Abstract
Background
Previous studies have suggested the potential association between air pollution and tuberculosis incidence, but this association remains inconclusive and evidence to assess causality is particularly lacking. We aimed to draw causal inference between fine particulate matter less than 2.5 μm in diameter (PM2.5) and tuberculosis in China.
Methods
Granger causality (GC) inference was performed within vector autoregressive models at levels and/or first-differences using annual national aggregated data during 1982–2019, annual provincial aggregated data during 1982–2019 and monthly provincial aggregated data during 2004–2018. Convergent cross-mapping (CCM) approach was used to determine the backbone nonlinear causal association based on the monthly provincial aggregated data during 2004–2018. Moreover, distributed lag nonlinear model (DLNM) was applied to quantify the causal effects.
Results
GC tests identified PM2.5 driving tuberculosis dynamics at national and provincial levels in Granger sense. Empirical dynamic modeling provided the CCM causal intensity of PM2.5 effect on tuberculosis at provincial level and demonstrated that PM2.5 had a positive effect on tuberculosis incidence. Then, DLNM estimation demonstrated that the PM2.5 exposure driven tuberculosis risk was concentration- and time-dependent in a nonlinear manner. This result still held in the multi-pollutant model.
Conclusions
Causal inference showed that PM2.5 exposure driving tuberculosis, which showing a concentration gradient change. Air pollutant control may have potential public health benefit of decreasing tuberculosis burden.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-16934-8.
Keywords: PM2.5, Tuberculosis, Causality, Empirical dynamic modeling, Eco-driver
Background
Tuberculosis (TB) is a chronic infectious disease and one of the leading causes of mortality worldwide. Mycobacterium tuberculosis (M. tb) infects approximately one quarter of the world’s population (latent TB infection, LTBI) [1], causing estimated 10.0 million symptomatic cases and 1.4 million death in 2019 [2]. In addition, post-TB sequelae add substantially to the overall disease burden [3]. China accounted for 8.5% (rank 3rd, after India and Indonesia) of global total TB cases in 2019, and was included in WHO’s three high TB burden country lists for the period 2016–2020 [2].
The linkage between poverty and TB has long been apparent. In China, prenatal and early-life exposure to malnutrition during the Great Famine of 1959–1961 increased the risk of tuberculosis in adulthood [4]. On the one hand, China’s economic growth, accompanied by improved nutrition and better healthcare programs, has become an integral component of national TB control efforts [5]. On the other, rapid urbanization and large flow of migrant workers might facilitate TB transmission and spatial diffusion [6]. Furthermore, there is evidence suggesting an association between ambient air pollution (especially particulate matter 2.5, PM2.5), a byproduct of economic activity, and TB development [7–10]. However, it is methodologically complex to establish causal link between air pollution and TB because TB changes during the past four decades are unlikely to have happened without changes in other environmental and socio-economic conditions [11].
Developments in epidemiologic and statistical methods have brought light to better causal inference in disease ecology [12]. Standard regression-based methods suffer from both omitted variable bias and errors-in-variable bias. As our study subject is large, complex, coupled human-natural system, it is probable that the overall resilience of the system cannot be reduced to a linear relationship. Both Granger causality (GC) and convergent cross mapping (CCM) tests are powerful methodological approaches that can help distinguish causality from spurious correlation in time series from stochastic or deterministic (chaotic) dynamical systems [13].
There was a demonstrable affirmative correlation between ambient PM2.5 levels and the incidence of newly diagnosed pulmonary tuberculosis in Jinan, China [14]. However, the situation in Beijing was characterized by equivocal evidence, with no definitive positive link observed [15]. A recent finding on the causal impact between major PM2.5 components and TB showed that PM2.5 components exposure was associated with increased TB burden [16]. Studies examining the long-term effects of ambient air pollution on the incidence of TB remain sparse, particularly in the context of causal inference. In the study, we focused on PM2.5, using combined modeling analysis on a large dataset covering 31 provinces in mainland China, to explore the population impact of air pollution on TB at national and provincial scale.
Methods
Data
The longitudinal data was retrieved from provincial and national TB prevalence surveys [5, 17, 18]. The time series data of annual reported number of pulmonary tuberculosis (PTB) in China during 1982–2019 was collected from online global TB database (https://worldhealthorg.shinyapps.io/tb_profiles/) [19]. The panel data of TB incidence in 31 provinces (annually during 1997–2018, and monthly during 2004–2018), was obtained from Chinese public health science data center (https://www.phsciencedata.cn/).
The air pollutant and whether data were retrieved from the modern-era retrospective analysis for research and applications version 2 (MERRA-2) released by national aeronautics and space administration (NASA) of USA [20].
The national and provincial-level data on annual birth rate, population density, per capita GDP, certified doctors and beds of medical institutions were extracted from the governmental statistical yearbooks (http://www.stats.gov.cn/tjsj/ndsj/).
Granger causality (GC) tests
GC tests are well-suited for rudimentary linear causality analysis, particularly in instances characterized by limited data length [21]. The GC analysis was conducted as an initial step to explore the causal relationship between PM2.5 and TB with the annual 1982–2019 time series data, using vector autoregressive (VAR) models [22] or vector error correction (VECM) models [23]. Then, the heterogeneous panel GC tests were applied to annual 1997–2018 and monthly 2004–2018 panel data, based on Monte-Carlo or Bootstrap simulation [24].
The analyses were performed using the standard modules (e.g., var, vec, vargranger, xtgcause) in Stata 17.0 (StataCorp, Texas, USA).
Convergent cross mapping (CCM) method
The Granger causality framework is inapplicable in scenarios where the segregation of information pertaining to variables from the broader system is unfeasible, particularly in cases where causal relationships exhibit weak to moderate strengths. Conversely, CCM presents a heightened utility in addressing intricate systems and data, exhibiting diminished susceptibility to the effects of noise and external factors [25]. Nonetheless, it is imperative to note that CCM necessitates the availability of time-series data of substantial duration for meaningful analysis. Thus, we used empirical dynamic modeling (EDM), a data-driven equation-free mechanistic approach [25], to model mechanisms forcing TB epidemics with monthly 2004–2018 panel data. Convergent cross-mapping (CCM) method was adopted to distinguish causality between pairs of time series from correlations. The basic idea of CCM is to look for the signature of X in Y’s time series [26].
