Abstract
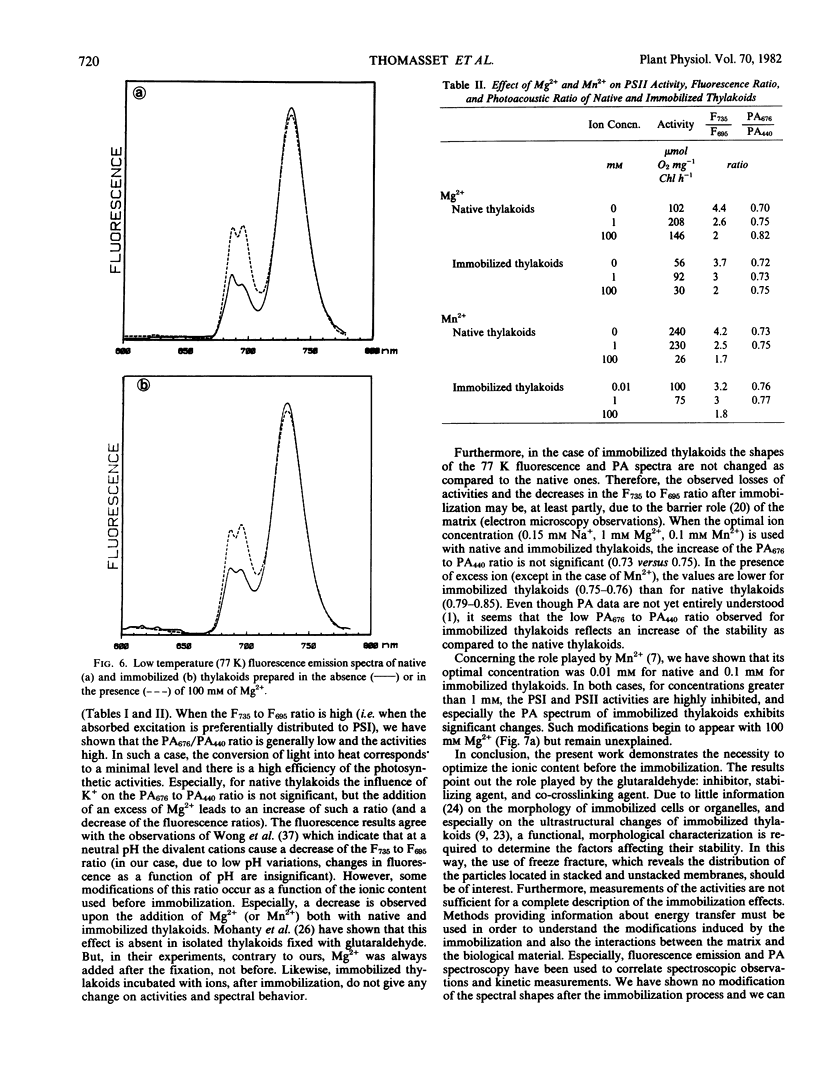
Immobilization of lettuce (Lactuca sativa) thylakoids has been performed by using glutaraldehyde and bovine serum albumin. Confirming previous reports, a stabilization of the O2 evolution activity of the photosystem II (PSII) under storage and functional conditions has been observed. The present work is devoted to the role played by mono-and divalent cations, during the immobilization process itself, on the O2 production. Four types of measurements have been employed: kinetic measurements, low temperature (77 K) fluorescence emission, photoacoustic (PA) spectroscopy, and electron microscopy observations. We show that the effect of glutaraldehyde is complex because it acts as an inhibitor, a stabilizing agent, and a cross-linking reactive. In the present studies, the thylakoids are immobilized within a polymeric insoluble albumin matrix. The highest activity yield and the best storage conditions are obtained when 0.15 mm Na+ (or K+), 1 mm Mg2+, and 0.1 mm Mn2+ are present in the resuspending media before the immobilization. Due to modifications of the ionic content during such a process, structural differences are observed on the stacking degree of thylakoids. No modification of the fluorescence and PA spectra after the immobilization are found. Furthermore, a correlation between activities and spectral changes have been shown: when the activities increase, the F735 to F695 ratio increases and the PA676 to PA440 ratio decreases.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahen D., Bults G., Garty H., Malkin S. Photoacoustics in life sciences. J Biochem Biophys Methods. 1980 Nov;3(5):293–310. doi: 10.1016/0165-022x(80)90010-x. [DOI] [PubMed] [Google Scholar]
- Cahen D., Malkin S., Lerner E. I. Photoacoustic spectroscopy of chloroplast membranes; listening to photosynthesis. FEBS Lett. 1978 Jul 15;91(2):339–342. doi: 10.1016/0014-5793(78)81205-8. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- Chow W. S., Thorne S. W., Duniec J. T., Sculley M. J., Boardman N. K. The stacking of chloroplast thylakoids. Effects of cation screening and binding, studied by the digitonin method. Arch Biochem Biophys. 1980 Apr 15;201(1):347–355. doi: 10.1016/0003-9861(80)90520-2. [DOI] [PubMed] [Google Scholar]
- Davis D. J., Armond P. A., Gross E. L., Arntzen C. J. Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch Biochem Biophys. 1976 Jul;175(1):64–70. doi: 10.1016/0003-9861(76)90485-9. [DOI] [PubMed] [Google Scholar]
