Abstract
Connective tissue growth factor (CTGF) is a distinct signaling molecule modulating many physiological and pathophysiological processes. This protein is upregulated in numerous fibrotic diseases that involve extracellular matrix (ECM) remodeling. It mediates the downstream effects of transforming growth factor beta (TGF-β) and is regulated via TGF-β SMAD-dependent and SMAD-independent signaling routes. Targeting CTGF instead of its upstream regulator TGF-β avoids the consequences of interfering with the pleotropic effects of TGF-β. Both CTGF and its upstream mediator, TGF-β, have been linked with the pathophysiology of glaucomatous optic neuropathy due to their involvement in the regulation of ECM homeostasis. The excessive expression of these growth factors is associated with glaucoma pathogenesis via elevation of the intraocular pressure (IOP), the most important risk factor for glaucoma. The raised in the IOP is due to dysregulation of ECM turnover resulting in excessive ECM deposition at the site of aqueous humor outflow. It is therefore believed that CTGF could be a potential therapeutic target in glaucoma therapy. This review highlights the CTGF biology and structure, its regulation and signaling, its association with the pathophysiology of glaucoma, and its potential role as a therapeutic target in glaucoma management.
Keywords: Antiglaucoma, CTGF, extracellular matrix, fibrosis, remodeling, TGF-β
Impact statement
Connective tissue growth factor (CTGF) over-expression is involved in the pathophysiology of fibrotic diseases involving extracellular matrix (ECM) remodeling leading to excessive ECM deposition. Increased intraocular pressure (IOP) in glaucomatous optic neuropathy has been associated with excessive expression of CTGF and its upstream signaling molecule transforming growth factor beta (TGF-β). Imbalance in ECM regulation increases resistance at the aqueous humor outflow pathway leading to elevation of IOP, which subsequently damages the optic nerve. Therefore, CTGF could potentially be a target for antiglaucoma agents. This article describes the biology of CTGF, its regulation, signaling, and association with the pathophysiology of glaucoma. The impact of this review article lies in the possibility of utilizing CTGF as a potential therapeutic target for glaucoma and other fibrotic diseases.
Introduction
Connective tissue growth factor (CTGF) is a unique signaling modulator that is involved in multiple physiological processes such as cell proliferation, differentiation, adhesion and survival, angiogenesis, wound healing, and production of extracellular matrix (ECM). In addition, it plays a crucial part in pathophysiological processes, including tumorigenesis, fibrotic disorders, atherosclerosis, rheumatoid arthritis, and cardiac failure. The involvement of CTGF in pathophysiological processes leading to fibrotic disorders has been demonstrated in various studies, particularly those that utilized small-interfering RNA (siRNA) or antisense-mediated knockdown of CTGF.1,2 Since CTGF is the downstream effector to transforming growth factor beta (TGF-β), a profibrotic cytokine, it may serve as a useful target for suppressing some of the TGF-β’s profibrotic actions without interfering with TGF-β’s other essential physiological functions.
From this standpoint, role of CTGF in glaucoma pathophysiology is important to investigate. Glaucoma causes irreversible blindness and is the worldwide second leading cause of visual loss and is characterized by optic nerve degeneration and visual field defects in affected individuals. Intraocular pressure (IOP) is regulated by maintaining the balance between aqueous humor (AH) formation and drainage. IOP is the most critical risk factor for the disease. Ciliary body produces AH and this colorless fluid passes from the posterior chamber to the anterior chamber and exits the eye via the trabecular meshwork (TM) into Schlemm’s canal. In primary open-angle glaucoma (POAG), there are no detectable structural abnormalities and IOP elevation is an outcome of the abnormally high resistance to AH outflow within TM. The IOP is also elevated in primary closed-angle glaucoma; however, this subtype of glaucoma often results from the structural abnormalities causing narrowing of iridocorneal angle and blocking the drainage of AH. POAG accounts for 74% of all cases of glaucoma. 3
The increased TM resistance in POAG patients is linked to an increase in the deposition of extracellular fibrillar material consisting of elastic fibers and fibronectin, the major ECM constituent in the TM tissue. A large percentage of these patients have elevated AH TGF-β2 level, indicating that TGF-β2 is part of the pathogenesis of increased resistance in TM and elevated IOP in POAG eyes.4–6 In vitro and ex vivo treatment using TGF-β2 increases the synthesis of ECM components and this effect has been shown to be largely mediated through CTGF.7–10 Notably, CTGF is abundantly expressed in the TM of human eyes11,12 and its expression is triggered by mechanical stimuli in a diverse range of cell types. Hence, increased IOP in glaucomatous eyes may serve as a mechanical stimulus inducing CTGF expression and the resultant pathological changes in TM. Therefore, CTGF could be a target for potential antiglaucoma drugs aiming for modifying the disease process in the TM of POAG eyes and other diseases associated with ECM remodeling.
In this brief review, we recapitulate recent knowledge relating to the regulation of CTGF expression in TM, its effects on the expression of ECM proteins, and its role in IOP elevation in glaucomatous eyes. We also highlight the possible role of CTGF as a therapeutic focus in the currently used antiglaucoma drugs and also for future drug development.
CTGF: biology and structure
CTGF was found by Bradham et al. 13 in 1991 as an ECM belonging to the CCN family, a simple acronym based on its three classical members “Connective tissue growth factor (CTGF), Cysteine-rich protein (Cyr61), and Nephroblastoma-overexpressed gene (NOV).” 14 These proteins were later renamed as cellular communication network (CCN). 15 There are a total of six CCN family members, including CCN2 (CTGF), Cyr61 (CCN1), NOV (CCN3), WISP1 (CCN4), WISP2 (CCN5), and WISP3 (CCN6). 16 The CCN family of proteins have a multimodular structure that enables them to communicate with other proteins in order to execute their functions. 17 These interactions include those with cytokines, growth factors, cell surface integrins, matrix metalloproteinases (MMPs), and ECM proteins such as fibronectin.18–20 The CCN family is involved in regulating cellular processes, such as cell proliferation, differentiation, death, survival, migration, and adhesion.17,21–23
CTGF is widely expressed and is pivotal in the embryonic development and wound healing process following injury. 24 The absence or lack CTGF expression has been implicated in neurodevelopmental disorders, including tuberous sclerosis, skeletal dysmorphism, and pulmonary hypoplasia,25–27 whereas over-expression or excessive amount of this protein is associated with malignancies, such as osteosarcoma, breast cancer, and hepatocellular carcinoma,28–31 and fibrotic disorders, such as glaucoma, cardiac and pulmonary fibrosis, as well as renal fibrosis.32–35
CTGF, like other members in the CCN group, consists of four cysteine-rich domains and is present in the cellular stroma.22,36 CTGF is a mosaic protein due to its modular structure consisting of five exons that code for 394 amino acid residues. Both of its N- and C-terminals are made up of two domains known as module. The N-terminal comprises domain 1, the insulin-like growth factor–binding protein (IGFBP), and domain 2, the von Willebrand factor type C (vWC). The C-terminal comprises domain 3, the thrombospondin-1 (TSP-1), and domain 4, the C-terminal cystine knot (CT).37,38 In between N- and C-terminals, there is a hinge, the site of proteolytic cleavage (Figure 1(B)). Proteases, such as elastase and plasmin, and MMP group of enzymes, such as MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, and MMP-13, cause cleavage at the hinge-breaking CTGF between N- and C-terminals. 39
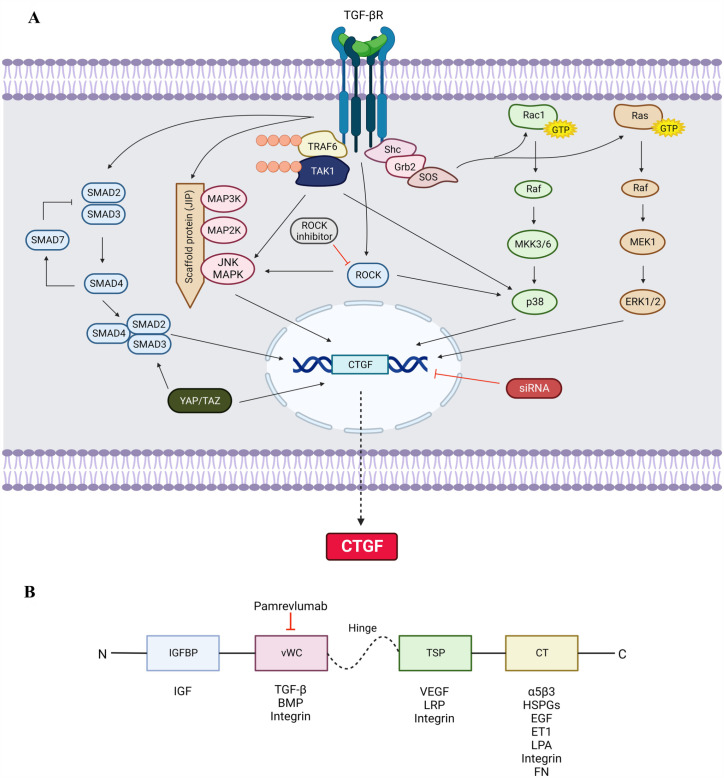
Figure 1.
