Abstract
Postmastectomy pain syndrome is a very common disorder in breast cancer survivors. The impact on the quality of patients’ lives is significantly adverse. The precise pathophysiology has not been determined as yet though various risk factors have been identified that make the patient vulnerable. Required preoperative work includes the identification and possible elimination of risk factors. Treatment is multidisciplinary involving surgical and non-surgical modalities. There is a great scope of research in this field.
Keywords: neuroma, neuromodulation, gabapentinoids, neuropathy, intercostobrachial nerve, antidepressants, postmastectomy pain syndrome, mastectomy
Introduction and background
Breast cancer is one of the most commonly diagnosed cancers among women and the leading cause of cancer-related death among them. The treatment modalities and the outcome of breast cancer have witnessed substantial improvement over the last few decades. However, over time, the adverse effects of treatment have also become increasingly obvious [1]. The treatment of this cancer depends on the stage at which it presents, and the primary treatment of nearly 37-40% of women with breast cancer is surgical resection that includes different variants of mastectomy with or without reconstruction like modified radical mastectomy, skin-sparing mastectomy, simple mastectomy, etc. [2]. After surgical procedures, a number of complications may arise. Postmastectomy pain syndrome (PMPS) is a common postoperative complication that affects 20-68% of women who have undergone mastectomy [3,4]. Wood first described persistent pain after mastectomy in 1978 [5], but despite reports of a high incidence of this complication, research on PMPS seems to be lacking. There is no clear consensus on the specific definition of PMPS, nor have the etiology and pathogenesis of PMPS been determined. PMPS has been documented to be extraordinarily burdensome and to have a detrimental effect on overall functional ability and quality of life (QOL) in women who survive long after mastectomy [6].
In light of the foregoing, this article has been compiled to review the current understanding of PMPS with an emphasis on epidemiology, etiopathogenesis, presentation, risk factors, prevention, and treatment options. The primary purpose of this article is to raise awareness of the disorder among medical professionals and encourage more research on the subject.
Review
Methods
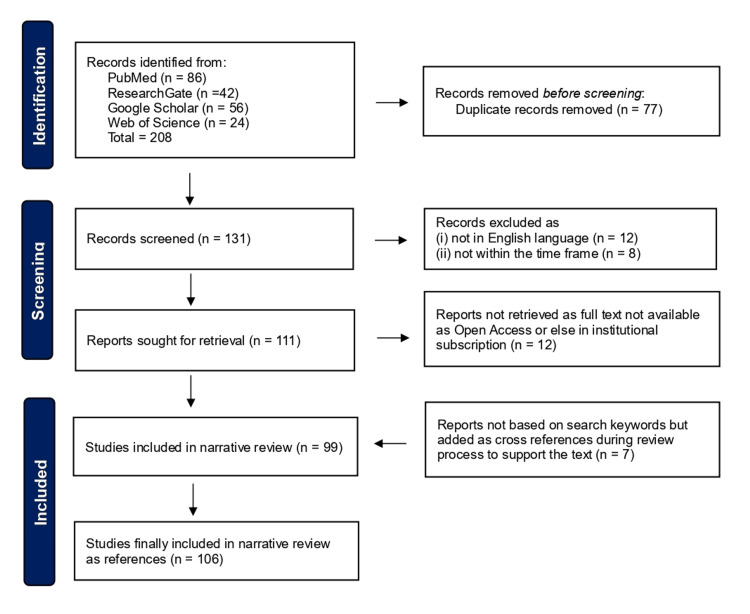
The databases of PubMed, ResearchGate, Google Scholar, and Web of Science were searched for observational studies, reviews, and case reports pertaining to PMPS. The last four authors (KAA, ASA, SAA, TMA) manually searched a single database each, and after comparing the results, duplicate entries were removed (Figure 1). No automation tools were utilized. The search was based on the keywords: ‘Postmastectomy pain syndrome’, ‘Postmastectomy neuropathy, ‘Postmastectomy pain’, and ‘Chronic mastectomy pain’. Publications written between 2000 and 2023 in the English language were included; articles published in other languages were excluded (Figure 1). Additionally, after analyzing the included papers, certain cross-references were accommodated if they provided historical context or else provided evidence that articles within the above timeframe did not provide.
Figure 1. Flowchart showing the selection process.
Image credit: Sajad Ahmad Salati (Author)
Epidemiology
According to reports in the literature, 20-68% of breast cancer survivors suffer from some degree of PMPS [7,8]. In a Danish study by Vilholm et al., the prevalence of PMPS was reported to be 24% in 258 surgically treated breast cancer patients [9]. It was determined that the odds ratio for developing PMPS was 2.9. In a study carried out in China by Gong et al., 560 (28.2%) cases of patients who had undergone surgical treatment for breast cancer experienced PMPS [10]. Gartner et al. found that of the 3253 patients, a total of 1543 (47%) reported PMPS, of whom 201 (13%) had severe pain, 595 (39%) had moderate pain, and 733 (48%) had light pain [11]. Couceiro et al. [12] found PMPS in 44.4% of patients, and in the series by Alves Nogueira Fabro et al. [13], 52% of the enrolled 174 breast cancer survivors suffered from PMPS.
Pathophysiology
The precise pathophysiology has not yet been determined. Although other explanations have been put forward, intercostobrachial nerve neuralgia is currently considered to be the most likely cause of PMPS. The intercostobrachial nerve is the cutaneous lateral branch of the second intercostal nerve that emerges through the serratus anterior muscle, providing sensation to the axilla and the internal portion of the upper arm.
During axillary dissection, the intercostobrachial nerve may get damaged to various degrees by stretching or compression during retraction (neuropraxia, axonotmesis) or by frank transection (neurotmesis). There is a possibility of indirect nerve injury intraoperatively or postoperatively. Intraoperatively, poor arm positioning can stress and compress the nerve, and postoperatively, hematoma, seroma, and scarring can result in stretch and compression injuries.
The nerve injury is followed by ectopic neural activity originating at the nerve injury site and dorsal root ganglion, resulting in increased sensitivity to chemical or mechanical stimuli and a subsequent increased perception of pain [14-16]. Other nerves related to PMPS include the medial and lateral pectoral, thoracodorsal, long thoracic, and intercostal nerves [17].
