Abstract
Pre-eclampsia (PE) is a severe pregnancy complication characterized by impaired trophoblast invasion and spiral artery remodeling and can have serious consequences for both mother and child. Protein phosphatase 1 regulatory subunit 3G (PPP1R3G) is involved in numerous tumor-related biological processes. However, the biological action and underlying mechanisms of PPP1R3G in PE progression remain unclear. We used western blotting and immunohistochemistry to investigate PPP1R3G expression in gestational age-matched pre-eclamptic and normal placental tissues. After lentivirus transfection, wound-healing, Transwell, cell-counting kit-8 (CCK-8), 5-ethynyl-2′-deoxyuridine (EdU), and TdT mediateddUTP Nick End Labeling (TUNEL) assays were used to assess trophoblast migration, invasion, proliferation, and apoptosis, respectively. The relative expression levels of PPP1R3G and the proteins involved in the Akt signaling pathway were determined using western blotting. The results showed that PPP1R3G levels were significantly lower in the placental tissues and GSE74341 microarray of the PE group than those of the healthy control group. We also found that neonatal weight and Apgar score were lower at birth, and peak systolic blood pressure and diastolic blood pressure were higher in the PE group than in the non-PE group. In addition, PPP1R3G knockdown decreased p-Akt/Akt expression and inhibited migration, invasion, and proliferation in HTR-8/SVneo trophoblasts but had no discernible effect on cell apoptosis. Furthermore, PPP1R3G positively regulated matrix metallopeptidase 9 (MMP-9), which was downregulated in placental tissues of pregnant women with PE. These results provided the first evidence that the reduced levels of PPP1R3G might contribute to PE by suppressing the invasion and migration of trophoblasts and targeting the Akt/MMP-9 signaling pathway.
Keywords: PPP1R3G, PE, trophoblast invasion, MMP-9
Impact Statement
Pre-eclampsia (PE) is a severe pregnancy complication characterized by impaired trophoblast invasion and spiral artery remodeling and can have serious consequences for both mother and child. Protein phosphatase 1 regulatory subunit 3G (PPP1R3G) in PE progression remain unclear. In this study, we find that PPP1R3G levels were significantly lower in the placental tissues and GSE74341 microarray of the PE group than those of the healthy control group. We also found that neonatal weight and Apgar score were lower at birth, and peak systolic blood pressure and diastolic blood pressure were higher in the PE group than in the non-PE group. In addition, PPP1R3G knockdown decreased p-Akt/Akt expression and inhibited migration, invasion, and proliferation in human trophoblast research-8 (HTR-8)/SVneo trophoblasts. Furthermore, PPP1R3G positively regulated matrix metallopeptidase 9 (MMP-9), which was downregulated in placental tissues of pregnant women with PE. Our findings provided the first evidence that the reduced levels of PPP1R3G might contribute to PE by suppressing the invasion and migration of trophoblasts and targeting the Akt/MMP-9 signaling pathway.
Introduction
PE, a complication of hypertensive disorders of pregnancy, is characterized by the development of proteinuria and hypertension after the 20th week of gestation, with or without multivisceral organ failure.1,2 PE affects 3–5% of pregnant women worldwide and is a significant risk factor for long-term conditions such as cardiovascular events, metabolic disorders, hypercholesterolemia, and renal problems.3,4 PE, the second leading obstetric cause of perinatal death worldwide, has surpassed postpartum infections and hemorrhage as the leading causes of maternal mortality. 5 Therefore, antenatal care and the early detection of PE are critical to maternal and neonatal health.
Serine/threonine protein phosphatase 1 (PP1) holoenzyme is fundamental in regulating multiple cellular functions, such as cell cycle, adhesion, migration, and apoptosis.6–9 PPP1R3 family members act as PP1 regulatory subunits to modulate lipid and glycogen metabolism, and also are involved in cancer progression. 10 PPP1R3G, a member of the PPP1R3 family, is implicated in multiple cellular biological processes, such as cellular proliferation, migration, apoptosis, and necroptosis.8,11,12 The Human Protein Atlas database confirmed the expression of PPP1R3G in the placenta at the gene and protein level. However, the role of PPP1R3G in PE has not yet been explored.
The trophoblast migration and invasion into the maternal decidual and penetrance to the uterine spiral artery wall are essential for normal pregnancy. 13 Impaired trophoblast migration and invasion have been recognized to be the major etiology of PE.14,15 However, the molecular mechanisms underlying the dysregulated trophoblast function remain obscured, and the etiology of PE still needs to be unveiled. Gene expression data from Gene Expression Omnibus (GEO) demonstrated PPP1R3G downregulation in PE. Our preliminary experiment confirmed the downregulation of PPP1R3G mRNA and protein expression in PE placental tissues as compared with that of normal pregnancy. Currently, we tried to explore the functional role of PPP1R3G in the regulation of trophoblast proliferation, migration, and invasion. A previous study demonstrated that PPP1R3G is a functional partner of Akt and may be crucial for Akt phosphorylation. 10 Coincidentally, the Akt signaling pathway has been discovered to be pivotal for maintaining the biological activities of trophoblasts, including cell migration, invasion, proliferation, and hyperplasia. 16 In addition, the loss of Akt activity has been implicated in the development of PE. 17 Studies have reported that the Akt/MMP-9 signaling pathway is involved in invasion of trophoblast cells.18–20 Therefore, we speculated that the Akt/MMP-9 signaling pathway mediated by PPP1R3G may contribute to the development of PE.
In this article, we sought to investigate the underlying role of PPP1R3G in the cellular biology of trophoblast and the potential molecular mechanisms. The results demonstrated that PPP1R3G regulated trophoblast cell invasion, migration, and proliferation by regulating the Akt/MMP-9 signaling pathway. PPP1R3G downregulation in placenta might play a role in contributing to the development of PE.
