Abstract
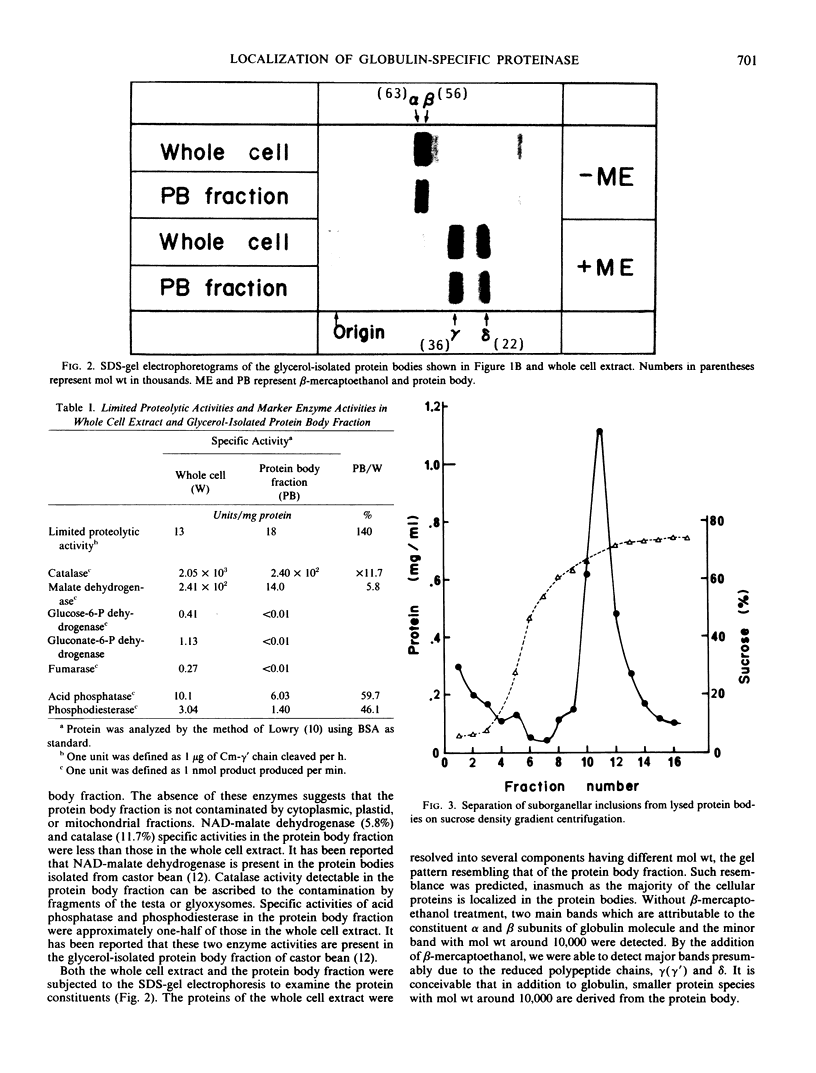
Protein bodies were prepared from the cotyledons of pumpkin (Cucurbita sp.) seeds by employing a nonaqueous isolation method. Both light micrographic examination and the marker enzyme assays have shown that the isolated protein bodies were intact and contamination with other cell organelles or cytoplasmic components was negligible. A proteolytic enzyme catalyzing the limited hydrolysis of carboxymethylated γ′ chain of globulin was found to be present in the protein bodies. The specific activity in the protein body (18 units per milligram protein) was higher than that in the whole cell extract (13 units per milligram protein), indicating that the limited proteolytic enzyme was localized in the protein body.
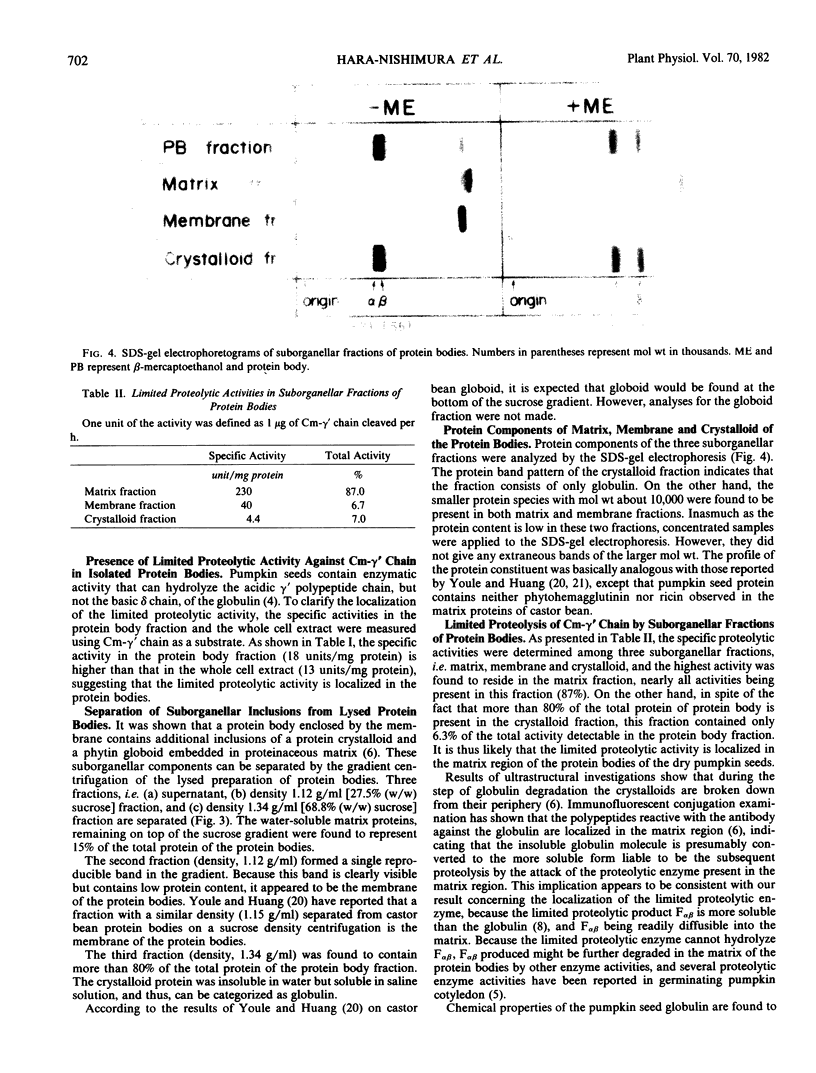
After lysis of the protein bodies using hypotonic buffer solution, the suborganellar components (matrix, membranes, and crystalloids) were separated by sucrose density gradient centrifugation. The crystalloid was composed of only globulin, a major seed protein. The major proteins of matrix and membrane fractions were shown to have mol wt of approximately 10,000. About 90% of the limited proteolytic activity was found in the matrix region.