Etiology
Although the exact etiopathogeneses are still unclear, many risk factors (Figure 2) that may play a part in the emergence of PMPS have been put forth [18,19].
Figure 2. Common characteristics of persistent postmastectomy syndrome (PMPS).
Image Credit: Sajad Ahmad Salati (Author)
Age < 40 Years
It has been discovered that younger age groups pose a risk. The reason for this association is not clear, though several possibilities have been suggested, including a higher histopathological tumor grade in younger patients with a requirement for adjuvant chemotherapy, generally decreased pain receptor sensitivity in older patients, and differences in estrogen receptor status [20-22]. In a series by Alves Nogueira Fabro et al., the mean age of 174 studied patients was 58 years, and it was found that younger women (<40 years) show a significantly increased risk of PMPS (relative risk (RR) = 5.23, 95%CI: 1.11-24.64) [13].
Type of Surgery
The type of surgery has been linked to the prevalence of PMPS, and it has been discovered that, in contrast to breast conservation procedures and sentinel lymph node biopsy, the more invasive breast operations like the different variants of mastectomy (e.g., radical mastectomy, modified radical mastectomy, skin-sparing mastectomy, simple mastectomy, etc.) and axillary lymph node dissection are more likely to damage sensory nerves in advanced cancers [23]. According to Alves Nogueira Fabro et al., patients who underwent axillary lymph node dissection (with more than 15 lymph nodes removed) had a noticeably higher chance of developing PMPS (RR = 2.01, 95%CI: 1.08-3.75) [13].
Psychosocial Factors
Numerous significant studies demonstrate that various psychological factors play a significant role in raising PMPS sensitivity. Miaskowski et al.'s longitudinal study of 410 cancer survivors revealed a substantial correlation between preoperative anxiety and depression and the eventual development of moderate-to-severe PMPS [24]. Belfer et al., in a cross-sectional cohort study, followed 611 patients for three years and concluded that anxiety, catastrophizing, sleep disturbance, and somatization were independently associated with PMPS [25].
A study by Meretoja et al. enrolled 970 patients and documented associations of both anxiety and depression with subsequent PPMP [26]. Ghadimi et al. investigated the possible relationship of PMPS with clinical and psychological variables and concluded that loneliness may be a significant risk factor [27].
Several epidemiological studies have suggested that body mass index (BMI) may be potentially associated with PMPS [26,28-30]. In order to assess the relationship between BMI and PMPS, Ding et al. first conducted a prospective study [29]. Then, using a meta-analysis, they synthesized the evidence from eight observational studies that were found by searching PubMed and Embase until February 2017. They concluded that BMI may be positively correlated with the risk of PMPS.
Along similar lines, the potential association of smoking with PMPS has also been analyzed in some studies. Aliyev and Asik, in a prospective study, observed that PMPS developed in 27 (90%) of 30 patients who smoked preoperatively and, hence, a statistically significant relationship was established between smoking and CPPS (p<0.001) [31]. Sipila et al. found that smoking induces changes at the neuronal level in the nervous system that stay for a long time and contribute to the emergence of PMPS [32]. Nicotine in tobacco products has been found to play a critical role in pain-related pathophysiology. It has proven acute analgesic effects but the smokers get exposed to it on long-term exposure, which leads to the development of tolerance and increased pain sensitivity due to desensitization of nicotinic acetylcholine receptors and neuronal plastic changes. Yamaguchi et al. drafted a consensus statement on smoking cessation in patients with pain of any origin and summarized the available evidence on the impact of smoking on pain [33]. They stressed that smoking cessation interventions not only reduce pain but also improve the outcomes of underlying pain-causing conditions and reduce the risks of tobacco-related disorders.
Preoperative Pain
It has been discovered that preoperative pain at the surgical site increases the risk of PPMP. Although the exact processes are unknown, it has been hypothesized that pain sensitivity and/or central sensitization may increase the susceptibility to persistent pain following surgery [34]. In their study, Roth et al. discovered that over half of the individuals had experienced preoperative pain to some extent [35]. They came to the conclusion that chronic pain following breast reconstruction might not always be related to pain from the operation.
Surgical Department Patient Turnover
Patients treated at low-volume healthcare facilities that undertake fewer than 100 mastectomy operations of different variants annually have a noticeably greater incidence of PMPS than patients operated on at surgical units that perform more than 100 mastectomies annually. According to Tasmuth et al., high turnover improves experience and aids surgical teams in honing their skills [36]. The more experienced teams frequently employ safer patient positioning and more precise surgical techniques, thereby ensuring that the dissection is carried out with greater care, minimizing the possibility of nerve injury during the procedure, and avoiding issues like hematoma, seroma, and dense scarring that could potentially result in stretch or compression of the nerves.
This is a trend that has been witnessed with many other surgical procedures. For example, Gorbea et al. found lesser overall perioperative complications in the hands of otolaryngologists who perform a high volume of total thyroidectomy than those with less volume, and the risk of temporary tracheostomy was particularly higher among low-volume surgeons [37]. These findings are consistent with previous studies of the effect of thyroidectomy volume on surgical complications. Hauch et al. also found that higher surgeon volume is associated with improved patient outcomes and fewer complications [38].
Sociodemographic Factors, Race/Ethnicity
According to data, race or ethnicity and sociodemographic characteristics affect the risk of developing PMPS [19]. Minorities, such as Latinas and African American women, exhibited a higher prevalence and intensity of PMPS, according to Eversley et al. [39]. There are a number of explanations given [11], including the fact that patients from ethnic minorities often receive diagnoses at later stages of the disease, necessitating extensive surgical procedures and more aggressive neoadjuvant and adjuvant therapies (such as chemotherapy and radiation therapy).
Chemotherapy
The breast cancer management has witnessed an increased use of systemic agents, including taxanes, platinum agents, and vinca alkaloids as part of chemotherapy regimens and aromatase inhibitors (AIs) as hormonal therapy. Many studies have found a potential relationship with the development of PMPS and specific terms like persistent pain in breast cancer treated with chemotherapy (PPBCC) and persistent pain in breast cancer hormonal therapy (PPBCHT) have been proposed [40,41].
