Abstract
The most common reasons seen for lack of asthma control include misconceptions about disease control, low controller treatment adherence, poor inhaler technique, and the resulting underuse of controllers and overuse of short-acting beta2 agonists (SABAs). Narrowing these care gaps may be achieved through well-designed patient education that considers the patient’s motivation, beliefs, and capabilities regarding their asthma and its management and empowers the patient to become an active participant in treatment decisions. Digital health technologies (DHTs) and digital therapeutic (DT) devices provide new opportunities to monitor treatment behaviors, improve communication between healthcare providers and patients, and generate data that inform educational interactions. DHT and DT have been proven effective in enhancing patient self-management in other chronic conditions, particularly diabetes. Accelerated integration of DHT and DT into the management of asthma patients is facilitated by the use of digital inhalers that employ sensor technology (“smart” inhalers). These devices efficiently provide real-time feedback on controller adherence, SABA use, and inhaler technique that have the strong potential to optimize asthma control.
Keywords: adherence, asthma, digital health technology, disease control, health outcomes, inhaler technique, patient education, patient engagement, patient-provider communications, smart inhaler
Introduction
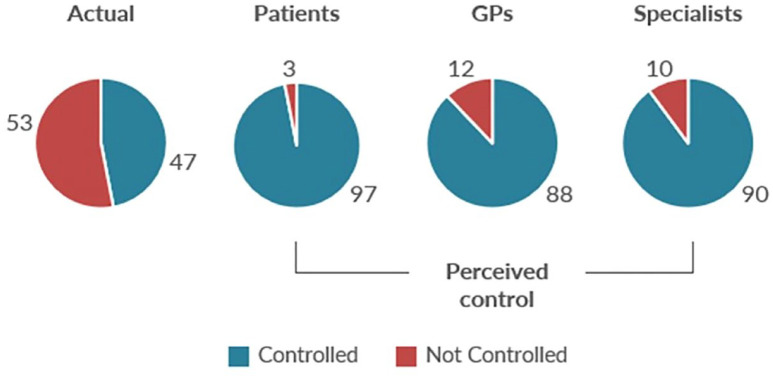
Despite advances in asthma classification, diagnosis, and inhalation therapy for both maintenance and exacerbations, asthma remains a significant global burden to the patient, healthcare providers (HCPs), and the healthcare system.1,2 The persistently high burden of asthma reflects poor control, which is an important risk factor for asthma exacerbations.3–5 Between 30% and 62% of patients in Europe and North America have uncontrolled asthma, as defined by guidelines criteria.6–11 Compounding the high frequency of uncontrolled asthma is the common perception among patients, general practitioners, and even respiratory specialists that asthma is controlled to a much greater degree than is actually the case (Figure 1).9,10
Figure 1.
Real and perceived asthma control. 10
A 2018 survey gathered feedback from online responses and semi-structured interviews with 234 Canadian healthcare professionals (HCPs) across four provinces (Alberta, British Columbia, Ontario, and Quebec). The responses provided insight into the factors underlying common gaps and challenges in asthma care within Canada (Table 1). 12 There were high levels of agreement with the statements that “Most patients with asthma do not proactively help themselves” and “HCPs believe they should be doing more to point out important gaps in both patient self-care and management.”
Table 1.
Canadian healthcare professional statements on asthma care. 12
| Level of agreement with. . . | % (n) of participants who reported agreement with the statement a | ||||||
|---|---|---|---|---|---|---|---|
| GP/FPs (n = 79) | Specialists (n = 18) | CREs (n = 21) | Nurses (n = 18) | Pharms (n = 54) | Total (n = 190) | Significant differences b | |
| I believe there are discrepancies between the Canadian guidelines and the international guidelines which create confusion of what to do in practice | 41% (n = 32) | 72% (n = 13) | 43% (n = 9) | 72% (n = 13) | 50% (n = 27) | 49% (n = 94) | p = 0.035 |
| Asthma spirometry test is not necessary to diagnose asthma | 43% c (n = 34) | 44% (n = 8) | 14% (n = 3) | 17% (n = 3) | 17% (n = 9) | 30% (n = 57) | p = 0.002 |
| Asthma can be diagnosed based on patient history, and response to a medication trial | 75% (n = 59) | 72% (n = 13) | 71% (n = 15) | 50% (n = 9) | 63% (n = 34) | 68% (n = 130) | NS |
| Most patients with asthma do not proactively help themselves | 56% (n = 44) | 67% (n = 12) | 48% (n = 10) | 33% (n = 6) | 61% (n = 33) | 55% (n = 105) | NS |
| Managing adult patients with asthma is time-consuming and frustrating | 35% (n = 28) | 72% (n = 13) | 33% (n = 7) | 39% (n = 7) | 39% (n = 21) | 40% (n = 76) | NS |
| I suspect there is more I should be doing in the care of patients with asthma | 72% (n = 57) | 67% (n = 12) | 81% (n = 17) | 89% (n = 16) | 87% (n = 47) | 78% (n = 149) | NV |
Source: Murray et al. 12 Reproduced with permission from the authors according to the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Participants were asked to indicate their level of agreement with the following statements. Data are the % of participants that selected 3 or 4 on a four-point scale (1 = completely disagree, 2 = slightly disagree, 3 = slightly agree, 4 = completely agree).
Significant differences between professions using chi square (p < 0.05).
Post hoc test indicated for statistical difference.
CRE, certified respiratory educator; FP, family physician; GP, general practitioner; NS, not significant; NV, Chi square not valid due to distribution; Pharm, community pharmacist.
Asthma control is influenced by a host of factors, including comorbidities, environment, medication, asthma status, and patient- and physician-related issues (Table 2).13–31 Guidelines from the Global Initiative for Asthma (GINA), the United States (US) National Asthma Education and Prevention Program, and Canadian Thoracic Society identify non-adherence and poor inhaler technique as two of the principal associations.13,14,32 High short-acting beta2 agonist (SABA) use and misconception of asthma control must also be addressed to significantly improve patients’ asthma status.
Table 2.
Factors influencing asthma control.
|
Patient related
• Adherence7,14–18 ○ Inadequate knowledge about asthma and its management ○ Mixing maintenance and rescue inhalers ○ Forced inhaler changes ○ Patient inertia ■ Low acceptance of asthma diagnosis or severity ■ Clinical fatigue/frustration over lack of improvement ■ Apathy to treat preventively • Inhaler technique13–15,17,19,20 ○ Education and reinforcement of proper technique ○ Type of inhaler ○ Motivation to master technique • Older age21,22 • Duration of asthma21,22 • Asthma severity14,21 • Smoking history14,15,22,23 • Female sex22,23 • Low socioeconomic status13,14,16 • Stress 14 • Medication access/cost24,25 Physician related • Lack of specialist care17,26 • Suboptimal adherence to asthma guidelines27–29 • Underestimation of asthma severity11,29,30 • Poor communication of asthma and its management to patients and caregivers • Clinical fatigue/frustration over lack of improvement • Apathy to treat preventively Respiratory related • Low FEV1 (especially < 60% predicted)13,14,15,23 • Elevated blood eosinophils 7 • Elevated FeNO 13 Other medical conditions • Obesity13–15,22,23 • Chronic obstructive pulmonary disease 22 • Chronic rhinosinusitis13,15 • Allergic rhinoconjunctivitis16,17 • Cardiovascular disease15,22 • Gastroesophageal reflux disease13,15 • Allergies13,14 • Major psychological issues13,14 • Alcohol or other substance abuse 15 • Pregnancy 13 Environmental • Air pollution 13 • Weather 31 • Exposure to dust, gas, vapor, fumes, or other allergens13,23 • Second-hand tobacco smoke, including e-cigarettes13,16–18 |
FeNO, fractional concentration of exhaled nitric oxide; FEV1, forced expiratory volume in 1 s.