Materials and methods
Placental specimens and data collection
A total of 30 PE patients and 30 age-matched healthy women with a normal singleton pregnancy during the same period were enrolled. The non-PE pregnancies had normal blood pressure, no proteinuria, and no medical or obstetric complications. Both groups of pregnant women underwent elective cesarean section. All patients were informed of the aims and details of this trial, and all signed an informed consent form after the study protocol was approved by the Ethics Committee of Xuzhou Cancer hospital (approval no. 2022-02-016-K01). PE was strictly diagnosed according to the 23rd edition of Williams Obstetrics’ definition: 21 (1) a systolic pressure (SBP) of 140 mmHg and/or a diastolic pressure (DBP) of 90 mmHg, from two recordings at ⩾20 weeks’ gestation without a history of pre-existing hypertension; (2) at least 1+ for proteinuria measurement or more than 300 mg of urinary protein in 24 h. After delivery of the placenta, nonfibrotic, noncalcified, and non-necrotic tissues (each 3 × 3 cm2 from the central maternal surface) were removed. These tissues were then separated into five tubes, briefly flash-frozen in liquid nitrogen, and maintained at −80°C until needed, after being rinsed in phosphate-buffered saline (PBS) to remove maternal blood. To prepare the placental tissues for subsequent procedures, they were immersed in 10% formaldehyde overnight. Baseline characteristics of all recruited subjects were listed in Table 1.
Table 1.
Clinical characteristics of the patients with normal (control) and PE pregnancies.
| Variable | PE (N = 30) | Control (N = 30) | p value (PE versus Control) |
|---|---|---|---|
| Maternal age (years) | 31.13 ± 0.89 | 30.37 ± 0.82 | >0.05 |
| Maternal weight (kg) | 77.77 ± 1.32 | 73.48 ± 1.74 | >0.05 |
| Systolic blood pressure (mmHg) | 154.90 ± 1.39 | 118.80 ± 2.00 | <0.0001 |
| Diastolic blood pressure (mmHg) | 100.20 ± 1.34 | 77.77 ± 1.35 | <0.0001 |
| Proteinuria (g/day) | 0.395 (0.27) | 0.21 ± 0.01 | <0.0001 |
| Neonatal birth weight (g) | 2952 ± 72.87 | 3407 ± 56.63 | <0.0001 |
| Apgar score | 9 (2) | 10 (0) | <0.001 |
| Neonatal weight z score | −0.24 ± 1.00 | −1.65 ± 0.99 | 0.05 |
PE: pre-eclampsia.
Acquisition of gene expression data
To investigate the dysfunctional genes in PE, the GEO (ncbi.nlm.nih.gov/geo/) gene expression microarray data of PE and normal full-term placental tissues were searched and downloaded. The GSE74341 dataset contains placental gene expression profiles from 15 pregnancies with PE and 10 pregnancies without PE as controls.
Cell culture and lentivirus treatment
For the study cohort, HTR-8/SVneo (an immortalized human trophoblast cell line) was purchased from the Zhongqiao Xinzhou Science and Technology Co. (Shanghai, China) and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Vicmed Biotech, Xuzhou, China) supplemented with 1% penicillin-streptomycin (Beyotime, Shanghai, China) and 10% fetal bovine serum (Gibco, Carlsbad, CA) at 37°C, 5% CO2. HTR-8/SVneo cells in logarithmic growth phase were collected (2.0 × 105 cells per well), dispersed in six-well plates, and passaged two to three times per week. Human PPP1R3G knockdown lentivirus (sh-PPP1R3G) and negative control lentivirus (sh-normal control (NC)) were purchased from Jikai Gene Chemical Technology (Shanghai, China). The virus stock was diluted in serum-free RPMI-1640 medium and then used (1.5 μL virus solution; 15 multiplicity of infection/cell) for the HTR-8/SVneo infection. The complete medium was replaced 12–16 h later. After 48 h of transfection, stable integrations were selected with puromycin (2 μg/mL) and harvested for amplification culture.
Quantitative reverse transcription PCR
Gene expression was analyzed by quantitative reverse transcription polymerase chain reaction (RT-qPCR): PPP1R3G (NCBI Accession number: NM_001145115.3), MMP-9 (NCBI Accession number: NM_004994), and β-actin (NM_001101.5) was used as a housekeeping gene. Total cellular ribonucleic acid (RNA) was extracted using TRIzol reagent (Sangon Biotech, Shanghai, China), chloroform, and isopropanol. After concentration and purification, the RNAs were reverse transcribed into complementary deoxyribonucleic acids (DNAs) using a Reverse Transcription Kit (Takara, Kyoto, Japan), and quantitative PCR was performed using the synergy brands Green real-time PCR method (Takara, Kyoto, Japan). Expression changes were calculated using the 2-ΔΔct strategy. The sequences of the PCR primers were as follows: PPP1R3G, forward, 5′-GCGCTACACCTTTACCGAGT-3′ and reverse, 5′-TGGCTCTTTCTTGGCATCCC-3′; MMP-9, forward, 5′-AGACCTGGGCAGATTCCAAAC-3′and reverse, 5′-CGGCAAGTCTTCCGAGTAGT-3′; β-Actin, forward, 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse, 5′-GCTGTCACCTTCACCGTTCC-3′.
Western blotting
Using radio immuno precipitation assay lysis buffer, total proteins from placental tissue samples or HTR-8/SVneo cells were extracted and electrophoresed. Protein samples were transferred to polyvinylidene difluoride (PVDF) membranes and then blocked with 5% skim milk for two hours to prevent nonspecific binding. The primary antibodies listed below were used to incubate the blots: primary antibodies: PPP1R3G (1:1000 dilution, abmart), p-Akt (1:100–1000 dilution, abmart), Akt (1:100–1000 dilution, abmart), MMP-9 (1:100–1000 dilution, santa), Bcl2 (1:500–1000 dilution, proteintech), Bax (1:100–1000 dilution, abmart), and β-actin (1:5000–10000 dilution, abmart) at 4°C overnight, followed by peroxidase-conjugated secondary antibodies. All the antibodies were diluted with (Vicmed Biotech Co., Ltd., Xuzhou, China) antibody dilute solution (VP6022, the generic type). The bands were visualized using the Odyssey CLx Infrared Imaging System(LI-COR Biosciences, Lincoln, NE). Image J software (version 1.8.0_112; Bethesda, MD) was used to analyze the gray value of each band.