Postoperative Factors
A major risk factor consistently linked to the emergence of PMPS is the presence of severe postoperative pain. In a study by Bruce et al. involving 362 postmastectomy patients, it was discovered that higher postoperative pain levels were associated with higher odds of PMPS at four months (OR = 1.34, 95%CI = 1.12-1.60) and at nine months (OR =1.17, 95%CI = 1.00-1.37) [42]. Andersen et al. discovered that higher postoperative pain levels raised the likelihood of moderate-to-severe PMPS after one year [43].
Radiation Therapy
PMPS risk has been observed to be greatly enhanced when neoadjuvant or adjuvant radiation therapy is delivered over the chest wall and/or axilla as a part of multimodality breast cancer treatment [44,45]. This is related to tissue fibrosis, which results in nerve entrapment and restricted shoulder joint movement [11]. Furthermore, skin reactions and lymphedema may add to the discomfort levels [45]. According to Gartner et al., adjuvant radiation was linked to a higher risk of PMPS (OR =1.5, 95%CI: 1.08-2.07, p = 0.03) [11].
Definition and clinical presentation
There is an ambiguity surrounding the definition of PMPS in literature, and several definitions have been used. PMPS is most commonly defined as a chronic pain that lasts beyond the normal healing time, when all other potential causes of pain, such as infection or recurrence, have been eliminated [46,47]. Vilholm et al. [9] defined PMPS as neuropathic pain with intensity greater than 4 on a 10-point numeric scale, lasting longer than six months following surgery, while Couceiro et al. [12] label pain persisting for longer than three months as PMPS.
Technically, the term PMPS is rather misleading, as the syndrome has been reported to occur even after breast-conserving surgical operations [13,48]. The pain is often located in the axilla, the shoulder, the arm, or the chest wall or breast region. PMPS is often described as a typical neuropathic pain (Figure 2) characterized by burning, painful cold, electrical shock sensations, stinging, numbness, tingling, stabbing, pulling, or pins and needles sensations [46,49]. Pain may be deep, blunt in character, and evoked by pressure. In a series by Kakati et al., pain was located in 120 patients over the anterior chest wall (41.8%), axilla (32.6%), and medial upper arm (25.6%) [4]. PMPS gets exacerbated by various factors, which include movement, straining, overhead activity, and exposure to cold [50].
The onset of pain is most likely to occur after surgery, though there may also be a new onset of symptoms after many months following adjuvant therapy such as chemotherapy or radiation therapy [51]. Kakati et al. prospectively followed up 120 women after mastectomy to identify the incidence and severity of PMPS along with its impact on QOL in Indian patients, by using the Brief Pain Inventory (BPI) questionnaire and the QOL questionnaire (QLQ) by the European Organization for Research and Treatment of Cancer (EORTC-QLQ 30) [4]. They found that 35.8% of patients suffered from PMPS at six months and the character of pain was described as dull aching by about 49%. About 56% of patients had mild-intensity pain, and they were attempting to manage and accept it as an inevitable component of their treatment. Macdonald et al. assessed the long-term outcome of PMPS at 7-12 years postoperatively and found that 52% of the patients continued to experience PMPS at a mean of nine years after surgery [3].
To prevent discrepancies in clinical as well as research settings, Waltho and Rockwell tried to establish a consensus for defining PMPS by identifying the various elements and proposing a new and complete definition [46]. Accordingly, they defined PMPS as a pain that occurs after any breast surgery, which is of at least moderate severity, possesses neuropathic qualities, is located in the ipsilateral breast/chest wall, axilla, and/or arm, lasts at least six months, occurs at least 50% of the time, and may be exacerbated by movements of the shoulder girdle.
Impact on QOL
PMPS has been found to exert a significantly negative impact on the overall QOL, even in patients in whom its severity is mild. PMPS may limit the range of motion (ROM) of the upper extremities, which would negatively impact the capacity to perform daily tasks and even basic occupational tasks. Furthermore, PMPS has been found to take a significant toll on mental health, emotional well-being, and social relationships [22,52].
Ferreira et al. assessed the impact of PMPS on the lives of 30 breast cancer survivors using the BPI and found that pain exerts a negative impact on mood, normal work, and sleep [53]. Caffo et al. explored the physical well-being, physical autonomy, relational life, and psychological well-being of patients undergoing surgical treatment for breast cancer and found that the patients who developed PMPS had significantly worse QOL scores on all of the subscales than those who did not develop PMPS [6]. Fontes et al. studied the impact of PMPS on patients' sleep quality and found a significant association between PMPD and deterioration in sleep quality during the first year of follow-up, more pronounced among those with good sleep quality (Pittsburgh Sleep Quality Index (PSQI) 5) than those with poor sleep quality at baseline (PSQI >5) [54]. Daytime dysfunction and sleep duration were found to be the most impaired components.
Bibi et al. employed descriptive correlational research design, using the BPI Short Form (BPI-SF) to assess PMPS and the Functional Assessment of Cancer Therapy-Breast (FACT-B) to measure the health-related QOL and found that there was a significant negative impact on the health-related QOL, particularly on the physical well-being [55]. Burckhardt et al. compared and contrasted the pain characteristics, symptom impact, health status, and QOL of post-breast cancer surgery patients with regional chronic pain with those with widespread chronic pain and concluded that the women who experienced widespread pain had significantly more severity of pain and pain impact, and lower physical health status than those with regional pain [56].
Prevention of PMPS
Patients with strong risk factors for PMPS need to be identified, and proper efforts need to be made for its prevention through the use of multimodal care involving psychotherapy, proper surgical technique, physiotherapy, and peripherally targeted or centrally acting pharmacotherapy [57]. Multiple studies have found that active preoperative psychological counseling and social support can reduce the severity of PMPS [58,59]. Hence, caregivers need to be aware of and make available interventions that strengthen resilience and provide social support. Liukas et al. retrospectively reviewed 101 electronic health records of patients with breast cancer, and 337 different expressions describing psychosocial factors associated with postoperative persistent pain were identified, which included psychological strength and resilience, social factors, emotional factors, anxiety, sleep-related factors, and depression [60]. They found that although psychosocial factors associated with PMPS were placed in the electronic health records, documentation about such factors was not found in all patients' records, nor was the documentation done in a systematic manner. The study concluded that there is potential to use electronic health records as an information source in the development of decision-support system algorithms to better support nurses in the identification of patients at risk of developing PMPS, provided quality standards are set and applied for comprehensive documentation.
