Abstract
Background
Shared decision-making is useful to facilitate cancer treatment decisions. However, it is difficult to make treatment decisions when physician and patient preferences are different. This review aimed to summarize and compare the preferences for cancer treatments between physicians and patients.
Methods
A systematic literature search was conducted on PubMed, Embase, PsycINFO, CINAHL and Scopus. Studies elicited and compared preferences for cancer treatments between physicians and patients were included. Information about the study design and preference measuring attributes or questions were extracted. The available relative rank of every attribute in discrete choice experiment (DCE) studies and answers to preference measuring questions in non-DCE studies were summarized followed by a narrative synthesis to reflect the preference differences.
Results
Of 12,959 studies identified, 8290 were included in the title and abstract screening and 48 were included in the full text screening. Included 37 studies measured the preferences from six treatment-related aspects: health benefit, adverse effects, treatment process, cost, impact on quality of life, and provider qualification. The trade-off between health benefit and adverse effects was the main focus of the included studies. DCE studies showed patients gave a higher rank on health benefit and treatment process, while physicians gave a higher rank on adverse effects. Non-DCE studies suggested that patients were willing to take a higher risk of adverse effects or lower health benefit than physicians when accepting a treatment.
Conclusions
Physicians and patients had important preference differences for cancer treatment. More sufficient communication is needed in cancer treatment decision-making.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-023-11598-4.
Keywords: Cancer treatment decision-making, Preference difference, Systematic review
Background
Cancer patients often need to choose from multiple treatment options with various health benefit and safety profiles. Patient preference thus plays an important role in such decision making [1]. Shared decision-making (SDM) explicitly considers patient preference and value and has been increasingly used in cancer care practice [2–4]. SDM involves the interaction and mutual information sharing between physicians and patients, where physicians provide evidence-based and rational treatment messages, and patients express their needs and preferences [5]. Through SDM, all useful information is considered and treatments are selected based on preferences, which helps to improve the treatment compliance and outcome [6, 7]. However, this decision-making process becomes difficult when physician and patient’s preferences differ.
A few reviews have investigated how patients’ preferences were different from those of physicians. Montgomery and Fahey suggested that discordant preferences between patients and physicians always existed, and the magnitude of the differences varied with disease conditions [8]. Muhlbach and Juhnke also reported mixed degrees of differences between the preferences of patients and the judgements of physicians, where the physician judgements were defined as their evaluation on patients which is different from physician preferences [9]. Harrison et al. reviewed studies using discrete choice experiment (DCE) to elicit both patient and healthcare provider preferences, and found that healthcare providers weighed more on treatment outcome (e.g., mortality) and treatment structure (e.g., organizational structures, human resources), while patients placed more weights on the treatment process (e.g., risk, treatment regimen, waiting time) [10].
However, there lacks a comprehensive comparison of treatment preferences between physicians and patients with cancer. Therefore, we conducted a systematic literature review aimed at comparing patient preferences for cancer treatment with those of physicians.
Methods
This review was structured in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
Search strategy
A systematic literature search was conducted on PubMed, EMBASE, PsycINFO, CINAHL and Scopus, from the inception of the databases to May 4, 2022. The search strategy combined Medical Subject Headings (MeSH) terms about “neoplasm” and free text pertaining to “cancer”, “physician”, “patient” and “preference”. Further search details can be found in Appendix 1. Reference lists of included papers were also manually searched.
Inclusion and exclusion criteria
Studies were included if they directly compared physician and patient preferences for cancer treatments using established methods, including DCE, conjoint analysis (CA), the threshold technique, time trade-off (TTO), trade-off method (TTM), standard gamble (SG), prospective measure of preference (PMP), and self-designed questionnaire. Patients were those who were having cancer and facing treatment decisions.
Studies were excluded if they measured preferences for other health conditions (e.g., cancer-related chronic pains); measured preferences for cancer screening or diagnosis, instead of cancer treatments; or elicited preference from proxies (e.g., general public or family members). Non-research articles, including conference abstracts, letters, and editorials, as well as reviews were also excluded.
Study selection
Both title and abstract and full text screenings were conducted independently and in duplicate by two reviewers (MZ and XH). Any discrepancies between reviewers were discussed and resolved through consensus. If necessary, a third reviewer (JW) was consulted to make the final decision.
Appraisal and quality assessment
The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force was used to assess the DCE and CA studies, and the Appraisal Tool for Cross-Sectional Studies (AXIS) was used to assess other studies (Appendix 2 and 3) [12, 13]. Two reviewers (MZ and XN) independently applied the guide/tool to each included study and recorded supporting information and justifications for assessments. Any discrepancies in judgements were resolved through consensus, with a third reviewer (JW) acting as an arbiter if necessary.
Data extraction and synthesis
A narrative synthesis of the included studies was conducted given the heterogeneity among these studies [14]. Basic information of each included study was extracted, including first author, publication year, study country, cancer type, elicitation technique, sample recruitment approach, sample size and mode of administration.
In DCE/CA studies, attributes and their levels were pre-defined, describing the alternative scenarios for participants, to investigate preferences, while in non-DCE/non-CA studies, generic or trade-off questions were used. These attributes and questions were abstracted and grouped into 6 categories in line with the systems-based framework which was used to assess the quality of healthcare [15] and operationalized in previous reviews in this area [9, 16, 17]: (1) health benefits –patients’ health outcomes and clinical benefits; (2) adverse effects – mainly treatment induced side effects; (3) treatment process – process-related factors (e.g., dosage form, dosing frequency, etc.); (4) cost – any types of treatment costs; (5) impact on quality of life - influences developed by treatment on patients’ daily activities and physical or psychological conditions; and (6) provider qualification – type of healthcare organization (e.g., specialist hospital, general hospital, etc.) and reputation of medical personnel.
Differences in preferences for cancer treatment between physicians and patients were summarized. For DCE/CA studies, the ranking of attributes, if reported, was extracted. For threshold technique/TTO/TTM studies, the threshold scores were extracted. For SG/PMP studies, the willingness-to-trade values were extracted. For questionnaire studies, the proportions of participants to specific question options were exacted. If statistic test was conducted to verify the significant difference between physicians and patients, corresponding P-values were extracted.