The convergent cross-mapping analysis, an EDM for detecting causality in nonlinear dynamic systems, [25] was composed of three parts here. First, the CCM causality between PM2.5 and TB incidence was tested based on univariate state-space reconstruction (SSR) according the modified methods described elsewhere [27, 28]. We examined whether the cross-map prediction skill (ρCCM, the Pearson correlation between observations and CCM prediction) increased and demonstrated convergence as the library length increased if causality existed for two variables. CCM for the real time series need to show higher prediction skill than 90% confidence intervals of surrogate time series.
Second, multivariate SSR (including stochastic causal variables as a coordinate in the state space) could improve the ability of nearest-neighbor prediction. For seasonal TB, PM2.5 could be considered stochastic because information about it may already be included in the univariate embedding [25]. We examined multivariate SSR forecast improvement, according to a modified method developed by a previous study [28].
Third, Scenario exploration with multivariate SSR was employed to investigate the effect of a small change in the potential driver (PM2.5) on TB incidence across different states of the system. The effect of ΔTB/ΔPM2.5 provided a way to understand the causality direction.
The analyses were performed using rEDM package version 0.7.5 of R software (R Foundation for Statistical Computing, Vienna, Austria).
Distributed lag nonlinear model (DLNM)
While CCM helped us to establishing the causal relationship (statistical significance) and the causal direction (temporality), it provided little information on the causal strength (exposure-response relationship). Thus, we further evaluated the exposure risks using distributed lag nonlinear models (DLNM) [29].The basic model of DLNM is generalized linear model (GLM). In the multivariate DLNM, temperature, precipitation and sunshine duration were included to control the potential confounders [9]. The cumulative relative risks (RRs) were calculated for different extents of exposure to PM2.5 within lag 0–15 months, as well as for every 10 µg/m3 of PM2.5. The reference values of PM2.5 was set as 15 µg/m3 according to WHO’s air quality guidelines (https://www.who.int/publications/i/item/9789240034228). In order to fit the nonlinear and delayed effects, we constructed “cross-basis” (bidimensional) function and depicted the effects of predictors and lags simultaneously. Moreover, we computed a three-dimensional model of PM2.5, lag months and risk of TB incidence into a hexahedron.
Sensitivity analysis was conducted by fitting multi-pollutant models to identify the robustness of the results. To avoid multicollinearity problem, the pollutant would be excluded if the Pearson correlation coefficient ≥ 0.7 [29].
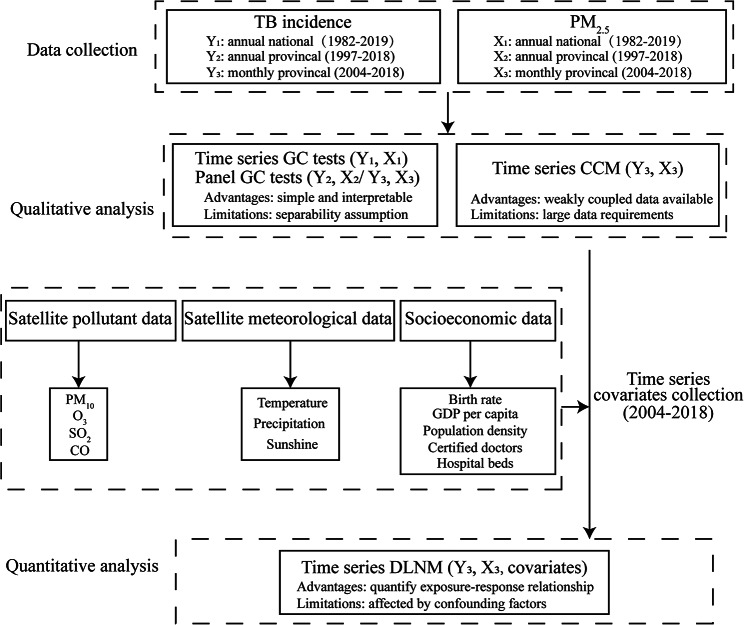
The analyses were performed using the package “dlnm” version 2.4.7 in R software (R Foundation for Statistical Computing, Vienna, Austria). Figure 1 showed the complete flow diagram.
Fig. 1.
Methodology flowchart of the causal inference study on PM2.5 and TB.
Data availability
The data that supports the findings of this study are available in the supplementary material.
Results
Economic development and environmental health trends
With the progress of society, both PM2.5 and TB have experienced three stages during 1982–2019: from slow increase, then rapid rise to moderate decline (SI Appendix Fig. S1A, SI Appendix Table S1). Real GDP per capita (pGDP) is utilized to extend the environmental Kuznets curve (EKC) hypothesis to the interrelationships among economic growth, environment and health, indicated by the inverted U-shaped curves (SI Appendix Fig. S1B). That is, the health gains obtained through improved incomes could be significantly negated by the environmental stress variable at the beginning. But after a threshold of economic development level, environmental health issues will decline [30].
GC analysis
We found positive associations between TB incidence and PM2.5 in most provinces during 1997–2018 (SI Appendix Fig. S2). Based on the VAR models using the non-stationary time series at difference, GC tests revealed a significant unidirectional causality from dPM2.5 to dTB (Wald F test, P = 0.026, Table 1, SI Appendix Table S2). The response of dTB to dPM2.5 reached its peak at 1-year and prevailed between 2 and 4 years (SI Appendix Fig. S3). Meanwhile, the GC analysis based on VECM also indicated a possible causal link from PM2.5 to TB, although the association did not reach statistical significance (P = 0.114) (Table 1).
Table 1.