Immunohistochemistry
In brief, the placental tissues were cut into 5 μm-thick paraffin sections, which were afterward deparaffinized with xylene and rehydrated in ethanol. Deparaffinized tissue sections were incubated with 10 mM sodium citrate buffer (pH 6.0, Beyotime, Shanghai, China) and heated for 20 min to retrieve antigens followed by cooling to room temperature. Following a 30-min period of blocking and permeabilization in PBS containing 10% Bovine Serum Albumin, sections were incubated with the PPP1R3G primary antibody (1:20–50 dilution, Abcepta) at 4°C overnight. After overnight incubation, horse radish peroxidase-conjugated goat anti-rabbit immunoglobin G (PV-9001, ZSGB-BIO, Beijing, China) and reaction enhancing solution were added dropwise for 20 min at room temperature. After PBS washing, the sections were incubated with diaminobezidin chromogenic solution (ZLI-9018, ZSGB-BIO, Beijing, China) for 5–10 min at room temperature until a tan color development was observed. Following this, the sections were then counterstained with hematoxylin, sealed, and photographed. Images were taken by the Olympus VS120 microscope (Olympus, Tokyo, Japan).
Cell-counting kit-8
To determine the effect of PPP1R3G on trophoblasts, a 96-microwell, flat-bottomed plate was inoculated with 3 × 104 exponentially proliferating HTR-8/SVneo cells per well in 100 µL of culture medium, and 100 mL of RPMI-1640 medium was provided for growth. Following the manufacturer’s recommendations, 10 µL of cell-counting kit-8 (CCK-8) suspension (Sigma, MO, USA) was added to each well after 24 h, and the color was then allowed to turn orange at 37°C away from light for 1–2 h. Absorbance was read at 450 nm two hours later.
Wound-Healing Test
To determine the role of PPP1R3G in the migration of HTR-8/SVneo cells, a horizontal line was drawn evenly on the back of the six-well plate every 0.5–1 cm with a marker bout. HTR-8/SVneo cells (5 × 105 cells per well) were cultured in RPMI-1640 medium for 24 h. The next day, a 10 μL gun head was used to create a vertical scratch which was perpendicular to the horizontal line at the bottom. RPMI-1640 medium was added; washed three times with PBS to remove the floating cells; and photographed at 0, 24, and 48 h.
5-ethynyl-2′-deoxyuridine assay
At a density of 3 × 104 cells per well, cells were seeded on a 48-well plate and stained for two hours at 37°C with 50 μmol/EdU (5-ethynyl-2′-deoxyuridine) (Ribobio, Guangzhou, China) to investigate the impact of PPP1R3G on HTR-8/SVneo cells proliferative ability. The cells were fixed in 4% paraformaldehyde for 30 min, incubated on a decolorizing shaker for 5 min, and then incubated in 0.5% TritonX-100 for 10 min. After washing with PBS, the cells were stained for 30 min in the dark with 1*Apollo® reaction solution (200 μL) to stain the EdU and for 30 min in the dark with 1*Hoechst 33342 (200 μL). Images were captured utilizing fluorescence microscopy Olympus IX73 (Olympus, Tokyo, Japan). EdU-positive cells were calculated in nine randomly different fields per sample, and the EdU-positive ratio was reported as EdU-positive cells/Hoechst-positive cells using Image J software.
Transwell assay
To investigate the involvement of PPP1R3G in HTR-8/SVneo cells invasion, cells were seeded into the upper chamber of a Transwell insert (Costar, Cambridge, USA) in serum-free medium at a density of 40,000 cells/well, and a volume of 0.6 mL complete medium was added to the lower chamber. The noninvasion cells were removed from the membrane surface with a moist cotton swab during a 24-h incubation period (37°C, 5% CO2). The membranes were fixed in methanol for 30 min and stained with crystal violet for 20 min, and the invasive cells were calculated.
TUNEL staining
Cells were cultured in six-well plates and then fixed with 4% formalin for 30 min to examine the effect of PPP1R3G on apoptosis of HTR-8/SVneo cells. Each well was treated with 50 μL TUNEL reaction mixture (meilunbio, Dalian, China) for 60 min and stained with 4′, 6-diamidino-2-phenylindole (DAPI, Beyotime Biotechnology, Shanghai, China) at 37°C without exposure to light. The cells were then washed in PBS and immediately photographed. Images were captured utilizing fluorescence microscopy Olympus IX73 (Olympus, Tokyo, Japan). Image J was used to count the TUNEL-positive cells in five randomly selected areas of the microscope.
Statistical analysis
The data analysis was performed using IBM SPSS Statistics version 19.0 software (IBM, Chicago, IL) and GraphPad Prism® 8.0 software (San Diego, CA, USA). The standard error of the mean (SEM) is used to express all quantitative data, and three replicates were performed for each experiment. Student’s t-test and one-way analysis of variance (ANOVA) (Tukey’s) were used to evaluate statistical differences between two groups. For all tests, the level of statistical significance was set at P < 0.05.
Results
Clinical characteristics of the subjects
The clinical characteristics of the study participants are summarized in Table 1. Maternal age, maternal weight, and neonatal weight z-scores were not significantly different between the PE (PE) and control (NC) groups. In addition, patients in the PE group had higher levels of SBP DBP, and proteinuria comparing with that of the control group. The PE group also had significantly lower neonatal birth weights and Apgar scores than the control group.
PPP1R3G expression is downregulated in PE placental tissues
After analyzing the dysregulated genes of 15 PE versus 10 normal human placental tissues in the GSE74341 database, we found that PPP1R3G expression was significantly downregulated in PE placental tissues compared with that in normal pregnancies and was the lowest in the same subfamily (Figure 1(A)). In addition, RT-qPCR and western blotting (WB) were performed to determine the levels of PPP1R3G mRNA and protein expression in the collected placental samples. PE placental tissues showed significant downregulation of PPP1R3G mRNA and protein expression compared with that in the control group (Figure 1(B) and (C)). Moreover, we further examined PPP1R3G protein levels in placental tissues by immunohistochemistry (IHC), and the results were consistent with those of the western blot experiments (Figure 1(D)).
Figure 1.