During operations on the breast, attempts are to be made to minimize the damage to the pectoralis major, pectoralis minor, and the surrounding fascia and if axillary exploration is undertaken, care should be taken to avoid injury to the intercostobrachial nerve. Studies suggest that nerve block during surgery may prevent PMPS, and a study by O’Brien et al. showed promising results with targeted muscle reinnervation, wherein transection of the lateral cutaneous intercostal branch was identified immediately and coaptation of the intercostal nerve to the target motor muscle was accomplished [61]. Gatherwright and Knackstedt have reported the use of cadaveric nerve grafts for targeted reinnervation of damaged intercostal nerves [62]. In their randomized control trial, Fujii et al. studied pectoral nerve-2 (PECS 2) block vs. serratus plane block for chronic pain after mastectomy [63]. The study found that a pectoralis nerve block decreased the rate of moderate to severe chronic pain at six months postoperatively compared with a serratus anterior block.
In the postoperative phase, early commencement of physiotherapy can play a helpful role in maintaining glenohumeral and scapular chest movement, strength, and normal neuromuscular recruitment patterns, thereby minimizing upper limb dysfunction and PMPS [64].
Pharmacologic agents, including gabapentinoids, memantine, and nefopam, administered in the perioperative period have been found to be safe and efficacious in reducing acute pain and the subsequent development of PMPS [65,66]. In their randomized control trial, Fassoulaki et al. evaluated the impact of multimodal analgesia on acute and chronic pain after breast surgery for cancer and found that by combining perioperative gabapentin and local anesthesia, a significant decrease in PMPS can be achieved over the long term, thereby supporting the importance of a multimodal approach to the prevention of PMPS [67].
Amr and Yousef prospectively investigated the analgesic efficacy of venlafaxine and gabapentin on pain associated with mastectomy for cancer [68]. A total of 150 patients scheduled for either partial or radical mastectomy with axillary dissection were randomized to receive extended-release venlafaxine (37.5 mg/d), gabapentin 300 mg/d, or placebo for 10 days starting the night before the operation. It was found that at six months post the operation, the incidence of chronic pain, its intensity, and the need for analgesics were reduced in venlafaxine compared to gabapentin and the placebo group, but the burning type of pain was more frequent in the control groups than in gabapentin.
Memantine is an NMDA receptor antagonist. Morel et al. carried out a randomized, pilot clinical trial enrolling 40 women undergoing mastectomy [69]. The aim was to evaluate whether memantine administered before and after mastectomy could prevent the development of PMPS and the impairment of cognition. Accordingly, memantine (5-20 mg/day; n = 20) or placebo (n = 20) was administered for four weeks, starting two weeks before surgery. The study found that, at three months, patients receiving memantine showed a significant difference in postmastectomy pain intensity, less rescue analgesia, and a better emotional state as compared to the placebo group.
Nefopam is a non-opioid, non-steroidal benzoxazocine analgesic that is believed to act by inhibiting the reuptake of serotonin, norepinephrine, and dopamine. Additionally, it modulates the calcium and sodium channels in the glutamatergic pathway, thereby decreasing the activation of N-methyl-D-aspartate (NMDA) receptors. Na et al. prospectively evaluated the efficacy of nefopam on acute and chronic postoperative pain after breast cancer surgery [70]. The patients were assigned to the nefopam (n = 41) or control (n = 42) groups. Before the start of the operation, 20 mg of nefopam was administered to the patients in the nefopam group, and normal saline was used in the control group. The study concluded that preventive nefopam reduced acute postoperative pain with reduced use of rescue analgesic drugs and contributed to a reduced incidence of PMPS at three months.
Proper control and alleviation of pain in the postoperative phase is important in other ways too, as studies have shown that improperly controlled pain increases hospitalization.
Treatment
PMPS involves chronic pain as well as psychosocial and functional disruption, and hence, a multidisciplinary approach can address all these domains. While adopting a multidisciplinary approach, the team may comprise a plastic surgeon, a pain specialist, a physical therapist, an oncologist, a radiologist, and a clinical psychologist. An important step in management is the exclusion of other nonneuropathic causes of pain, such as infection, musculoskeletal pain, or oncologic recurrence [8]. Lack of awareness in patients about treatment possibilities is a big factor that leads to prolonged suffering, and hence, management should involve proper education regarding common postmastectomy sequelae and the many available treatment options.
The different modalities of PMPS treatment described in the contemporary literature include psychological interventions, pharmacological interventions, interventional therapy, physical therapy, and surgery [71].
Psychological Interventions
Implementation of psychological interventions helps with chronic pain control as well as allows patients to effectively cope during this difficult phase of their lives [72]. Jassim et al. studied 28 randomized controlled trials involving 3940 breast cancer patients and evaluated the effectiveness of psychological interventions [73]. They found that psychological intervention, in particular cognitive behavioral therapy, produced highly favorable effects on anxiety, depression, and mood disturbance. In a meta-analysis by Haller et al., the effectiveness of mindfulness-based stress reduction was examined in 1709 breast cancer survivors, and a significant improvement was found in health-related quality of life, fatigue, sleep, stress, anxiety, and depression [74].
Pharmacological Therapy
A wide range of medications have been shown to be effective in PMPS, and an appropriate selection depends upon patient co-morbidities, drug adverse-effect profiles, potential long-term consequences, and cost-effectiveness. Various medication choices by class are briefly outlined below:
Antidepressants: Tricyclic antidepressants (TCAs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are commonly prescribed to treat PMPS at lower doses than those needed for anti-depressant effects. TCAs such as amitriptyline and nortriptyline inhibit the re-uptake of neurotransmitters including norepinephrine, serotonin, and adenosine at nerve endings, thereby increasing their concentration, which, by unclear mechanisms, plays a role in the analgesic effects [75].