Granger causality between PM2.5 and tuberculosis
| Test | Null hypothesis (H0) | Lag | Statistic | P | Conclusion |
|---|---|---|---|---|---|
| 1982–2019 annual country-level data | |||||
| VAR-based GC | ΔPM2.5 does not G-cause ΔTB | 1 Y | 4.925 | 0.026 | PM2.5 G-causes TB |
| ΔTB does not G-cause ΔPM2.5 | 1 Y | 0.578 | 0.447 | TB does not G-cause PM2.5 | |
| VECM-based GC | PM2.5 does not G-cause TB | 2 Y | 2.490 | 0.114 | PM2.5 does not G-cause TB |
| TB does not G-cause PM2.5 | 2 Y | 0.880 | 0.348 | TB does not G-cause PM2.5 | |
| 1997–2018 annual province-level data | |||||
| PVAR-based GC (cross-sectional) | PM2.5 does not G-cause TB | 3 Y |
W-Bar:7.57 Z-Bar:10.39 |
0.060 | PM2.5 G-causes TB for at least one province |
| TB does not G-cause PM2.5 | 3 Y |
W-Bar:5.72 Z-Bar:6.18 |
0.214 | TB does not G-cause PM2.5 | |
| 2004–2018 monthly province-level data | |||||
| PVAR-based GC (cross-sectional) | PM2.5 does not G-cause TB | 9 M |
W-Bar:47.71 Z-Bar:50.80 |
< 0.001 | PM2.5 G-causes TB for at least one province |
| TB does not G-cause PM2.5 | 9 M |
W-Bar:45.57 Z-Bar:48.00 |
< 0.001 | TB G-causes PM2.5 for at least one province | |
VAR, vector autoregression model; Δ, 1st difference; G-cause, Granger-cause; GC, Granger causality test; VECM, vector error correction model; PVAR, panel vector autoregression model
Based on the panel data of from 1997 to 2018 (SI Appendix Table S3), the heterogeneous GC tests based on panel vector autoregressive model (PVAR) suggested unidirectional G-causality between PM2.5 and TB (Z-Bar 10.39, P = 0.060, Table 1, SI Appendix Table S4).
For the monthly data during 2004–2018 (SI Appendix Table S5), although pooled panel regression showed negative association between PM2.5 and TB incidence (SI Appendix Fig. S4), the meta-analysis of Pearson correlation coefficients (R) demonstrated positive association between them (overall R = 0.12, 95%CI 0.07–0.17, P < 0.001) (SI Appendix Fig. S5). The panel GC tests based on the cross-sectional Wald statistic suggested bidirectional G-causality between PM2.5 and TB (both P < 0.001) (Table 1, SI Appendix Table S6), although the converse scenario could not be true because TB cannot cause air pollution. This result was not surprising because the data duration was shorter and the threshold for rejecting the null hypothesis was causal relation in Granger sense for at least one province.
CCM causal testing
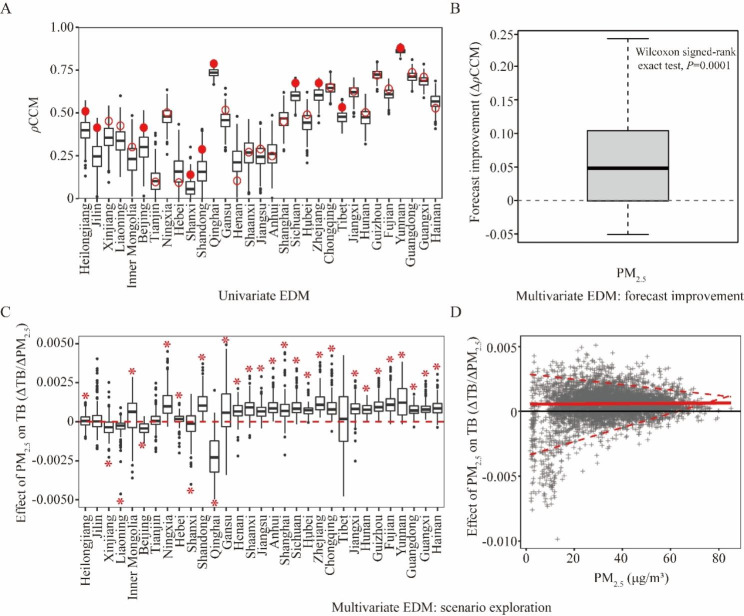
The seasonality of TB and PM2.5 was distinct at country level, with peaks in winter and spring respectively (SI Appendix Fig. S6). In addition, the heatmaps showed substantial temporal and geospatial variation of TB seasonality (SI Appendix Fig. S7). The mutual seasonality of TB and PM2.5 makes it especially important to distinguish causal interactions from spurious correlation. First, we performed univariate state-space reconstruction (SSR) with optimized CCM model parameters (SI Appendix Figs. S8, S9). The hypothesis was: if CCM prediction of TB for the observational PM2.5 was significantly better than it was for the null surrogates which had the same seasonal cycle as PM2.5 yet with randomized anomalies, the causal forcing of PM2.5 on TB would be established (SI Appendix Fig. S10). The box-and-whisker plot (Fig. 2A) demonstrated that PM2.5 be causal forcing for TB in 10 provinces, indicated by the measured cross-map skill (ρCCM) with significant P values (≤ 0.1). The results had very high metasignificance (Fisher’s method) for PM2.5: P < 4.2 × 10− 5. Second, we used the multivariate SSR to look for improvement in forecasting. That is, if the multivariate SSR containing the potential driving variable PM2.5 produced better forecasts of TB than without, then PM2.5 causally influenced TB in the CCM sense. It turned out that including PM2.5 led to significant improvement on forecast skill of TB (Fig. 2B). Third, we conducted scenario exploration with multivariate SSR. By predicting the change in TB (ΔTB) that result from a small change in PM2.5 (ΔPM2.5), we demonstrated that PM2.5 had a positive effect on TB incidence (positive values for ΔTB/ΔPM2.5) for 22 provinces individually (Fig. 2C) and for the whole group (Fig. 2D). Nevertheless, the combined results of the correlation and CCM analysis are provided in Table 2.
Fig. 2.