Rates of non-adherence to asthma therapy have been shown to range between 30% and 70%, irrespective of the metric used to measure adherence, and several studies show the significant impact of non-adherence on asthma control.33–35 Non-adherence is defined in the GINA guidelines as the failure to take medication as agreed upon between the patient and HCP; 13 in actuality, patients leaving their physician’s office with a prescription for asthma medication do not necessarily understand or agree to its usage, even if they have been instructed about the importance of taking it as prescribed. Adherence may be considered with respect to treatment initiation, implementation, and/or persistence and discontinuation. Causes of non-adherence are multifactorial (Table 2) and adherence can vary within the same patient across therapies for different conditions.33,36 Non-adherence may be intentional or unintentional.13,37 Intentional non-adherence refers to when patients actively decide to disregard treatment recommendations, arising from factors such as concern about treatment, perceived lack of efficacy, beliefs about illness, treatment fatigue, perceived need, or a lack of resources. Unintentional non-adherence is characterized by patients who cannot follow their prescribed treatment regimen due to factors beyond their control. Examples of these factors are forgetfulness, complex dosing, or incorrect administration. Poor communication between the HCP and the patient is a significant contributor to unintentional non-adherence, leading to the patient misunderstanding instructions. Miscommunication is multifactorial and often involves family and belief systems.
Improper inhaler technique is another principal factor in poor asthma control. According to a systematic review (N = 144 articles), the rate of correct technique was 31% (28–35%) and another 31% (27–36%) had poor technique, and there has been no significant improvement in technique over the past 40 years. 38 The most common errors identified for both metered-dose and dry-powder inhalers include inadequate inhaler preparation, incorrect tilting of the head, failure to empty lungs prior to inhalation of medication, insufficient inspiratory effort, and breath not held for at least 3 s following inhalation.19,38,39
Principles of asthma education
To address the factors reducing asthma control – notably non-adherence, poor inhaler technique, SABA overuse, and misconception of asthma control – proper patient education tailored to the patient’s age and health literacy level is essential. The GINA guidelines stress the importance of patient education that focuses on self-monitoring of symptoms and/or peak flow, a written asthma action plan, and regular review of asthma control, treatment, and skills in inhaler use, which has been shown to reduce asthma morbidity in adults and children. 13
To bring about lasting behavior change, patient education should follow the principles of adult learning, accounting for the patient’s level of knowledge about asthma, literacy and education levels, desire to learn about and/or address their conditions, and ethnic/cultural perspectives. Effective patient education can be provided by the treating physician, nurses, pharmacists, and trained asthma educators. 13 All HCPs involved in asthma education must be capable of providing information in a way that considers the patient’s motivation, beliefs, and capabilities. HCPs must remain updated about the most recent developments in asthma diagnosis, classification, monitoring, and management, including daily care and response to exacerbations, and be prepared to change their management strategies according to new information and guidance. Readiness to change clinical practice behavior applies to HCPs as well as patients. HCP education should also include the use of enhanced patient engagement strategies focused on behavior change, such as motivational interviewing and shared decision-making, particularly among those who are intentionally non-adherent.40–42 The key principles of motivational interviewing are to guide and empower patients who are ambivalent to behavior change by helping them discover both their needs and capacity to change. 43 Shared decision-making entails ensuring that patients feel well informed and part of the treatment team, helping them to identify their preference among the therapeutic options and offering professional guidance as needed. 42
Regular reinforcement of correct technique is essential to optimize patients’ proper self-administration.19,20 Rather than “testing” technique, which patients may perceive as judgmental, educational reinforcement should be encouraging and motivating. Contributors to incorrect inhaler technique include improper instruction by the HCP, physical limitations, and changes in device type. Although teaching inhaler device technique is a core competency in asthma management, HCPs may lack the skills to correctly demonstrate inhaler techniques.39,44,45 This instruction is a shared responsibility of all HCPs involved in care of the patient with asthma, and detailed communication is necessary to ensure that it is performed properly.
Asthma educators play an important role in helping patients and their caregivers manage asthma, reduce the risk of exacerbations, respond appropriately to exacerbations, and improve their overall quality of life.46,47 Educators will optimally engage with the rest of the HCP team to ensure that these messages are reinforced at all points of care.
Roles of digital health technology
For approximately 30 years, digital health technology (DHT) has been growing in use throughout healthcare systems and transforming patient care.48–50 DHT comprises the spectrum of electronically delivered care, from online patient documents and telemedicine to wearable devices and artificial intelligence (AI)-based systems. Beyond improving healthcare systems and delivery of care, DHT has a prominent and growing role in HCP and patient education. Patient education has traditionally comprised general information about the patient’s condition and its management; however, advances in the ability of DHT to monitor disease markers are increasingly providing individualized education about their current health and self-management. 50 DHT is frequently discussed as a homogeneous entity, even in studies. It is a broad term that covers generalized, patient-specific non-interactive, and patient-specific interactive elements. 51
Generalized DHT represents content that is provided to patients that is not patient specific or bidirectional. Examples include reminders for prescription refills or appointments and non-interactive educational/motivational content such as brochures or pamphlets.
Patient-specific non-interactive content involves data that are collected from patients or shared with them that is not intended to facilitate self-management, support tools that do not provide feedback, and static patient education.
Interactive patient-specific activities are bidirectional, including data collection that provides feedback to patients, interactive telemedicine, digital therapeutics (DTs) devices, and platforms.
DT is an emerging category of DHT including regulatory-approved devices and software that have been demonstrated with clinical and real-world evidence to prevent, manage, or treat a medical condition.52,53
Discussion about DHT must recognize the wide spectrum of what it encompasses and to what degree the user is interacting with the content. Digitally mediated models of care are intended to contribute to improved health outcomes through the encouragement of consistent evidence-based management. They also enable patients and their caregivers by helping to provide more timely, accessible, and appropriate care, whether from an HCP or via self-management.
The adoption of DHT is facilitated by the widespread global use of portable digital technology such as smartphones. There are an estimated 5.29 billion unique mobile phone users, or 67.1% of the world’s population. 54 Approximately 30.74 million Canadians (81.8%) were smartphone users in 2019.55,56 Digital health apps remain a growing market. Between 350,000 and 500,000 medical, health, and fitness apps are available for download around the world,57,58 up from 325,000 in 2017. 59 More than 91,000 new apps were introduced in 2020 alone. 57 Apps that support health condition management have grown in relative number, from 27% of all health apps in 2015 to 47% in 2020. 57 Among the 2580 health condition management apps, those dealing with mental health and behavioral disorders were the most common (22% of total), followed by diabetes (15%), heart and circulatory system (10%), digestive system (8%), and respiratory system (7%). 57 While the number of apps continues to increase, their quality is less certain as no standardized evaluation has been established. A project to develop guidance that will effectively assess the quality and reliability of health apps was initiated by the European Committee for Standardization with collaboration from the International Organization for Standard-ization and the International Electrotechnical Commission. 60
There are several challenges to the continued progress of DHT in healthcare systems. Increasing reliance on DHT for patient information and self-management risks the expansion of social and economic digital divisions.61–65
Internet access can be considered critical to health literacy. In Canada, limited access to reliable Internet, due to remote geography and/or lower socioeconomic and marginalized community status, has created a digital divide. DHT, therefore, should be developed with the goals of maximum accessibility, meaningful interaction, and lasting behavior change.
There is a high level of mistrust of healthcare and DHT, particularly among ethnic minorities and other marginalized populations.65–67
A large proportion of DHT is incomplete or not integrated into current medical record-keeping, limiting its usefulness.
Rapid facilitation of data collection and sharing allows unprecedented big-data analysis that enhances medical research and provision of care; however, the ethics of data transfer remains unclear with respect to privacy, data protection, and informed consent.68–70
The roles of AI continue to expand in diagnosis, treatment, and prediction of clinical outcomes; however, biomedical AI technologies are subject to bias, 71 few established standards exist for AI validation and several AI systems operate as “black box” techniques; that is, inputs and outputs may be observed but not the internal workings. 72
AI is associated with multiple medicolegal implications. Physicians may depend too much on technology and defer to it rather than their own clinical decision-making. Other potential factors that may increase a healthcare professional’s legal liability include the temptation to copy and paste patient information rather than conducting a full examination at each visit, claims of negligence if the physician overlooks a patient email or does not reply promptly, and not acting on the additional quantity of available patient data. 73 App-specific barriers include a lack of physician compensation and no model for reimbursement of staff time to support patients using data-gathering apps nor insurance coverage for costs related to the technology. 74
Optimal expansion of DHT solutions in clinical use must continue to build on the advantages while addressing the real and potential barriers.