PPP1R3G expression is downregulated in human PE placentas. (A) Differentially expressed genes of the PPP1R3 family in PE and normal pregnancy placental tissues in GSE74341. (B) Relative mRNA level of PPP1R3G in PE and normal pregnancy placental tissues collected in our hospital. (C) Representative western blot bands and quantitative analysis of PPP1R3G in PE and normal pregnancy placental tissues. (D) Representative IHC images and quantitative analysis of PPP1R3G in PE and normal pregnancy placental tissues. Bar = 50 μm. Data are expressed as means ± SEM (n = 3), Student’s t-test, **P < 0.01 versus NC group.
PPP1R3G knockdown inhibits trophoblast proliferation
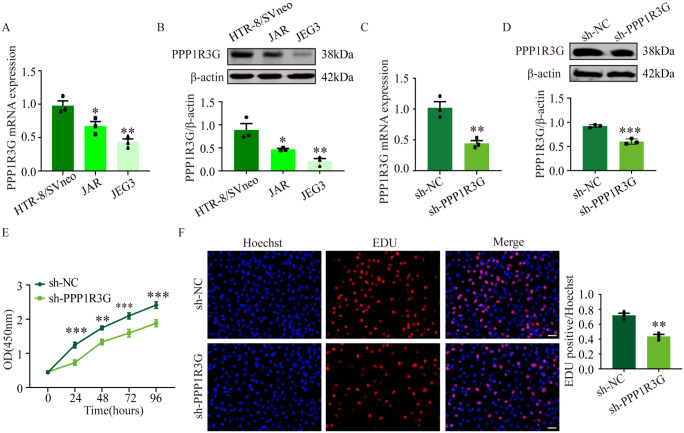
First, the levels of PPP1R3G expression were measured in HTR-8/SVneo, Jusikiniai Eglei Geliu-3 choriocarcinoma cell line, and Jegathesan choriocarcinoma cell line cells. The results showed that PPP1R3G expression was noticeably higher in HTR-8/SVneo cells than in other cell types (Figure 2(A) and (B)). Next, we transfected HTR-8/SVneo cells with an shRNA lentivirus to silence PPP1R3G. The protein expression and mRNA expression of PPP1R3G were significantly decreased in sh-PPP1R3G cells compared with that in sh-NC cells (Figure 2(C) and (D)). In this study, we explored the role of PPP1R3G in trophoblast proliferation. The CCK-8 assay demonstrated that PPP1R3G knockdown significantly reduced trophoblast proliferation compared with that in control cells 24 h later (Figure 2(E)). Meanwhile, EdU assays also validated a lower number of EdU-positive cells in the sh-PPP1R3G group (Figure 2(F)). These results indicated that PPP1R3G knockdown inhibited the proliferation of HTR-8/SVneo cells.
Figure 2.
PPP1R3G regulates trophoblast proliferation. Relative PPP1R3G mRNA level in HTR-8/SVneo, JAR, and JEG3 cells. (B) Representative western blot bands and quantitative analysis of PPP1R3G in HTR-8/SVneo, JAR, and JEG3 cells. Data are expressed as means ± SEM (n = 3), one-way ANOVA (Tukey’s), *P < 0.05, **P < 0.01 versus HTR-8/SVneo group. (C–D) The mRNA and protein expression of PPP1R3G in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. (E) CCK-8 assay showed the viability in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC at 24, 48, 72, and 96 h. (F) The merged images of EdU-positive cells (red) and nuclei (blue) in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC and quantitative analysis. Bar = 50 μm. Data are expressed as means ± SEM (n = 3), Student’s t-test, **P < 0.01, ***P < 0.001 versus sh-NC group.
PPP1R3G knockdown significantly inhibits trophoblast invasion and migration
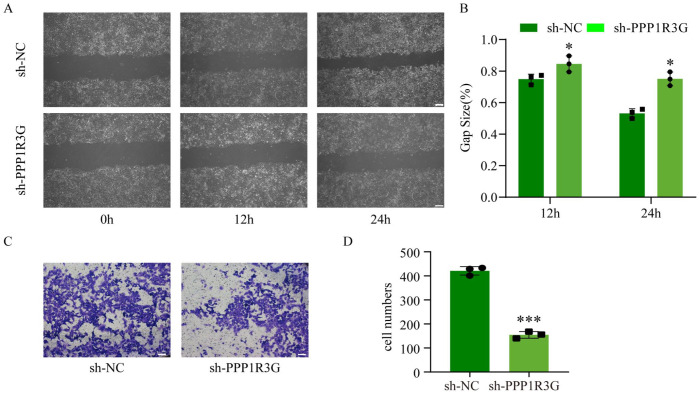
We investigated whether PPP1R3G knockdown affected cell invasion and migration by suppressing trophoblast cell proliferation. The invasion and migration of HTR-8/SVneo cells were examined using Transwell and wound-healing assays, respectively. Our results indicated that PPP1R3G knockdown markedly reduced the migratory ability of HTR-8/SVneo cells (Figure 3(A) and (B)) and decreased the number of invading cells (Figure 2(C) and (D)). Altogether, these data indicate that PPP1R3G knockdown inhibits the migration and invasion of HTR-8/SVneo cells.
Figure 3.
PPP1R3G knockdown significantly inhibits trophoblast invasion and migration.
(A–B) Representative images of wound-healing assay and quantitative analysis of HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC at 0, 12, and 24 h. (C–D) Representative images of transwell assay and quantitative analysis of HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. Bar = 50 μm. Data are expressed as means ± SEM (n = 3), Student’s t-test, *P < 0.05, ***P < 0.001 versus sh-NC group.
PPP1R3G knockdown has no noticeable effect on trophoblast apoptosis
TUNEL staining and western blot were used to analyze the effect of PPP1R3G on HTR-8/SVneo cell apoptosis. Interestingly, PPP1R3G knockdown had no discernible effect on the percentage of TUNEL-positive cells (Figure 4(A) and B). Similarly, WB revealed no significant differences in the protein expression of Bcl-2 and Bax between the two groups (Figure 4(C)). Cumulatively, these results confirm that PPP1R3G knockdown has no significant effect on HTR-8/SVneo cell apoptosis.