Cross-map causality of PM2.5 on tuberculosis. (A) Cross-map causality beyond shared seasonality of ambient PM2.5 on tuberculosis based on univariate SSR. The box-and-whisker plots show the null distributions for cross-map skill (ρCCM) expected from random surrogate time series which share the same seasonality as the true PM2.5 concentration. Red circles demonstrate the unlagged ρCCM for observed TB predicting purported PM2.5. Filled circles indicate the significant ρCCM (P ≤ 0.1). Provinces are ordered according to their latitudes. (B) Forecast improvement with multivariate SSR is quantified using ΔρCCM = ρCCM (with PM2.5) - ρCCM (without PM2.5). Wilcoxon signed-rank exact test reveals a significant difference. (C) Effect of PM2.5 on TB (ΔTB/ΔPM2.5) for each province. In the scenario analysis, PM2.5 shows a positive effect on TB incidence for 22 provinces (P ≤ 0.1). (D) Range of ΔTB/ΔPM2.5 as a function of PM2.5 grouped over all provinces. SSR, state-space reconstruction; CCM, convergent cross-mapping; ρCCM, the Pearson correlation between observations and CCM prediction
Table 2.
Correlation and CCM causal analysis results between PM2.5 concentration and TB incidence across 31 provinces in China during 2004–2018
| Province | Pearson correlation (R) | PM2.5 causes TB (ρ) | TB causes PM2.5 (ρ) | Causal direction# |
|---|---|---|---|---|
| Heilongjiang | 0.0068 | 0.5101* | 0.4036*** | PM2.5↔TB |
| Jilin | 0.1834** | 0.4143** | 0.0879 | PM2.5→TB |
| Xinjiang | 0.1722** | 0.4527 | 0.1126 | Neutrality |
| Liaoning | 0.2709*** | 0.4256 | 0.3248 | Neutrality |
| Inner Mongolia | 0.1138 | 0.3014 | 0.0187 | Neutrality |
| Beijing | 0.2158*** | 0.4147* | -0.0413 | PM2.5→TB |
| Tianjin | -0.0326 | 0.0977 | -0.0166 | Neutrality |
| Ningxia | 0.2847*** | 0.5019 | 0.3743 | Neutrality |
| Hebei | 0.0321 | 0.0937 | -0.2149 | Neutrality |
| Shanxi | 0.1552** | 0.1397** | 0.0913 | PM2.5→TB |
| Shandong | 0.0974 | 0.2879* | 0.3084 | PM2.5→TB |
| Qinghai | 0.2644*** | 0.7879*** | 0.5165* | PM2.5↔TB |
| Gansu | 0.3895*** | 0.5176 | 0.3645** | TB→PM2.5 |
| Henan | -0.0090 | 0.1044 | 0.1777 | Neutrality |
| Shaanxi | 0.1264* | 0.2712 | 0.1077 | Neutrality |
| Jiangsu | 0.0327 | 0.2896 | 0.4116*** | TB→PM2.5 |
| Anhui | 0.0789 | 0.2491 | 0.3672** | TB→PM2.5 |
| Shanghai | 0.0102 | 0.4501 | 0.1720 | Neutrality |
| Sichuan | 0.1928*** | 0.6745** | 0.4451* | PM2.5↔TB |
| Hubei | 0.0619 | 0.4903 | 0.4764** | TB→PM2.5 |
| Zhejiang | 0.0716 | 0.6748** | 0.4381*** | PM2.5↔TB |
| Chongqing | 0.1054 | 0.6511 | 0.4271 | Neutrality |
| Tibet | 0.4404*** | 0.5331* | 0.3843 | PM2.5→TB |
| Jiangxi | 0.0736 | 0.6263 | 0.3711 | Neutrality |
| Hunan | 0.0165 | 0.5033 | 0.6260*** | TB→PM2.5 |
| Guizhou | 0.1276* | 0.7201 | 0.5867* | TB→PM2.5 |
| Fujian | 0.1479** | 0.6415 | 0.1869 | Neutrality |
| Yunnan | 0.1828** | 0.8796* | 0.4377 | PM2.5→TB |
| Guangdong | -0.0493 | 0.7374 | 0.6069*** | TB→PM2.5 |
| Guangxi | 0.0227 | 0.7098 | 0.5783** | TB→PM2.5 |
| Hainan | -0.0929 | 0.5281 | 0.4971** | TB→PM2.5 |
*P ≤ 0.1, **P ≤ 0.05, ***P ≤ 0.01
#The converse scenario could not be true because tuberculosis does not cause air pollution, that is, the shadow attractor constructed using PM2.5 data should not contain information to accurately reconstruct past TB incidence
The exposure–response effects of air pollutants on TB risk
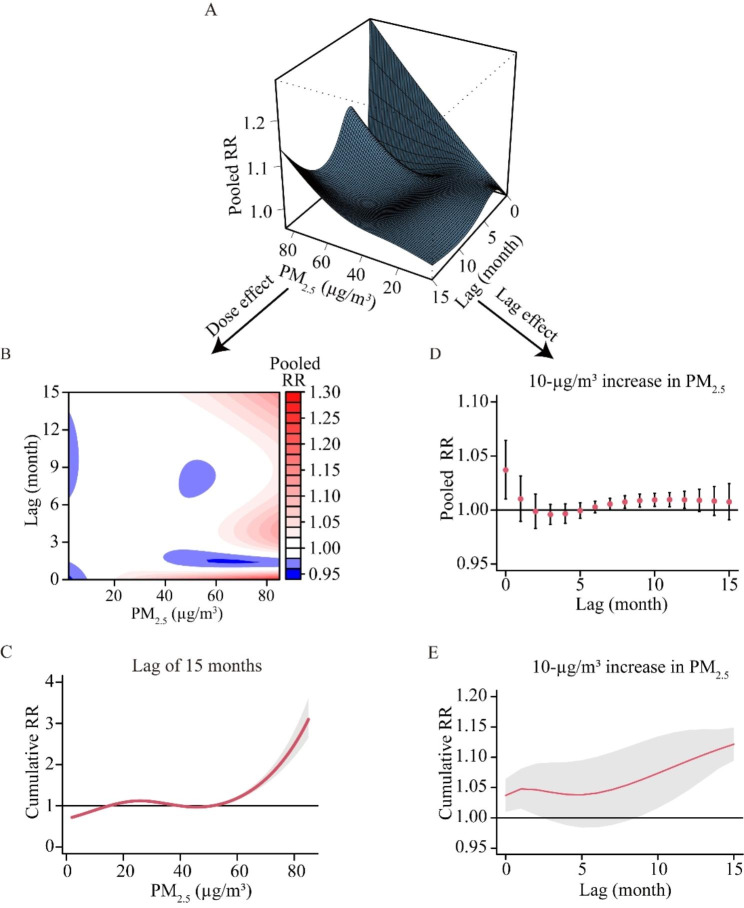
Based on the multivariate DLNM model, the three-dimensional graph vividly depicted the overall effects of PM2.5 on TB incidence, calculated as relative risks (RRs) (Fig. 3A). In the contour plot, acute effects (lag 0–1 months) were observed under exposure to high levels of PM2.5 (with the maximum pooled [lag-specific] RR of 1.28 under exposure to 85 µg/m3 of PM2.5 at the current month), while delayed effects were seen under exposure to high levels of PM2.5 at lag 2–15 months (Fig. 3B). The cumulative (15 months) effects of PM2.5 on TB incidence were demonstrated in the exposure-response curve (Fig. 3C). Besides, the pooled and cumulative (throughout lags of 0–15 months) RRs associated with 10-µg/m3 increase in PM2.5 were shown in Fig. 3D and E respectively.