Impact of digital solutions on chronic disease management
DHT has been successfully incorporated into the routine care of patients with several chronic diseases. Diabetes requires daily patient self-management coordinated among the patient, caregivers, and the healthcare team to reduce the risks of short- and long-term complications. Proper glycemic control to avoid both hyper- and hypoglycemia necessitates regular measurement of blood glucose and appropriate response if the glucose levels are outside of the desired range. Fear of hypoglycemia can be so profound that some patients intentionally remain hyperglycemic by reducing or skipping administration of their insulin or oral agents.75,76 Continuous glucose monitoring (CGM) is gradually replacing capillary blood glucose monitoring as a convenient and accurate glucose measurement. Key to the increased use of CGM has been the development of DTs, hardware, and software that measure, analyze, and display feedback as well as consensus documents on the interpretation of large amounts of data through ambulatory glucose profiles and time-in-range evaluation.77–81 Significantly lower A1c levels were achieved with CGM versus traditional self-monitoring and among patients with more versus less frequent scan rates.82–84 CGM was also associated with significant reductions in the incidence of severe hypoglycemia, 84 and patients reported higher levels of satisfaction with their glucose monitoring. 85 Smart insulin pens work with associated smartphone apps to measure and store glucose levels, track active insulin, calculate personalized doses, and give dose reminders. Randomized trials and real-world studies have shown that smart insulin pens are associated with improvements in glycemic control, adherence, life expectancy, and increased confidence in the ability to self-manage among patients with T1DM and T2DM.86–89
Beyond diabetes, DHT and DT are increasingly being integrated into clinical practice for many other chronic conditions, such as cardiovascular disease,90–93 chronic kidney disease, 94 ophthalmology,95,96 and mental health.97,98 DHT is also able to improve healthcare in developing nations.96,99–103 As one example, a team of eye care professionals used a Peek Retina attachment on a smartphone to take images of the lens and fundus of 1460 participants in the Nakuru Eye Disease Cohort in Kenya, which were shared with Moorfields Eye Hospital Reading Centre (London, UK). 96 No observable difference was found between images taken by an experienced retinal photographer and a lay photographer with no healthcare experience. A survey of mobile health information access in 10 Asian countries (N = 9086) found that smartphones are effective in bridging the digital divide between countries with varying health expenditures; however, these devices also expanded the divide among individuals by socioeconomic status. 104
Future of DHT in asthma in the primary care setting
Acceptability, impact, design features: what we have learned to date?
As with other chronic diseases, DHT has the capability to transform the management of the patients with asthma by providing data and patient education that can improve adherence, inhaler technique, and an overall ability to self-manage. A machine learning tool for the prediction of exacerbations was developed using data generated from 360 patients with poorly controlled asthma over 12 weeks. 105 According to this model, the mean number of daily inhalations over 4 days had the highest predictive weight of all features that were assessed. DHT employing motivational interviewing techniques on a mobile or electronic health platform was reported in a systematic review to achieve improvements in targeted health behaviors among patients with chronic diseases, and increased engagement in health behaviors or improvements in health outcomes were reported among patients from marginalized racial or ethnic groups in several studies. 106 DHT-delivered motivational interviewing was also found to be accepted positively by patients, who expressed the belief that they were supported through this approach. 107 To achieve optimal usage and outcomes among asthma patients and HCPs, key learnings to date about DHT must be implemented in terms of acceptability, impact, and design features (Table 3).
Table 3.
Key findings for DHT in management of the patient with asthma.
|
Adherence and outcomes
• Significant improvement in asthma control with high-frequency (more often than once monthly) behavioral support using DHT versus low-frequency (less often or equal to once monthly) HCP-directed behavioral support, HCP-directed educational support, or usual care 108 • Higher achievement of clinically meaningful asthma improvement versus standard of care inhaler 109 • Increase in HCP-reported patient interactions 109 • Improved adherence110,111 Environmental scan and quality review • Several asthma management apps have demonstrated the ability to change user behaviour 112 User-specific perspectives • Patients and physicians generally have a positive view of DHT51,113 User-centered design • Most important design criteria: 51 ○ Simplicity of use for both inhaler device and software ○ Interface similar to smartphone technology with which users are already accustomed ○ Software should inform, engage, and motivate users ○ Device should be unobtrusive, affordable, and provide accurate and objective measurements ○ Interface language/terminology should be concrete and understandable by persons at a Grade 6–7 reading level 114 |
DHT, Digital health technology; HCP, healthcare provider.
Impact of DHT on asthma adherence and outcomes
Several studies have demonstrated the benefits of DHT in the improvement of asthma control and adherence. A systematic review and network meta-analysis including 35 trials (N = 5195 patients) found that behavioral support using high-frequency (i.e. more than once monthly) DHT was found to be associated with significant improvement in asthma control in relation to low-frequency HCP-directed behavioral or educational support or usual care, and the probability of high-frequency DHT being the best option for asthma control was determined by surface under the cumulative ranking curve to be 97.6%. 108 A Cochrane review of 40 parallel randomized controlled trials involving adults and children with asthma (N = 15 207) found low-certainty evidence of increased adherence to maintenance medication among patients receiving digital interventions, which the investigators found likely to be clinically significant among patients with poor baseline adherence levels. 115 Significantly better adherence was observed for electronic monitoring devices [+23% versus control; 95% confidence interval (CI) 10.84–34.16%; seven studies] and with short message services (+12% versus control; 95% CI 6.22–18.03%; four studies). A community-based Italian study investigated the impact of the Turbu+ program on adherence to budesonide and formoterol (Symbicort® AstraZeneca plc, Cambridge, England) Turbuhaler® in 661 patients with physician-diagnosed asthma. 116 This program uploaded inhaler use data to a smartphone app and provided reminders, a display of medication use, and motivational nudge messages. The average medication adherence for maintenance (1 or 2 inhalations bid) or maintenance + reliever use was 70.2% and the proportion of adherent days was 56.6%.
The CONNECT1 feasibility study (N = 333 US participants aged ⩾13 years with suboptimal asthma control) determined that the use of an albuterol digital inhaler – which included a mobile app, cloud-based digital health platform, and a web interface – was associated with a higher achievement of clinically meaningful improvement in asthma control versus standard of care (SoC) albuterol reliever inhalers. 109 A Bayesian statistical analytical approach determined that the albuterol digital inhaler had an 85.3% probability of greater odds of achieving a clinically meaningful response than those treated with SoC. The CONNECT1 group also found that the albuterol digital inhaler was effective in prompting interactions between patients and HCPs, particularly regarding inhaler technique and/or adherence (107 versus 44 discussions; difference in mean number of events 0.95; 95% CI 0.476–1.634). 109 Parallel studies assessed the effect of a Breezhaler® (Novartis AG, Basel, Switzerland) digital companion (sensor and smartphone app) in adult European and Japanese patients with asthma.110,111 Mean daily adherence levels – number of puffs taken/number of puffs prescribed × 100% (maximum 100%) – in the European cohorts were 85.1% and 85.6% at 1 month and 78.9% and 74.5% at 3 months. Mean (SD) adherence was lower in the Japanese cohort: 69.0% (39.3%) and 62.0% (42.8%) at 1 and 3 months, respectively. In the first study, the proportion of patients with well-controlled asthma [Asthma Control Test (ACT) score > 19] rose from 22.7 to 43.7%, while those with very poorly controlled asthma (ACT score ⩽ 15) declined from 53.4% at baseline to 32.0% at follow-up.
It should be noted that nearly all studies of digital inhalers were conducted in specialty practices. Data from use in primary care are essential to demonstrate benefits in routine clinical care.
Environmental scan and quality review
In a 2019 systematic evaluation, asthma management apps were assessed for their ability to change user behavior. 112 The investigators identified 23 asthma apps according to the definition of behavior change technique (BCT) and determined quality ratings for each according to the Mobile App Rating Scale (MARS). 117 Abraham and Michie defined 26 BCTs to reflect theoretical aspects of behavior change; examples of techniques include providing information on consequences, providing general encouragement, setting graded tasks, and prompting self-monitoring of behavior. 118 The 23 selected apps were reviewed by raters trained in Abraham and Michie’s coding of BCT taxonomy. Quality ratings were determined based on the MARS, which comprises 23 items in 5 domain areas – engagement, functionality, aesthetics, information quality, and subjective quality – rated on a five-point scale (1 = inadequate; 5 = excellent). The mean number of BCTs used in these apps was four (range 1–11), and three apps used ⩾8 BCTs. The most commonly used BCTs were instruction, behavior-health links, self-monitoring, feedback, teaching to use prompts/cues, consequences, and others’ approval. The average MARS score for app quality was 3.32 out of 5 (range 2.45–4.50): four apps scored above 4.0 and five had a score between 3.5 and 4.0.