(A) TGF-β signaling via SMAD-dependent and SMAD-independent pathways to regulate CTGF-mediated ECM production. TGF-β signaling is initiated by binding of the ligand with the receptors. In SMAD pathway, the activated receptors phosphorylate SMAD 2 and SMAD 3 together with SMAD 4 to promote CTGF expression. SMAD 7 acts as inhibitory mediator for further stimulation of SMAD signaling. In SMAD-independent pathway, TGF-β activates MAP3K at scaffold protein, JNK-interacting protein-1, which facilitates the activation of MAP2K and JNK MAPK. JNK can also be activated by TAK1 upon polyubiquitylation with TRAF6 at the ligand-bound TGF-βR. Binding of the TGF-βR causing autophosphorylation of tyrosine residues recruits Shc and Grb2. The Shc-Grb2 complex binds to SOS that activates Rac1 and Ras GTPases. Rac1 activates Raf to phosphorylate MKK3/6 and stimulate p38. Ras activates Raf to phosphorylate MEK1 which stimulates ERK1/2. TGF-βR also activates ROCK signaling leading to phosphorylation of JNK and p38. The activation of these MAPK signaling molecules JNK, p38, and ERK1/2 induces the expression of CTGF for ECM synthesis. YAP/TAZ involves binding with the SMAD 2/3/4 complex and directly translocates into the nucleus. The siRNA inhibits the transcription of CTGF gene. The transcription of CTGF gene promotes the CTGF protein synthesis and secretes extracellularly. TGF-β: transforming growth factor β; TGF-βR: TGF-c receptor; SMAD: suppressor of mothers against decapentaplegic; MAP: mitogen-activated protein; MAPK: mitogen-activated protein kinase; JNK: c-Jun amino terminal kinase; TRAF6: tumor necrosis factor: TNF receptor–associated factor 6; TAK1: TGF-β-associated kinase 1; Shc: Src homology domain 2–containing protein; Grb2: growth factor receptor–binding protein 2; SOS: son of sevenless; GTP: guanosine triphosphate; MKK3/6: MAP kinase kinase 3/6; ERK: extracellular signal–regulated kinases; MEK1: MAP/ERK kinase 1; ROCK: RhoA/Rho-associated protein kinase; YAP/TAZ: yes-associated protein 1/transcriptional co-activator with PDZ-binding motif; siRNA: short-interfering RNA; CTGF: connective tissue growth factor. (B) Schematic representation of CTGF protein structure showing four structurally distinct domains and the proteins or growth factors that interact with specific domain. IGFBP: insulin-like growth factor–binding protein; vWC: von Willebrand factor type C; TSP: thrombospondin-1; CT: C-terminal cystine knot; IGF: insulin-like growth factor; TGF-β: transforming growth factor-β; BMP: bone morphogenetic protein; VEGF: vascular endothelial growth factor; LRP: lipoprotein receptor–related protein; HSPGs: heparan sulfate proteoglycans; EGF: epidermal growth factor; ET1: endothelin 1; LPA: lysophosphatidic acid; FN: fibronectin. Created with BioRender.com.
Pointed black arrows refer to stimulatory and red blunt arrows refer to inhibitory.
The regulators of CTGF expression
Diverse variables modulate CTGF expression at the transcriptional, post-transcriptional, and post-translational stages. The extracellular stimuli including growth factors; cytokines, for example, TGF-β and bone morphogenetic protein (BMP)-2; hormones including angiotensin II and glucocorticoids, monocyte chemotactic protein (MCP)-1, and interferon (IFN)-γ promote CTGF expression.40–42
TGF-β
TGF-β is considered a master regulator of tissue growth, regeneration, remodeling, and fibrosis. Most TGF-β responses, including fibrosis, wound healing, ECM remodeling, fibroblasts proliferation, involve CTGF activity.43–45 The intracellular signaling involving TGF-β and CTGF varies according to different kind of cell and the physiological or pathological outcomes. The TGF-β comprises isoforms, including TGF-β1, TGF-β2, and TGF-β3, and exerts its effects via intracellular signaling that is largely mediated via the SMAD-dependent (canonical) and the non-SMAD-dependent (non-canonical) pathways. TGF-β ligands exert their action via binding to TGF-β receptor type II (TGF-βRII), a transmembrane protein with protein kinase domain. This binding promotes TGF-β type I receptor (TGF-βRI) and phosphorylates it to form a ligand receptor complex. 46 Thereafter, downstream signaling involves either SMAD proteins of the canonical pathway or the mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K)/AKT, and Rho-like GTPase of the non-canonical pathway (Figure 1(A)).
TGF-β interacts with CTGF by binding at the vWC domain (Figure 1(B)). CTGF serves as a chaperone to transport TGF-β to its receptors by binding TGF-β with a low affinity and thus creating a positive feedback loop, which magnifies the TGF-β effects inducing higher CTGF expression.47,48 TGF-β signaling via canonical or non-canonical pathways may lead to CTGF expression depending on the different cell types, tissues, or intended functional outcome. For example, the CTGF expression in normal fibroblasts is induced via the SMAD-dependent signaling, whereas in scleroderma fibroblasts, the induction of CTGF expression is SMAD-independent. 49
SMAD-dependent signaling in TGF-β-mediated expression of CTGF
The SMAD-dependent downstream signaling is activated after the TGF-β ligands stimulate the phosphorylation of TGF-βRI. The SMAD pathway comprises series of phosphorylation of several SMAD proteins belonging to three functional classes: these include the receptor-regulated SMAD (R-SMAD), the co-mediator SMAD (Co-SMAD), and the inhibitory SMAD (I-SMAD). The ligand–receptor complex phosphorylates and then actuates R-SMADs (SMAD 2 and 3), which heterodimerize and form a complex with Co-SMAD (SMAD 4). The activated complexes then translocate into the nucleus, where transcription of target genes will be regulated by binding of the complex directly to DNA, interacting with diverse DNA-binding proteins, and employing either transcriptional co-activators or co-repressors. The I-SMADs block phosphorylation of R-SMADs50,51 (Figure 1(A)).
There are a total of nine SMAD proteins including SMAD 1 to SMAD 9. The R-SMADs include SMAD 1, 2, 3, 5, 8, 9; the SMAD 4 is Co-SMAD, and I-SMADs include SMAD 6 and 7. SMAD 2 and 3 specifically mediate the TGF-β signaling pathway that phosphorylates after receptor activation at the cell membrane. SMAD 4 functions as a central sensor, forming heteromeric multimeric complexes with activated R-SMADs, and regulates transcription of target genes. SMAD 7, originally localized in the nucleus, inhibits TGF-β signaling as it blocks the formation and activation of R-SMAD and Co-SMAD complexes by adhering to the TGF-βRI at the cell membrane.
SMAD proteins conduct key functions in signal conduction and regulation of downstream target genes in the canonical TGF-β pathway. 52 CTGF has been recognized as one of these target genes that mediates the development of fibrosis, including myofibroblast activation, excessive ECM synthesis, and ECM degradation inhibition. CTGF reinforces TGF-β receptor by binding directly to the TGF-β receptor type III (TGF-βRIII). Thus, blockade of TGF-β/SMAD-dependent expression of CTGF is likely to be a more reasonable therapeutic target in preventing fibrogenesis rather than the upstream blockade of TGF-β/SMAD that is susceptible to interfere with a wide range of physiological functions. 53
SMAD 2/3 phosphorylation has been reported to be stimulated by TGF-β in human lung fibroblasts 54 and orbital fibroblasts. 55 Aberrations of TGF-β1 have been shown to upregulate the gene expression of ECM proteins, α-smooth muscle actin (α-SMA), 56 as well as the downstream CTGF proteins 57 via SMAD 2/3 phosphorylation. Moreover, inhibition of TGF-β/SMAD signaling was shown to decrease CTGF expression, leading to reduction of the fibrotic response in endometrial fibrosis. 53 Similarly, it was observed that inhibition of SMAD 2/3 led to the reduction in TGF-β1-induced increase in CTGF expression in human granulosa cells. 58 Interestingly, the rise of CTGF expression in rat progenitor cells activated by TGF-β1 was not mediated by the SMAD signaling pathway. 59 This finding was also supported by O’Donovan et al., 60 who have observed elevated CTGF level and reduced SMAD 2/3 phosphorylation in renal mesangial cells in streptozotocin (STZ)-induced diabetic mice. These findings insinuate that TGF-β/SMAD signaling regulates CTGF expression differently depending on the cell types, the species, and the experimental conditions.
SMAD-independent signaling in TGF-β-mediated expression of CTGF
The stimulation of SMAD-independent or the non-canonical pathway following the activation of TGF-β receptors occurs via sequential phosphorylation or direct interaction of signaling proteins. These non-canonical signaling largely includes MAPK pathways leading to the activation of extracellular signal–regulated kinase (ERK)1/2, p38 MAPK, and c-jun N-terminal kinase (JNK) via Ras superfamily of small GTPase. In addition, TGF-β signaling may also activate Rho subfamily of small GTPase which has been shown to crosstalk with some constituents of MAPK 61 (Figure 1(A)).