Figure 4.
PPP1R3G knockdown has no significant effect on trophoblast apoptosis. (A) TUNEL staining of HTR-8/SVneo cells and the percentage of TUNEL-positive cells (red) were quantified. (B) Representative western blot bands and quantitative analysis of Bcl-2/Bax in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. Bar = 50 μm. Data are expressed as means ± SEM (n = 3), Student’s t-test.
PPP1R3G regulates Akt phosphorylation and MMP-9 expression in trophoblasts
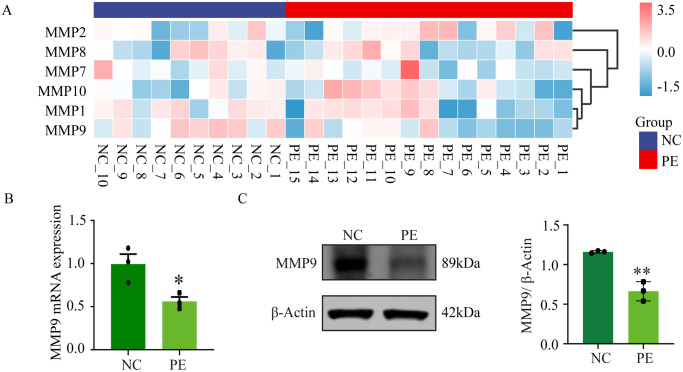
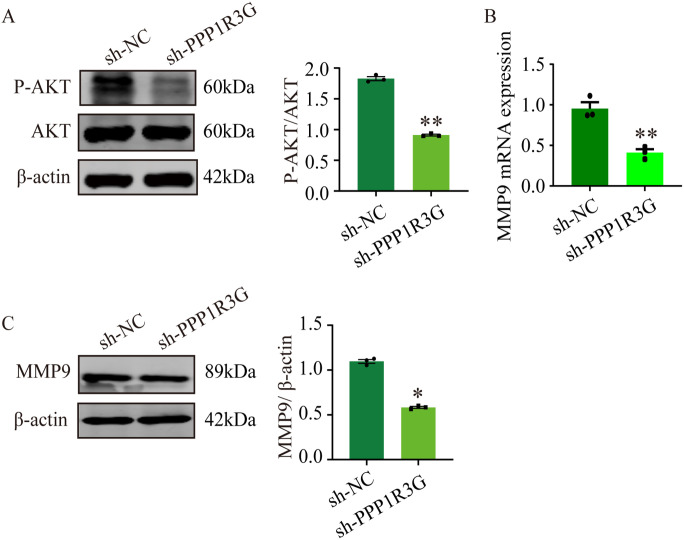
Further experiments revealed the molecular mechanisms underlying the effects of PPP1R3G on trophoblasts. MMP-9 plays an essential role in cell invasion and contributes substantially to the onset of PE. In this study, placental tissues from patients at our hospital and the GSE74341 database revealed that MMP-9 was downregulated in PE patients compared with normal pregnancies (Figure 5(A)– (C)). After PPP1R3G knockdown in the trophoblasts, western blot analysis was performed to evaluate Akt signaling pathway expression. In contrast to the sh-NC group, the expression of p-Akt/Akt was significantly reduced in the sh-PPP1R3G group (Figure 6(A)). Moreover, MMP-9 levels in HTR-8/SVneo cells were dramatically reduced by PPP1R3G knockdown at both the mRNA and protein levels (Figure 6(B) and (C)). These results demonstrate that PPP1R3G knockdown in HTR-8/SVneo cells results in decreased Akt phosphorylation and MMP-9 protein expression.
Figure 5.
MMP-9 expression is downregulated in human PE placentas. (A) Differentially expressed genes of the MMP family in PE and normal pregnancy placental tissues in GSE74341. (B) Relative mRNA level of MMP-9 in PE and normal pregnancy placental tissues collected in our hospital. (C) Representative western blot bands and quantitative analysis of MMP-9 in PE and normal pregnancy placental tissues. Data are expressed as means ± SEM (n = 3), Student’s t-test, *P < 0.05, **P < 0.01 versus NC group.
Figure 6.
PPP1R3G regulates the Akt, and MMP-9 signaling pathway in trophoblasts. (A) Representative western blot bands and quantitative analysis of p-Akt/Akt in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. (B) Relative mRNA expression of MMP-9 in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. (C) Representative western blot bands and quantitative analysis of MMP-9 in HTR-8/SVneo cells transfected with sh-PPP1R3G or sh-NC. Data are expressed as means ± SEM (n = 3), Student’s t-test, *P < 0.05, **P < 0.01 versus NC group.
Discussion
PE is a multisystem disorder of pregnancy with uncertain etiology and pathophysiology, and its primary clinical presentation is hypertension and proteinuria after 20 weeks of gestation. 22 It affects 5–8% of all pregnancies worldwide and is an independent significant risk factor for both maternal and fetal morbidity.23,24 Generalized arteriolar vasospasm is a key factor in PE. 25 Abnormal remodeling of the uterine spiral arteries is considered a critical initial step in the pathophysiology of PE.26–28 However, the molecular mechanisms underlying these processes are not completely understood. The remodeling of uterine spiral arteries is strongly influenced by trophoblast invasion, and the trophoblast invasion process is precisely controlled by a large variety of molecules. 29 For the first time, we present evidence that PPP1R3G regulates human trophoblast invasion through the involvement of MMP-9.