Additionally, TCAs antagonize the N-methyl-D-aspartate (NMDA) receptors. In the context of pain, NMDA receptor activation is believed to contribute to the development of the wind-up phenomenon, wherein the intensity of pain increases over time for a given stimulus and causes central sensitization. NMDA receptor antagonism results in a decrease in sustained neuronal depolarization, thereby decreasing excitatory transmission along afferent pain pathways and resulting in the alleviation of pain. Larsson et al. systematically reviewed the treatment modalities and found that antidepressants lead to a statistically significant reduction of PMPS symptoms [17]. SNRIs, including duloxetine and venlafaxine, also inhibit neurotransmitter reuptake and have been explored with varying degrees of success in PMPS [76].
Gabapentinoids: Pregabalin and gabapentin function by modulating voltage-activated calcium channels, which inhibit nerve conduction in pain-transmitting neurons and central sensitization [77]. These drugs share structural similarities with the gamma-aminobutyric acid (GABA) neurotransmitter. They might also play a part in the central nervous system's activation of noradrenergic pain-inhibitory pathways. In a retrospective analysis of 89 PMPS patients, de Miguel-Jimeno et al. discovered that 80% of patients had successfully acquired a significant long-term reduction in pain [78]. However, 10% stopped receiving medication because of the negative effects.
Pregabalin has the added benefit of being almost completely absorbed in the body, unlike gabapentin, and hence, with about three times the absorption, it reaches peak blood concentrations within one hour after ingestion. Kaur et al. conducted an open-label, single-arm, prospective study to evaluate the efficacy of Pregabalin in relieving PMPS and found that it brought about significant reductions in pain (visual analog scale (VAS) scores; baseline 5.50 ± 1.197, end of one month 2.40 ± 1.430, end of two months 2.10 ± 1.370) and thereby significantly improved the QOL [79].
Topical medications: Topical medications are preferred by some patients to avoid the adverse events associated with oral pharmaceutical treatments. Of the various topical agents reported, capsaicin appears to be the most promising. Capsaicin is believed to act by leading to the attenuation of cutaneous hypersensitivity through a process described as "defunctionalization" [80].
Dupoiron et al. reported an important or complete effect for 70.7% and 56.0% of applications in patients with PMPS existing for less than 12 months and greater than 10 years, respectively [81]. The adverse effects noticed included application site reactions in 54.4% of patients (mainly burning sensation or pain, 45.9%, or erythema, 30.8%) and high blood pressure in 7.2%. Flother et al. reported pain relief without tolerance development with the application of capsaicin 8% patches in a 62-year-old female with intense PMPS that had persisted chronically over months and had not sufficiently responded to oral medication including tapentadol and pregabalin [82].
Opioids are the cornerstone of therapy for acute or chronic pain of moderate to severe intensity. They act on three types of opioid receptors: µ, δ, and κ (MOR, DOR, and KOR) in the central nervous system, but studies have suggested that these receptors also occur in other tissues, thereby creating a possibility for the topical use of opioids to achieve local analgesia without systemic adverse effects [83]. Accordingly, Mohamed et al. investigated the effect of topical morphine (in one of three doses: 5, 10, or 15 mg) and found that topical morphine controlled acute postmastectomy pain in a dose-dependent manner and reduced the incidence and severity of PMPS [84].
Interventional Therapy
Nerve blocks: The problematic nerves are identified by a thorough history and physical exam, and after real-time visualization under ultrasound, they are directly targeted for perineural injections with local anesthetics, steroids, or neurolytic substances such as phenol or alcohol. If the pain is not consistent with a specific nerve or otherwise appears to involve multiple nerves, various regional or interfascial plane blocks are used as options for pain relief.
Wijayasinghe et al. assessed the feasibility of intercostobrachial nerve (ICBN) blockade and its effects on pain and sensory function in 16 patients with PMPS [85]. They found that the second intercostal space could be visualized on ultrasound and that an ICBN block could be adequately performed, thereby achieving significant pain relief. Even the pre-existing areas of hypoesthesia decreased in most of the participants. In another study, Wijayasinghe et al. examined the effects of pectoral nerve block (Pecs block) on summed pain intensity (SPI) and sensory function (through quantitative sensory testing) in eight patients with PMPS [86]. The study suggested that the pectoral nerves play a role in the maintenance of PMPS and, hence, further research in the field was suggested. Takimoto et al. suggested that serratus plane block (SPB) has the potential to emerge as a treatment modality for PMPS as it can be performed more safely and easily than neuraxial approaches [87].
Neuromodulation: Neuromodulation attempts to change or disrupt the firing patterns of nerves via electrical stimulation. Dorsal root ganglion stimulation is one such modality that involves the placement of electrodes directly on the dorsal root ganglion and focused stimulation of the first-order neuron. Morgalla reported a case wherein the procedure was well tolerated and the pain score dropped from an 8/10 to a 4/10 for four years with a 50% reduction in the medication regimen [88]. However, the patient, due to local pain, required the transfer of the generator from the right gluteal region to the right abdominal region, but she was able to resume her job, and her functional status returned to baseline. Hetta et al. evaluated and compared the analgesic efficacy of pulsed radiofrequency (PRF) when delivered either on the thoracic dorsal root ganglion (DRG) of intercostobrachial nerves (thoracic DRG 2, 3, and 4) or their corresponding thoracic paravertebral nerves (PVNs) [89]. They enrolled 64 patients with PMPS for the study and concluded that PRF of both the thoracic DRG and the thoracic PVN are both effective treatments; however, PRF of the DRG provided a better long-term analgesic effect. They recommend that PRF of the DRG be reserved for cases that failed to gain adequate response to PRF of the thoracic PVN in conjunction with medical treatment due to the inherent risk of performing thoracic foraminal interventions and the technical difficulty of targeting the thoracic DRG.
Scrambler therapy is another neuromodulation modality based on non-invasive electrical neurocutaneous stimulation. The device generates 16 different types of waveforms that resemble action potentials and delivers them to the surface receptors of the c-fibers, thereby transmitting "non-pain" information along the damaged pathways to reduce central sensitization. In a series by Smith et al., patients achieved significant (over 75%) and sustained (months) reductions of allodynia, hyperalgesia, and pain, thereby reporting marked improvements in their QOL and functional status [90]. No participant reported any side effects.