ERK1/2
The MAPK pathway is activated when TGF-β ligand binds to TGF-βRII, causing phosphorylation of tyrosine residues. Following this, Src homology domain 2–containing protein (Shc) and growth factor receptor–binding protein 2 (Grb2) are recruited by TGF-βRII, bringing forth the stimulation of ERK1/2 through sequential activation of Ras, Raf, and MEK1/2.62,63 ERK1/2 activation leads to phosphorylation of transcription factors that modulate gene expression. Interestingly, ERK1/2 signaling may also interact with the TGF-β/SMAD pathway. 64 In TM cells, TGF-β-activated ERK signaling increases expression of plasminogen activator inhibitor-1 (PAI-1), a tissue plasminogen activator (tPA) primary inhibitor. 65 Inhibition of tPA prevents the activation of pro-MMP and that inhibits ECM degradation causing accumulation of ECM within the TM tissue. In human lung epithelial cells, TGF-β has been proven to induce CTGF expression via the ERK signaling pathway. 66 Inhibiting CTGF in dextran sulfate sodium-induced ulcerative colitis could improve inflammatory response via blocking ERK signaling pathway. 67
p38 MAPK
The GTPase Rac1 activates p38 MAPK pathway, thus stimulating the expression of Secreted Protein Acidic and Rich in Cysteine (SPARC) in TM cells. 68 The binding of SPARC to ECM proteins regulates the growth factor efficacy and MMP expression. 69 Upon activation of TGF-β receptors, tumor necrosis factor receptor–associated factor 6 (TRAF6), an E3 ubiquitin ligase, binds with TGF-βRI.70,71 This subsequently recruits TGF-β-activated kinase 1 (TAK1) through association and induced activation allowing the phosphorylation of MAP kinase kinase (MAP2K) MKK3/6. This resulted in the activation of the p38 MAPK pathway.72,73 p38 MAPK mediates expression of CTGF, ECM proteins such as fibronectin, as well as MMP-2.74–76 SB203580, a p38 MAPK inhibitor, has been demonstrated to attenuate type I collagen production induced by TGF-β2 in human TM cells. 77
JNK
TGF-β is capable in inducing the activation of the MAP kinase kinase kinase (MAP3K) family. Components of the MAP3K, the MAP2K family members, and JNK are retained closely by the scaffold protein, JNK-interacting protein-1. This enables a quick series of phosphorylation that leads to the JNK active site phosphorylation. 78 JNK can also be activated via TAK1 through phosphorylation. 63 Stimulation of the JNK signaling pathway can initiate the SMAD 3 phosphorylation, translocation to nuclei, which finally lead to recruitment of SMAD 3 to the CTGF promoter, inducing CTGF expression in both human and orbital fibroblasts.79–81
Rho GTPase/Rho kinase signaling
TGF-β can stimulate Rho-like GTPases pending on different cell types. Studies have shown that in epithelial cells and primary keratinocytes, TGF-β actuates Rho A in its GTP-bound state, an activation that is likely to be independent of SMAD 2 or SMAD 3.63,82,83 JNK and p38 MAPK can directly be activated by Rho GTPases. Rho kinase may also mediate TGF-β-induced CTGF expression by phosphorylating ERK1/2 and JNK. 84 Hence, TGF-β and CTGF may also increase the ECM component expressions such as fibronectin, type I collagen, and MMP-2 by stimulating the RhoA/Rho-associated protein (ROCK) signaling pathway. 85
Other regulators of CTGF
CTGF expression may also be regulated by multiple ligands and extracellular signaling molecules since specific receptors of CTGF have not been identified. These ligands include BMP, α5β3 integrin, insulin-like growth factor (IGF) 1, vascular endothelial growth factor (VEGF), low-density lipoprotein receptor–related protein 1 (LRP-1), heparan sulfate proteoglycans (HSPGs), decorin, epidermal growth factor (EGF), hemodynamic endothelin (ET) 1, secreted frizzled-like protein-2 (sFRP-2), antiproliferative factor, thrombin, histamine, serotonin, and prostaglandins.41,86–89
Hypoxia induces CTGF expression via p38 signaling.90,91 In fact, TGF-β was shown to synergize with hypoxia for induction of CTGF expression and muscle fibrosis. 92 Mechanical stresses, such as mechanical stretch and fluid flow, can cause CTGF elevation in different types of the cells.93–95 CTGF is elevated in mesangial cells and myofibroblasts cultivated under high-glucose conditions, and this is regulated by TGF-β.96–98 In addition, CTGF expression was found to be increased by metabolic factors, such as advanced glycation end-products (AGE) and free fatty acids.99,100 Another inducer of CTGF is lysophosphatidic acid (LPA). LPA is a potent bioactive phospholipid that plays a significant role in development of fibrosis including that in skeletal muscle by inducing CTGF expression and fibroblasts activation and proliferation.101–103
Tumor necrosis factor α (TNF-α) is a known powerful inhibitor of CTGF in cells of mesenchymal origin. 104 In a recent study, level of CTGF in TNF-α-exposed osteoblasts was reduced in a concentration- and time-dependent manner. 105 CTGF expression in non-involved fibroblasts in chronic wounds of human tissue was also reduced by TNF-α. 106 TNF-α was reported to inhibit CTGF-promoter activity in endothelial cells of human lung in a dose-dependent manner. 107 Nitric oxide was also shown to downregulate CTGF in rat mesangial cells.108,109
Another signal transduction pathway, the yes-associated protein 1/transcriptional co-activator with PDZ-binding motif (YAP/TAZ) signaling pathway, regulates cell proliferation, differentiation, and organ size.110–112 These two are closely related transcriptional co-activators and are activated by various extracellular signals, including TGF-β, LPA, integrins, mechanical cues, and many others. Activation of YAP/TAZ pathway by TGF-β is mediated through activation of Rho GTPase signaling and SMAD complex, which associate with YAP/TAZ and translocate into the nucleus. 113 Importantly, one of the key target genes of YAP/TAZ pathway is CTGF as YAP/TAZ binds directly to the promoter region of CTGF and enhances its downstream signaling including ECM production and cell proliferation. 114 (Regulators of CTGF expression are summarized in Figure 2.)
Figure 2.
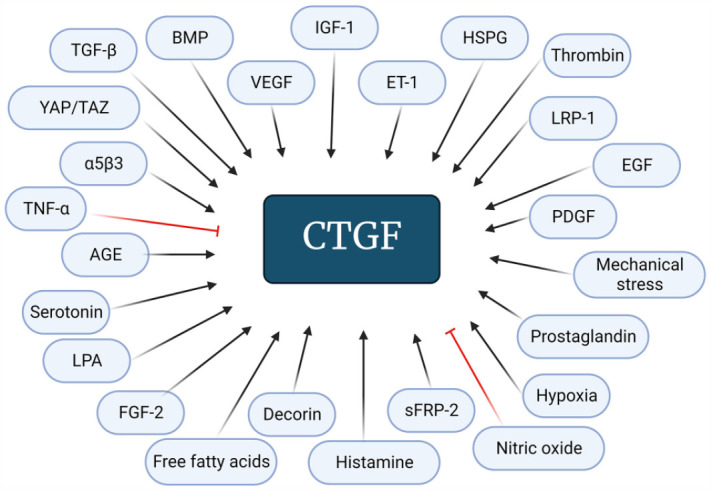
Factors involved in the regulation of CTGF. TGF-β: transforming growth factor-β; BMP: bone morphogenetic protein; VEGF: vascular endothelial growth factor; IGF-1: insulin-like growth factor 1; ET1: endothelin 1; HSPGs: heparan sulfate proteoglycans; LRP: lipoprotein receptor–related protein; EGF: epidermal growth factor; PDGF: platelet-derived growth factor; sFRP-2: secreted frizzled-like protein-2; FGF-2: fibroblast growth factor 2; LPA: lysophosphatidic acid; AGE: advanced glycation end-products; TNF-α: tumor necrosis factor α; YAP/TAZ: yes-associated protein 1/transcriptional co-activator with PDZ-binding motif.
Pointed black arrows refer to stimulatory and red blunt arrows refer to inhibitory. Created with BioRender.com.
CTGF mediates tissue remodeling and fibrosis
The regulatory actions of CTGF result from its binding with several receptors, including integrins, HSPGs, lipoprotein receptor–related proteins (LRPs), and tyrosine kinase (TK) receptors, to initiate signal transduction. 115 In liver fibrosis, CTGF binds to integrin α5β3 in hepatic stellate cells and with HSPGs acting as co-receptors. 116 In pancreatic fibrosis, CTGF joined to α5β1 and HSPG regulates pancreatic stellate cells’ adhesion, propagation, migration, and collagen synthesis. 117 CTGF also binds to a receptor known as IGF-2 receptor leading to proliferation of human corneal fibroblasts. 118
CTGF may also directly bind to cytokines such as VEGFs, fibroblast growth factor 2 (FGF-2), platelet-derived growth factor B (PDGFB), TGF-β, and BMP for regulating their activity.47,119,120 In addition, it adheres to ECM elements such as fibronectin. The binding of CTGF to fibronectin boosts the fibronectin affinity to fibrin for ECM accumulation in wound healing and tissue fibrosis. 121 Over-expression of CTGF in skeletal muscle is associated with skeletal muscle dystrophy, such as Duchenne muscular dystrophy (DMD), leading to increased collagen deposition and muscle fibrosis, which eventually impairs muscle function and reduces myocyte survival.122–124
CTGF in the eye
CTGF is normally expressed in the ocular tissue. 123 Studies have elucidated the presence of CTGF in healthy ocular tissues such as the corneal endothelium, lens subcapsular epithelium, vasculature of iris and retina of mice and rabbits, 124 tear fluid of horses, 125 and the rat retinas. 126 Presence of CTGF-immunoreactivity (CTGF-IR) was shown by van Setten et al. 127 confirming the presence of CTGF in various structures of the human eye. This study detected CTGF in the cornea (majority in the basal layers), stromal keratinocytes, and endothelial cells. The sphincter and dilator muscle of the iris and conjunctiva epithelium also displayed a positive CTGF-IR. 124 These findings supported the presence of CTGF in human tear fluid. 128 A weak CTGF-IR was observed in the ciliary body but it was prominent in the vascular endothelium which may point to the origin of CTGF in the AH. 123 Further studies detected the presence of CTGF in the choroid mainly at the choriocapillaris, retinal nerve fiber layer, optic nerve head, lamina cribrosa, and endothelium of the central retinal artery in healthy human eye. 127
The presence of CTGF in many parts of the normal eye is well known; its increased expression, however, is frequently linked to pathological conditions involving the anterior and/or posterior eye segments. These include pseudoexfoliation glaucoma, corneal and conjunctival scarring, Graves’ ophthalmology, age-related macular degeneration, diabetic retinopathy, and proliferative vitreoretinopathy.129–134 In patients with age-related macular degeneration, CTGF has been expressed in endothelial cells and retinal pigment epithelial cells of choroidal neovascular membranes. 135 CTGF expression in retinal microglia and pericytes has also been shown in patients with diabetic retinopathy. 136 In addition, CTGF gene and protein expressions were found to be significantly upregulated in Graves’ ophthalmology orbital fibroblasts when compared to normal fibroblasts. 134