Results
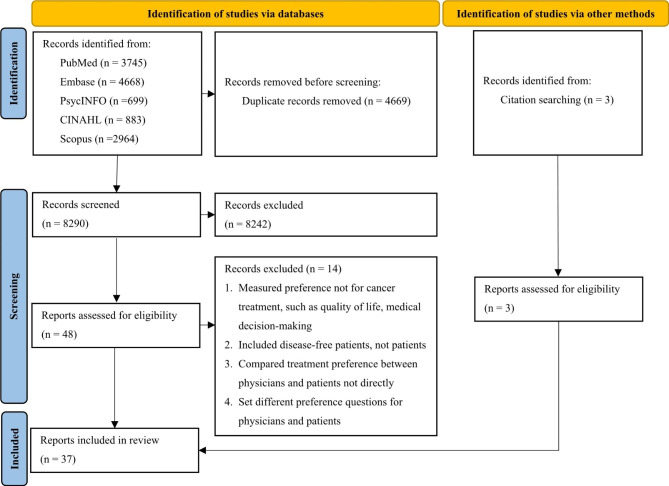
The review identified 12,959 publications. After removing 4,669 duplicates, the title and abstract of 8,290 publications were screened for eligibility and 8,242 were excluded. A full-text screening was conducted on the remaining 48 publications, of which 34 were included in the review. Additional 3 studies were included through reviewing the reference lists of identified publications. The detailed selection process is shown in Fig. 1.
Fig. 1.
Flow chart of the study selection
In the quality assessment, for DCE and CA studies, the construction of choice tasks and clarification of preference elicitation were often partially reported. For other studies, sample size justification and non-respondent information were often not reported. The final assessment tables were in supplementary Tables 1–2.
Basic Information
As shown in Table 1, the first study was published in 2003 and the number of studies has increased since 2017 with 11 (30%) conducted in the US. A total of thirteen types of cancer were the target conditions among these studies, including melanoma (n = 6, 16%), breast cancer (n = 6, 16%) and lymphoma (n = 5, 14%). DCE was the most frequently used preference elicitation technique (n = 23, 62%). Convenient samples were most frequently used (n = 20, 54%). The sample size of physicians ranged from 18 to 363 and that of patients varied from 30 to 456. In terms of the mode of administration, online survey was most common (n = 14, 38%), followed by face-to-face (n = 5, 14%) and postal survey (n = 3, 8%). Twelve studies (32%) used two modes, and in ten studies (27%), multiple modes was used for physicians and patients.
Table 1.
Basic characteristics of included studies
| First Author | Year | Country | Cancer type | Elicitation technique | Sample recruitment approach | Sample size | Mode of administration | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Physicians | Patients | Physicians | Patients | Physicians | Patients | ||||||
| Amin, S. | 2022 | US | Breast cancer | DCE | Marketing survey company | 117 | 169 | Online | |||
| Fernández, O. | 2022 | Spain | Renal cell carcinoma | DCE | Sponsor company | Hospital | 67 | 105 | Online/Paper | ||
| Stellato, D. | 2021 | Canada | Breast cancer | DCE | Sponsor company | Patient advocacy groups | 21 | 62 | Online | ||
| Post, C. C. B. | 2021 | Netherlands | Endometrial cancer | TTM | Physician groups | Primary physicians | 63 | 171 | Online/Paper | ||
| Le, H. | 2021 | US | Lymphocytic Leukemia | DCE | Marketing survey company | Patient advocacy groups/ physician referral/online communities | 151 | 220 | Online | ||
| Beusterien, K. | 2021 | US | Breast cancer | DCE | Marketing survey company | 200 | 300 | Online | |||
| Maculaitis, M. C. | 2021 | US | Breast cancer | DCE&BWS | Marketing survey company | 209 | 304 | Online | |||
| Hauber, B. | 2020 | US | NSCLC | DCE | Marketing survey company | 102 | 200 | Online | |||
| van der Valk, M. J. M. | 2020 | Netherlands | Rectal cancer | DCE | Hospital | 128 | 94 | Online | |||
| Weiss, J. | 2020 | German | Melanoma | Questionnaire | Hospital | 27 | 30 | Postal | |||
| Fifer, S. J. | 2019 | Australia | Multiple Myeloma | DCE | Specialist healthcare research panels | Patient advocacy groups | 28 | 124 | Online | ||
| Stenehjem, D. D. | 2019 | US | Melanoma | DCE | Registration database | 20 | 233 | E-mail/Postal | |||
| Stellato, D. | 2019 | Canada | Melanoma | DCE | Marketing survey company | Patient advocacy groups | 18 | 39 | Online | ||
| Ivanova, J. | 2019 | US | Soft Tissue Sarcoma | DCE | Marketing survey company | Physician referral/Patient advocate group | 160 | 76 | Online | ||
| Nakayama, M. | 2018 | Japan | Prostate Cancer | DCE | Marketing survey company | 127 | 103 | Online | |||
| Gonzalez, J. M. | 2018 | US | Renal Cell Carcinoma | DCE | Marketing survey company | 142 | 201 | Online | |||
| Bröckelmann, P. J. | 2018 | France, German, UK | Classical Hodgkin Lymphoma | DCE | Marketing survey company | 281 | 289 | Online | |||
| Kennedy, E. D. | 2018 | Canada | Low Rectal Cancer | Threshold Technique | Physician registration database | Hospital | 363 | 50 | Postal | Face to face | |
| Kahler, K. C. | 2018 | German | Melanoma | Threshold Technique | Hospital | 108 | 130 | Face to face | |||
| Liu, F. X. | 2017 | US | Melanoma | DCE | Marketing survey company | 150 | 200 | Online | |||
| Lee, J. Y. | 2017 | Korea | Endometrial Cancer | DCE&TTO | Physician list | Hospital | 56 | 103 | Online | Face to face | |
| Gonzalez, J. M. | 2017 | US | Colorectal Cancer | DCE | Marketing survey company | 127 | 150 | Online | |||
| Vaz-Luis, I. | 2017 | US | Breast Cancer | Questionnaire | Clinical trial | 175 | 456 | Online | Telephone/Postal | ||
| Pacchiana, M. V. | 2017 | Italy | NSCLC | Questionnaire | NA | 37 | 92 | Face to face | |||
| Landfeldt, E. | 2016 | German, Sweden | Lymphocytic Leukemia | CA | Marketing survey company | Patient list | 72 | 44 | Online | Online/Telephone | |
| Blinman, P. | 2016 | Australia, New Zealand | Endometrial Cancer | TTO | Clinical trial | 44 | 83 | Face to face | |||
| Blinman, P. | 2015 | Australia | Non-Small-cell Lung Cancer | TTO | Hospital | 82 | 122 | Face to face | |||
| Kunneman, M. | 2014 | Netherlands | Endometrial Cancer | TTM | Physician list | Hospital | 77 | 95 | Online | Face to face | |
| Krammer, R. | 2014 | German | Melanoma | Questionnaire | Hospital | 30 | 30 | Face to face | |||
| de Bekker-Grob, E. W. | 2013 | New Zealand | Prostate Cancer | DCE | Physician list | Hospital | 50 | 110 | Postal | ||
| Park, M. H. | 2012 | Korea | Renal Cell Carcinoma | DCE | Hospital | 272 | 172 | Face to face | |||
| Thrumurthy, S. G. | 2011 | UK | Esophagogastric Cancer | DCE | Hospital | 90 | 81 | Face to face | Postal | ||
| Shafey, M. | 2011 | Canada | Follicular Lymphoma | DCE | Physician list | Hospital | 48 | 81 | Postal | ||
| Muhlbacher, A. C. | 2011 | German | Follicular Lymphoma | DCE | Physician registration database | Patient organization | 243 | 282 | E-mail/Postal | ||
| Gandhi, S. | 2011 | Canada | Breast Cancer | Questionnaire | NA | Hospital | 40 | 153 | Face to face | ||
| Harrison, J. D. | 2008 | Australia | Rectal Cancer | PMP | Physician association | Hospital | 264 | 103 | Postal | Face to face | |
| Solomon, M. J. | 2003 | Australia | Colorectal Cancer | SG&TTO | Physician association | Hospital | 146 | 110 | Postal | Face to face | |
DCE: Discrete Choice Experiment; TTO: Time Trade-off; TTM: Trade-off Method; PMP: Prospective Measure of Preference Method; SG: Standard Gamble; CA: Conjoint Analysis.