Multiple studies have confirmed that abnormal lipid metabolism during pregnancy plays a critical role in the pathogenesis of PE.30–32 This complicated process involving multiple redundant signaling pathways and molecular mechanisms regulating trophoblasts remain unclear. It has been demonstrated that PPP1R3G, which is expressed in several tissues including the placenta, plays a significant role in lipid homeostasis.33–35 According to previous studies, PPP1R3G is downregulated in the liver after feeding, and its hepatic knockdown slows postprandial blood glucose clearance. 36 More importantly, PPP1R3G is necessary for various biological processes including type I necroptosis and RIPK1-dependent apoptosis. 8 A recent study found that PPP1R3G correlates with immune infiltration and poor prognosis in lung adenocarcinoma. 12 In this study, the expression of PPP1R3G was decreased in the placental tissues of patients with PE. Thus, we tried to explore the functional role of PPP1R3G in the pathogenesis of PE. Implantation, in which the embryo attaches and invades the underlying maternal endometrium, is similar to tumor invasion. 37 Current opinions support the view that trophoblast cell invasion is crucial for the development of PE.38–40 The current experimental results demonstrated that PPP1R3G knockdown suppressed the proliferation, invasion, and migration abilities, but had no discernible effect on apoptosis in HTR-8/SVneo trophoblast cells. Thus, it can be speculated that PPP1R3G might be involved in the pathogenesis of PE through modulating trophoblast cell behavior.
MMP-9 has been known positively regulate trophoblast migration and invasion.41–43 Other studies demonstrate the involvement of Akt signaling in regulation of MMP-9 expression. 44 Notably, MMP-9 also plays a critical role in the regulation of uterine remodeling and placental vasodilation. 45 The results of this study showed that MMP-9 expression was decreased in the placental tissues of PE patients. These findings are consistent with those of Yang et al. 46 who have reported that downregulation of MMP-9 caused insufficient trophoblast invasion, thereby inducing PE. Previously, Wu et al. 47 demonstrated that CXCR2 modulates trophoblast invasiveness by regulating MMP-9 and phosphorylated Akt. Similarly, the invasion and migration of breast cancer cells 48 as well as bladder tumorigenesis 49 were induced by the Akt/MMP-9 signaling pathway.
In liver, PPP1R3G functions as a substrate and downstream signaling of Akt, thereby mediating the activation of insulin signaling. 10 Interestingly, p-Akt/Akt level was significantly downregulated in the trophoblast with PPP1R3G knockdown, suggesting a regulatory role on Akt phosphorylation in trophoblast. Moreover, decreased expression of MMP-9 was also observed in the trophoblast with PPP1R3G knockdown. Overall, PPP1R3G knockdown inhibited the invasion and migration of trophoblast, possibly by suppressing Akt phosphorylation MMP-9 expression.
In conclusion, this study identified novel biological functions of PPP1R3G. In contrast to healthy pregnancies, PPP1R3G was downregulated in the placentas of PE pregnancies, and PPP1R3G knockdown suppressed trophoblast proliferation, invasion, and migration. Mechanistically, PPP1R3G might participate in the regulation of MMP-9 expression dependent on Akt signaling. These findings provided depth insights into the biological function of PPP1R3G and the potential mechanisms underlying the development of PE.
However, our study has several limitations that should be identified. First, there was a lack of in vivo data to support the presented in vitro results. Still, we recognize the need for in vivo studies to explore the functional role of PPP1R3G in the pathophysiology of PE. Second, we used only one cell line. In a recent review, it was noted that HTR-8/SVneo cell line contains a mixed population of trophoblast and stromal cells. 50 HTR-8/SVneo displayed an expression profile which was characteristic of cells which have undergone epithelial-to-mesenchymal transition (EMT) to acquire an invasive phenotype. 51 It has also been postulated that HTR-8/SVneo can be potentially used as an in vitro model system to investigate cellular invasion capacity in trophoblasts. 52 Future studies should explore other trophoblast cell lines, such as JEG-3, BeWo, JAR, and Swan-71, or primary human extravillous trophoblast cells, which would allow us to identify the heterogeneity in trophoblast cells.
Supplemental Material
Supplemental material, sj-docx-1-ebm-10.1177_15353702231182214 for Decreased PPP1R3G in pre-eclampsia impairs human trophoblast invasion and migration via Akt/MMP-9 signaling pathway by Huimin Shi, Renyu Kong, Xu Miao, Lingshan Gou, Xin Yin, Yuning Ding, Xiliang Cao, Qingyong Meng, Maosheng Gu and Feng Suo in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: HMS, RYK, and XM contributed equally to this work. FS and MSG designed the study. RYK and XM performed the experiments and data analysis. HMS, XY, and QYM collected placental tissues. HMS, LSG, XLC, YND, and FS finalized the article. All authors of this article contributed to the interpretation of the data and agreed to the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Xuzhou “Pengcheng Talent” Youth Medical Reserve Talent Project (grant no XWRCHT20220014), the Key Medical Talents Training Project of Xuzhou (grant no XWRCHT20220060), the Jiangsu Commission of Health Research Project (H2019007), the Science and Technology Planning Project of Traditional Chinese Medicine of Jiangsu (grant no YB2020050), and the Science and Technology projects of Xuzhou City (grant no KC21061).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Feng Suo  https://orcid.org/0000-0002-7203-9591
https://orcid.org/0000-0002-7203-9591
References