Glaucoma
Glaucoma is an optic neuropathy and the major risk factor associated with this condition is the elevated IOP. 137 IOP fluctuations lead to mechanical stress on the posterior ocular structures, and this in turn triggers activation of apoptotic pathways leading to degeneration of the retinal ganglion cells. 138 A critical balance between AH production and drainage maintains the IOP in its normal range (10–21 mmHg). The drainage of AH from the anterior segment at the iridocorneal angle occurs largely through TM and to a little extent via alternative uveoscleral pathway. 139
In the TM of POAG patients, excessive deposition of extracellular fibrillar material also known as “sheath-derived plaque material” has been detected. The plaque material consists of ECM elements such as elastic fibers, fibronectin, and collagen type IV.140–142 The TM cells also have a distinct actin-myosin skeleton with contractile ability. A dysfunction of TM cell skeleton leads to their relaxation, which results in increased AH outflow resistance. 143
CTGF in the pathogenesis of glaucoma
TGF-β2 is commonly associated in the pathogenesis of glaucoma. Studies have revealed that patients with POAG have higher TGF-β2 level in their AH in contrast to healthy subjects.4,144 TGF-β2 treatment of TM cells heightens the ECM component synthesis, including fibronectin and collagen type IV.11,145 TM contractility was also shown to be affected following TGF-β1 treatment due to the development of actin stress fibers and increased α-SMA expression. 146 TGF-β target gene, CTGF, is a majorly expressed genes in the human TM. 12 It has been described as a significant mediator of TGF-β2-induced ECM accumulation in the human TM. Synthesis of fibrillar ECM such as fibronectin, collagen types III, IV, and VI was increased following CTGF and TGF-β2 treatment of human TM cells. 11 However, the expression of collagen I was found to be induced by CTGF but not by TGF-β2.11,147 The effects of TGF-β2 on ECM were shown to be mediated by CTGF as it interacts with human TM cells surface molecules to form cell–matrix adhesions. 11 CTGF is upregulated when TM cells are exposed to mechanical stretch or high-pressure perfusion. 93 Increase of CTGF by abnormally activated TGF-β2 can result in over-expression of ECM in the TM, which contributes to the elevated AH outflow resistance. As TGF-β2 downstream mediator of ECM remodeling, CTGF has no effect on the proteolytic system such as MMP2, MMP9, and PAI-1. 11
CTGF as target for the treatment of glaucoma
Prostaglandin analogues and β-blockers
The current management of glaucoma is largely based on the reduction of IOP within the target range using IOP-lowering medications, including prostaglandin analogues and β-blockers. Prostaglandin F2α receptors are extensively scattered in the eye especially in the ciliary muscle bundle, the site of unconventional route of AH outflow. Prostaglandin analogues such as latanoprost reduce IOP by increasing AH drainage through uveoscleral pathway, a non-pressure-dependent pathway unlike the conventional TM pathway. These analogues stimulate prostaglandin F2α receptors in the ciliary muscles and increase the release of MMPs. MMPs are enzymes that increase the degradation of ECM deposited within the ciliary muscle and hence reduce the non-conventional AH outflow resistance.
Timolol maleate and levobunolol are examples of β-adrenoceptor antagonist (β-blockers) and are clinically used topically to lower IOP. It has been proposed that β-blockers have a dual mechanism of action in which they lower AH production and augment the AH drainage. β-blockers block the β-adrenergic receptors which cause a reduction in the intracellular second messenger, cAMP. cAMP elevation in the ciliary epithelial cells increases chloride ion (Cl−) efflux, attracting water and hence increases AH production. These effects are blocked by β-blockers. Studies have also shown that timolol enhances AH outflow; the precise mechanism, however, remains unclear. 148
Based on a meta-analysis by Li et al., 149 prostaglandin analogues such as bimatoprost and latanoprost have the highest efficacy to lower IOP followed by β-blockers. A fixed prostaglandin analogues and β-blockers combination is commonly used in treating glaucoma patients when more than one antiglaucoma agents are needed to achieve the target IOP. 150 Recent studies have demonstrated that treatment with combined prostaglandin analogues and β-blockers more effectively suppressed the profibrotic genes expression in TM of patients with POAG compared to treatment with prostaglandin analogues alone or β-blockers alone. The study by Tejwani et al. 151 observed that combination of bimatoprost and timolol lowered the TGFβ1, TGFβ2, CTGF, FN gene expression. It was also observed that CTGF expression was lower in β-blockers alone group compared to prostaglandin-alone group. Furthermore, the messenger RNA (mRNA) expression of these genes was validated by quantification of the respective proteins showing reduced levels of CTGF and fibronectin in TM treated with combination of prostaglandin and β-blockers compared to the individual drug. The study also observed that the effects on profibrotic markers were modulated by inhibiting the phosphorylation of SMAD 3. 151
Rho Kinase (ROCK) inhibitor
Rho GTPase proteins are from the Ras superfamily and consist of monomeric small GTP-binding proteins. 152 Rho GTPases and its effector Rho kinase (Rho-associated coiled-coil-containing protein kinase [ROCK]) are known to be linked in the pathophysiology of glaucoma. ROCK has two isoforms, that is, ROCK1 (ROKb/P160) and ROCK2 (ROKa). Activation of Rho GTPase stimulates ROCK to phosphorylate numerous intracellular substrates for cellular responses. ROCK controls the actomyosin contraction, actin cytoskeletal dynamics, cell morphology, cell adhesion, cell stiffness, and cell and ECM reorganization.152,153 Ripasudil and netarsudil are two ROCK inhibitors currently approved as glaucoma therapy. Ripasudil was approved in 2014, whereas netarsudil was approved in 2017.154,155
Several studies have investigated the effects of ROCK inhibitors on TM cells, AH outflow, and IOP. ROCK inhibitors enhance the conventional outflow of AH by relaxing the smooth muscle, reducing deposition of ECM in TM and therefore attenuating AH outflow resistance. Both TGF-β and CTGF are known to upregulate the expression of ECM elements via the ROCK signaling pathway (Figure 1(A)). Inhibition of ROCK signaling inhibited CTGF-induced expression of ECM molecules including fibronectin. 85 In TM cells, the inhibition of ROCK was found to be dose- and time-dependent. They induce changes in cell shape associated with decreased actin stress fibers reversibly, focal adhesions, and cell–cell interactions.156–158 ROCK signaling also modulates the expression of ECM and fibrotic cytokines, including TGF-β and CTGF.159,160
SiRNA
Short-interfering RNAs (siRNAs) are small molecules of double-stranded RNA requiring a minimum length between 21 and 23 nucleotides to affect the target gene expression. 161 The siRNAs in the cytoplasm of eukaryotic cells form RNA-induced silencing complex, a multimeric RNA–protein complex that identifies the complementary mRNA and thus blocks the production of specific proteins. 162 SiRNAs can be introduced exogenously to mimic the endogenous RNA interference (RNAi) and these could silence the targeted gene at any stage of cellular process in specific regions without affecting other non-targeted regions 163 (Figure 1(A)).
Despite the potential application of siRNA as pharmacotherapy, several challenges remain unresolved. These include reducing off-target effects, ensuring stability of siRNAs, and establishing their route of administration. 162 One of the most challenging off-target effects is to prevent activation of innate immunity. This can be achieved with siRNA chemical structure modification or using shorter siRNA duplexes. 164 Chemical modifications of siRNA also improve the stability of siRNA. 165 Improvement of the bioavailability of siRNA-based drugs can be achieved with the use of cell-penetrating peptides which has been shown to improve the ocular delivery of siRNA. 166 The last decade has seen the advancement of viral and non-viral carriers for siRNA delivery systems and their extensive evaluation. 167
Junglas et al. 168 showed that RNAi used to deplete CTGF in cultured human TM cells attenuates actin cytoskeleton leading to reduction of outflow resistance. The study utilized pSiCTGF, a vector coding for a short-hairpin RNA against CTGF mRNA. The normal human TM cells transfected with pSiCTGF showed significantly reduced CTGF mRNA expression compared to non-transfected cells. They further observed that this reduction was associated with decreased CTGF level and its target gene, the fibronectin.
A recent study by Dillinger et al. 169 evaluated effects of siRNA administration targeting CTGF. They used different types of siRNA nanoparticles layer-by-layer coated with polyethylenimine and hyaluronic acid. They observed that human TM cells treated with hyaluronic acid (siRNA) nanoparticles led to reduction of CTGF expression by 50%. This study also attempted to examine the role of siRNA in reducing the expression of tight junction proteins in the Schlemm’s canal. It was observed that the reduction of tight junction proteins using siRNA was associated with IOP reduction; however, this effect lasted only for a short duration. 8 siRNA targeting CTGF has high potential as antiglaucoma agents. Development of improved formulation can further enhance delivery of the therapeutic agent to tissues of the AH outflow pathway with high efficiency.