Physical Therapy
The function of the upper extremities and shoulder can be hindered by chronic discomfort in the upper arm, axilla, and chest, which results in persistent stiffness, limited ROM, and frequently worsening pain. Physical and occupational therapy may therefore be involved. De Groef et al. reviewed the effectiveness of various postoperative physical therapy modalities and the timing of physical therapy after treatment of breast cancer on pain and impaired ROM of the upper limb [91]. They suggested that multifactorial physical therapy and active exercises were effective in treating pain and impaired ROM. However, they emphasized the need for more in-depth research in this area.
Surgical Interventions
Surgical treatment for PMPS is an option reserved for patients who exhaust other modalities and fail to show an adequate response. Surgical operations carry a potential risk of worsening the symptoms and, hence, before the adoption of this option, the possibility of failure to improve symptoms needs to be discussed as part of informed consent, and a viable backup plan must be kept in place should the operation fail.
Exploration
The areas of maximal point tenderness are carefully marked. Exploration is performed, and scar tissue, a neuroma, or a mechanical obstruction (i.e., surgical clips or sutures) may be encountered. If a nerve is found entering the scar bed, then dissection is carried out until healthy nerve bundles are freed proximally and distally. Nerve ends are coapted, and if there are no suitable distal nerve targets, the proximal nerves are buried into non-scarred tissue. Alternatively, allogeneic nerve grafting to regional nerves, nerve conduits, or allograft nerves is conducted.
Hart et al. conducted a retrospective review of seven PMPS patients who had intercostal neurectomy procedures along with muscle or dermal wrapping of the resected nerve's proximal end [92]. With the help of local anesthetic nerve blocks, the suspected nerves were located, and each patient had an average of 3.14 neurectomies (with a range of 1-6). Following surgery, pain scores significantly decreased, and 86% of patients reported being pain-free or "considerably improved" on long-term follow-up. Broyles et al. retrospectively reviewed 10 patients with PMPS in whom affected intercostal nerves were identified with a diagnostic local anesthetic block and then dissected and implanted into local muscle [93]. Most of the patients experienced long-lasting relief from pain.
Fat Grafting
Recent research has outlined the advantages of fat grafting for PMPS patients with painful scar retraction and nerve entrapment. Juhl et al. conducted a controlled, randomized experiment to ascertain whether fat grafting had any analgesic effects on PMPS and whether it remodeled the mastectomy scar [94]. The study analyzed a total of 15 patients (fat grafted: 8, seven control: 7), and the average amount of grafted fat was 71 ± 24.6 mL. Fat grafting significantly decreased pain (55%), according to the study. Furthermore, health-related QOL and scar quality also showed statistically significant improvements. Maione et al. performed autologous fat grafting in 59 patients and compared the outcomes with those of a control group of 37 patients who underwent no additional surgical procedures [95]. A significant reduction in pain was detected in patients treated with autologous fat grafting, and it was concluded that due to its safety, efficacy, and optimal tolerability, the procedure can be considered useful in treating PMPS. Caviggioli et al. similarly came to a comparable conclusion after using autologous fat grafts to treat 72 PMPS patients [96]. However, Sollie et al. failed to find that fat grafting was in any way more effective than a placebo for treating PMPS [97].
Laser Therapy
Recent years have seen a rise in the use of high-intensity laser therapy (HILT) as a treatment for pain of musculoskeletal origin [98]. The use of laser therapy is a particularly under-researched strategy, even within the relatively scant PMPS literature [69]. In a prospective study, Ebid and El-Sodany enrolled 61 women (30 in the laser group and 31 in the placebo laser group) to explore the potential utility of this tool in PMPS patients [99]. In contrast to the placebo laser group, which received placebo HILT plus routine physical therapy program, the laser group received HILT three times per week for four weeks. The laser group had a significant improvement in QOL outcomes, a decrease in pain scores, and a significant increase in shoulder ROM after four weeks of treatment and after 12 weeks of follow-up. Infrared laser and light-emitting diode (LED) were used in combination by Trelles and Calderhead for the treatment of PMPS in a 44-year-old female, and they reported reasonably encouraging results [100].
Relation of uncontrolled pain with patient outcomes and healthcare costs
Poorly controlled postoperative pain has been found to adversely impact patient outcomes, and increase the length of hospitalization and overall healthcare costs. Hence, there is a need to adopt a consistent, systematic, evidence-based, and holistic approach to acute pain management [101], and this shift would expectedly translate into reduction of PMPS in the long term.
Recent studies have suggested that chronic pain typically begins as acute poorly controlled postoperative pain, but gradually transitions into a persistent pain condition with neuropathic features that are unresponsive to opioids [102]. Research into the pathogenesis of this transition has led to a stronger appreciation of the use of more effective and safer analgesic regimens [103,104].
Uncontrolled pain has been found to trigger a complex neurohormonal cascade that negatively impacts nearly every organ system and results in renal and gastrointestinal dysfunction, increased incidence of infection, cardiopulmonary and thrombotic complications, impairment of wound healing, adverse psychological effects, and poorer functional recovery and QOL [105].
Algorithmic, Multidisciplinary Approach
The research on PMPS is still very limited and there is no unified line of action that may be projected as a gold standard. However, certain high-volume centers have devised their management protocols and published them in recent years for ease and possible adoption by healthcare providers. Yang et al. from Memorial Sloan Kettering Cancer Center, New York have proposed an algorithm to provide a stepwise application for selecting the appropriate therapies in the management of PMPS [106].
Beederman and Bank from the University of Chicago Medicine, Chicago, have proposed an algorithm (Figure 3), in which stress was laid upon the creation of a proper multidisciplinary treatment team for the management of PMPS [8]. Their team comprised plastic surgeons, physical therapists, and pain specialists, and they showed that local blocks can be used both for diagnosis and for symptom management and can be performed either by the plastic surgeon or by the pain specialist. Surgical intervention has been mentioned as a last resort when other, less-invasive options have been attempted.
Figure 3. An algorithmic, multidisciplinary approach to the care of patients with PMPS.
Image Source: Beederman and Bank, 2021 [8]; Open Access, Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND)
PMPS: postmastectomy pain syndrome
Limitations
The authors have relied on articles whose full text has been made available as Open-Access or else on literature subscribed by Qassim University Library and Saudi Digital Library. Hence, there is a possibility that some articles of relevance may not have been analyzed while drafting this narrative review.