Attribute identification
Of 24 DCE/CA studies (including 23 DCE studies and 1 CA study), a total of 142 attributes were identified (including duplicated attributes) with 3–8 attributes per study. Various qualitative methods were used to generate the attributes. Twenty studies (54%) developed the attribute list through a literature review and then confirmed them through clinical specialist and/or patient interview. Three studies (8%) only used the interview [18–20], one literature review only [21]; and two (5%) quantitative methods (i.e., principal component analysis, factor analysis and analyses of variance to finalize the attributes) [22, 23]. Sixteen studies (43%) conducted the pilot test to further refine the attributes.
Amongst these attributes, 39 attributes (27%) were about health benefits, 63 adverse effects (44%), 25 treatment process (18%), 5 cost (4%), 8 impact on quality of life (6%), and 2 the provider qualification (1%) (Table 2). All but one study included the attributes of health benefit and adverse effects. Health benefits were commonly measured using survival outcomes, including progression-free survival (PFS, n = 10, 26%), and overall survival (OS, n = 6, 15%). Seven studies (29%) used a generic term for all types of adverse effects such as degree of side effect, and others defined disease-specific adverse effects, including gastrointestinal perforation for kidney cell carcinoma [24] and permanent urinary incontinence for prostate cancer [25]. Mode and frequency of administration, dosing schedule/regimen and further therapies/monitoring were frequently used in the category of treatment process. Five studies (21%) measured the attribute of cost, with three on the cost paid by patients [26–28] and two on the cost by healthcare systems [29, 30]. Six studies (25%) included the attribute about the impact on quality of life. Only one study measured provider qualification [31].
Table 2.
Classification of attributes in 24 DCE/CA studies
| Health benefit N = 39* |
Adverse effect N = 63 |
Treatment process N = 25 |
Cost N = 5 |
Impact on quality of life N = 8 |
Provider qualification N = 2 |
|
|---|---|---|---|---|---|---|
| Amin, S. | Median OS, Median PFS | Risk of neuropathy, Risk of neutropenia, Risk of nausea, Risk of alopecia, Risk of immune-related AE | ||||
| Fernández, O. | Progression survival gain | Risk of SAE | Mode of administration | Monthly cost (healthcare system) | HRQoL | |
| Stellato, D. | Chance of progression-free over 24 months | Improvement in pain, Chance of hot flashes, Chance of neutropenia, Chance of nausea | Dosing regimen, Monitoring | |||
| Le, H. | Chance of 2-year PFS | Risk of atrial fibrillation, Risk of infection, Risk of tumor lysis syndrome, Risk of bleeding, Risk of arthralgia/myalgia/ musculoskeletal pain, Risk of discontinue due to AES | Duration and administration | |||
| Beusterien, K. | Chance of 5-Y invasive DFS | Risk of nausea, Risk of diarrhea, Risk of neutropenia, Risk of alopecia | Dosing schedule, Electrocardiogram monitoring | |||
| Maculaitis, M. C. | Risk of dose reduction due to AES, Risk of diarrhea, Risk of abdominal (belly) pain, Risk of III/IV neutropenia | Regimen, Dosing schedule, Electrocardiogram monitoring | ||||
| Hauber, B | Expected survival, Best-case survival, Worst-case survival | Degree of fecal fatigue, Degree of nausea, Risk of febrile neutropenia | ||||
| van der Valk, M. J. M. | DFS | Degree of fecal incontinence, Degree of urinary dysfunction, Degree of sexual dysfunction | Further therapies | Worry about cancer recurrence | ||
| Fifer, S. J. | OS, Remission period | Risk of SE | Mode & frequency of administration | Out of pocket (annual) | ||
| Stenehjem, D. D. | OS | Risk of immunotherapy-related SE, Risk of skin toxicity, Risk of gastrointestinal toxicity | Mode of administration | Out of pocket (month) | ||
| Stellato, D. | Chance of cancer-free for 21 months, Chance of free of distant metastases for 21 months, Chance of alive for 36 months | Risk of fever (≥ 39℃), Risk of diarrhea (4–6 episodes daily), Risk of thyroid problems with symptoms | Dosing regimen | Difficulties with work and daily activities | ||
| Ivanova, J. | OS, PFS, ORR | Risk of hospitalization due to SE | Treatment schedule | |||
| Nakayama, M. | Effect to keep disease stable | Degree of SE | Convenience of treatment | QoL | ||
| Gonzalez, J. M. | PFS, 3Y-PL | Degree of skin reactions, Degree of fatigue | Mode & frequency of administration | Co-payment (month) | ||
| Bröckelmann, P. J. | 5Y-OS, 5Y-PFS | Risk of SE requiring treatment, Risk of peripheral neuropathy, Risk of infertility, Risk of permanent pulmonary toxicity | ||||
| Liu, F. X. | MDT, ORR, PFS, OS | Risk of III/IV SE | Mode of administration, Dosing regimen | |||
| Lee, J. Y. | 5Y-recurrence rate | Risk of lymphedema, Surgery-related systemic morbidity | ||||
| Gonzalez, J. M. | PFS | Risk of severe papulopustular rash, Risk of serious hemorrhage, Risk of cardiopulmonary arrest | ||||
| Landfeldt, E. | OS, PFS | Degree of fatigue, Degree of nausea, Risk of serious infections | Mode & frequency of administration | |||
| de Bekker-Grob, E. W. | Effect of cure | Risk of permanent urinary incontinency, Risk of permanent erectile dysfunction, Risk of other permanent side effects | Frequency of PSA testing with a risk of new prostate biopsies | |||
| Park, M. H. | PFS | Risk of bone marrow suppression, Risk of hand-foot skin reaction, Risk of gastrointestinal perforation, Risk of bleeding | Mode of administration | |||
| Thrumurthy, S. G. | Mortality, Morbidity, Cure rate | QoL | Hospital type, Surgeon’s reputation | |||
| Shafey, M. | Median PFS & 5Y-PFS | Degree of SE | Mode & frequency of administration | Health cost (healthcare system) | ||
| Muhlbacher, A. C. | Increase in life-span | Degree of SE | Further therapies, Self-medication, Breaks in treatment | Emotional situation, Physical situation, Social situation |
QoL, Quality of Life; HRQoL, Health related quality of life, SE, Side Effect; AE, Adverse effect, SAE, Serious adverse effect, PFS, Progression-free Survival; 3Y-PL, Probability of Living at Least 3 Years; 5Y-OS, 5 Years Overall Survival; 5Y-PFS, 5 Years Progression-free Survival; MDT, Median Duration of Therapy; ORR, Objective Response Rate; OS, Overall Survival; III/IV SE, III/IV side effects
* N was the number of attributes
Questions in non-DCE/CA studies were mostly self-developed based on clinical evidence and pretested. They focused on two main categories, namely health benefit and adverse effects, where health benefit was more about survival rate and life years, while adverse effects were about the risk of cancer recurrence. For instance, physicians and patients were asked to consider how much the extra chance of survival or the potential risk of local regrowth was, a certain treatment could be accepted [32, 33].