Pamrevlumab (FG-3019)
FG-3019 is a full human recombinant DNA–derived CTGF-reactive monoclonal IgG1/K antibody, isolated and cloned from genetically engineered mice immunized with recombinant human CTGF and expressing human immunoglobulin transgenes. 170 This agent recognizes and binds to CTGF at the vWC domain, 171 thus intercepting the cytokine interactions with CTGF and subsequently inhibiting the downstream signaling (Figure 1(B)). Multiple studies have demonstrated the ability of pamrevlumab to diminish fibrotic response in conditions such as idiopathic pulmonary fibrosis, 172 diabetic kidney disease, 173 and DMD. 174 Experiments using FG-3019 as potential treatment for these conditions have reached clinical trials, and for idiopathic pulmonary fibrosis and chronic DMD, its evaluation has progressed to phase III. 175
This agent has also been on the radar of researchers for glaucoma. The glaucomatous TM cells showed reduction of profibrotic genes and protein expressions of α-SMA and collagen 1A1 after treatment with FG-3019. 9 Unfortunately, due to the limitation imposed by the suitability of the route of administration, the effects of FG-3019 on the IOP remain unknown. This monoclonal antibody is administered parenterally since oral administration is unsuitable due to its large molecular size and vulnerability to degradation in the gastrointestinal system. 176 Furthermore, drug administration to the eye has always been a major challenge for drug delivery scientists due to the unique ocular anatomy and physiology. Since the target site to reduce IOP is in the anterior chamber of the eye, it is unlikely that the parenterally administered agent can reach target region in sufficient concentration. The bioavailability of drugs reaching the ocular tissue via oral/systemic delivery is often less than 2%. 177
Search for drugs targeting CTGF has come a long way for the treatment for fibrotic diseases. Despite favorable data from various preclinical and clinical studies, their progression to clinical application is still awaited. Anti-CTGF strategies that reduce CTGF including siRNA, such as RXI-109, OLX 101, and OLX 201, or monoclonal antibody, such as pamrevlumab (FG-3019), 48 although are known to target CTGF expression and reduce fibrosis; their effectiveness for the same in clinical setting remains unclear. Furthermore, development of dosage forms suitable for clinical use remains challenging.
Conclusions
CTGF is an attractive target for future antiglaucoma agents. Its expression is regulated via several upstream regulators, of which TGF-β remains the prominent one. It increases the expression of several ECM proteins in TM causing an increase in AH outflow resistance. Better understanding of the involvement of CTGF in the IOP elevation in glaucomatous disease has led to development of new agents targeting CTGF in improving the drainage of AH through the TM tissue. Targeting CTGF only can avoid inhibition of its upstream regulators such as TGF-β that have pleotropic effects. This is expected to reduce the potential adverse drug reactions. Currently available drugs such as prostaglandin analogues, β-blockers, and ROCK inhibitors have been shown affect CTGF expression. However, contribution of this effect to their IOP-lowering capacity remains unclear. CTGF as a direct target rather than its targeting via upstream regulators could yield clinically useful antiglaucoma agents.
Acknowledgments
We thank Mohamad Hazwan Mohd Adi for his assistance with designing the illustrations that we visualized. The illustrations were created by using BioRender with publication license.
Footnotes
Authors’ Contributions: All authors participated in writing and review of the manuscript. The idea conceptualization of this review was made by MDSH and NR. MDSH took the lead in writing the manuscript and was supervised by NR. The preparation of the original draft was written by MDSH, NR, ASAB, and NFAH. The manuscript was compiled by MDSH in consultation with NR and RA for critical review, commentary, revision, and approval of the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We would like to acknowledge the funding from the Ministry of Higher Education, Malaysia under the Fundamental Research Grant Scheme, FRGS/1/2019/SKK08/UITM/02/18.
ORCID iDs: Mohammad Daniel Shafiq Hassan  https://orcid.org/0000-0002-9743-132X
https://orcid.org/0000-0002-9743-132X
Norhafiza Razali  https://orcid.org/0000-0002-8439-6713
https://orcid.org/0000-0002-8439-6713
Amy Suzana Abu Bakar  https://orcid.org/0000-0002-7760-7308
https://orcid.org/0000-0002-7760-7308
Noor Fahitah Abu Hanipah  https://orcid.org/0000-0001-6985-3632
https://orcid.org/0000-0001-6985-3632
Renu Agarwal  https://orcid.org/0000-0002-3050-7449
https://orcid.org/0000-0002-3050-7449
References
- 1. Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): from pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal 2009;3:5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J 2007;21:3355–68 [DOI] [PubMed] [Google Scholar]
- 3. Kapetanakis VV, Chan MPY, Foster PJ, Cook DG, Owen CG, Rudnicka AR. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): a systematic review and meta-analysis. Br J Ophthalmol 2016;100:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agarwal P, Daher AM, Agarwal R. Aqueous humor TGF-β2 levels in patients with open-angle glaucoma: a meta-analysis. Mol Vis 2015;21:612–20 [PMC free article] [PubMed] [Google Scholar]
- 5. Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-β2 in patients with glaucoma. Int Ophthalmol 2004;25:19–22 [DOI] [PubMed] [Google Scholar]
- 6. Picht C, Welge-Luessen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor β2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefe’s Arch Clin Exp Ophthalmol 2001;239:199–207 [DOI] [PubMed] [Google Scholar]
- 7. Fujimoto T, Inoue T, Maki K, Inoue-Mochita M, Tanihara H. Vascular endothelial growth factor: a increases the aqueous humor outflow facility. PLoS ONE 2016;11:e0161332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tam LCS, Reina-Torres E, Sherwood JM, Cassidy PS, Crosbie DE, Lütjen-Drecoll E, Flügel-Koch C, Perkumas K. Enhancement of outflow facility in the murine eye by targeting selected tight-junctions of Schlemm’s canal endothelia. Sci Rep 2017;7:40717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wallace DM, Clark AF, Lipson KE, Andrews D, Crean JK, O’Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Investig Ophthalmol Vis Sci 2013;54:7836–48 [DOI] [PubMed] [Google Scholar]
- 10. Wang K, Read AT, Sulchek T, Ethier CR. Trabecular meshwork stiffness in glaucoma. Exp Eye Res 2017;158:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Junglas B, Yu AHL, Welge-Lüssen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp Eye Res 2009;88:1065–75 [DOI] [PubMed] [Google Scholar]
- 12. Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci 2003;44:2588–96 [DOI] [PubMed] [Google Scholar]
- 13. Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product. J Cell Biol 1991;114:1285–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brigstock DR, Goldschmeding R, Katsube KI, Lam SCT, Lau LF, Lyons K, Naus C. Proposal for a unified CCN nomenclature. Mol Pathol 2003;56:127–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takigawa M. CCN proteins (cellular communication network factors): expanding their repertoire toward a new concept. Methods Mol Biol 2023;2582:1–10 [DOI] [PubMed] [Google Scholar]
- 16. Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci 2008;33:461–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim H, Son S, Shin I. Role of the CCN protein family in cancer. BMB Rep 2018;51:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci 2006;119:4803–10 [DOI] [PubMed] [Google Scholar]
- 19. Ono M, Inkson CA, Kilts TM, Young MF. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res 2011;26:193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone 2006;38:671–7 [DOI] [PubMed] [Google Scholar]
- 21. Barreto SC, Ray A, Ag Edgar P. Biological characteristics of CCN proteins in tumor development. J BUON 2016;21:1359–67 [PubMed] [Google Scholar]
- 22. Henrot P, Truchetet ME, Fisher G, Taïeb A, Cario M. CCN proteins as potential actionable targets in scleroderma. Exp Dermatol 2018;28:11–8 [DOI] [PubMed] [Google Scholar]
- 23. Katsube KI, Sakamoto K, Tamamura Y, Yamaguchi A. Role of CCN, a vertebrate specific gene family, in development. Dev Growth Differ 2009;51:55–67 [DOI] [PubMed] [Google Scholar]
- 24. Friedrichsen S, Heuer H, Christ S, Winckler M, Brauer D, Bauer K, Raivich G. CTGF expression during mouse embryonic development. Cell Tissue Res 2003;312:175–88 [DOI] [PubMed] [Google Scholar]
- 25. Baguma-Nibasheka M, Kablar B. Pulmonary hypoplasia in the connective tissue growth factor (Ctgf) null mouse. Dev Dyn 2008;237:485–93 [DOI] [PubMed] [Google Scholar]
- 26. Lambi AG, Pankratz TL, Mundy C, Gannon M, Barbe MF, Richtsmeier JT, Popoff SN. The skeletal site-specific role of connective tissue growth factor in prenatal osteogenesis. Dev Dyn 2012;241:1944–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ercan E, Han JM, Di Nardo A, Winden K, Han MJ, Hoyo L, Saffari A, Leask A, Geschwind DH, Sahi M. Neuronal CTGF/CCN2 negatively regulates myelination in a mouse model of tuberous sclerosis complexCTGF/CCN2 regulates myelination. J Exp Med 2017;214:681–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai HC, Su HL, Huang CY, Fong YC, Hsu CJ, Tang CH. CTGF increases matrix metalloproteinases expression and subsequently promotes tumor metastasis in human osteosarcoma through down-regulating miR-519d. Oncotarget 2014;5:3800–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou CH, Yang RS, Tsao YT. Connective tissue growth factor stimulates osteosarcoma cell migration and induces osteosarcoma metastasis by upregulating VCAM-1 expression. Biochem Pharmacol 2018;155:71–81 [DOI] [PubMed] [Google Scholar]