Conclusions
In breast cancer survivors, PMPS is a fairly common but little-discussed complication. Intercostobrachial nerve-related neuropathy is the most commonly recognized pathogenesis. The disorder has a negative effect on QOL. Before the management of breast cancer is undertaken, a multidisciplinary team should thoroughly investigate a patient to reduce the risk factors and then formulate a logical treatment plan. If a patient ends up developing PMPS, a holistic plan of management should be chalked out. It is imperative that healthcare professionals remain updated and that patients are adequately informed about the available preventative and therapeutic choices.
Acknowledgments
The authors thank the journal ‘Plastic and Reconstructive Surgery—Global Open’ for making available the article containing Figure 3 of our article as an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), making it permissible to download and share the work provided it is properly cited. The work has not been changed in any way or used commercially.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Mariyyah H. Alharbi, Sajad Ahmad Salati, Lamees Alsulaim, Abdulsalam S. Alsuhaibani, Khalid A. Albaqami, Shoug A. Alaodah, Norah H. Alharbi, Thana M. Alsenaid
Acquisition, analysis, or interpretation of data: Mariyyah H. Alharbi, Sajad Ahmad Salati, Lamees Alsulaim, Abdulsalam S. Alsuhaibani, Khalid A. Albaqami, Shoug A. Alaodah, Norah H. Alharbi, Thana M. Alsenaid
Drafting of the manuscript: Mariyyah H. Alharbi, Sajad Ahmad Salati, Lamees Alsulaim, Abdulsalam S. Alsuhaibani, Khalid A. Albaqami, Shoug A. Alaodah, Norah H. Alharbi, Thana M. Alsenaid
Critical review of the manuscript for important intellectual content: Mariyyah H. Alharbi, Sajad Ahmad Salati, Lamees Alsulaim, Abdulsalam S. Alsuhaibani, Khalid A. Albaqami, Shoug A. Alaodah, Norah H. Alharbi, Thana M. Alsenaid
Supervision: Sajad Ahmad Salati, Lamees Alsulaim
References
- 1.Treating persistent pain after breast cancer surgery. Khan JS, Ladha KS, Abdallah F, Clarke H. Drugs. 2020;80:23–31. doi: 10.1007/s40265-019-01227-5. [DOI] [PubMed] [Google Scholar]
- 2.Early postoperative outcomes in lumpectomy versus simple mastectomy. Chatterjee A, Pyfer B, Czerniecki B, Rosenkranz K, Tchou J, Fisher C. J Surg Res. 2015;198:143–148. doi: 10.1016/j.jss.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 3.Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Br J Cancer. 2005;92:225–230. doi: 10.1038/sj.bjc.6602304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post mastectomy pain syndrome at an Indian tertiary cancer centre and its impact on quality of life. Kakati B, Nair N, Chatterjee A. https://journals.lww.com/indianjcancer/fulltext/2023/60020/post_mastectomy_pain_syndrome_at_an_indian.23.aspx. Indian J Cancer. 2023;60:275–281. doi: 10.4103/ijc.ijc_861_21. [DOI] [PubMed] [Google Scholar]
- 5.Intercostobrachial nerve entrapment syndrome. Wood KM. https://pubmed.ncbi.nlm.nih.gov/663696/ South Med J. 1978;71:662–663. doi: 10.1097/00007611-197806000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Pain and quality of life after surgery for breast cancer. Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. https://link.springer.com/article/10.1023/A:1024435101619. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]
- 7.Post-mastectomy pain syndrome: an up-to-date review of treatment outcomes. Chappell AG, Yuksel S, Sasson DC, Wescott AB, Connor LM, Ellis MF. JPRAS Open. 2021;30:97–109. doi: 10.1016/j.jpra.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Post-breast surgery pain syndrome: shifting a surgical paradigm. Beederman M, Bank J. Plast Reconstr Surg Glob Open. 2021;9:0. doi: 10.1097/GOX.0000000000003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. Br J Cancer. 2008;99:604–610. doi: 10.1038/sj.bjc.6604534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevalence of postmastectomy pain syndrome and associated risk factors: a large single-institution cohort study. Gong Y, Tan Q, Qin Q, Wei C. Medicine (Baltimore) 2020;99:0. doi: 10.1097/MD.0000000000019834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prevalence of and factors associated with persistent pain following breast cancer surgery. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 12.Prevalence of post-mastectomy pain syndrome and associated risk factors: a cross-sectional cohort study. Couceiro TC, Valença MM, Raposo MC, Orange FA, Amorim MM. Pain Manag Nurs. 2014;15:731–737. doi: 10.1016/j.pmn.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Post-mastectomy pain syndrome: incidence and risks. Alves Nogueira Fabro E, Bergmann A, do Amaral E Silva B, et al. Breast. 2012;21:321–325. doi: 10.1016/j.breast.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Regional block and mexiletine: the effect on pain after cancer breast surgery. Fassoulaki A, Sarantopoulos C, Melemeni A, Hogan Q. Reg Anesth Pain Med. 2001;26:223–228. doi: 10.1053/rapm.2001.23205. [DOI] [PubMed] [Google Scholar]
- 15.Traumatic neuroma as a rare cause of intractable neuropathic breast pain following cancer surgery: Management and review of the literature. Salemis NS. Intractable Rare Dis Res. 2018;7:185–190. doi: 10.5582/irdr.2018.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Post-mastectomy pain syndrome: defining perioperative etiologies to guide new methods of prevention for plastic surgeons. Chappell AG, Bai J, Yuksel S, Ellis MF. World J Plast Surg. 2020;9:247–253. doi: 10.29252/wjps.9.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The post-mastectomy pain syndrome-a systematic review of the treatment modalities. Larsson IM, Ahm Sørensen J, Bille C. Breast J. 2017;23:338–343. doi: 10.1111/tbj.12739. [DOI] [PubMed] [Google Scholar]
- 18.Persistent pain following breast cancer surgery: correlates and determinants. Montazeri A. Womens Health (Lond) 2010;6:175–177. doi: 10.2217/whe.10.6. [DOI] [PubMed] [Google Scholar]