Fig. 3.
Exposure-response relationship between PM2.5 and tuberculosis incidence in single-pollutant DLNM model. (A) Three-dimensional plot: the height of the hexahedron represents RR for the association between TB incidence and ambient PM2.5 exposure, while two bottom edges represent the full range of monthly mean PM2.5 concentration and the number of months delayed. (B) Contour plot: the red color gradient represents RR > 1, and the blue gradient represents RR < 1. (C) Cumulative effects of PM2.5 exposure for 15 months. (D-E) Pooled and cumulative effects with 10 µg/m3 increase in PM2.5 throughout 0–15 months. The reference level of PM2.5 is set as 15 µg/m3. Monthly mean temperature, precipitation and sunshine duration, and annual population density, GDP per capita, certified doctors and beds of medical institutions are added as time-varying local control variables. TB, tuberculosis; RR, relative risk
The pooled exposure–response effects of air pollutants on TB risk in both the single-pollutant and two-pollutant models were shown in Table 3. In single pollutant model, each 10 µg/m3 increase in PM2.5 concentrations was significantly positively associated with the TB incidence, with RR of 1.121 (95% CI:1.095, 1.149). Moreover, there was no substantial change in the results when conducting the multi-pollutant models.
Table 3.
Cumulative association between tuberculosis incidence and 10 µg/m3 increase in PM2.5.
| Model | Pollutant(s) | RR (95%CI) for TB (associated with 10 µg/m3 increase in PM2.5) |
|---|---|---|
| Single-pollutant | PM2.5 | 1.121 (1.095, 1.149)* |
| Multi-pollutant | PM2.5 + PM10 | 1.209 (1.149, 1.273)* |
| PM2.5 + CO | 1.112 (1.085, 1.139)* | |
| PM2.5 + O3 | 1.292 (1.257, 1.327)* | |
| PM2.5 + PM10 + CO | 1.267 (1.189, 1.350)* | |
| PM2.5 + PM10 + O3 | 1.208 (1.144, 1.277)* | |
| PM2.5 + CO + O3 | 1.307 (1.271, 1.344)* | |
| PM2.5 + PM10 + CO + O3 | 1.183 (1.108, 1.263)* |
TB, tuberculosis; PM2.5, particulate matter of < 2.5 μm; PM10, particulate matter of < 10 μm; CO, carbon monoxide; O3, ozone. *P ≤ 0.05
Discussion
Evaluating the influence of PM2.5exposure on TB occurrence holds substantial relevance in the realm of public health, serving as the initial phase in formulating environmental strategies aimed at alleviating the tuberculosis burden within the context of China. Several empirical studies have addressed the potential relation between air pollution and TB incidence, but this issue remains controversial and inconclusive, deserving further investigation [31]. The information presented here refers to situations within China, but environmental health and protection are known without boundaries. The causal inference framework may be valuable for the identification of other air pollution-associated adverse health impacts.
Ambient air pollution is one of the leading environmental risk factors to human health. Short-term air pollution exposure is found to be causally related to acute adverse respiratory health effects and exacerbation of preexisting chronic airway diseases, while long-term exposure may be a causal factor for new-onset airway diseases such as childhood asthma [32]. PM2.5 (also called alveolar fraction) accounts for 96% of particles observed in human pulmonary system [33]. The toxicity of PM is inversely linked to particle size, with smaller particles contributing to greater inflammatory effects [34]. There are biological mechanisms by which PM2.5 could plausibly affect individual’s susceptibility to TB infection or reactivation. First, PM2.5 could directly attack the respiratory tract and suppress antimicrobial activity by down-regulating airway antimicrobial proteins and peptides (AMPs) which are important for airway innate immunity [35]. Second, it may disrupt the synthesis and secretion of inflammatory cytokines and impair anti-mycobacterial T cell immune responses to M tb [36]. Third, increased iron availability provided by PM2.5 may create a favorable environment for mycobacterial proliferation [37, 38]. Based on the above, PM2.5 served as the best indicator of all air pollutants here. Our findings support that exposure to air pollutants above a certain level may increase their susceptibility to M. tb infection or reactivation.