User-specific perspectives for all asthma stakeholders
A recent literature search and scoping review of digital interventions in asthma found that patients and physicians had a positive view of DHT, including digital inhaler technology. 51 Recruitment packages for the MAGNIFY trial of Breezhaler with adherence support technology were accepted by 96.1% of patients aged 40 years with chronic obstructive pulmonary disease and ⩽50% adherence to mono/dual therapy. 113 The majority of those who declined did so for practical reasons, including a small proportion of individuals who did not own a smartphone.
Several key considerations should be taken into account for all stakeholders involved in asthma management from a “what’s in it for me?” perspective (Table 4).
Table 4.
Factors to consider in the use of DHT for all asthma stakeholders.
| Patients • Typically assume incorrectly that they have excellent control • Adherence is less than optimal • Comfort and desire to use this technology • Cost and insurance coverage for asthma DHT Physicians • Have the most patient-specific information • Time and comfort with technology • Are generally reluctant to seek assistance with aspects of DHT use Asthma educators • Most comfortable with education • Have the least amount of patient-specific information • DHT may help to address the information gap with other HCPs Pharmacists • Moderate amount of patient-specific information • Focus on device and monitoring • Monitoring with the DHT can improve and simplify patient education |
DHT, digital health technology; HCP, healthcare provider.
Patients and caregivers
Although patients are generally open to DHT, not all technology will produce a positive result for each individual. It must be sufficiently engaging for the patient to want to use it consistently. Developers of educational content have increasingly employed “gamification,” which incorporates elements of gameplay – for example, point scoring, attractive graphics, and competition with others – to engage the user and increase use. Data must also be actionable, so the patient is motivated to respond to results in the maintenance of positive or alteration of negative behaviors. It should not be punitive – that is, patients feel as though they are “in trouble” with the physician – or the patient will be demotivated to use the technology. Rather, the technology should be perceived by the patient as supportive of their communication with HCPs, their overall care, and their ability to self-manage. As with any proposed change in the patient’s lifestyle, the HCP should assess the patient’s phase of readiness to change – pre-contemplation, contemplation, preparation, action, and maintenance – and tailor communication to that phase. A randomized controlled trial of an educational Alzheimer’s disease-related mobile phone app (Gray Matters) based on the stages of behavior change found positive impacts on behavior among healthy subjects aged 40–64 years and significant improvements in anthropometric measures and blood-based biomarkers. 119
Healthcare providers
For physicians and other HCPs, DHT should provide clear, accurate, evidence-based clinical information that contributes to therapeutic decision-making for the individual patient. It should not add to their workload for the same level of care. It should be easily integrated into the HCP’s current workflow and readily shared with the healthcare and administrative team, without the need to learn or incorporate additional systems. This can be a challenge with the continuous introduction of new technologies and in the absence of established DHT standards. The interpretation of results should be understandable by the HCP, which raises concerns regarding the training necessary to understand and interpret new technologies. Having a patient present with data readings that are not readily interpretable will likely lead to the physician discontinuing the use of the technology. Consideration must be given to who will train both the patient and HCP on this new technology. HCPs can also benefit from DHT and often require behavior change toward adopting DHT as an integral part of their daily clinical practice.
Asthma educators are the best-equipped HCPs to assist patients with how DHT can improve their understanding of their asthma and its management. This typically leads to a favorable impact on adherence and technique. The educator’s role is likewise assisted by DHT through more accurate dosing data – frequency, technique, etc. – than what a patient will self-report. The greatest barrier to effective educator interaction is the limited access to asthma educators.
Pharmacists have a perceived responsibility to provide education about their asthma devices, especially for the initial prescription. As such, they are also in the position to monitor the patient’s SABA use, which can make them feel like the “asthma police.” Pharmacists more commonly and consistently use DHT than other healthcare professionals, and they are generally willing to train patients on the use of new devices as long as they have received training on the salient points of use and interpretation beforehand. Pharmacists are commonly called upon to assist with understanding DHT from both medication and technological perspectives.
Table 5 lists the most common barriers to the use of DHT or incorporating it into clinical practice. Adherence refers to not only the medication but also the technology.
Table 5.
Common barriers to incorporating DHT into clinical practice.
| • Time • Training • Interpretation of information obtained from the device • Lack of consensus or standards for interpretation of DHT data • Adherence to the technology • Resistance to change • Fear of being watched, monitored, and/or reprimanded • Lack of perceived need for technology as the belief that patient is well controlled • No compensation for potentially more work • Overwhelmed with technology • Cost and insurance coverage for the device and technology • Medicolegal concerns |
DHT, digital health technology.
User-centered design
In the literature search and scoping review by Mosnaim et al., 51 the most important design criteria included simplicity of use for both inhaler device and software, with an interface similar to smartphone technology with which users are already accustomed. The software should inform, engage, and motivate users to develop and maintain healthy behaviors that help to control asthma and be customizable to integrate into the user’s daily routine. The device should be unobtrusive, affordable to patients across the spectrum of health insurance coverage, and provide accurate and objective measurements. The language/terminology of the interface must be concrete and understandable by persons at a Grade 6–7 reading level. 114 All components should work together seamlessly to evaluate symptoms and asthma control. 51 The only intervention type that resulted in improvements in both adherence and asthma burden was interactive with two-way responsive patient communication. 51 General interventions involving non-individualized content sent to patients were able to increase adherence to inhaled corticosteroids but did not improve asthma burden, and data-gathering interventions had no effect on either component. One limitation in this review was that the majority of patients in the studies included for analysis were monitored for ⩽12 months, and most commonly for 3–6 months, so it is impossible to interpret the long-term effects of these interventions. Few studies accounted for seasonality or provided contextual environment information. A 2017 systematic review concluded that DHT interventions − including mobile health, telemedicine, electronic health records, and digital app interventions − were associated with improved asthma management and control as well as patient acceptance and satisfaction with their treatment regimen in both adults and children. 120 There was, however, a high degree of heterogeneity in study design and endpoints.
DTs − smart inhalers
Although inhaler devices with the capability to objectively monitor patient usage have been evolving over the past 40 years, digital inhalers whose sensor technology is connected to an external device for data capture and analysis – or “smart” inhalers – are only now becoming available to asthma patients. Smart inhalers are equipped to collect data on the administration of doses and transmit them to a smartphone or other device by Bluetooth® (Bluetooth Special Interest Group Inc., Kirkland, Washington, United States) or near-field wireless technology. Most smart inhalers are paired with smartphone apps that effectively share gathered data on dashboards intended to make the information understandable and actionable for the patient. Data may also be stored on the cloud for ease of remote access by physicians and other HCPs. Flow sensors, either built into the smart inhaler or as a clip-on, are able to assess technique by measurement of peak inspiratory flow rate and inhaled volume. 121
The key advantage of smart inhalers is patient-directed feedback on adherence and inhalation technique. Several studies have shown that smart inhalers are effective in improving patient adherence. 122 They can address components of both intentional non-adherence (perceived lack of efficacy) and unintentional non-adherence (forgetfulness, complex dosing, and incorrect administration) and increase patient learning about their condition and its management. Smart inhalers also provide the opportunity to address the two key issues identified by Canadian HCPs: assist asthma patients to proactively help themselves and do more in the care of their patients with asthma. 12 The Symbicort Given as Needed in Mild Asthma (SYGMA) one trial comparing efficacy and safety of as-needed budesonide–formoterol with as-needed terbutaline and twice-daily budesonide plus as-needed terbutaline in patients with mild asthma employed an inhaler monitor to track use and an electronic diary to remind patients about taking their medications and to record morning and evening peak expiratory flow, asthma symptoms, and nocturnal awakenings due to asthma. 123 Patient adherence was 79.0% across the three treatment groups over 52 weeks of treatment. The SYGMA two trial comparing as-needed budesonide–formoterol with twice-daily budesonide plus as-needed terbutaline also used a smart inhaler but not the electronic diary and medication reminder. 124 Mean 52-week adherence was 63.4%.
Improvements in adherence have been shown with smart inhalers through data provision to both patients and physicians as well as electronic reminders.122,125–128 Other advantages offered by smart inhalers include the following:
Accuracies of dose counts and timing were found to be above 95% in most studies. 122
Understanding patterns of medication use is important in the evaluation of short- and long-term usage. 122 Patients frequently increase their inhaler use immediately before and/or after a physician visit but then taper off over time.
