Abstract
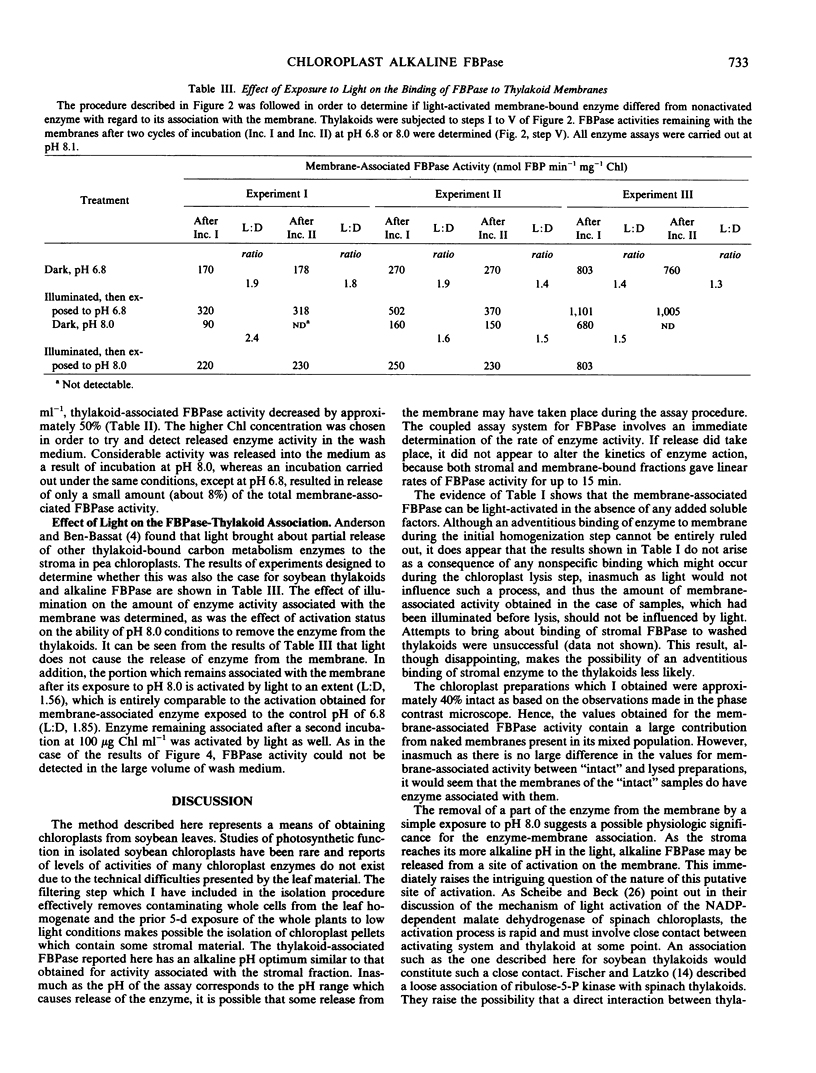
An alkaline fructose 1,6-bisphosphatase activity associated with soybean (Glycine max cv Beeson) chloroplasts appears to be membrane-bound. The pH optimum of the membrane-associated activity corresponds to that found for activity associated with the stroma. Illumination of washed thylakoids results in an increase in alkaline fructose 1,6-bisphosphatase activity in the absence of any added stromal factors. Exposure to pH 8.0 results in a partial release of enzyme activity from the membrane. The activation status of the enzyme does not appear to alter its association with the membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Chin H. M., Gupta V. K. Modulation of Chloroplast Fructose-1,6-bisphosphatase Activity by Light. Plant Physiol. 1979 Sep;64(3):491–494. doi: 10.1104/pp.64.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Brennan T., Anderson L. E. Thioredoxin-like Activity of Thylakoid Membranes: THIOREDOXIN CATALYZING THE REDUCTIVE INACTIVATION OF GLUCOSE-6-PHOSPHATE DEHYDROGENASE OCCURS IN BOTH SOLUBLE AND MEMBRANE-BOUND FORM. Plant Physiol. 1980 Oct;66(4):605–608. doi: 10.1104/pp.66.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Buchanan B. B., Wolosiuk R. A. Photosynthetic regulatory protein found in animal and bacterial cells. Nature. 1976 Dec 16;264(5587):669–670. doi: 10.1038/264669a0. [DOI] [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Properties of freshly purified and thiol-treated spinach chloroplast fructose bisphosphatase. Biochem J. 1980 Mar 1;185(3):689–693. doi: 10.1042/bj1850689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K. H., Latzko E. Chloroplast ribulose-5-phosphate kinase: light-mediated activation, and detection of both soluble and membrane-associated activity. Biochem Biophys Res Commun. 1979 Jul 12;89(1):300–306. doi: 10.1016/0006-291x(79)90978-1. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Zimmermann G., Latzko E. Light induced activation of fructose-1, 6-bisphosphatase in isolated intact chloroplasts. Biochem Biophys Res Commun. 1976 May 3;70(1):193–199. doi: 10.1016/0006-291x(76)91127-x. [DOI] [PubMed] [Google Scholar]
- Krause G. H. Light-induced movement of magnesium ions in intact chloroplasts. Spectroscopic determination with Eriochrome Blue SE. Biochim Biophys Acta. 1977 Jun 9;460(3):500–510. doi: 10.1016/0005-2728(77)90088-3. [DOI] [PubMed] [Google Scholar]
- Lara C., de la Torre A., Buchanan B. B. A new protein factor functional in the ferredoxin-independent light activation of chloroplast fructose 1,6-bisphosphatase. Biochem Biophys Res Commun. 1980 Mar 28;93(2):544–551. doi: 10.1016/0006-291x(80)91111-0. [DOI] [PubMed] [Google Scholar]
- Latzko E., Zimmermann G., Feller U. Evidence for a hexosediphosphatase from the cytoplasm of spinach leaves. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):321–326. doi: 10.1515/bchm2.1974.355.1.321. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. Exclusion of inulin from solutions of hyaluronic acid. Nature. 1960 Sep 17;187:1024–1024. doi: 10.1038/1871024a0. [DOI] [PubMed] [Google Scholar]
- Scheibe R., Beck E. On the Mechanism of Activation by Light of the NADP-dependent Malate Dehydrogenase in Spinach Chloroplasts. Plant Physiol. 1979 Nov;64(5):744–748. doi: 10.1104/pp.64.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]