Two earlier cohort studies reported potential association between PM2.5 and TB in Los Angeles city, USA and Taiwan province, China respectively [39, 40]. The results from time series studies on this issue contradicted one another. The inconsistent evidence may partly be due to the different methods, variable selection and time frames. A recent meta-analysis claimed that PM2.5 had neither long-term nor short-term TB risk (RR, 1.030; 95%CI, 0.996–1.065 and RR, 1.031; 95%CI, 0.981–1.083 respectively) [31]. However, this study argues that, the existing studies were restricted to a partial view of the phenomenon. In this respect, our study departs from the literature by taking into consideration the information from both the province (piece) and country (whole puzzle) sides, relative to their characteristics, heterogeneous settings and common trend. To do so, we analyzed the data from 31 provinces in China. Our findings could be convincing given the country’s sheer size and the allowance for temporal diversity.
Moving beyond correlation, we evaluated the causality between PM2.5 and TB with complementary strategies. To determine whether X causes Y: GC compares “knowledge about Yt” vs. “knowledge about Xt and Yt” in prediction of Yt+1 (forward looking) [41], while CCM compares “knowledge about MY” vs. “no knowledge about MY” in prediction of Xt (backward looking) [27]. GC can perform relatively well on short time series, while CCM generally prefer for longer time series (≥ 30 observations) [25]. The two seemingly opposite methods can yield similar causal inference in spite of the different assumptions [13]. Herein, GC or CCM (or both) were decided according to the aims and data characteristics, rather than “linear vs. nonlinear model” gradient. We re-enforced the causal effect of PM2.5 on TB by employing GC and CCM on the long panel dataset. Our approach has an advantage over the standard approach based on regression as it is free from issues concerning the exposure-confounders-morbidity modeling and does not involve extrapolation.
It is worth noting that, from exposure-response relationship perspective, PM2.5 was positively associated with both TB incidence, with RR of 1.12 (95% CI: 1.03, 1.22) per 10 µg/m3 increase, which was consistent with our results [42]. A regional study also demonstrated that long-term exposure to PM2.5 was significantly associated with higher TB incidence [43]. Increased exposure to PM2.5 contributed to a faster bacterial replication rate, indicating that M. tb exhibits increased reproductive activity, thus accelerating within-host endogenous reactivation [44]. Elevated concentrations of PM2.5 may exert pressure on healthcare systems through an augmentation in TB incidence and associated treatment expenditures.
During 2006–2012, China’s new air pollution policies which interact with political incentives were introduced in the 11th Five-Year Plan. These policies have been effective in cutting pollutants emission. After the winter-long “PM2.5 crisis” in eastern China in 2013, the standards for air pollution control have been updated and further strengthened [45]. The observed co-movement between PM2.5 and TB incidence suggest a possible link between the air pollution control policies and health risk reduction. Therefore, TB prevention should not only focus on interrupting TB transmission, but also on monitoring air pollutants such as PM2.5. Establish real-time air quality monitoring systems to notify the public and policymakers of elevated pollution levels, encouraging precautionary measures. Allocate healthcare resources efficiently in regions with significant burdens of TB and elevated PM2.5 levels.
This study has several limitations. First, the estimate for exposure-response relationship should be interpreted with caution. It cannot be extended to concentrations beyond the support of the data. Second, the effect of air pollution control policies on TB has not been tested. The counterfactual models such as difference-in-differences (DID) may be helpful for policy evaluation. Third, although the effects of PM2.5 to drive TB may be different for new infection and reactivation, we could not test the hypothesis. It is usually difficult to judge whether an active TB case is from LTBI or uninfected individuals in routine practice. Last, we analyzed the impacts of PM2.5 at the province level, yet different cities and counties might be heterogeneous even within one province. Prospective spatially oriented causal research endeavors have the potential to yield novel insights for elucidating heterogeneity.
In summary, we demonstrate that ambient PM2.5 exposure and tuberculosis incidence had a linkage which (1) is causal and ecologically important; (2) is independently detected in different provinces; and (3) follows an exposure-response gradient. The take-home message is clear: to fight tuberculosis, we must also fight air pollution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors’ contributions
G.Q., J.-F.M. and B.Z. conceived and designed the research. J.-J.M., C.-H.L., H.-L.C. and G.Q. performed the data analyses. J.-W.L., Y.-Y.G. and J.F. produced the figures. J.-J.M. and C.-H.L. wrote the first draft of the manusrcipt. B.Z., J.-F.M. and G.Q. made the key revision. All authors contributed to the scientific discussions and preparation of the manuscript.
Funding
G.Q. acknowledges the support from National Natural Science Foundation of China (NSFC, grant 81370520), Science and Technology Support Program of Jiangsu Province, China (grant BE2015655), Chinese Society of Academic Degrees and Graduate Education (grant 2020ZDB55), Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant SJCX22_1638). B.Z. acknowledges the support from Science and Technology Project of Nantong City, China (grant HS2020001).
Data Availability
The datasets supporting the conclusions of this article are available in the Data-center of China Public Health Science, http://www.phsciencedata.cn/Share/en/index.jsp. The codes are available at 10.5281/zenodo.10020179.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Gang Qin is an editorial board member of BMC Public Health. The remaining authors have no conflicts of interest to declare.
Footnotes
Using aggregate data for 31 provinces in China covering 1982-2019, we demonstrate the causal association between PM2.5 and tuberculosis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun-Jie Mao, Hong-Lin Chen and Chun-Hu Li contributed equally to this work.
Contributor Information
Bin Zhang, Email: binzane@163.com.
Jun-Feng Ma, Email: majunfengql@163.com.
Gang Qin, Email: tonygqin@ntu.edu.cn.