Concordances and discordances in preference between physicians and patients
The ranks of attributes by physicians vs. patients in 20 DCE/CA studies are plotted in Fig. 2 (the original ranks were shown in supplementary Table 3). Among all categories, health benefit was valued most with the first rank by both physicians and patients (n = 10, 50%). Treatment process, cost and provider qualification were less important indicated by lower ranks.
Fig. 2.
Relative rank of every attribute in 20 DCE/CA studies. Note: Every scatter indicates an attribute and the size of it is proportional to the number of studies with corresponding ranks
Table 3.
Threshold scores in 8 threshold technique/TTO/TTM studies
| Preferences measurement question | Objective point | Threshold scores | Consistency (P-value) |
||
|---|---|---|---|---|---|
| Physicians | Patients | ||||
| Post, C. C. B. | 1 Accept chemoradiotherapy VS. radiotherapy alone |
5Y-survival rate (over baseline rate) |
5% | 10% | 0.024 |
| Kennedy, E. D. | 1 Accept nonoperative management VS. abdominal perineal resection | 5Y-local regrowth rate | 5.0% | 20.0% | NA |
| 2 Accept nonoperative management VS. abdominal perineal resection | 5Y-survival rate | 75.0% | 60.0% | NA | |
| Kahler, K. C. | 1 Accept interferon alfa-2a and 2b (IFN) with mild-to-moderate side effects VS. without any IFN treatment | 5Y-DFS rate | 42.3% | 59.6% | < 0.001 |
| 2 Accept interferon alfa-2a and 2b (IFN) with severe side effects VS. without any IFN treatment | 53.6% | 69.8% | < 0.001 | ||
| 3 Accept mild-to-moderate side effects for adjuvant IFN | Risk of recurrence | 46.0% | 65.0% | 0.001 | |
| Lee, J. Y. | 1 Accept no lymphadenectomy VS. routine lymphadenectomy | Risk of recurrence | 2.75% | 3% | 0.620 |
| Blinman, P. | 1 Accept the addition of adjuvant chemotherapy to pelvic radiotherapy VS. no treatment (5 life years) | Life years | 6 years | 6 years | 0.400 |
| 2 Accept the addition of adjuvant chemotherapy to pelvic radiotherapy VS. no treatment (8 life years) | 9 years | 9 years | |||
| 3 Accept the addition of adjuvant chemotherapy to pelvic radiotherapy VS. no treatment (50% survival rate at 5 years) | Survival rate | 57% | 55% | 0.030 | |
| 4 Accept the addition of adjuvant chemotherapy to pelvic radiotherapy VS. no treatment (65% survival rate at 5 years) | 75% | 69% | |||
| Blinman, P. | 1 Accept the adjuvant chemotherapy VS. no treatment (3 life years) | Life years | 3.9 years | 3.9 years | NA |
| 2 Accept the adjuvant chemotherapy VS. no treatment (5 life years) | 5.9 years | 5.9 years | |||
| 3 Accept the adjuvant chemotherapy VS. no treatment (50% survival rate at 5 years) | Survival rate | 55% | 55% | ||
| 4 Accept the adjuvant chemotherapy VS. no treatment (65% survival rate at 5 years) | 70% | 70% | |||
| Kunneman, M. | 1 Accept surgery only VS. vaginal brachy therapy and surgery | 5Y-recurrence rate | 10% | 2% | < 0.001 |
|
Solomon, M. J. (TTO Part) |
1 Accept laparoscopic surgery VS. open surgery | Mortality risk | 3.20% | 5% | NA |
| 2 Accept local resection VS. colostomy | 13.40% | 17.20% | |||
| 3 Accept surgery alone VS. surgery and chemotherapy | 12.70% | 21.40% | |||
| 4 Accept chemoradiotherapy and no surgery VS. surgery and colostomy | 16.65% | 6.10% | |||
The preferences measurement questions refer to the trade-offs between two listed treatments. Taking the example of the Post’s study, the question would be “the desired 5-year overall survival benefit over the baseline rate to accept chemoradiotherapy relative to radiotherapy alone”. Then the value to this question, namely, the threshold score of physicians was 5%, lower than the corresponding rate of patients as 10%.
Among 20 attributes of health benefit with different ranks from patients and physicians, patients gave higher ranks in 11 attributes (55%) than their physicians did, including OS, PFS, ORR, cure rate, remission period, morbidity, chance of cancer-free and chance of distant metastases free. Patients also gave higher ranks among 11 out of 16 attributes (69%) for treatment process. While physicians placed higher importance on adverse effects. Among 38 attributes of adverse effects with different ranks, physicians valued 25 attributes (66%) higher than patients did. Due to the limited number of attributes on cost, impact on quality of life and provider qualification, no meaningful difference could be summarized.