- 30. Makino Y, Hikita H, Kodama T, Shigekawa M, Yamada R, Sakamori R, Eguchi H, Morii E, Yokoi H, Mukoyama M, Hiroshi S, Tatsumi T, Takehara T. CTGF mediates tumor–stroma interactions between hepatoma cells and hepatic stellate cells to accelerate HCC progression. Cancer Res 2018;78:4902–14 [DOI] [PubMed] [Google Scholar]
- 31. Kim H, Son S, Ko Y, Lee JE, Kim S, Shin I. YAP, CTGF and Cyr61 are overexpressed in tamoxifen-resistant breast cancer and induce transcriptional repression of ERα. J Cell Sci 2021;134:jcs256503 [DOI] [PubMed] [Google Scholar]
- 32. Toda N, Mukoyama M, Yanagita M, Yokoi H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm Regen 2018;38:14–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yin Q, Liu H. Connective tissue growth factor and renal fibrosis. Adv Exp Med Biol 2019;1165:365–80 [DOI] [PubMed] [Google Scholar]
- 34. Kono M, Nakamura Y, Suda T, Kato M, Kaida Y, Hashimoto D, Inui N, Hamada E, Miyazaki O, Kurashita S. Plasma CCN2 (connective tissue growth factor; CTGF) is a potential biomarker in idiopathic pulmonary fibrosis (IPF). Clin Chim Acta 2011;412:2211–5 [DOI] [PubMed] [Google Scholar]
- 35. Koshman YE, Sternlicht MD, Kim T, O’Hara CP, Koczor CA, Lewis W, Seeley TW, Lipson KE, Samarel AM. Connective tissue growth factor regulates cardiac function and tissue remodeling in a mouse model of dilated cardiomyopathy. J Mol Cell Cardiol 2015;89:214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perbal B. CCN proteins: A centralized communication network. J Cell Commun Signal 2013;7:169–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett 1993;327:125–30 [DOI] [PubMed] [Google Scholar]
- 38. de Winter P, Leoni P, Abraham D. Connective tissue growth factor: Structure-function relationships of a mosaic, multifunctional protein. Growth Factors 2008;26:80–91 [DOI] [PubMed] [Google Scholar]
- 39. Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem 2002;277:36288–95 [DOI] [PubMed] [Google Scholar]
- 40. Gu J, Liu X, Wang Q, Tan H, Guo M, Jiang W, Zhou L. Angiotensin II increases CTGF expression via MAPKs/TGF-β1/TRAF6 pathway in atrial fibroblasts. Exp Cell Res 2012;318:2105–15 [DOI] [PubMed] [Google Scholar]
- 41. Kubota S, Takigawa M. The CCN family acting throughout the body: recent research developments. Biomol Concepts 2013;4:477–94 [DOI] [PubMed] [Google Scholar]
- 42. Laug R, Fehrholz M, Schütze N, Kramer BW, Krump-Konvalinkova V, Speer CP, Kunzmann S. IFN-γ and TNF-α synergize to inhibit CTGF expression in human lung endothelial cells. PLoS ONE 2012;7:e45430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mendes FA, Coelho Aguiar JM, Kahn SA, Reis AH, Dubois LG, Romão LF, Ferreira LS, Chneiweiss H, Moura Neto V, Abreu JG. Connective-tissue growth factor (CTGF/CCN2) induces astrogenesis and fibronectin expression of embryonic neural cells in vitro. PLoS ONE 2015;10:e0133689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cho Y, Silverstein R, Geisinger MT, Martinkovich S, Corkill H, Cunnick JM, Planey SL, Arnott JA. AFAP1 is a novel downstream mediator of TGF-β1 for CCN2 induction in osteoblasts. PLoS ONE 2015;10:e0136712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xue X, Chen Q, Zhao G, Zhao JY, Duan Z, Zheng PS. The overexpression of TGF-β and CCN2 in intrauterine adhesions involves the NF-κB signaling pathway. PLoS ONE 2015;10:e0146159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts AB. TGF-β signaling from receptors to the nucleus. Microbes Infect 1999;1:1265–73 [DOI] [PubMed] [Google Scholar]
- 47. Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol 2002;4:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Z, Zhang N, Chu HY, Yu Y, Zhang ZK, Zhang G, Zhang BT. Connective tissue growth factor: from molecular understandings to drug discovery. Front Cell Dev Biol 2020;8:593269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holmes A, Abraham DJ, Sa S, Xu S, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 2001;276:10594–601 [DOI] [PubMed] [Google Scholar]
- 50. Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003;113:685–700 [DOI] [PubMed] [Google Scholar]
- 51. Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol 2002;191:1–16 [DOI] [PubMed] [Google Scholar]
- 52. Luo K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol 2017;9:a022137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li J, Du S, Sheng X, Liu J, Cen B, Huang F, He Y. MicroRNA-29b inhibits endometrial fibrosis by regulating the Sp1-TGF-β1/Smad-CTGF axis in a rat model. Reprod Sci 2016;23:386–94 [DOI] [PubMed] [Google Scholar]
- 54. Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 2014;58:13–9 [DOI] [PubMed] [Google Scholar]
- 55. Chung SA, Jeon BK, Choi YH, Back KO, Lee JB, Kook KH. Pirfenidone attenuates the IL-1β–induced hyaluronic acid increase in orbital fibroblasts from patients with thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci 2014;55:2276–83 [DOI] [PubMed] [Google Scholar]
- 56. Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol-Cell Physiol 2013;304:C216–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun YW, Zhang YY, Ke XJ, Wu XJ, Chen ZF, Chi P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur J Pharmacol 2018;822:199–206 [DOI] [PubMed] [Google Scholar]
- 58. Cheng JC, Chang HM, Fang L, Sun YP, Leung PC. TGF-β1 Up-regulates connective tissue growth factor expression in human granulosa cells through Smad and ERK1/2 Signaling Pathways. PLoS ONE 2015;10:e0126532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ding ZY, Jin GN, Liang HF, Wang W, Chen WX, Datta PK, Zhang MZ, Zhang B, Chen XP. Transforming growth factor β induces expression of connective tissue growth factor in hepatic progenitor cells through Smad independent signaling. Cell Signal 2013;25:1981–92 [DOI] [PubMed] [Google Scholar]
- 60. O’Donovan HC, Hickey F, Brazil DP, Kavanagh DH, Oliver N, Martin F, Godson C, Crean J. Connective tissue growth factor antagonizes transforming growth factor-β1/Smad signalling in renal mesangial cells. Biochem J 2012;441:499–510 [DOI] [PubMed] [Google Scholar]
- 61. Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J 2001;20:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pervan CL. Smad-independent TGF-β2 signaling pathways in human trabecular meshwork cells. Exp Eye Res 2017;158:137–45 [DOI] [PubMed] [Google Scholar]
- 63. Zhang YE. Non-Smad signaling pathways of the TGF-β family. Cold Spring Harb Perspect Biol 2017;9:a022129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen X, Xiao W, Wang W, Luo L, Ye S, Liu Y. The complex interplay between ERK1/2, TGFβ/Smad, and Jagged/Notch signaling pathways in the regulation of epithelial-mesenchymal transition in retinal pigment epithelium cells. PLoS ONE 2014;9:e96365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuchshofer R, Welge-Lussen U, Lütjen-Drecoll E. The effect of TGF-β2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res 2003;77:757–65 [DOI] [PubMed] [Google Scholar]
- 66. Ou SC, Bai KJ, Cheng WH, Chen JY, Lin CH, Wen HC, Chen BC. TGF-β induced CTGF expression in human lung epithelial cells through ERK, ADAM17, RSK1, and C/EBPβ pathways. Int J Mol Sci 2020;21:9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Song ZM, Liu F, Chen YM, Liu YJ, Wang XD, Du SY. CTGF-mediated ERK signaling pathway influences the inflammatory factors and intestinal flora in ulcerative colitis. Biomed Pharmacother 2019;111:1429–37 [DOI] [PubMed] [Google Scholar]
- 68. Gauthier AC, Liu J. Epigenetics and signaling pathways in glaucoma. Biomed Res Int. 2017;2017: 5712341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 2001;107:1049–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sorrentino A, Thakur N, Grimsby S, Marcusson A, Von Bulow V, Schuster N, Zhang S, Heldin CH, Landström M. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 2008;10:1199–207 [DOI] [PubMed] [Google Scholar]
- 71. Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol 2010;42:585–9 [DOI] [PubMed] [Google Scholar]
- 72. Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res 2012;347:11–20 [DOI] [PubMed] [Google Scholar]
- 73. Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates smad-independent activation of JNK and p38 by TGF-β. Mol Cell 2008;31:918–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Nagai N, Klimava A, Lee WH, Izumi-Nagai K, Handa JT. CTGF is increased in basal deposits and regulates matrix production through the ERK (p42/p44mapk) MAPK and the p38 MAPK signaling pathways. Invest Ophthalmol Vis Sci 2009;50:1903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cao YL, Duan Y, Zhu LX, Zhan YN, Min SX, Jin AM. TGF-β1, in association with the increased expression of connective tissue growth factor, induce the hypertrophy of the ligamentum flavum through the p38. Int J Mol Med 2016;38:391–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee YS, Kim JA, Kim KL, Jang HS, Kim JM, Lee JY, Shin IS, Lee JS, Suh W, Choi JH, Jeon ES, Byun J, Kim DK. Aldosterone upregulates connective tissue growth factor gene expression via p38 MAPK pathway and mineralocorticoid receptor in ventricular myocytes. J Korean Med Sci 2004;19:805–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Inoue-Mochita M, Inoue T, Fujimoto T, Kameda T, Awai-Kasaoka N, Ohtsu N, Kimoto K, Tanihara H. P38 MAP kinase inhibitor suppresses transforming growth factor-β2- induced type 1 collagen production in trabecular meshwork cells. Plos One 2015;10:e0120774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grynberg K, Ma FY, Nikolic-Paterson DJ. The JNK signaling pathway in renal fibrosis. Front Physiol 2017;8:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lin C-H, Shih C-H, Lin Y-C, Yang Y-L, Chen B-C. MEKK1, JNK, and SMAD3 mediate CXCL12-stimulated connective tissue growth factor expression in human lung fibroblasts. J Biomed Sci 2018;25:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hou T-Y, Wu S-B, Kau H-C, Tsai C-C. JNK and p38 inhibitors prevent transforming growth factor-β1-induced myofibroblasts transdifferentiation in Graves’ orbital fibroblasts. Int J Mol Sci 2021;22:2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee HS, Hua HS, Wang CH, Yu MC, Chen BC, Lin CH. Mycobacterium tuberculosis induces connective tissue growth factor expression through the TLR2-JNK-AP-1 pathway in human lung fibroblasts. FASEB J 2019;33:12554–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell 2001;12:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J Biol Chem 2001;276:46707–13 [DOI] [PubMed] [Google Scholar]