References
- 1.Houben RM, Dodd PJ. The global burden of latent Tuberculosis Infection: a re-estimation using Mathematical Modelling. PLoS Med. 2016;13(10):e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, Kapata N, Mfinanga S, Hasnain SE, Katoto P, et al. Global Tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021;113(Suppl 1):7–S12. doi: 10.1016/j.ijid.2021.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menzies NA, Quaife M, Allwood BW, Byrne AL, Coussens AK, Harries AD, Marx FM, Meghji J, Pedrazzoli D, Salomon JA, et al. Lifetime burden of Disease due to incident Tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health. 2021;9(12):e1679–87. doi: 10.1016/S2214-109X(21)00367-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng Q, Trangucci R, Nelson KN, Fu W, Collender PA, Head JR, Hoover CM, Skaff NK, Li T, Li X, et al. Prenatal and early-life exposure to the great Chinese famine increased the risk of Tuberculosis in adulthood across two generations. Proc Natl Acad Sci U S A. 2020;117(44):27549–55. doi: 10.1073/pnas.2008336117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, Chen M, Zhao Y, Jiang S, Du X, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–64. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 6.Bele S, Jiang W, Lu H, You H, Fan H, Huang L, Wang Q, Shen H, Wang J. Population aging and migrant workers: bottlenecks in Tuberculosis control in rural China. PLoS ONE. 2014;9(2):e88290. doi: 10.1371/journal.pone.0088290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Mao X, Liu Q, Song H, Ji Y, Xu D, Qiu B, Tian D, Wang J. Long-term effect of exposure to ambient air pollution on the risk of active Tuberculosis. Int J Infect Dis. 2019;87:177–84. doi: 10.1016/j.ijid.2019.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Xiang H, Yang W, Zhu Z, Tian L, Deng S, Zhang T, Lu Y, Liu F, Li X, et al. Short-term effect of Air Pollution on Tuberculosis based on Kriged Data: a time-series Analysis. Int J Environ Res Public Health. 2020;17(5):1522. doi: 10.3390/ijerph17051522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang K, Ding K, Yang XJ, Hu CY, Jiang W, Hua XG, Liu J, Cao JY, Zhang T, Kan XH, et al. Association between short-term exposure to ambient air pollutants and the risk of Tuberculosis outpatient visits: a time-series study in Hefei, China. Environ Res. 2020;184:109343. doi: 10.1016/j.envres.2020.109343. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Tian C, Wang W, Luo X. Temporal cross-correlations between Ambient Air pollutants and Seasonality of Tuberculosis: a Time-Series Analysis. Int J Environ Res Public Health. 2019;16(9):1585. doi: 10.3390/ijerph16091585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Gong J, Zhou J, Zhao Y, Tan J, Ibrahim AN, Zhou Y. A spatial, social and environmental study of Tuberculosis in China using statistical and GIS technology. Int J Environ Res Public Health. 2015;12(2):1425–48. doi: 10.3390/ijerph120201425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plowright RK, Sokolow SH, Gorman ME, Daszak P, Foley JE. Causal inference in Disease ecology: investigating ecological drivers of Disease emergence. Front Ecol Environ. 2008;6(8):420–9. doi: 10.1890/070086. [DOI] [Google Scholar]
- 13.Barraquand F, Picoche C, Detto M, Hartig F. Inferring species interactions using Granger causality and convergent cross mapping. Theoretical Ecol. 2021;14(1):87–105. doi: 10.1007/s12080-020-00482-7. [DOI] [Google Scholar]
- 14.Liu Y, Cui L, Hou L, Yu C, Tao N, Liu J, Li Y, Zhou C, Yang G, Li H. Ambient Air Pollution exposures and newly diagnosed pulmonary Tuberculosis in Jinan, China: a Time Series Study. Sci Rep 2018, 8. [DOI] [PMC free article] [PubMed]
- 15.Sun S, Chang Q, He J, Wei X, Sun H, Xu Y, Magalhaes RJS, Guo Y, Cui Z, Zhang W. The association between air pollutants, meteorological factors and Tuberculosis cases in Beijing, China: a seven-year time series study. Environ Res 2023, 216. [DOI] [PubMed]
- 16.Wang S, Wu G, Du Z, Wu W, Ju X, Yimaer W, Chen S, Zhang Y, Li J, Zhang W et al. The causal links between long-term exposure to major PM2.5 components and the burden of Tuberculosis in China. Sci Total Environ 2023, 870. [DOI] [PubMed]
- 17.Hsu-Yu H. The epidemiologic status of lung Tuberculosis in China 1979 and 1984/85. The result of the 2d nationwide randomized prevalence study. Pneumologie. 1993;47(7):450–5. [PubMed] [Google Scholar]
- 18.Dai ZC, Xiao DL, Wan LY. A history of Tuberculosis Control in China. Beijing: People’s Medical Publishing House; 2013. [Google Scholar]
- 19.Schwoebel V. Surveillance of Tuberculosis. Indian J Tuberc. 2020;67(4S):33–S42. doi: 10.1016/j.ijtb.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Gelaro R, McCarty W, Suárez MJ, Todling R, Molod A, Takacs L, Randles C, Darmenov A, Bosilovich MG, Reichle R, et al. The Modern-Era Retrospective Analysis for Research and Applications, Version 2 (MERRA-2) J Clim. 2017;30(Iss 13):5419–54. doi: 10.1175/JCLI-D-16-0758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan AE, Shou W. Data-driven causal analysis of observational biological time series. Elife 2022, 11. [DOI] [PMC free article] [PubMed]
- 22.Rodrigues SD, Ueda RM, Barreto AC, Zanini RR, Souza AM. How atmospheric pollutants impact the development of Chronic Obstructive Pulmonary Disease and Lung cancer: a var-based model. Environ Pollut. 2021;275:116622. doi: 10.1016/j.envpol.2021.116622. [DOI] [PubMed] [Google Scholar]
- 23.Alimi S. Export - Led growth or growth – driven exports? Evidence from Nigeria. Br J Econ Manage Trade. 2013;3(2):89–100. doi: 10.9734/BJEMT/2013/2386. [DOI] [Google Scholar]