Of 8 threshold technique/TTO/TTM studies (Table 3), one study showed the same threshold scores for both physicians and patients [34], and one study showed mixed results that physicians and patients held the same threshold scores towards life years but different scores towards survival rate [32]. The remaining 6 studies all showed discordance between physicians and patients with 3 reaching statistical significance [35–37]. Among 5 studies measuring the trade-off towards the risk of regrowth rate or recurrence rate, 4 studies reported higher threshold scores in patients than physicians [33, 36–39]. Among 4 studies that valued the minimum survival to accept the treatment and with different threshold scores, patients had higher scores than physicians in two studies [35, 36] while lower in the other two [32, 33].
Five self-designed questionnaire studies showed physicians expected more health benefits from treatment, while patients rather accepted a treatment even with smaller health benefits (Table 4). Taking the example of Vaz-Luis’s study, only 18% of physicians considered 6 months of chemotherapy worthwhile for 1-month survival benefit, while 42% of patients considered so [40]. In addition, Krammer et al. showed that patients and physicians differed in their trade-off between survival and side effects [41]. When choosing from 16 weeks survival with moderate side effects and 8 weeks survival with mild side effects, 83% of physicians preferred the former, while 56% of patients did so [41]. Similarly, the willingness to trade in 2 SG/PMP studies revealed that patients preferred to use more remaining life years to avoid the treatment risk and treatment-related side effect on daily life (Table 5) [39, 42].
Table 4.
Results of 5 questionnaire studies
| Preferences measurement question | Proportion | Consistency (P-value) |
||
|---|---|---|---|---|
| Physicians | Patients | |||
| Weiss, J. | 1 19 months living with combination immunotherapy and severe side effects in 36% VS. 9 months living with standard immunotherapy and severe side effects in 15% | 70%/30% | 45%/55% | 0.050 |
| 2 12 months living with combination immunotherapy and severe side effects in 36% VS. 11 months living with standard immunotherapy and severe side effects in 15% | 15%/85% | 17%/83% | NA | |
| 3 24 months living with combination immunotherapy and severe side effects in 36% VS. 3 months of pain-free living without tumor therapy with palliative therapy | 80%/20% | 50%/50% | 0.018 | |
| 4 Agree to a treatment with many side effects at any time and with the very low prospect of prolonging life | 60% | 30% | NA | |
| 5 Prefer to receive the infusions every three weeks rather than every two weeks with the equivalent effect | 92% | 83% | ||
| 6 Prefer early palliative therapy to a therapy rich in side effects if there is no prospect of healing | 59% | 57% | ||
| Vaz-Luis, I. | 1 Whether 6 months of chemotherapy would be worthwhile for a 1-, 2-, 6-, 9-, 12-, and 24-month survival benefit |
1-month: 18% 2-month: 37% 6-month: 86% 9-month: 93% 12-month: 97% 24-month: 97% |
1-month: 42% 2-month: 57% 6-month: 79% 9-month: 87% 12-month: 93% 24-month: 96% |
NA |
| Pacchiana, M. V. | 1 Whether interested in maintenance therapy, rather than treatment-free | 97% | 75% | 0.003 |
| 2 Whether interested in maintenance therapy if improving life expectancy by about 1 Month, 3 Month, 6 Month, 1 Year |
1-month: 14% 3-month: 62% 6-month: 89% 1 Year: 100% |
1-month: 46% 3-month: 61% 6-month: 76% 1 Year: 88% |
1-month: <0.001 3-month: 0.910 6-month: 0.080 1 Year: 0.030 |
|
| 3 Whether interested in maintenance therapy if providing no survival benefit but would result in symptom control | 78% | 74% | 0.630 | |
| 4 Whether interested in maintenance therapy if providing no survival benefit but would result in radiologic tumor stabilization | 38% | 62% | 0.010 | |
| Krammer, R. | 1 16 weeks survival with moderate side effects with ipilimumab VS. 8 weeks survival with mild side effects with chemotherapy | 83%/17% | 56%/44% | NA |
| 2 3 months survival with mild side effects with chemotherapy VS. 3 months survival free of symptoms with palliative care | 10%/90% | 32%/68% | ||
| 3 Spending €100.000 for ipilimumab VS. palliative care VS. skin screening VS. primary prevention | 3%/21%/10%/66% | 4%/4%/46%/46% | ||
| Gandhi, S. | 1 Minimum overall survival required to continue aromatase inhibitor 5 years |
<1%: 0% 1–2%: 45% 2–5%: 37.5% 5–10%: 12.5% 10–15%: 0% 15–20%: 2.5% >20%: 0% |
<1%: 30.1% 1–2%: 14.4% 2–5%: 11.8% 5–10%: 12.4% 10–15%: 3.9% 15–20%: 3.9% >20%: 17.0% |
NA |
| 2 Minimum decrease in risk of cancer recurrence required to continue aromatase inhibitor 5 years |
<1%: 0% 1–2%: 2.5% 2–5%: 37.5% 5–10%: 35.0% 10–15%: 12.5% 15–20%: 0% >20%: 2.5% |
<1%: 27.5% 1–2%: 14.4% 2–5%: 13.1% 5–10%: 14.4% 10–15%: 4.6% 15–20%: 5.9% >20%: 14.4% |
||
Table 5.
Willingness-to-trade in 2 SG/PMP studies
| Preferences measurement question | Willingness-to-trade | Consistency (P-value) |
|||
|---|---|---|---|---|---|
| Physicians | Patients | ||||
| Harrison, J. D. | Proportion of remaining life expectancy could be traded to | 1 Avoid abdominoperineal resection | 14.3% | 34.0% | NA |
| 2 Avoid anterior resection and chemoradiotherapy | 9.7% | 24.0% | |||
| 3 Avoid anterior resection and chemotherapy | 8.0% | 20.0% | |||
| 4 Avoid anterior resection and preoperative radiotherapy | 8.3% | 17.0% | |||
| 5 Avoid anterior resection and postoperative radiotherapy | 12.7% | 20.0% | |||
|
Solomon, M. J. (SG Part) |
1 Accept laparoscopic surgery relative to open surgery | 1.50% | 0.80% | NA | |
| 2 Accept local resection relative to colostomy | 9.50% | 2.70% | |||
| 3 Accept surgery alone relative to surgery and chemotherapy | 5.85% | 2.50% | |||
| 4 Accept chemoradiotherapy and no surgery relative to surgery and colostomy | 9.35% | 0.80% | |||
Discussion
This study systematically reviewed the discrepancies in preferences for cancer treatment between physicians and patients. Health benefit and adverse effects were key drivers of treatment preferences, and the trade-offs between them were the primary focus of the included studies. Compared to physicians, patients valued health benefit more and were willing to take on more risks of adverse effects. Patients also placed a higher weight on the treatment process than physicians did. The preference differences between physicians and patients varied across studies.