Full text
PDF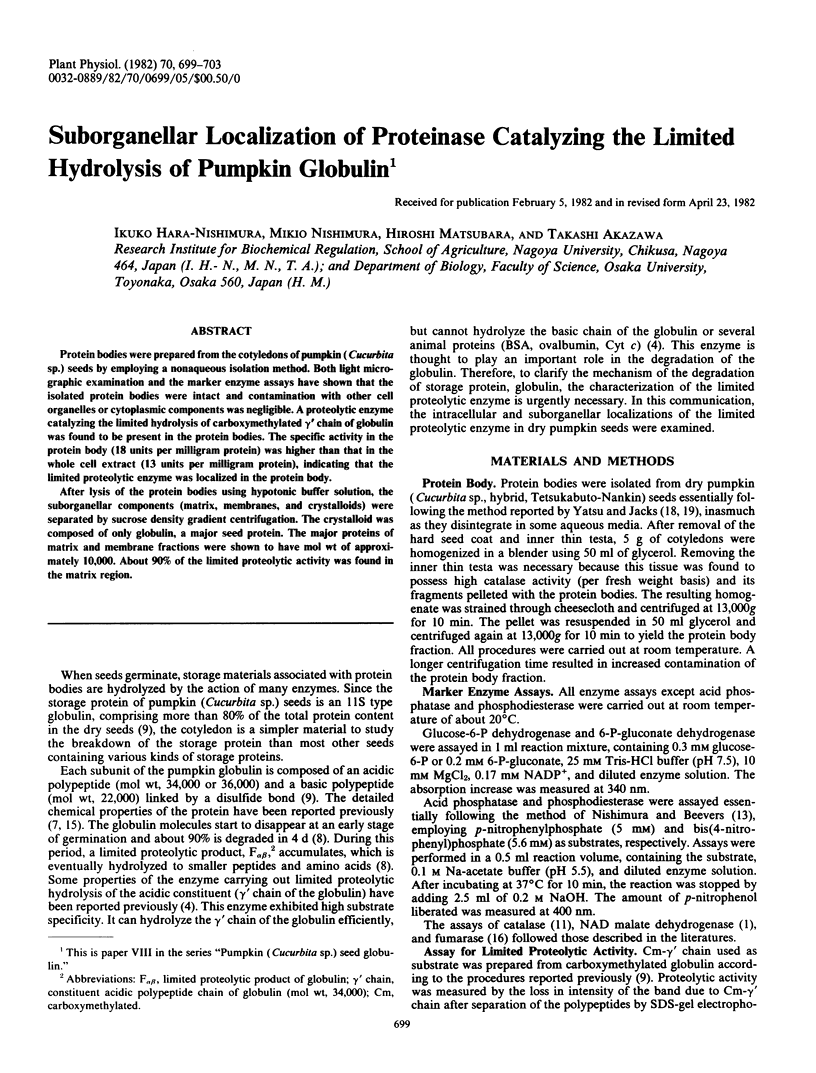


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahi T., Nishimura M. Regulatory function of malate dehydrogenase isoenzymes in the cotyledons of mung bean. J Biochem. 1973 Feb;73(2):217–225. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Isolation and characterization of the protein body membrane of castor beans. Plant Physiol. 1979 Sep;64(3):506–511. doi: 10.1104/pp.64.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Albumin storage proteins in the protein bodies of castor bean. Plant Physiol. 1978 Jan;61(1):13–16. doi: 10.1104/pp.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Huang A. H. Protein Bodies from the Endosperm of Castor Bean: Subfractionation, Protein Components, Lectins, and Changes during Germination. Plant Physiol. 1976 Dec;58(6):703–709. doi: 10.1104/pp.58.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]





