B. Calcium channel blockers for inhibiting preterm labour and birth. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD002255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flenady 2014a
- Flenady V, Reinebrant HE, Liley HG, Tambimuttu EG, Papatsonis DNM. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004452.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaunekar 2004
- Naik Gaunekar N, Raman P, Bain E, Crowther CA. Maintenance therapy with calcium channel blockers for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD004071.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldenberg 2008
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371(9606):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gáspár 2013
- Gáspár R, Hajagos‐Tóth J. Calcium channel blockers as tocolytics: principles of their actions, adverse effects and therapeutic combinations. Pharmaceuticals (Basel) 2013;6(6):689‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagberg 2001
- Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991‐94. Acta Paediatrica 2001;90(3):271‐7. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katz 2013
- Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small‐for‐gestational‐age infants in low‐income and middle‐income countries: a pooled country analysis. Lancet 2013;382(9890):417‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2005
- King JF, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
Lawn 2009
- Lawn JE, Kerber K, Enweronu‐Laryea C, Cousens S. 3.6 million neonatal deaths—what is progressing and what is not?. Seminars in Perinatology 2010;34(6):371‐86. [DOI] [PubMed] [Google Scholar]
Marlow 2005
- Marlow N, Wolke D, Bracewell MA, Samara M, EPICure Study Group. Neurologic and developmental disability at six years of age after extremely preterm birth. New England Journal of Medicine 2005;352(1):9‐19. [DOI] [PubMed] [Google Scholar]
Noble 2009
- Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144:S11‐S19. [DOI] [PubMed] [Google Scholar]
O'Connor 2007
- O'Connor AR, Wilson CM, Fielder AR. Ophthalmological problems associated with preterm birth. Eye (London) 2007;21(10):1254‐60. [DOI] [PubMed] [Google Scholar]
Plunkett 2008
- Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Annals of Medicine 2008;40:167‐95. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
- Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Talge 2010
- Talge N, Holzman C, Wang J, Lucia V. Late‐preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics 2010;126(6):1124‐31. [DOI] [PubMed] [Google Scholar]
Teune 2011
- Teune MJ, Bakhuizen S, Gyamfi Bannerman C, Opmeer BC, Kaam AH, Wassenaer AG, et al. A systematic review of severe morbidity in infants born late preterm. American Journal of Obstetrics and Gynecology 2011;205(4):374.e1‐374.e9. [DOI] [PubMed] [Google Scholar]
van Baar 2009
- Baar ALA, Vermaas JJ, Knots EE, Kleine MJKM, Soons PP. Functioning at school age of moderately preterm children born at 32 to 36 weeks' gestational age. Paediatrics 2009;124(1):251‐7. [DOI] [PubMed] [Google Scholar]
Wray 2003
- Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir‐Bishty E, et al. Calcium signalling and uterine contractility. Journal of the Society for Gynecologic Investigation 2003;10:252‐64. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nardin 2006
- Nardin JM, Carroli G, Alfirevic Z. Combination of tocolytic agents for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006169] [DOI] [PMC free article] [PubMed] [Google Scholar]