Poor technique may be identified through a second administration within 15–30 s of the initial dose.129,130 Patients may have inhaled too quickly and/or did not hold their breath post-administration for a sufficient amount of time.
Multiple uses prior to appointments in rapid succession may signal “dose dumping” where the patient activates the inhaler in an attempt to show adherence.
Although most randomized, controlled trials showed no significant difference in symptom scores between smart inhaler users and controls, some studies reported improvements in symptom-free days compared with baseline. 122
Patient-reported outcomes improved in most studies conducted with smart inhalers in both children and adults.51,125,128,131–134
A number of challenges remain for optimal adoption and use of smart inhalers.
Although ease of use is expected to be improved from traditional to smart inhalers, patients are required to perform additional tasks with these new devices, such as downloading the app, pairing the app to the device, setting dates, times, and reminders, as well as maintaining and charging the device, and sometimes transferring data. 122
Ease of use among physicians may be impaired by access to data, particularly when patients fail to bring their devices with them. 122 As previously stated, physicians are also concerned with the time and workload required to understand device usage and to download and examine data, consensus recommendations on data reporting and interpretation, and the potential medicolegal issues associated with data gathering.
Few current smart inhalers integrate or synchronize with electronic medical records, which reduces ease of use for health systems. 122
There is a paucity of data regarding the accuracy of the assessment of inhaler technique by smart inhalers. 122 A few devices that use acoustic measurement or detection of inspiratory flow rates have demonstrated good assessment ability.
Cost and coverage of DHT in asthma
Several studies have found that increases in asthma symptom control and adherence to asthma therapies are associated with reductions in healthcare costs.135,136 While the ability of DHT to improve these factors points toward their cost-effectiveness, additional clinical data are necessary to support this association. A systematic review of telemedicine and mobile digital applications toward improved access to asthma care found that the limited evidence was favorable for the cost-effectiveness of these strategies. 137 In their narrative review, Jansen et al. anticipated that smart inhalers would be particularly cost-effective in asthma subgroups, such as those with severe asthma and/or are eligible for additional therapy (i.e. oral corticosteroids or biologics), patients who experience frequent exacerbations, working-age patients, and those who overuse beta2 agonists. 138
Nevertheless, economic factors may be a barrier to the implementation of DHT, particularly in the primary-care setting. Asthma DHT is presently not covered by Canadian public or private insurers, and there is currently no payor coverage for DHT in Canada and no remuneration for the time spent by HCPs learning and incorporating this technology into their practices.
Limitations
For this review, the authors applied a narrative 139 approach to synthesize key findings from the literature and evidence in asthma treatment, and specifically to provide a synopsis of the issues and challenges for the integration of DHT into asthma management. The approach was guided by the experience and expertise of the authors, not by a systematic search process or by a rigorous inclusion/exclusion procedure. As such, this review and its findings may not be reproducible, and the citations that are selected are subject to the authors’ bias.140,141 To mitigate the risk of bias, this review is collaborative in nature and incorporates input from multiple domains of expertise, including family medicine, respirology, pharmacy, health education, and behavioral sciences. A systematic review of the literature on asthma treatment and implications for DHT, including explicitly identified search criteria, is recommended.
Conclusion
Asthma control remains low despite improvements in management over the past several decades. Lack of asthma control is primarily a consequence of widespread treatment non-adherence and poor inhaler technique, with significant contributions from high SABA use and misconception of asthma control. Improved education among patients, their caregivers, and healthcare professionals is an important step in addressing non-adherence and poor technique. Education initiatives should follow principles of adult learning and be adapted to the individual patient, including age, desire, literacy and educational levels, and ethnic/cultural identity.
DHT has been shown to improve patient outcomes and satisfaction in a number of disease states. The widespread accessibility and use of devices such as smartphones can be leveraged to provide important healthcare information, either on its own or paired with DHT devices. DHT is also key to resolving enduring asthma care challenges and improving disease control. Smart inhalers have shown the ability to improve adherence, and a growing number are equipped with sensors to assess inhaler technique. Additional study data are required to more fully understand the role of smart inhalers in improving markers of asthma control and to optimize ease of use for patients, physicians, and the healthcare system.
Acknowledgments
The authors wish to thank Jeff Alexander (SNELL Medical Communication) for writing assistance, which was made available through funding by Teva Canada.
Footnotes
Availability of data and materials: Not applicable.
Contributor Information
Alan Kaplan, Department of Family and Community Medicine, University of Toronto, 14872 Yonge Street, Aurora, Toronto, ON L4G 1N2, Canada; Family Physician Airways Group of Canada, Markham, ON, Canada.
Michael Boivin, CommPharm Consulting Inc., Barrie, ON, Canada.
Jacques Bouchard, Faculty of Medicine, Université Laval, Quebec, QC, Canada.
James Kim, Faculty of Medicine, University of Calgary, Calgary, AB, Canada.
Sean Hayes, Cohaesio Inc., Montreal, QC, Canada.
Christopher Licskai, Division of Respirology, Department of Medicine, Western University, London, ON, Canada.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Alan Kaplan: Conceptualization; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Michael Boivin: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Jacques Bouchard: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
James Kim: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Sean Hayes: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Christopher Licskai: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial assistance with the preparation of this manuscript was funded by Teva Canada.
Dr. AK declares that he has received consulting fees from AstraZeneca, Bellus, Covis Pharma, Eisai, GlaxoSmithKline, Pfizer, Merck Frost, Sanofi, Teva, Trudel, and Valeo. He has received honoraria from AstraZeneca, Boehringer Ingelheim, Covis Pharma, Cipla, Eisai, GlaxoSmithKline, Merck Frosst, Novo Nordisk, Moderna, Pfizer, Sanofi, Teva, Trudel, and Valeo. He received a FENO machine from Sanofi as part of a severe asthma survey. Mr. MB declares that he has received honoraria as a speaker and consultant for CHE activities from Pfizer, Novo Nordisk, Khiron, Johnson & Johnson, AbbVie, Teva, Biosyent, Boehringer Ingelheim, Moderna, Canopy, and Valneva. He has also been a consultant for CHE activities on advisory boards for Novo Nordisk, Emergent BioSolutions, Pfizer, and Novamax. Dr. JB declares that he has no competing interests in association with this manuscript. Dr. JK declares that he received grants/contracts from Novo Nordisk. He received consulting fees from Abbott, AbbVie, AstraZeneca, Bayer, BD, Boerhinger Ingelheim, Eisai, Embecta, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk, Miravo Pharm, Teva, Takeda, and Sanofi. He has received honoraria from Abbott, AbbVie, AstraZeneca, Boerhinger Ingelheim, Eisai, Embecta, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk, Miravo Pharm, Moderna, Pfizer Takeda, Sanofi, and Otsuka. Dr. SH declares that he received an honorarium as a workshop facilitator for Teva. Dr. CL declares that his institution has received grants/contracts from AstraZeneca, the Ontario Ministry of Health, Canadian Institutes of Health Research, and the Federal Economic Development Agency for Southern Ontario. His company has received payment/honoraria from AstraZeneca, Boerhinger Ingelheim, Covis Pharma, GlaxoSmithKline, Novartis, and Valeo. He has participated in advisory boards for AstraZeneca, GlaxoSmithKline, Novartis, Sanofi Genzyme, and Teva.
References
- 1. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ismaila AS, Sayani AP, Marin M, et al. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulm Med 2013; 13: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pavord ID, Mathieson N, Scowcroft A, et al. The impact of poor asthma control among asthma patients treated with inhaled corticosteroids plus long-acting β2-agonists in the United Kingdom: a cross-sectional analysis. NPJ Prim Care Respir Med 2017; 27: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Racine G, Forget A, Moullec G, et al. Predictors of asthma control and exacerbations: a real-world study. J Allergy Clin Immunol Pract 2021; 9: 2802–2811.e2. [DOI] [PubMed] [Google Scholar]
- 5. Haselkorn T, Fish JE, Zeiger RS, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol 2009; 124: 895–902.e1-4. [DOI] [PubMed] [Google Scholar]
- 6. Sundbom F, Malinovschi A, Lindberg E, et al. Effects of poor asthma control, insomnia, anxiety and depression on quality of life in young asthmatics. J Asthma 2016; 53: 398–403. [DOI] [PubMed] [Google Scholar]
- 7. Sadatsafavi M, McTaggart-Cowan H, Chen W, et al. Quality of life and asthma symptom control: room for improvement in care and measurement. Value Health 2015; 18: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 8. Sadatsafavi M, Chen W, Tavakoli H, et al. Saving in medical costs by achieving guideline-based asthma symptom control: a population-based study. Allergy 2016; 71: 371–377. [DOI] [PubMed] [Google Scholar]
- 9. Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 2014; 24: 14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FitzGerald JM, Boulet LP, McIvor RA, et al. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J 2006; 13: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. AsthmaStats: uncontrolled asthma among adults, https://www.cdc.gov/asthma/asthma_stats/uncontrolled-asthma-adults-2019.htm (2019, accessed 13 March 2023).