Current preference measurement studies focused on the trade-off between health benefit and adverse effects [16, 43]. Existing threshold technique, such as TTO, TTM, SG or PMP, could only evaluate the tradeoffs between two attributes. Although DCE or CA can include more attributes, achieving clinical relevance might require detailed attributes, thereby increase the possible combinations of all attributes and the complexity of the experiment design, which could discourage researchers [44]. Further studies could consider other methods that can incorporate more attributes and be flexible in supporting real-world decisions. For example, adaptive conjoint analysis can include more attributes and customize the preference elicitation based on prior responses [45].
An important difference was that generally, patients placed a higher value on health benefit and physicians on adverse effects. As health care providers, physicians are process-oriented and focus on the whole treatment including safety. In contrast, patients are result-oriented, for whom survival benefit is the most important. The included studies showed that whether in active treatment aiming to keep functioning in the long term, or adjuvant treatment or maintenance therapy aiming to lower cancer recurrence risk, patients always expect survival benefits. Moreover, patients often preferred to seek active treatment and wanted to make sure they have tried every treatment option [46, 47]. Patients also tended to behave as risk-takers and overlooked the concerns of having adverse effects [48]. When seeking treatments, patients may assume their own survival gains are more favorable and exceed the population average gain [49, 50]. This “value of hope” also drives them to accept a higher risk [51]. While adverse effects of cancer treatment may have non-negligible impact on patient preferences [39, 42]. For some patients, survival is preferred but conditional upon no worsening in quality of life [52]. Prior longitudinal research also found that patients with advanced cancer placed stronger emphasis on quality of life (vs. survival) as the treatment goal [53].
Patients also concerned more on treatment process than physicians, which were closely related to their daily life. The review conducted by Harrison et al. also reported that medication safety, delivery and timing of treatment, and treatment accessibility were more important to patients [10]. As a part of patient experience, treatment process is one of the most common indicators used to evaluate the healthcare services [54].
The differences in treatment preferences between physicians and patients varied across various studies. For example, both Stellato’s and Liu’s studies examined the preferences for melanoma treatment, and Stellato et al. concluded that the physician and patient had the same preferences [55], while Liu et al., showed physicians valued adverse effect most while patients valued survival most [56]. Even in DCE or CA studies, physician and patient preferences for the same attribute had heterogeneity across studies. Studies by Gonzalez et al., Brockelmann et al. and Park et al. all showed that physicians valued PFS more than patients [24, 28, 57], while the study by Landfeldt et al. indicated that patients valued PFS more [58]. And Liu et al. showed that physicians and patients weighed PFS similarly [56]. The preference differences may be correlated with the individual characteristics. Current preference studies focused on the aggerated level that revealed the sample average preference, other than the individual level and personal preference. Individual preference heterogeneity is remaining a salient topic [59]. The preference differences may be also impacted by patient’s treatment experience. Patients who have survived or recovered from previous treatments may develop positive experiences about the treatment and therefore tend to favor the choice they were offered rather than the alternative [60, 61]. This generally resulted from normal psychologic processes called cognitive dissonance reduction and adaptation mechanisms [62]. Another possible explanation for this difference could be that in different studies, patients and physicians had different understandings of the survival or risk statistics in the questions. Using standard decision aids and consistent illustrations for statistics might help form the common understanding and increase the comparability across the studies.
The difference in cancer treatment preferences between physicians and patients may have important implications on treatment decision making. As physicians and patients are mainly concern about the benefit-risk trade-off, and always have different preferences on it, evidence on these two attributes should be carefully discussed in SDM [63, 64]. Further to facilitate SDM, physicians may also need to master the ability of communicating evidence in a clear, understandable, and non-misleading manner [65]. Training physicians with sufficient SDM knowledge or skill is essential [66]. In addition, some tools have been developed to assist physicians with implementing SDM into their practice, like SHARE approach developed by the AHRQ (Agency for Healthcare Research and Quality) [67]. Moreover, patients should be encouraged to actively convey preferences and understand the importance of their participation [68]. The educational material could be distributed to improve the awareness and importance of SDM among patients [66]. Furthermore, the development of clinical practice guidelines that should take into account the discordance in preference between physicians and patients and discuss the implications on SDM.
There are some limitations in this review. First, qualitative studies were excluded. Second, non-quantitative synthesis was done due to the heterogeneity of included studies.
Conclusion
This review found that there were important differences in treatment preferences between physicians and cancer patients. Patients placed a higher weight on health benefit and treatment process, while physicians placed higher weight on adverse effects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not available.
Abbreviations
- SDM
Shared decision-making
- DCE
Discrete choice experiment
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CA
Conjoint analysis
- TTO
Time trade-off
- TTM
Trade-off method
- SG
Standard gamble
- PMP
Prospective measure of preference
- ISPOR
International Society for Pharmacoeconomics and Outcomes Research
- AXIS
Appraisal Tool for Cross-Sectional Studies
- AHRQ
Agency for Healthcare Research and Quality
Authors’ contributions
All authors contributed to the overall conceptualization and design of this study. Study selection, quality assessment and narrative synthesis were conducted by X.H and M.Z, with regular review by J.W and F.X. Manuscript was drafted by M.Z, and J.W, F.X, X.H provided critical revision. All authors contributed to manuscript preparation and have approved this version of the manuscript. J.W and X.H acted as the overall guarantor.
Funding
This study was funded by National Natural Science Foundation of China (No. 71804122, No. 71673197, and No. 72174142).
Data Availability
The data analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not available.
Consent for publication
Not available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaoning He, Email: hexn@tju.edu.cn.
Jing Wu, Email: jingwu@tju.edu.cn.