- Epel B. L., Neumann J. The mechanism of the oxidation of ascorbate and MN2+ by chloroplasts. The role of the radical superoxide. Biochim Biophys Acta. 1973 Dec 14;325(3):520–529. doi: 10.1016/0005-2728(73)90211-9. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Yoshii F., Kaetsu I. Stabilization of Photosystem II (O(2) Evolution) of Spinach Chloroplasts by Radiation-induced Immobilization. Plant Physiol. 1981 Feb;67(2):351–354. doi: 10.1104/pp.67.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E. L., Prasher S. H. Correlation between monovalent cation-induced decreases in chlorophyll a fluorescence and chloroplast structural changes. Arch Biochem Biophys. 1974 Oct;164(2):460–468. doi: 10.1016/0003-9861(74)90056-3. [DOI] [PubMed] [Google Scholar]
- Hall D. O. Photobiological energy conversion. FEBS Lett. 1976 Apr 15;64(1):6–16. doi: 10.1016/0014-5793(76)80236-0. [DOI] [PubMed] [Google Scholar]
- Hardt H., Kok B. Stabilization by glutaraldehyde of high-rate electron transport in isolated chloroplasts. Biochim Biophys Acta. 1976 Oct 13;449(1):125–135. doi: 10.1016/0005-2728(76)90012-8. [DOI] [PubMed] [Google Scholar]
- Isaakidou J., Papageorgiou G. C. Functional effects of chemical modification of unstacked and stacked chloroplasts with glutaraldehyde. Arch Biochem Biophys. 1979 Jul;195(2):280–287. doi: 10.1016/0003-9861(79)90354-0. [DOI] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Butler W. L. Microencapsulation of chloroplast particles. Plant Physiol. 1976 May;57(5):746–750. doi: 10.1104/pp.57.5.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosencwaig A. Photoacoustic spectroscopy. Annu Rev Biophys Bioeng. 1980;9:31–54. doi: 10.1146/annurev.bb.09.060180.000335. [DOI] [PubMed] [Google Scholar]
- Shioi Y., Sasa T. Immobilization of photochemically-active chloroplasts onto diethylaminoethyl-cellulose. FEBS Lett. 1979 May 15;101(2):311–315. doi: 10.1016/0014-5793(79)81032-7. [DOI] [PubMed] [Google Scholar]
- Véjux A. M., Bae P. Photoacoustic spectrometry of macroporous hemoglobin particles. J Opt Soc Am. 1980 May;70(5):560–562. doi: 10.1364/josa.70.000560. [DOI] [PubMed] [Google Scholar]
- Wasserman A. R., Fleischer S. The stabilization of chloroplast function. Biochim Biophys Acta. 1968 Jan 15;153(1):154–169. doi: 10.1016/0005-2728(68)90156-4. [DOI] [PubMed] [Google Scholar]
- Wong D., Govindjee, Merkelo H. Effects of bulk pH and of monovalent and divalent cations on chlorophyll a fluorescence and electron transport in pea thylakoids. Biochim Biophys Acta. 1980 Oct 3;592(3):546–558. doi: 10.1016/0005-2728(80)90099-7. [DOI] [PubMed] [Google Scholar]