- 1. Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci 2018;19:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012;125:911–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu J, Park K, Chandrasekhar J, Kalkman DN, Johnson JA, Wild RA, Dobies D, Thomas L, Skelding KA, Ahmed B, Barber KR, Mungee S, Mehran R. Feasibility and utility of a cardiovascular risk screening tool in women undergoing routine gynecology evaluation. J Womens Health (Larchmt) 2020;29:1150–9 [DOI] [PubMed] [Google Scholar]
- 4. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension 2005;46:1243–9 [DOI] [PubMed] [Google Scholar]
- 5. Singla A, Rajaram S, Mehta S, Radhakrishnan G. A Ten year audit of maternal mortality: millennium development still a distant goal. Indian J Community Med 2017;42:102–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bollen M, Peti W, Ragusa MJ, Beullens M. The extended PP1 toolkit: designed to create specificity. Trends Biochem Sci 2010;35:450–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhowmick R, Thakur RS, Venegas AB, Liu Y, Nilsson J, Barisic M, Hickson ID. The RIF1-PP1 axis controls abscission timing in human cells. Curr Biol 2019;29:1232–42.e5 [DOI] [PubMed] [Google Scholar]
- 8. Du J, Xiang Y, Liu H, Liu S, Kumar A, Xing C, Wang Z. RIPK1 dephosphorylation and kinase activation by PPP1R3G/PP1gamma promote apoptosis and necroptosis. Nat Commun 2021;12:7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y, Kotian N, Aranjuez G, Chen L, Messer CL, Burtscher A, Sawant K, Ramel D, Wang X, McDonald JA. Protein phosphatase 1 activity controls a balance between collective and single cell modes of migration. Elife 2020;9:e52979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Q, Zhao Q, Zhang J, Zhou L, Zhang W, Chua B, Chen Y, Xu L, Li P. The protein phosphatase 1 complex is a direct target of AKT that links insulin signaling to hepatic glycogen deposition. Cell Rep 2019;28:3406–22.e7 [DOI] [PubMed] [Google Scholar]
- 11. Li F, Niu Y, Zhao W, Yan C, Qi Y. Construction and validation of a prognostic model for lung adenocarcinoma based on endoplasmic reticulum stress-related genes. Sci Rep 2022;12:19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhuo X, Chen L, Lai Z, Liu J, Li S, Hu A, Lin Y. Protein phosphatase 1 regulatory subunit 3G (PPP1R3G) correlates with poor prognosis and immune infiltration in lung adenocarcinoma. Bioengineered 2021;12:8336–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romero R, Chaiworapongsa T. Preeclampsia: a link between trophoblast dysregulation and an antiangiogenic state. J Clin Invest 2013;123:2775–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension 2008;51:970–5 [DOI] [PubMed] [Google Scholar]
- 15. Zadora J, Singh M, Herse F, Przybyl L, Haase N, Golic M, Yung HW, Huppertz B, Cartwright JE, Whitley G, Johnsen GM, Levi G, Isbruch A, Schulz H, Luft FC, Muller DN, Staff AC, Hurst LD, Dechend R, Izsvak Z. Disturbed placental imprinting in preeclampsia leads to altered expression of DLX5, a human-specific early trophoblast marker. Circulation 2017;136:1824–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C, Liang X, Wang J, Zheng Q, Zhao Y, Khan MN, Liu S, Yan Q. Protein O-fucosyltransferase 1 promotes trophoblast cell proliferation through activation of MAPK and PI3K/Akt signaling pathways. Biomed Pharmacother 2017;88:95–101 [DOI] [PubMed] [Google Scholar]
- 17. Cudmore MJ, Ahmad S, Sissaoui S, Ramma W, Ma B, Fujisawa T, Al-Ani B, Wang K, Cai M, Crispi F, Hewett PW, Gratacós E, Egginton S, Ahmed A. Loss of Akt activity increases circulating soluble endoglin release in preeclampsia: identification of inter-dependency between Akt-1 and heme oxygenase-1. Eur Heart J 2012;33:1150–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, Yu L, Ding Y, Peng M, Deng Y. Progesterone enhances the invasion of trophoblast cells by activating PI3K/AKT signaling pathway to prevent preeclampsia. Cell Transplant 2023;32:9636897221145682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan M, Dong L, Meng Y, Wang Y, Zhen J, Qiu J. Leptin promotes HTR-8/SVneo cell invasion via the crosstalk between MTA1/WNT and PI3K/AKT pathways. Dis Markers 2022;2022:7052176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sui X, Zhang L, Zhang XF, Zhang Y. TRIB3-regulated Akt signal pathway affects trophoblast invasion in the development of preeclampsia. Am J Perinatol. Epub ahead of print 22 September 2021. DOI: 10.1055/s-0041-1735872. [DOI] [PubMed] [Google Scholar]
- 21. Zhu H, Kong L. LncRNA CRNDE regulates trophoblast cell proliferation, invasion, and migration via modulating miR-1277. Am J Transl Res 2019;11:5905–18 [PMC free article] [PubMed] [Google Scholar]
- 22. Gao Q, Fan X, Xu T, Li H, He Y, Yang Y, Chen J, Ding H, Tao J, Xu Z. Promoter methylation changes and vascular dysfunction in pre-eclamptic umbilical vein. Clin Epigenetics 2019;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu K, Fu Q, Liu Y, Wang C. An integrative bioinformatics analysis of microarray data for identifying hub genes as diagnostic biomarkers of preeclampsia. Biosci Rep 2019;39:BSR20190187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y, Liu Z, Tian M, Hu X, Wang L, Ji J, Liao A. The altered PD-1/PD-L1 pathway delivers the “one-two punch” effects to promote the Treg/Th17 imbalance in pre-eclampsia. Cell Mol Immunol 2018;15:710–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cowley E, Thompson JP, Sharpe P, Waugh J, Ali N, Lambert DG. Effects of pre-eclampsia on maternal plasma, cerebrospinal fluid, and umbilical cord urotensin II concentrations: a pilot study. Br J Anaesth 2005;95:495–9 [DOI] [PubMed] [Google Scholar]
- 26. Circulation: clinical summaries. Circulation 2015;131:687–8 [DOI] [PubMed] [Google Scholar]