References
- 1.Monzani D, et al. Patient preferences for Lung Cancer treatments: a study protocol for a Preference Survey using Discrete Choice Experiment and Swing Weighting. Front Med (Lausanne) 2021;8:689114. doi: 10.3389/fmed.2021.689114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood DE, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2018;16(4):412–41. doi: 10.6004/jnccn.2018.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB, et al. NCCN Roundtable:value-Based decision-making at the Bedside. J Natl Compr Canc Netw. 2015;13(5 Suppl):659–61. doi: 10.6004/jnccn.2015.0196. [DOI] [PubMed] [Google Scholar]
- 4.Sheridan SL, Harris RP, Woolf SH. Shared decision making about screening and chemoprevention. A suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(1):56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Kane HL, et al. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin. 2014;64(6):377–88. doi: 10.3322/caac.21245. [DOI] [PubMed] [Google Scholar]
- 6.Arora NK, et al. Physicians’ decision-making style and psychosocial outcomes among cancer survivors. Patient Educ Couns. 2009;77(3):404–12. doi: 10.1016/j.pec.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Committee on Improving the Quality of Cancer Care: Addressing the Challenges of an Aging, P. and S.I.o.M. Board on Health Care, Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis, L. Levit, et al., Editors et al. 2013, National Academies Press (US): Washington (DC). [PubMed]
- 8.Montgomery AA, Fahey T. How do patients’ treatment preferences compare with those of clinicians? Qual Health Care. 2001;10(Suppl 1):i39–43. doi: 10.1136/qhc.0100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11(3):163–80. doi: 10.1007/s40258-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 10.Harrison M, et al. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017;7(5):e014719. doi: 10.1136/bmjopen-2016-014719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bridges JF, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health. 2011;14(4):403–13. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Downes MJ, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ open. 2016;6(12):e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Chandler TJ, Cumpston J, Li M, Page T, Welch MJ VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021; Available from: www.training.cochrane.org/handbook.
- 15.Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–8. doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
- 16.Bien DR, et al. Patients’ preferences for outcome, process and cost attributes in Cancer Treatment: a systematic review of Discrete Choice experiments. Patient. 2017;10(5):553–65. doi: 10.1007/s40271-017-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donabedian A. Evaluating the Quality of Medical Care. 2005;83(4):691–729. doi: 10.1111/j.1468-0009.2005.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stellato D, et al. Preferences of Canadian patients and Physicians for treatment of HR+/HER2- advanced Breast Cancer. Curr Oncol. 2021;28(1):491–508. doi: 10.3390/curroncol28010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le H, et al. Oncologist and patient preferences for Novel agents in First-Line treatment for chronic lymphocytic Leukemia: commonalities and disconnects. Patient Prefer Adherence. 2021;15:99–110. doi: 10.2147/PPA.S289139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beusterien K, et al. Patient, oncologist, and payer preferences for adjuvant endocrine therapy and CDK4/6 inhibitor regimens in early-stage Breast Cancer: a Discrete Choice Experiment. Patient Prefer Adherence. 2021;15:611–23. doi: 10.2147/PPA.S298670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanova J, et al. Patient and oncologist preferences for the treatment of adults with Advanced Soft tissue sarcoma: a Discrete Choice Experiment. Patient. 2019;12(4):393–404. doi: 10.1007/s40271-019-00355-0. [DOI] [PubMed] [Google Scholar]
- 22.Nakayama M, et al. Patient preferences and urologist judgments on Prostate Cancer therapy in Japan. Am J Mens Health. 2018;12(4):1094–101. doi: 10.1177/1557988318776123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlbacher AC, Nubling M. Analysis of physicians’ perspectives versus patients’ preferences: direct assessment and discrete choice experiments in the therapy of Multiple Myeloma. Eur J Health Econ. 2011;12(3):193–203. doi: 10.1007/s10198-010-0218-6. [DOI] [PubMed] [Google Scholar]
- 24.Park MH, et al. A comparison of preferences of targeted therapy for metastatic renal cell carcinoma between the patient group and health care professional group in South Korea. Value Health. 2012;15(6):933–9. doi: 10.1016/j.jval.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 25.de Bekker-Grob EW, et al. Patients’ and urologists’ preferences for Prostate cancer treatment: a discrete choice experiment. Br J Cancer. 2013;109(3):633–40. doi: 10.1038/bjc.2013.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fifer SJ, et al. Alignment of preferences in the treatment of Multiple Myeloma - a discrete choice experiment of patient, carer, physician, and nurse preferences. BMC Cancer. 2020;20(1):546. doi: 10.1186/s12885-020-07018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenehjem DD, et al. Immunotargeted therapy in Melanoma: patient, provider preferences, and willingness to pay at an academic cancer center. Melanoma Res. 2019;29(6):626–34. doi: 10.1097/CMR.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González JM, et al. Comparing the relative importance of attributes of metastatic renal cell carcinoma treatments to patients and Physicians in the United States: a discrete-choice experiment. PharmacoEconomics. 2018;36(8):973–86. doi: 10.1007/s40273-018-0640-7. [DOI] [PubMed] [Google Scholar]
- 29.Fernández O, et al. Preferences for renal cell carcinoma pharmacological treatment: a Discrete Choice experiment in patients and oncologists. Front Oncol. 2021;11:773366. doi: 10.3389/fonc.2021.773366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shafey M, et al. Preferences of patients and physicians concerning treatment options for relapsed follicular Lymphoma: a discrete choice experiment. Bone Marrow Transplant. 2011;46(7):962–9. doi: 10.1038/bmt.2010.225. [DOI] [PubMed] [Google Scholar]
- 31.Thrumurthy SG et al. Discrete-choice preference comparison between patients and doctors for the surgical management of oesophagogastric cancer Br J Surg, 2011. 98(8): p. 1124-31; discussion 1132. [DOI] [PubMed]
- 32.Blinman P, et al. Patients’ and clinicians’ preferences for adjuvant chemotherapy in endometrial cancer: an ANZGOG substudy of the PORTEC-3 intergroup randomised trial. Br J Cancer. 2016;115(10):1179–85. doi: 10.1038/bjc.2016.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy ED, et al. Patient and physician preferences for Nonoperative Management for low rectal Cancer: is it a reasonable treatment option? Dis Colon Rectum. 2018;61(11):1281–9. doi: 10.1097/DCR.0000000000001166. [DOI] [PubMed] [Google Scholar]
- 34.Blinman P, et al. Patients’ and doctors’ preferences for adjuvant chemotherapy in resected non-small-cell Lung cancer: what makes it worthwhile? Eur J Cancer. 2015;51(12):1529–37. doi: 10.1016/j.ejca.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Post CCB et al. Patients’ and clinicians’ preferences in adjuvant treatment for high-risk endometrial cancer: Implications for shared decision making. Gynecol Oncol, 2021. [DOI] [PubMed]
- 36.Kahler KC, et al. The outweigh of toxicity versus risk of recurrence for adjuvant interferon therapy: a survey in German Melanoma patients and their treating physicians. Oncotarget. 2018;9(40):26217–25. doi: 10.18632/oncotarget.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunneman M, et al. Treatment preferences and involvement in treatment decision making of patients with endometrial cancer and clinicians. Br J Cancer. 2014;111(4):674–9. doi: 10.1038/bjc.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JY, et al. Treatment preferences for routine lymphadenectomy Versus No Lymphadenectomy in Early-Stage Endometrial Cancer. Ann Surg Oncol. 2017;24(5):1336–42. doi: 10.1245/s10434-016-5729-7. [DOI] [PubMed] [Google Scholar]
- 39.Solomon MJ, et al. What do patients want? Patient preferences and surrogate decision making in the treatment of Colorectal cancer. Dis Colon Rectum. 2003;46(10):1351–7. doi: 10.1007/s10350-004-6749-0. [DOI] [PubMed] [Google Scholar]
- 40.Vaz-Luis I, et al. Survival benefit needed to undergo chemotherapy: patient and physician preferences. Cancer. 2017;123(15):2821–8. doi: 10.1002/cncr.30671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krammer R, Heinzerling L. Therapy preferences in Melanoma treatment–willingness to pay and preference of quality versus length of life of patients, physicians and healthy controls. PLoS ONE. 2014;9(11):e111237. doi: 10.1371/journal.pone.0111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison JD, et al. Patient and physician preferences for surgical and adjuvant treatment options for rectal cancer. Arch Surg. 2008;143(4):389–94. doi: 10.1001/archsurg.143.4.389. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt K, et al. Preferences of Lung cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care (Engl) 2016;25(4):580–91. doi: 10.1111/ecc.12425. [DOI] [PubMed] [Google Scholar]
- 44.Brown L, et al. Applying stated-preference methods to improve health systems in sub-saharan Africa: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2017;17(5):441–58. doi: 10.1080/14737167.2017.1375854. [DOI] [PubMed] [Google Scholar]
- 45.Sawtooth, Software, editors. Inc., ACA system for adaptive conjoint analysis. ACA Manual.