- 12. Murray S, Labbé S, Kaplan A, et al. A multi-stakeholder perspective on asthma care in Canada: findings from a mixed methods needs assessment in the treatment and management of asthma in adults. Allergy Asthma Clin Immunol 2018; 14: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Global Initiative for Asthma. Global strategy for asthma management and prevention, http://www.ginasthma.org (2022, accessed 13 March 2023).
- 14. Yang CL, Hicks EA, Mitchell P, et al. Canadian Thoracic Society 2021 Guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can J Respir Crit Care Sleep Med 2021; 5: 348–361. [Google Scholar]
- 15. Tomisa G, Horváth A, Tamási L. Prevalence and impact of risk factors for poor asthma outcomes in a large, specialist-managed patient cohort: a real-life study. J Asthma Allergy 2019; 12: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuti BP, Omole KO, Kuti DK. Factors associated with childhood asthma control in a resource-poor center. J Family Med Prim Care 2017; 6: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aschalew A, Kebed RA, Demie TG, et al. Assessment of level of asthma control and related factors in children attending pediatric respiratory clinics in Addis Ababa, Ethiopia. BMC Pulm Med 2022; 22: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGhan SL, MacDonald C, Befus AD. Factors associated with poor asthma control in children aged five to 13 years. Can Respir J 2006; 13: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008; 102: 593–604. [DOI] [PubMed] [Google Scholar]
- 20. Ovchinikova L, Smith L, Bosnic-Anticevich S. Inhaler technique maintenance: gaining an understanding from the patient’s perspective. J Asthma 2011; 48: 616–624. [DOI] [PubMed] [Google Scholar]
- 21. Gosavi S, Nadig P, Haran A. Factors contributing towards poor asthma control in patients on regular medication. J Clin Diagn Res 2016; 10: OC31–OC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tomisa G, Horváth A, Sánta B, et al. Epidemiology of comorbidities and their association with asthma control. Allergy Asthma Clin Immunol 2021; 17: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abrahamsen R, Gundersen GF, Svendsen MV, et al. Possible risk factors for poor asthma control assessed in a cross-sectional population-based study from Telemark, Norway. PLoS ONE 2020; 15: e0232621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bloomberg GR, Banister C, Sterkel R, et al. Socioeconomic, family, and pediatric practice factors that affect level of asthma control. Pediatrics 2009; 123: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laba TL, Jan S, Zwar NA, et al. Cost-related underuse of medicines for asthma-opportunities for improving adherence. J Allergy Clin Immunol Pract 2019; 7: 2298–2306.e12. [DOI] [PubMed] [Google Scholar]
- 26. Håkansson KEJ, Backer V, Ulrik CS. Socioeconomic biases in asthma control and specialist referral of possible severe asthma. Eur Respir J 2021; 58: 2100741. [DOI] [PubMed] [Google Scholar]
- 27. de Marco R, Cazzoletti L, Cerveri I, et al. ; ISAYA Study Group. Are the asthma guideline goals achieved in daily practice? A population-based study on treatment adequacy and the control of asthma. Int Arch Allergy Immunol 2005; 138: 225–234. [DOI] [PubMed] [Google Scholar]
- 28. Akinbami LJ, Salo PM, Cloutier MM, et al. Primary care clinician adherence with asthma guidelines: the National Asthma Survey of Physicians. J Asthma 2020; 57: 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braido F, Baiardini I, Alleri P, et al. Asthma management in a specialist setting: results of an Italian respiratory society survey. Pulm Pharmacol Ther 2017; 44: 83–87. [DOI] [PubMed] [Google Scholar]
- 30. Halterman JS, Yoos HL, Kaczorowski JM, et al. Providers underestimate symptom severity among urban children with asthma. Arch Pediatr Adolesc Med 2002; 156: 141–146. [DOI] [PubMed] [Google Scholar]
- 31. Asthma and Allergy Foundation of America. Asthma: Weather, https://www.aafa.org/weather-triggers-asthma (2017, accessed 13 March 2023).
- 32. Berko JK, Brown S, Elward KS, et al. 2020 Focused Updates to the Asthma Management Guidelines. NIH Publication No. 20-HL-8140, 2020. [Google Scholar]
- 33. Lindsay JT, Heaney LG. Nonadherence in difficult asthma – facts, myths, and a time to act. Patient Pref Adher 2013; 7: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Partridge MR, van der Molen T, Myrseth S-E, et al. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med 2006; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bårnes CB, Ulrik CS. Asthma adherence to inhaled corticosteroids: current status and future perspectives. Respir Care 2015; 60: 455–468. [DOI] [PubMed] [Google Scholar]
- 36. Dima AL, van Ganse E, Stadler G, et al. Does adherence to inhaled corticosteroids predict asthma-related outcomes over time? A cohort study. Eur Respir J 2019; 54: 1900901. [DOI] [PubMed] [Google Scholar]
- 37. NICE Clinical Guideline 76. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence, https://www.nice.org.uk/guidance/cg76 (2009, accessed 13 March 2023). [PubMed]
- 38. Sanchis J, Gich I, Pedersen S; Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest 2016; 150: 394–406. [DOI] [PubMed] [Google Scholar]
- 39. Price DB, Román-Rodríguez M, McQueen RB, et al. Inhaler errors in the CRITIKAL Study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract 2017; 5: 1071–1081. [DOI] [PubMed] [Google Scholar]
- 40. Lussier M-T, Richard C. The motivational interview. Can Fam Physician 2007; 53: 2117–2118. [PMC free article] [PubMed] [Google Scholar]
- 41. Miller WR, Moyers TB. Motivational interviewing and the clinical science of Carl Rogers. J Consult Clin Psychol 2017; 85: 757–766. [DOI] [PubMed] [Google Scholar]
- 42. Elwyn G, Dehlendorf C, Epstein RM, et al. Shared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Ann Fam Med 2014; 12: 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motivational Interviewing Network of Trainers. Understanding motivational interviewing, https://motivationalinterviewing.org/understanding-motivational-interviewing (2019, accessed 13 March 2023).
- 44. Swami V, Cho J-G, Smith T, et al. Confidence of nurses with inhaler device education and competency of device use in a specialised respiratory inpatient unit. Chron Respir Dis 2021; 18: 14799731211002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Prasad S, Moore M, Sathyamurthy R. Confidence and aptitude of healthcare professionals at demonstrating inhaler technique. Thorax 2018; 73(Suppl. 4): A204. [Google Scholar]
- 46. Gregory K. Important role of asthma educators. Paper presented at 5 Things to Know: the Important Role of the Asthma Educator. Allergy & Asthma Network, https://allergyasthmanetwork.org/?s=asthma+educator&id=1769409 (2020, accessed 13 March 2023). [Google Scholar]
- 47. Asthma Initiative of Michigan. Asthma educators, https://getasthmahelp.org/asthma-educator.aspx (accessed 13 March 2023).
- 48. Health Canada. eHealth, https://www.canada.ca/en/health-canada/services/health-care-system/ehealth.html#shr-pg0 (2010, accessed 13 March 2023).
- 49. Cummins N, Schuller BW. Five crucial challenges in digital health. Front Digit Health 2020; 2: 536203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kuwabara A, Su S, Krauss J. Utilizing digital health technologies for patient education in lifestyle medicine. Am J Lifestyle Med 2020; 14: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mosnaim G, Safioti G, Brown R, et al. Digital health technology in asthma: a comprehensive scoping review. J Allergy Clin Immunol Pract 2021; 9: 2377–2398. [DOI] [PubMed] [Google Scholar]
- 52. Smart Patient. What are digital therapeutics? Definitions and terminology explained, https://www.smartpatient.eu/blog/what-are-digital-therapeutics-dtx-definition-and-terminology (2020, accessed 13 March 2023).