- 84. Nagai Y, Kawanami D, Matoba K, Takeda Y, Akamine T, Ishizawa S, Kanazawa Y, Yokota T, Utsunomiya K. Rho-Kinase induces CTGF expression through actin dynamics in mesangial cells. Diabetes 2018;67:4989 [Google Scholar]
- 85. Zhu J, Nguyen D, Ouyang H, Zhang XH, Chen XM, Zhang K. Inhibition of RhoA/Rho-kinase pathway suppresses the expression of extracellular matrix induced by CTGF or TGF-β in ARPE-19. Int J Ophthalmol 2013;6:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kular L, Pakradouni J, Kitabgi P, Laurent M, Martinerie C. The CCN family: a new class of inflammation modulators. Biochimie 2011;93:377–88 [DOI] [PubMed] [Google Scholar]
- 87. Matika CA, Wasilewski M, Arnott JA, Planey SL. Antiproliferative factor regulates connective tissue growth factor (CTGF/CCN2) expression in T24 bladder carcinoma cells. Mol Biol Cell 2012;23:1976–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bai KJ, Chen BC, Pai HC, Weng CM, Yu CC, Hsu MJ, Yu MC, Ma HP, Wu CH, Hong CY, Kuo ML, Lin CH. Thrombin-induced CCN2 expression in human lung fibroblasts requires the c-Src/JAK2/STAT3 pathway. J Leukoc Biol 2011;93:101–12 [DOI] [PubMed] [Google Scholar]
- 89. Vial C, Gutiérrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with connective tissue growth factor (CTGF)/CCN2 by LRR12 inhibiting its biological activity. J Biol Chem 2011;286:24242–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol 2006;146:362–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ponticos M, Holmes AM, Shi-wen X, Leoni P, Khan K, Rajkumar VS, Hoyles RK, Bou-Gharios G, Black CM, Denton CP, Abraham DJ, Leask A, Lindahl GE. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum 2009;60:2142–55 [DOI] [PubMed] [Google Scholar]
- 92. Valle-Tenney R, Rebolledo DL, Lipson KE, Brandan E. Role of hypoxia in skeletal muscle fibrosis: Synergism between hypoxia and TGF-β signaling upregulates CCN2/CTGF expression specifically in muscle fibers. Matrix Biol 2020;87:48–65 [DOI] [PubMed] [Google Scholar]
- 93. Chudgar SM, Deng P, Maddala R, Epstein DL, Rao PV. Regulation of connective tissue growth factor expression in the aqueous humor outflow pathway. Mol Vis 2006;12:1117–26 [PubMed] [Google Scholar]
- 94. Furumatsu T, Kanazawa T, Miyake Y, Kubota S, Takigawa M, Ozaki T. Mechanical stretch increases Smad3-dependent CCN2 expression in inner meniscus cells. J Orthop Res 2012;30:1738–45 [DOI] [PubMed] [Google Scholar]
- 95. Honjo T, Kubota S, Kamioka H, Sugawara Y, Ishihara Y, Yamashiro T, Takigawa M, Takano-Yamamoto T. Promotion of Ccn2 expression and osteoblastic differentiation by actin polymerization, which is induced by laminar fluid flow stress. J Cell Commun Signal 2012;6:225–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol 2000;11:25–38 [DOI] [PubMed] [Google Scholar]
- 97. Vivar R, Anfossi R, Humeres C, Catalán M, Reyes C, Cárdenas S, Conteras A, Aránguiz P, González F, Diaz-Araya G. FoxO1 is required for high glucose-dependent cardiac fibroblasts into myofibroblast phenoconversion. Cell Signal 2021;83:109978. [DOI] [PubMed] [Google Scholar]
- 98. Shao J, Xu H, Wu X, Xu Y. Epigenetic activation of CTGF transcription by high glucose in renal tubular epithelial cells is mediated by myocardin-related transcription factor A. Cell Tissue Res 2020;379:549–59 [DOI] [PubMed] [Google Scholar]
- 99. Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol 2009;297:C1490–500 [DOI] [PubMed] [Google Scholar]
- 100. Yan L, Chaqour B. Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of ocular neovascular and fibrovascular disease therapy. J Cell Commun Signal 2013;7:253–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Riquelme-Guzmán C, Contreras O, Brandan E. Expression of CTGF/CCN2 in response to LPA is stimulated by fibrotic extracellular matrix via the integrin/FAK axis. Am J Physiol-Cell Physiol 2018;314:C415–27 [DOI] [PubMed] [Google Scholar]
- 102. Nathan S, Zhang H, Andreoli M, Leopold PL, Crystal RG. CREB-dependent LPA-induced signaling initiates a pro-fibrotic feedback loop between small airway basal cells and fibroblasts. Respir Res 2021;22:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Córdova-Casanova A, Cruz-Soca M, Chun J, Casar JC, Brandan E. Activation of the ATX/LPA/LPARs axis induces a fibrotic response in skeletal muscle. Matrix Biol 2022;109:121–39 [DOI] [PubMed] [Google Scholar]
- 104. Lin J, Liliensiek B, Kanitz M, Schimanski U, Böhrer H, Waldherr R, Martin E, Kauffmann G, Ziegler R, Nawroth PP. Molecular cloning of genes differentially regulated by TNF-α in bovine aortic endothelial cells, fibroblasts and smooth muscle cells. Cardiovasc Res 1998;38:802–13 [DOI] [PubMed] [Google Scholar]
- 105. Ni Y, Zhang H, Li Z, Li Z. Connective tissue growth factor (CCN2) inhibits TNF-α-induced apoptosis by enhancing autophagy through the Akt and Erk pathways in osteoblasts. Pharmazie 2020;75:213–7 [DOI] [PubMed] [Google Scholar]
- 106. Elliott CG, Forbes TL, Leask A, Hamilton DW. Inflammatory microenvironment and tumor necrosis factor alpha as modulators of periostin and CCN2 expression in human non-healing skin wounds and dermal fibroblasts. Matrix Biol 2015;43:71–84 [DOI] [PubMed] [Google Scholar]
- 107. Laug R, Fehrholz M, Schütze N, Kramer BW, Krump-Konvalinkova V, Speer CP, Kunzmann S. IFN-c and TNF-a synergize to inhibit CTGF expression in human lung endothelial cells. PLoS ONE 2012;7:e45430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Keil A, Blom IE, Goldschmeding R, Rupprecht HD. Nitric oxide down-regulates connective tissue growth factor in rat mesangial cells. Kidney Int 2002;62:401–11 [DOI] [PubMed] [Google Scholar]
- 109. Tran CM, Fujita N, Huang BL, Ong JR, Lyon LM, Shapiro IM, Risbud MV. Hypoxia- inducible factor (HIF)-1α and CCN2 form a regulatory circuit in hypoxic nucleus pulposus cells: CCN2 suppresses HIF-1α level and transcriptional activity. J Biol Chem 2013;288:12654–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005;122:421–34 [DOI] [PubMed] [Google Scholar]
- 111. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 2007;17:2054–60 [DOI] [PubMed] [Google Scholar]
- 112. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev 2014;94:1287–312 [DOI] [PubMed] [Google Scholar]
- 113. Li H, Raghunathan V, Stamer WD, Ganapathy PS, Herberg S. Extracellular matrix stiffness and TGFβ2 regulate YAP/TAZ activity in human trabecular meshwork cells. Front Cell Dev Biol 2022;10:844342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Noguchi S, Saito A, Nagase T. YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci 2018;19:3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 2011;10:945–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gao R, Brigstock DR. Connective tissue growth factor (CCN2) induces adhesion of rat activated hepatic stellate cells by binding of its C-terminal domain to integrin αvβ3 and heparan sulfate proteoglycan. J Biol Chem 2004;279:8848–55 [DOI] [PubMed] [Google Scholar]
- 117. Gao R, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin α5β1 as a novel CCN2 receptor. Gastroenterology 2005;129:1019–30 [DOI] [PubMed] [Google Scholar]
- 118. Blalock TD, Gibson DJ, Duncan MR, Tuli SS, Grotendorst GR, Schultz GS. A connective tissue growth factor signaling receptor in corneal fibroblasts. Invest Ophthalmol Vis Sci 2012;53:3387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pi L, Shenoy AK, Liu J, Kim S, Nelson N, Xia H, Hauswirth WW, Petersen BE, Schultz GS, Scott EW. CCN2/CTGF regulates neovessel formation via targeting structurally conserved cystine knot motifs in multiple angiogenic regulators. FASEB J 2012;26:3365–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nishida T, Kubota S, Aoyama E, Janune D, Maeda A, Takigawa M. Effect of CCN2 on FGF2-induced proliferation and MMP9 and MMP13 productions by chondrocytes. Endocrinology 2011;152:4232–41 [DOI] [PubMed] [Google Scholar]
- 121. Yoshida K, Munakata H. Connective tissue growth factor binds to fibronectin through the type I repeat modules and enhances the affinity of fibronectin to fibrin. Biochim Biophys Acta 2007;1770:672–80 [DOI] [PubMed] [Google Scholar]
- 122. Vial C, Zúñiga LM, Cabello-Verrugio C, Cañón P, Fadic R, Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol 2008;215:410–21 [DOI] [PubMed] [Google Scholar]
- 123. van Setten GB, Blalock TD, Grotendorst G, Schultz GS. Detection of connective tissue growth factor in human aqueous humor. Ophthalmic Res 2002;34:306–8 [DOI] [PubMed] [Google Scholar]
- 124. Gibson DJ, Pi L, Sriram S, Mao C, Peterson BE, Scott EW, Leask A, Schultz GS. Conditional knockout of CTGF affects corneal wound healing. Invest Ophthalmol Vis Sci 2014;55:2062–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ollivier FJ, Brooks DE, Schultz GS, Blalock TD, Andrew SE, Komaromy AM, Cutler TJ, Lassaline ME, Kallberg ME, Van Setten GB. Connective tissue growth factor in tear film of the horse: Detection, identification and origin. Graefes Arch Clin Exp Ophthalmol 2004;242:165–71 [DOI] [PubMed] [Google Scholar]