- 46.Chapple A, et al. Is ‘watchful waiting’ a real choice for men with Prostate cancer? A qualitative study. BJU Int. 2015;90(3):257–64. doi: 10.1046/j.1464-410X.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 47.Charles C, et al. Doing nothing is no choice: Lay Constructions of Treatment decision-making among women with early-stage Breast Cancer. Volume 20. Sociology of Health & Illness; 2010. pp. 71–95. 1.
- 48.Chu D-T, et al. Patient attitudes towards chemotherapy as assessed by patient versus physician: a prospective observational study in advanced non-small cell Lung cancer. Lung Cancer. 2007;56(3):433–430169. doi: 10.1016/j.lungcan.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 49.Lakdawalla DN, et al. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood) 2012;31(4):676–82. doi: 10.1377/hlthaff.2011.1300. [DOI] [PubMed] [Google Scholar]
- 50.Bruhn, John G. Therapeutic value of hope. South Med J. 1984;77(2):215–9. doi: 10.1097/00007611-198402000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Johnson FR, et al. Something is Better Than nothing: the value of active intervention in stated preferences for treatments to Delay Onset of Alzheimer’s Disease symptoms. Value Health. 2019;22(9):1063–9. doi: 10.1016/j.jval.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Mertz S, et al. Progression-free survival and quality of life in metastatic Breast cancer: the patient perspective. Breast. 2022;65:84–90. doi: 10.1016/j.breast.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douglas S, et al. Patient–physician discordance in goals of care for patients with advanced cancer. Curr Oncol. 2019;26(6):370–9. doi: 10.3747/co.26.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rapport F, et al. What do patients really want? An in-depth examination of patient experience in four Australian hospitals. BMC Health Serv Res. 2019;19(1):38. doi: 10.1186/s12913-019-3881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stellato D, et al. Preferences of Canadian patients and physicians for adjuvant treatments for Melanoma. Curr Oncol. 2019;26(6):e755–65. doi: 10.3747/co.26.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu FX, et al. Patient and oncologist preferences for attributes of treatments in advanced Melanoma: a discrete choice experiment. Patient Prefer Adherence. 2017;11:1389–99. doi: 10.2147/PPA.S140226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bröckelmann PJ et al. Patient and physician preferences for first-line treatment of classical Hodgkin Lymphoma in Germany, France and the United Kingdom. Br J Haematol, 2018. [DOI] [PMC free article] [PubMed]
- 58.Landfeldt E, et al. Patient, physician, and general population preferences for treatment characteristics in relapsed or refractory chronic lymphocytic Leukemia: a conjoint analysis. Leuk Res. 2016;40:17–23. doi: 10.1016/j.leukres.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Deal K. Segmenting patients and physicians using preferences from discrete choice experiments. Patient. 2014;7(1):5–21. doi: 10.1007/s40271-013-0037-9. [DOI] [PubMed] [Google Scholar]
- 60.Ludwig H, et al. Patient preferences for interferon alfa in Multiple Myeloma. J Clin Oncol. 1997;15(4):1672–9. doi: 10.1200/JCO.1997.15.4.1672. [DOI] [PubMed] [Google Scholar]
- 61.Slevin ML, et al. Attitudes to chemotherapy: comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ. 1990;300(6737):1458–60. doi: 10.1136/bmj.300.6737.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiggelbout AM, De Haes JCJM. Patient preference for cancer therapy: an overview of measurement approaches. J Clin Oncol. 2001;19(1):220–30. doi: 10.1200/JCO.2001.19.1.220. [DOI] [PubMed] [Google Scholar]
- 63.Kaul S, Stockbridge N, Butler J. Benefit-Risk tradeoffs in Assessment of New Drugs and devices. Circulation. 2020;142(20):1974–88. doi: 10.1161/CIRCULATIONAHA.120.048933. [DOI] [PubMed] [Google Scholar]
- 64.Jen W-Y, et al. Qualitative study of factors affecting patient, caregiver and physician preferences for treatment of Myeloma and Indolent Lymphoma. Patient Prefer Adherence. 2020;14:301–8. doi: 10.2147/PPA.S241340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann TC, Lewis J, Maher CG. Shared decision making should be an integral part of physiotherapy practice. Physiotherapy. 2020;107:43–9. doi: 10.1016/j.physio.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Légaré F, et al. Training health professionals in shared decision-making: an international environmental scan. Patient Educ Couns. 2012;88(2):159–69. doi: 10.1016/j.pec.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Faiman B, Tariman JD. Shared decision making: improving patient outcomes by understanding the benefits of and barriers to Effective Communication. Clin J Oncol Nurs. 2019;23(5):540–2. doi: 10.1188/19.CJON.540-542. [DOI] [PubMed] [Google Scholar]
- 68.Longtin Y, et al. Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc. 2010;85(1):53–62. doi: 10.4065/mcp.2009.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study available from the corresponding author on reasonable request.