- 53. Golata P. Wearable healthcare: digital therapeutics and the future of medical technology, https://www.tti.com/content/ttiinc/en/resources/marketeye/categories/new-technology/me-golata-20220210.html (2022, accessed 13 March 2023).
- 54. Datareportal. Digital around the world, https://datareportal.com/global-digital-overview (2021, accessed 13 March 2023).
- 55. Statista. Smartphone users in Canada 2018–2024, https://www.statista.com/statistics/467190/forecast-of-smartphone-users-in-canada (2023, accessed 13 March 2023).
- 56. Statistics Canada. Canada’s population estimates: age and sex, July 1, 2019, https://www150.statcan.gc.ca/n1/daily-quotidien/190930/dq190930a-eng.htm (2019, accessed 13 March 2023).
- 57. IQVIA Institute. Digital health trends 2021, https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/digital-health-trends-2021/iqvia-institute-digital-health-trends-2021.pdf?_=1642643073822 (2021, accessed 13 March 2023).
- 58. IEEE Standards Association. Mobile health applications. Version 1.0, https://standards.ieee.org/content/dam/ieee-standards/standards/web/governance/iccom/IC21-003-Mobile_Health_Applications.pdf (2021, accessed 13 March 2023).
- 59. Research 2 Guidance. 325,000 mobile health apps available in 2017 – Android now the leading mHealth platform, https://research2guidance.com/325000-mobile-health-apps-available-in-2017 (2017, accessed 13 March 2023).
- 60. NEN. Health and wellness apps: new international guidelines to help to sort the best from the rest, https://www.nen.nl/en/health-and-welness-apps (2022, accessed 13 March 2023).
- 61. Makri A. Bridging the digital divide in health care. Lancet Digital Health 2019; 1: e204–e205. [Google Scholar]
- 62. Taylor K, Silver L; Pew Research Center. Smartphone ownership is growing rapidly around the world, but not always equally, https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally (2019, accessed 13 March 2023).
- 63. Neter E, Brainin E. eHealth literacy: extending the digital divide to the realm of health information. J Med Internet Res 2012; 14: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. International Telecommunication Union. Measuring digital development: facts and figures, https://www.itu.int/en/ITU-D/Statistics/Documents/facts/FactsFigures2021.pdf (2021, accessed 13 March 2023).
- 65. Lee MK, Rich K. Who is included in human perceptions of AI?: Trust and perceived fairness around healthcare AI and cultural mistrust. In: CHI conference on human factors in computing systems (CHI ’21). Yokohama, Japan, May 8–13 2021, p. 14. New York, NY, USA: ACM. [Google Scholar]
- 66. Canadian Race Relations Foundation. Race relations in Canada 2021: a survey of Canadian public opinion and experience, https://crrf-fcrr.ca/2021/11/race-relations-in-canada-2021-a-survey-of-canadian-public-opinion-and-experience/ (2021, accessed 13 March 2023).
- 67. Anderson A, Arthur A, Billioux A, et al. The path to techquity: an introduction to key issues impacting equitable design & deployment of technology in the US healthcare system, https://www.ipsos.com/sites/default/files/2022_03_07_Ipsos_HLTH_Techquity_Whitepaper.pdf (2022, accessed 13 March 2023).
- 68. Sharon T. The Googlization of health research: from disruptive innovation to disruptive ethics. Pers Med 2016; 13: 563–574. [DOI] [PubMed] [Google Scholar]
- 69. Schmietow B, Marckmann G. Mobile health ethics and the expanding role of autonomy. Med Health Care Philos 2019; 22: 623–630. [DOI] [PubMed] [Google Scholar]
- 70. Brall C, Schröder-Bäck P, Maeckelberghe E. Ethical aspects of digital health from a justice point of view. Eur J Public Health 2019; 29(Suppl. 3): 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cirillo D, Catuara-Solarz S, Morey C, et al. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPI Digit Med 2020; 3: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Voigt P, Von dem Bussche A. The EU general data protection regulation (GDPR): a practical guide. 1st ed. Cham: Springer, 2017. [Google Scholar]
- 73. Paterick ZR, Patel NJ, Paterick TE. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent) 2018; 31: 558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. May SG, Huber C, Roach M, et al. Adoption of digital health technologies in the practice of behavioral health: qualitative case study of glucose monitoring technology. J Med Internet Res 2021; 23: e18119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goebel-Fabbri AE, Fikkan J, Franko DL, et al. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care 2008; 31: 415–419. [DOI] [PubMed] [Google Scholar]
- 76. Di Battista AM, Hart TA, Greco L, et al. Type 1 diabetes among adolescents: reduced diabetes self-care caused by social fear and fear of hypoglycemia. Diabetes Educ 2009; 35: 465–475. [DOI] [PubMed] [Google Scholar]
- 77. Dicembrini I, Cosentino C, Monami M, et al. Effects of real-time continuous glucose monitoring in type 1 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetologica 2021; 58: 401–410. [DOI] [PubMed] [Google Scholar]
- 78. Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with Type 2 diabetes. Diabetes Ther 2020; 11: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dunn TC, Xu Y, Hayter G, et al. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract 2018; 137: 37–46. [DOI] [PubMed] [Google Scholar]
- 80. Al Hayek AA, Robert AA, Al Dawish MA. Evaluation of FreeStyle Libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin Med Insights Endocrinol Diabetes 2017; 10: 1179551417746957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes – 2022. Diabetes Care 2022; 45(Supp. 1): S83–S96. [DOI] [PubMed] [Google Scholar]
- 82. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019; 42: 1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020; 43: 1146–1156. [DOI] [PubMed] [Google Scholar]
- 84. Kröger J, Reichel A, Siegmund T, et al. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol 2020; 14: 586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Matthaei S, Dealaiz RA, Bosi E, et al. Consensus recommendations for the use of ambulatory glucose profile in clinical practice. Br J Diabetes 2014; 14: 153–157. [Google Scholar]
- 86. Adolfsson P, Hartvig NV, Kaas A, et al. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther 2020; 22: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Galindo RJ, Ramos C, Cardona S, et al. Efficacy of a smart insulin pen cap for the management of patients with uncontrolled type 2 diabetes: a randomized cross-over trial. J Diabetes Sci Technol 2023; 17: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jendle J, Ericsson Å, Gundgaard J, et al. Smart insulin pens are associated with improved clinical outcomes at lower cost versus standard-of-care treatment of type 1 diabetes in Sweden: a cost-effectiveness analysis. Diabetes Ther 2021; 12: 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Heinemann L, Schnell O, Gehr Bm, Schloot N, et al. Digital diabetes management: a literature review of smart insulin pens. J Diabetes Sci Technol 2022; 16: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ramkumar S, Nerlekar N, D’Souza D, et al. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open 2018; 8: e024178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Spaulding EM, Marvel FA, Lee MA, et al. Corrie health digital platform for self-management in secondary prevention after acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2019; 12: e005509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Marvel FA, Spaulding EM, Lee MA, et al. Digital health intervention in acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2021; 14: e007741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shan R, Ding J, Weng D, et al. Early blood pressure assessment after acute myocardial infarction: insights using digital health technology. Am J Prev Cardiol 2020; 3: 100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. O’Hara DV, Yi TW, Lee VW, et al. Digital health technologies to support medication adherence in chronic kidney disease. Nephrology (Carlton) 2022; 27: 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012; 96: 614–618. [DOI] [PubMed] [Google Scholar]
- 96. Bastawrous A, Giardini ME, Bolster NM, et al. Clinical validation of smartphone based adapter: Peek Retina for optic disc imaging in Kenya. JAMA Ophthalmol 2016; 134: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Anton MT, Greenberger HM, Andreopoulos E, et al. Evaluation of a commercial mobile health app for depression and anxiety (AbleTo Digital+): retrospective cohort study. JMIR Form Res 2021; 5: e27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prescott MR, Sagui-Henson SJ, Welcome Chamberlain CE, et al. Real world effectiveness of digital mental health services during the COVID-19 pandemic. PLoS One 2022; 17: e0272162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Griffee K, Martin R, Chory A, et al. A systematic review of digital interventions to improve ART adherence among youth living with HIV in sub-Saharan Africa. AIDS Res Treat 2022; 2022: 9886306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bilal W, Mohanan P, Rahmat ZS, et al. Improving access to maternal care in Africa through telemedicine and digital health. Int J Health Plann Manage 2022; 37: 2494–2500. [DOI] [PubMed] [Google Scholar]