- 126. Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Zheli. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-β2, elucidated by treatment with CTGFsiRNA. Acta Ophthalmol 2010;88:652–9 [DOI] [PubMed] [Google Scholar]
- 127. van Setten GB, Trost A, Schrödl F, Kaser-Eichberger A, Bogner B, van Setten M, Heindl LM, Grabner G, Reitsamer HA. Immunohistochemical detection of CTGF in the human eye. Curr Eye Res 2016;41:1571–9 [DOI] [PubMed] [Google Scholar]
- 128. van Setten GB, Blaloc TD, Grotendorst G, Schultz GS. Detection of connective tissue growth factor (CTGF) in human tear fluid: preliminary results. Acta Ophthalmol Scand 2003;81:51–3 [DOI] [PubMed] [Google Scholar]
- 129. Browne JG, Ho SL, Kane R, Oliver N, Clark AF, O’Brien CJ, Crean JK. Connective tissue growth factor is increased in pseudoexfoliation glaucoma. Investig Ophthalmol Vis Sci 2011;52:3660–6 [DOI] [PubMed] [Google Scholar]
- 130. Daftarian N, Baigy O, Suri F, Kanavi MR, Balagholi S, Aski SA, Moghaddasi A, Nourini R, Abtahi SH, Ahmadieh H. Intravitreal connective tissue growth factor neutralizing antibody or bevacizumab alone or in combination for prevention of proliferative vitreoretinopathy in an experimental model. Exp Eye Res 2021;208:108622. [DOI] [PubMed] [Google Scholar]
- 131. Esson DW, Neelakantan A, Iyer SA, Blalock TD, Balasubramanian L, Grotendorst GR, Schultz GS, Sherwood MB. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest Ophthalmol Vis Sci 2004;45:485–91 [DOI] [PubMed] [Google Scholar]
- 132. Huang Y, Qian C, Zhou J, Xue J. Investigation of expression and influence of CTGF and HO-1 in rats with diabetic retinopathy. Exp Ther Med 2020;19:2291–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Khaw PT, Bouremel Y, Brocchini S, Henein C. The control of conjunctival fibrosis as a paradigm for the prevention of ocular fibrosis-related blindness. “Fibrosis has many friends”. Eye 2020;34:2163–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tsai CC, Wu SB, Chang PC, Wei YH. Alteration of connective tissue growth factor (CTGF) expression in orbital fibroblasts from patients with Graves’ ophthalmopathy. PLoS ONE 2015;10:e0143514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. He S, Jin ML, Worpel V, Hinton DR. A role for connective tissue growth factor in the pathogenesis of choroidal neovascularization. Arch Ophthalmol 2003;121:1283–8 [DOI] [PubMed] [Google Scholar]
- 136. Kuiper EJ, Witmer AN, Klaassen I, Oliver N, Goldschmeding R, Schlingemann RO. Differential expression of connective tissue growth factor in microglia and pericytes in the human diabetic retina. Br J Ophthalmol 2004;88:1082–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Michael B, Laura D, Milena C, Fatimah P, Cordeiro MF. Glaucoma: the retina and beyond. Acta Neuropathol 2016;132:807–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311:1901–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ahmad SS, Abdul Ghani S, Singh D, Wah LP. The dynamics of aqueous humor outflow—a major review. US Opthalmic Rev 2014;7:137–42 [Google Scholar]
- 140. Hann CR, Springett MJ, Wang X, Johnson DH. Ultrastructural localization of collagen IV, fibronectin, and laminin in the trabecular meshwork of normal and glaucomatous eyes. Ophthalmic Res 2001;33:314–24 [DOI] [PubMed] [Google Scholar]
- 141. Lütjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res 1999;18:91–119 [DOI] [PubMed] [Google Scholar]
- 142. Tektas OY, Hammer CM, Danias J, Candia O, Gerometta R, Podos SM, Lütjen-Drecoll E. Morphologic changes in the outflow pathways of bovine eyes treated with corticosteroids. Invest Ophthalmol Vis Sci 2010;51:4060–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Tian B, Gabelt BT, Geiger B, Kaufman PL. The role of the actomyosin system in regulating trabecular fluid outflow. Exp Eye Res 2009;88:713–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-β2 levels in aqueous humor of glaucomatous eyes. Graefe’s Arch Clin Exp Ophthalmol 2001;239:109–13 [DOI] [PubMed] [Google Scholar]
- 145. Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res 2009;88:683–8 [DOI] [PubMed] [Google Scholar]
- 146. Nakamura Y, Hirano S, Suzuki K, Seki K, Sagara T, Nishida T. Signaling mechanism of TGF-β1–induced collagen contraction mediated by bovine trabecular meshwork cells. Invest Ophthalmol Vis Sci 2002;43:3465–72 [PubMed] [Google Scholar]
- 147. Fuchshofer R, Yu AHL, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-β2 in human trabecular meshwork cells. Investig Ophthalmol Vis Sci 2007;48:715–26 [DOI] [PubMed] [Google Scholar]
- 148. Kazemi A, McLaren JW, Trese MGJ, Toris CB, Gulati V, Fan S, Reed DM, Kristoff T, Gilbert J, Moroi SE, Sit AJ. Effect of timolol on aqueous humor outflow facility in healthy human eyes. Am J Ophthalmol 2019;202:126–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, Wormald R, Dickersin K. Comparative effectiveness of first-line medications for primary open-angle glaucoma: A systematic review and network meta-analysis. Opthalmology 2016;123:129–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cheng JW, Cheng SW, Gao LD, Lu GC, Wei RL. Intraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: a systematic review and meta-analysis. PLoS ONE 2012;7:e45079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tejwani S, Machiraju P, Nair AP, Ghosh A, Das RK, Ghosh A, Sethu S. Treatment of glaucoma by prostaglandin agonists and beta-blockers in combination directly reduces pro-fibrotic gene expression in trabecular meshwork. J Cell Mol Med 2020;24:5195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rao PV, Pattabiraman PP, Kopczynski C. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Exp Eye Res 2017;158:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Thumkeo D, Watanabe S, Narumiya S. Physiological roles of rho and rho effectors in mammals. Eur J Cell Biol 2013;92:303–15 [DOI] [PubMed] [Google Scholar]
- 154. Garnock-Jones KP. Ripasudil: First global approval. Drugs 2014;74:2211–5 [DOI] [PubMed] [Google Scholar]
- 155. Hoy SM. Netarsudil ophthalmic solution 0.02%: first global approval. Drugs 2018;78:389–96 [DOI] [PubMed] [Google Scholar]
- 156. Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of Rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci 2001;42:137–44 [PubMed] [Google Scholar]
- 157. Inoue T, Tanihara H. Rho-associated kinase inhibitors: a novel glaucoma therapy. Prog Retin Eye Res 2013;37:1–12 [DOI] [PubMed] [Google Scholar]
- 158. Rao VP, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs 2007;21:167–77. [DOI] [PubMed] [Google Scholar]
- 159. Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol–Lung Cell Mol Physiol 2015;308:L344–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am J Physiol Cell Physiol 2008;295:C1057–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence-and target-independent angiogenesis suppression by siRNA via TLR3. Nature 2008;452:591–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Guzman-Aranguez A, Loma P, Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br J Ophthalmol 2013;170:730–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Keaney J, Campbell M, Humphries P. From RNA interference technology to effective therapy: how far have we come and how far to go? Ther Deliv 2011;2:1395–406 [DOI] [PubMed] [Google Scholar]
- 164. Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, Albuquerque RJ, Hirano Y, Terasaki H, Kondo M, Fujita T, Ambati BK, Tarallo V, Gelfand BD, Bogdanovich S, Baffi JZ, Ambati J. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther 2012;20:101–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gaglione M, Messere A. Recent progress in chemically modified siRNAs. Mini Rev Med Chem 2010;10:578–95 [DOI] [PubMed] [Google Scholar]
- 166. Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther 2008;16:107–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cai B, Wu J, Yu X, Su XZ, Wang RF. FOSL1 inhibits type I interferon responses to malaria and viral infections by blocking TBK1 and TRAF3/ TRIF interactions. Mbio 2017;8:e02161–10216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bösl M, Bosserhoff A, Köstler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol 2012;180:2386–403 [DOI] [PubMed] [Google Scholar]
- 169. Dillinger AE, Guter M, Froemel F, Weber GR, Perkumas K, Stamer WD, Ohlmann A, Fuchshofer R, Breunig M. Intracameral delivery of layer-by-layer coated siRNA nanoparticles for glaucoma therapy. Small 2018;14:e1803239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Brenner MC, Krzyzanski W, Chou JZ, Signore PE, Fung CK, Guzman D, Li D, Zhang W, Olsen DR, Nguyen VT, Koo CW, Sternlicht MD, Lipson KE. FG-3019, a human monoclonal antibody recognizing connective tissue growth factor, is subject to target-mediated drug disposition. Pharm Res 2016;33:1833–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zajac M, Gomez G, Benitez J, Martínez-Delgado B. Molecular signature of response and potential pathways related to resistance to the HSP90 inhibitor, 17AAG, in breast cancer. BMC Med Genomics 2010;3:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Sgalla G, Franciosa C, Simonetti J, Richeldi L. Pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Expert Opin Investig Drugs 2020;29:771–7 [DOI] [PubMed] [Google Scholar]
- 173. Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, Li D, Neff TB, Urquilla PR, Sewell KL. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol 2010;5:1420–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet 2013;22:4938–51 [DOI] [PubMed] [Google Scholar]
- 175. Van Ruiten H, Bushby K, Guglieri M. State-of-the-art advances in Duchenne muscular dystrophy. Eur Med J 2017;2:90–9 [Google Scholar]
- 176. Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010;49:493–507 [DOI] [PubMed] [Google Scholar]
- 177. Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther 2003;3:45–56 [DOI] [PubMed] [Google Scholar]