- 24.Dumitrescu E-I, Hurlin C. Testing for Granger non-causality in heterogeneous panels. Econ Model. 2012;29(4):1450–60. doi: 10.1016/j.econmod.2012.02.014. [DOI] [Google Scholar]
- 25.Sugihara G, May R, Ye H, Hsieh CH, Deyle E, Fogarty M, Munch S. Detecting causality in complex ecosystems. Science. 2012;338(6106):496–500. doi: 10.1126/science.1227079. [DOI] [PubMed] [Google Scholar]
- 26.Schiecke K, Pester B, Feucht M, Leistritz L, Witte H. Convergent Cross Mapping: Basic concept, influence of estimation parameters and practical application. Annu Int Conf IEEE Eng Med Biol Soc 2015, 2015:7418–7421. [DOI] [PubMed]
- 27.Deyle ER, Maher MC, Hernandez RD, Basu S, Sugihara G. Global environmental drivers of Influenza. Proc Natl Acad Sci U S A. 2016;113(46):13081–6. doi: 10.1073/pnas.1607747113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nova N, Deyle ER, Shocket MS, MacDonald AJ, Childs ML, Rypdal M, Sugihara G, Mordecai EA. Susceptible host availability modulates climate effects on dengue dynamics. Ecol Lett. 2021;24(3):415–25. doi: 10.1111/ele.13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Ding H, Chang ST, Lu R, Zhong H, Zhao N, Lin TH, Bao Y, Yap L, Xu W, et al. Exposure to air pollution and scarlet Fever resurgence in China: a six-year surveillance study. Nat Commun. 2020;11(1):4229. doi: 10.1038/s41467-020-17987-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grossman GM, Krueger AB. Economic growth and the Environment. Q J Econ. 1995;110(2):353–77. doi: 10.2307/2118443. [DOI] [Google Scholar]
- 31.Xiang K, Xu Z, Hu YQ, He YS, Dan YL, Wu Q, Fang XH, Pan HF. Association between ambient air pollution and Tuberculosis risk: a systematic review and meta-analysis. Chemosphere. 2021;277:130342. doi: 10.1016/j.chemosphere.2021.130342. [DOI] [PubMed] [Google Scholar]
- 32.Thurston GD, Balmes JR, Garcia E, Gilliland FD, Rice MB, Schikowski T, Van Winkle LS, Annesi-Maesano I, Burchard EG, Carlsten C, et al. Outdoor Air Pollution and New-Onset Airway Disease. An official American thoracic Society Workshop Report. Ann Am Thorac Soc. 2020;17(4):387–98. doi: 10.1513/AnnalsATS.202001-046ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churg A, Brauer M. Human lung parenchyma retains PM2.5. Am J Respir Crit Care Med. 1997;155(6):2109–11. doi: 10.1164/ajrccm.155.6.9196123. [DOI] [PubMed] [Google Scholar]
- 34.Gillespie P, Tajuba J, Lippmann M, Chen LC, Veronesi B. Particulate matter neurotoxicity in culture is size-dependent. Neurotoxicology. 2013;36:112–7. doi: 10.1016/j.neuro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Huo X, Zhang Y, Lu X, Xu C, Xu X. The association of PM2.5 with airway innate antimicrobial activities of salivary agglutinin and surfactant protein D. Chemosphere. 2019;226:915–23. doi: 10.1016/j.chemosphere.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Ibironke O, Carranza C, Sarkar S, Torres M, Choi HT, Nwoko J, Black K, Quintana-Belmares R, Osornio-Vargas A, Ohman-Strickland P et al. Urban Air Pollution particulates suppress human T-Cell responses to Mycobacterium Tuberculosis. Int J Environ Res Public Health 2019, 16(21). [DOI] [PMC free article] [PubMed]
- 37.Ghio AJ. Particle exposures and Infections. Infection. 2014;42(3):459–67. doi: 10.1007/s15010-014-0592-6. [DOI] [PubMed] [Google Scholar]
- 38.Rivas-Santiago CE, Sarkar S, Cantarella Pt, Osornio-Vargas A, Quintana-Belmares R, Meng Q, Kirn TJ, Ohman Strickland P, Chow JC, Watson JG, et al. Air pollution particulate matter alters antimycobacterial respiratory epithelium innate immunity. Infect Immun. 2015;83(6):2507–17. doi: 10.1128/IAI.03018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jassal MS, Bakman I, Jones B. Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive Tuberculosis. Public Health. 2013;127(3):268–74. doi: 10.1016/j.puhe.2012.12.030. [DOI] [PubMed] [Google Scholar]
- 40.Lai TC, Chiang CY, Wu CF, Yang SL, Liu DP, Chan CC, Lin HH. Ambient air pollution and risk of Tuberculosis: a cohort study. Occup Environ Med. 2016;73(1):56–61. doi: 10.1136/oemed-2015-102995. [DOI] [PubMed] [Google Scholar]
- 41.Tang Q, Gong K, Xiong L, Dong Y, Xu W. Can El Niño-Southern Oscillation increase respiratory infectious Diseases in China? An empirical study of 31 provinces. Int J Environ Res Public Health. 2022;19(5):2971. doi: 10.3390/ijerph19052971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu S, Wu Y, Wang Q, Gao LJ, Chen L, Zeng FF, Yang P, Gao YH, Yang J. Long-term exposure to ambient air pollution and greenness in relation to pulmonary Tuberculosis in China: a nationwide modelling study. Environ Res 2022, 214. [DOI] [PubMed]
- 43.Popovic I, Magalhaes RJS, Yang YR, Yang SK, Yang BY, Dong GH, Wei XL, Fox GJ, Hammer MS, Martin RV et al. Effects of long-term ambient air pollution exposure on township-level pulmonary Tuberculosis notification rates during 2005–2017 in Ningxia, China. Environ Pollut 2023, 317. [DOI] [PubMed]
- 44.Tao BL, Li ZQ, Wang YT, Wu JZ, Shi XL, Shi JY, Liu Q, Wang JM. Environment pollutants exposure affects the endogenous activation of within-host Mycobacterium tuberculosis. Environ Res 2023, 227. [DOI] [PubMed]
- 45.Jin Y, Andersson H, Zhang S. Air Pollution Control policies in China: a retrospective and prospects. Int J Environ Res Public Health. 2016;13(12):1219. doi: 10.3390/ijerph13121219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material.
The datasets supporting the conclusions of this article are available in the Data-center of China Public Health Science, http://www.phsciencedata.cn/Share/en/index.jsp. The codes are available at 10.5281/zenodo.10020179.