- 101. Moodley N, Velen K, Saimen A, et al. Digital chest radiography enhances screening efficiency for pulmonary tuberculosis in primary health clinics in South Africa. Clin Infect Dis 2022; 74: 1650–1658. [DOI] [PubMed] [Google Scholar]
- 102. Semakula-Katende NS, Andronikou S, Susan Lucas S. Digital platform for improving non-radiologists’ and radiologists’ interpretation of chest radiographs for suspected tuberculosis – a method for supporting task-shifting in developing countries. Pediatr Radiol 2016; 46: 1384–1391. [DOI] [PubMed] [Google Scholar]
- 103. Rojas G, Martínez V, Martínez P, et al. Improving mental health care in developing countries through digital technologies: a mini narrative review of the Chilean case. Front Public Health 2019; 7: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang X, Shi J, Lee KM. The digital divide and seeking health information on smartphones in Asia: survey study of ten countries. J Med Internet Res 2022; 24: e24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lugogo N, DePietro M, Reich M, et al. A predictive machine learning tool for asthma exacerbations: results from a 12-week, open-label study using an electronic multi-dose dry powder inhaler with integrated sensors. J Asthma Allergy 2022; 15: 1623–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pedamallu H, Ehrhardt MJ, Maki J, et al. Technology-delivered adaptations of motivational interviewing for the prevention and management of chronic diseases: scoping review. J Med Internet Res 2022; 24: e35283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shingleton RM, Palfai TP. Technology-delivered adaptations of motivational interviewing for health-related behaviors: a systematic review of the current research. Patient Educ Couns 2016; 99: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dhippayom T, Wateemongkollert A, Mueangfa K, et al. Comparative efficacy of strategies to support self-management in patients with asthma: a systematic review and network meta-analysis. J Allergy Clin Immunol Pract 2022; 10: 803–814. [DOI] [PubMed] [Google Scholar]
- 109. Hoyte F, Mosnaim G, Rodgers L, et al. Effectiveness of a digital inhaler system for patients with asthma: a 12-week, open-label, randomized study (CONNECT1). J Allergy Clin Immunol Pract 2022; 10: 2579–2587. [DOI] [PubMed] [Google Scholar]
- 110. Woehrle H, Mastoridis P, Stempel DA, et al. Medication adherence and asthma control with once-daily indacaterol/glycopyrronium/mometasone (IND/GLY/MF) Breezhaler® digital companion: interim analysis from Europe. Paper presented at the European Respiratory Society International Congress, 4–6 September 2022, Barcelona, Spain. [Google Scholar]
- 111. Woehrle H, Mastoridis P, Stempel DA, et al. Medication adherence and asthma control with once-daily indacaterol/glycopyrronium/mometasone (IND/GLY/MF) Breezhaler® digital companion: interim analysis from Japan and Germany. Paper presented at the European Respiratory Society International Congress, 4–6 September 2022, Barcelona, Spain. [Google Scholar]
- 112. Ramsey RR, Caromody JK, Voorhees SE, et al. A systematic evaluation of asthma management apps examining behavior change techniques. J Allergy Clin Immunol Pract 2019; 7: 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dickens AP, Halpin D, Carter V, et al. Patient-reported barriers to accepting a technological adherence package in the MAGNIFY trial. Paper presented at the European Respiratory Society International Congress, 4–6 September 2022, Barcelona, Spain. [Google Scholar]
- 114. Hutchinson N, Baird GL, Garg M. Examining the reading level of Internet medical information for common internal medicine diagnoses. Am J Med 2016; 129: 637–639. [DOI] [PubMed] [Google Scholar]
- 115. Chan A, De Simoni A, Wileman V, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database Syst Rev 2022; 6: CD013030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rumi G, Canonica GW, Foster JM, et al. Digital coaching using smart inhaler technology to improve asthma management in patients with asthma in Italy: community-based study. JMIR Mhealth Uhealth 2022; 10: e25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stoyanov SR, Hides L, Kavanagh DJ, et al. Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR MHealth UHealth 2015; 3: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008; 27: 379–387. [DOI] [PubMed] [Google Scholar]
- 119. Hartin PJ, Nugent CD, McClean SI, et al. The empowering role of mobile apps in behavior change interventions: the Gray Matters randomized controlled trial. JMIR Mhealth Uhealth 2016; 4: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bonini M. Electronic health (e-health): emerging role in asthma. Curr Opin Pulm Med 2017; 23: 21–26. [DOI] [PubMed] [Google Scholar]
- 121. Chrystyn H, Saralaya D, Shenoy A, et al. Investigating the accuracy of the Digihaler, a new electronic multidose dry-powder inhaler, in measuring inhalation parameters. J Aerosol Med Pulm Drug Deliv 2022; 35: 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tay TR, van Boven JFM, Chan A, et al. Electronic inhaler monitoring for chronic airway disease: development and application of a multidimensional efficacy framework. J Allergy Clin Immunol Pract 2022; 10: 1189–1201.e1. [DOI] [PubMed] [Google Scholar]
- 123. O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 2018; 378: 1865–1876. [DOI] [PubMed] [Google Scholar]
- 124. Bateman ED, Reddel HK, O’Byrne PM, et al. As-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378: 1877–1887. [DOI] [PubMed] [Google Scholar]
- 125. Sulaiman I, Greene G, MacHale E, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. Eur Respir J 2018; 51: 1701126. [DOI] [PubMed] [Google Scholar]
- 126. Foster JM, Usherwood T, Smith L, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol 2014; 134: 1260–1268. [DOI] [PubMed] [Google Scholar]
- 127. Moore A, Preece A, Sharma R, et al. A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients. Eur Respir J 2021; 57: 2003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the Propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract 2016; 4: 455–463. [DOI] [PubMed] [Google Scholar]
- 129. Anderson WC, Gondalia R, Hoch HE, et al. Screening for inhalation technique errors with electronic medication monitors. J Allergy Clin Immunol 2019; 7: 2065–2067. [DOI] [PubMed] [Google Scholar]
- 130. de Oliveira PD, Baptista Menezes AM, Bertoldi AD, et al. Assessment of inhaler techniques employed by patients with respiratory diseases in southern Brazil: a population-based study. J Bras Pneumol 2014; 40: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chan AH, Stewart AW, Harrison J, et al. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respir Med 2015; 3: 210–219. [DOI] [PubMed] [Google Scholar]
- 132. Barrett MA, Humblet O, Marcus JE, et al. Effect of a mobile health, sensor-driven asthma management platform on asthma control. Ann Allergy Asthma Immunol 2017; 119: 415–421. [DOI] [PubMed] [Google Scholar]
- 133. Morton RW, Elphick HE, Rigby AS, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax 2017; 72: 347–354. [DOI] [PubMed] [Google Scholar]
- 134. O’Dwyer S, Greene G, MacHale E, et al. Personalized biofeedback on inhaler adherence and technique by community pharmacists: a cluster randomized clinical trial. J Allergy Clin Immunol 2020; 8: 635–644. [DOI] [PubMed] [Google Scholar]
- 135. Khaw SM, Li SC, Mohd Tahir NA. A systematic review of the cost-effectiveness of medication adherence-enhancing intervention for asthma. J Asthma 2022; 59: 697–711. [DOI] [PubMed] [Google Scholar]
- 136. Zafari Z, Sadatsafavi M, Chen W, et al. The projected economic and health burden of sub-optimal asthma control in Canada. Respir Med 2018; 138: 7–12. [DOI] [PubMed] [Google Scholar]
- 137. Codispoti CD, Greenhawt M, Oppenheimer J. The role of access and cost-effectiveness in management asthma: a systematic review. J Allergy Clin Immunol Pract 2022; 10: 2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jansen EM, van de Hei SJ, Dierick BJH, et al. Global burden of medication non-adherence in chronic obstructive pulmonary disease (COPD) and asthma: a narrative review of the clinical and economic case for smart inhalers. J Thorac Dis 2021; 13: 3846–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Turin T, Ahmed S, Vaska M. Conducting a literature review in health research: basics of the approach, typology and methodology. JNHFB 2016; 5: 44–51. [Google Scholar]
- 140. Sukhera J. Narrative reviews in medical education: key steps for researchers. J Grad Med Educ 2022; 14: 418–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sullivan GM. Why are medical education literature reviews so hard to do? J Grad Med Educ 2018; 10: 481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]