- 27. Thadhani R. Inching towards a targeted therapy for preeclampsia. Hypertension 2010;55:238–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monteagudo-Sanchez A, Sanchez-Delgado M, Mora JRH, Santamaria NT, Gratacos E, Esteller M, de Heredia ML, Nunes V, Choux C, Fauque P, de Nanclares GP, Anton L, Elovitz MA, Iglesias-Platas I, Monk D. Differences in expression rather than methylation at placenta-specific imprinted loci is associated with intrauterine growth restriction. Clin Epigenetics 2019;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakraborty D, Cui W, Rosario GX, Scott RL, Dhakal P, Renaud SJ, Tachibana M, Rumi MA, Mason CW, Krieg AJ, Soares MJ. HIF-KDM3A-MMP12 regulatory circuit ensures trophoblast plasticity and placental adaptations to hypoxia. Proc Natl Acad Sci U S A 2016;113:E7212–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oe Y, Ko M, Fushima T, Sato E, Karumanchi SA, Sato H, Sugawara J, Ito S, Takahashi N. Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase. Sci Rep 2018;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang C, Austin MA, Edwards KL, Farin FM, Li N, Hsu L, Srinouanprachanh SL, Williams MA. Functional variants of the lipoprotein lipase gene and the risk of preeclampsia among non-Hispanic Caucasian women. Clin Genet 2006;69:33–9 [DOI] [PubMed] [Google Scholar]
- 32. Oggero S, Austin-Williams S, Norling LV. The contrasting role of extracellular vesicles in vascular inflammation and tissue repair. Front Pharmacol 2019;10:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Gu J, Wang L, Zhao Z, Pan Y, Chen Y. Ablation of PPP1R3G reduces glycogen deposition and mitigates high-fat diet induced obesity. Mol Cell Endocrinol 2017;439:133–40 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, Xu D, Huang H, Chen S, Wang L, Zhu L, Jiang X, Ruan X, Luo X, Cao P, Liu W, Pan Y, Wang Z, Chen Y. Regulation of glucose homeostasis and lipid metabolism by PPP1R3G-mediated hepatic glycogenesis. Mol Endocrinol 2014;28:116–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu J, Zhang Y, Xu D, Zhao Z, Zhang Y, Pan Y, Cao P, Wang Z, Chen Y. Ethanol-induced hepatic steatosis is modulated by glycogen level in the liver. J Lipid Res 2015;56:1329–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo X, Zhang Y, Ruan X, Jiang X, Zhu L, Wang X, Ding Q, Liu W, Pan Y, Wang Z, Chen Y. Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes 2011;60:1435–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo C, Kong F, Lv Y, Gao N, Xiu X, Sun X. CDC20 inhibitor Apcin inhibits embryo implantation in vivo and in vitro. Cell Biochem Funct 2020;38:810–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Li J, Yang D. ADAM7 promotes the proliferation and invasion in trophoblast cells. Exp Mol Pathol 2021;121:104659. [DOI] [PubMed] [Google Scholar]
- 39. Penailillo R, Acuna-Gallardo S, Garcia F, Monteiro LJ, Nardocci G, Choolani MA, Kemp MW, Romero R, Illanes SE. Mesenchymal stem cells-induced trophoblast invasion is reduced in patients with a previous history of preeclampsia. Int J Mol Sci 2022;23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Varberg KM, Soares MJ. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021;113:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong H, Diao H, Zhao Y, Xu H, Pei S, Gao J, Wang J, Hussain T, Zhao D, Zhou X, Lin D. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Prolif 2019;52:e12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jang HY, Hong OY, Youn HJ, Kim MG, Kim CH, Jung SH, Kim JS. 15d-PGJ2 inhibits NF-kappaB and AP-1-mediated MMP-9 expression and invasion of breast cancer cell by means of a heme oxygenase-1-dependent mechanism. BMB Rep 2020;53:212–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jung CH, Han AR, Chung HJ, Ha IH, Um HD. Linarin inhibits radiation-induced cancer invasion by downregulating MMP-9 expression via the suppression of NF-kappaB activation in human non-small-cell lung cancer A549. Nat Prod Res 2019;33:3582–6 [DOI] [PubMed] [Google Scholar]
- 44. Xian J, Shao H, Chen X, Zhang S, Quan J, Zou Q, Jin H, Zhang L. Nucleophosmin mutants promote adhesion, migration and invasion of human leukemia THP-1 cells through MMPs up-regulation via Ras/ERK MAPK signaling. Int J Biol Sci 2016;12:144–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sundrani D, Narang A, Mehendale S, Joshi S, Chavan-Gautam P. Investigating the expression of MMPs and TIMPs in preterm placenta and role of CpG methylation in regulating MMP-9 expression. IUBMB Life 2017;69:985–93 [DOI] [PubMed] [Google Scholar]
- 46. Yang ZM, Luo X, Bai B, Qi HB. [Expression of KLF-8 and MMP-9 in placentas and their relationship with the pathogenesis of preeclampsia]. Zhonghua Fu Chan Ke Za Zhi 2013;48:755–8 [PubMed] [Google Scholar]
- 47. Wu D, Hong H, Huang X, Huang L, He Z, Fang Q, Luo Y. CXCR2 is decreased in preeclamptic placentas and promotes human trophoblast invasion through the Akt signaling pathway. Placenta 2016;43:17–25 [DOI] [PubMed] [Google Scholar]
- 48. Li J, Zhang J, Wang Y, Liang X, Wusiman Z, Yin Y, Shen Q. Synergistic inhibition of migration and invasion of breast cancer cells by dual docetaxel/quercetin-loaded nanoparticles via Akt/MMP-9 pathway. Int J Pharm 2017;523:300–9 [DOI] [PubMed] [Google Scholar]
- 49. Chen YT, Yang CC, Shao PL, Huang CR, Yip HK. Melatonin-mediated downregulation of ZNF746 suppresses bladder tumorigenesis mainly through inhibiting the AKT-MMP-9 signaling pathway. J Pineal Res 2019;66:e12536 [DOI] [PubMed] [Google Scholar]
- 50. Abou-Kheir W, Barrak J, Hadadeh O, Daoud G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017;50:1–7 [DOI] [PubMed] [Google Scholar]
- 51. Msheik H, Azar J, El Sabeh M, Abou-Kheir W, Daoud G. HTR-8/SVneo: a model for epithelial to mesenchymal transition in the human placenta. Placenta 2020;90:90–7 [DOI] [PubMed] [Google Scholar]
- 52. Liao J, Zheng Y, Hu M, Xu P, Lin L, Liu X, Wu Y, Huang B, Ye X, Li S, Duan R, Fu H, Huang J, Wen L, Fu Y, Kilby MD, Kenny LC, Baker PN, Qi H, Tong C. Impaired sphingosine-1-phosphate synthesis induces preeclampsia by deactivating trophoblastic YAP (Yes-Associated Protein) through S1PR2 (Sphingosine-1-Phosphate Receptor-2)-induced actin polymerizations. Hypertension 2022;79:399–412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ebm-10.1177_15353702231182214 for Decreased PPP1R3G in pre-eclampsia impairs human trophoblast invasion and migration via Akt/MMP-9 signaling pathway by Huimin Shi, Renyu Kong, Xu Miao, Lingshan Gou, Xin Yin, Yuning Ding, Xiliang Cao, Qingyong Meng, Maosheng Gu and Feng Suo in Experimental Biology and Medicine