- 19.Acute and persistent postoperative pain after breast surgery. Fecho K, Miller NR, Merritt SA, Klauber-Demore N, Hultman CS, Blau WS. Pain Med. 2009;10:708–715. doi: 10.1111/j.1526-4637.2009.00611.x. [DOI] [PubMed] [Google Scholar]
- 20.Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. Bruce J, Thornton AJ, Scott NW, et al. Br J Cancer. 2012;107:937–946. doi: 10.1038/bjc.2012.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Factors influencing the effect of age on prognosis in breast cancer: population based study. Kroman N, Jensen MB, Wohlfahrt J, Mouridsen HT, Andersen PK, Melbye M. BMJ. 2000;320:474–478. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A retrospective study of postmastectomy pain syndrome: incidence, characteristics, risk factors, and influence on quality of life. Meijuan Y, Zhiyou P, Yuwen T, Ying F, Xinzhong C. ScientificWorldJournal. 2013;2013:159732. doi: 10.1155/2013/159732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuropathic pain after breast cancer treatment: characterization and risk factors. Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes J, Lunet N. J Pain Symptom Manage. 2017;54:877–888. doi: 10.1016/j.jpainsymman.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. Miaskowski C, Cooper B, Paul SM, et al. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Persistent postmastectomy pain in breast cancer survivors: analysis of clinical, demographic, and psychosocial factors. Belfer I, Schreiber KL, Shaffer JR, et al. J Pain. 2013;14:1185–1195. doi: 10.1016/j.jpain.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Clinical prediction model and tool for assessing risk of persistent pain after breast cancer surgery. Meretoja TJ, Andersen KG, Bruce J, et al. J Clin Oncol. 2017;35:1660–1667. doi: 10.1200/JCO.2016.70.3413. [DOI] [PubMed] [Google Scholar]
- 27.Predictors of postoperative pain six months after breast surgery. J Ghadimi D, Looha MA, Akbari ME, Akbari A. Sci Rep. 2023;13:8302. doi: 10.1038/s41598-023-35426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socio-demographic, treatment-related, and health behavioral predictors of persistent pain 15 months and 7-9 years after surgery: a nationwide prospective study of women treated for primary breast cancer. Johannsen M, Christensen S, Zachariae R, Jensen AB. Breast Cancer Res Treat. 2015;152:645–658. doi: 10.1007/s10549-015-3497-x. [DOI] [PubMed] [Google Scholar]
- 29.Body mass index and persistent pain after breast cancer surgery: findings from the women's healthy eating and living study and a meta-analysis. Ding YY, Yao P, Wu L, Han ZK, Hong T, Zhu YQ, Li HX. Oncotarget. 2017;8:43332–43343. doi: 10.18632/oncotarget.17948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.High body mass index is a potential risk factor for persistent postoperative pain after breast cancer treatment. van Helmond N, Timmerman H, van Dasselaar NT. https://www.painphysicianjournal.com/linkout?issn=&vol=20&page=E661. Pain Physician. 2017;20:661–671. [PubMed] [Google Scholar]
- 31.Postoperative chronic pain syndrome and risk factors in patients with breast surgery. Aliyev D, Asik I. https://pubmed.ncbi.nlm.nih.gov/37300253/ J Coll Physicians Surg Pak. 2023;33:609–615. doi: 10.29271/jcpsp.2023.06.609. [DOI] [PubMed] [Google Scholar]
- 32.Development of a screening instrument for risk factors of persistent pain after breast cancer surgery. Sipilä R, Estlander AM, Tasmuth T, Kataja M, Kalso E. Br J Cancer. 2012;107:1459–1466. doi: 10.1038/bjc.2012.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Consensus statement on smoking cessation in patients with pain. Iida H, Yamaguchi S, Goyagi T, et al. https://doi.org/10.1007/s00540-022-03097-w. J Anesth. 2022;36:671–687. doi: 10.1007/s00540-022-03097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preoperative breast pain predicts persistent breast pain and disability after breast cancer surgery. Langford DJ, Schmidt B, Levine JD, et al. J Pain Symptom Manage. 2015;49:981–994. doi: 10.1016/j.jpainsymman.2014.11.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Is chronic postsurgical pain surgery-induced? A study of persistent postoperative pain following breast reconstruction. Roth RS, Qi J, Hamill JB, Kim HM, Ballard TN, Pusic AL, Wilkins EG. Breast. 2018;37:119–125. doi: 10.1016/j.breast.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chronic post-treatment symptoms in patients with breast cancer operated in different surgical units. Tasmuth T, Blomqvist C, Kalso E. Eur J Surg Oncol. 1999;25:38–43. doi: 10.1053/ejso.1998.0597. [DOI] [PubMed] [Google Scholar]
- 37.The impact of surgeon volume on total thyroidectomy outcomes among otolaryngologists. Gorbea E, Goldrich DY, Agarwal J, Nayak R, Iloreta AM. Am J Otolaryngol. 2020;41:102726. doi: 10.1016/j.amjoto.2020.102726. [DOI] [PubMed] [Google Scholar]
- 38.Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Hauch A, Al-Qurayshi Z, Randolph G, Kandil E. Ann Surg Oncol. 2014;21:3844–3852. doi: 10.1245/s10434-014-3846-8. [DOI] [PubMed] [Google Scholar]
- 39.Post-treatment symptoms among ethnic minority breast cancer survivors. Eversley R, Estrin D, Dibble S, Wardlaw L, Pedrosa M, Favila-Penney W. Oncol Nurs Forum. 2005;32:250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 40.Persistent pain after breast cancer treatment. Smith HS, Wu SX. Ann Palliat Med. 2012;1:182–194. doi: 10.3978/j.issn.2224-5820.2012.10.13. [DOI] [PubMed] [Google Scholar]
- 41.Persistent pain, sensory disturbances and functional impairment after adjuvant chemotherapy for breast cancer: cyclophosphamide, epirubicin and fluorouracil compared with docetaxel + epirubicin and cyclophosphamide. Andersen KG, Jensen MB, Kehlet H, Gärtner R, Eckhoff L, Kroman N. Acta Oncol. 2012;51:1036–1044. doi: 10.3109/0284186X.2012.692884. [DOI] [PubMed] [Google Scholar]
- 42.Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Bruce J, Thornton AJ, Powell R, et al. Pain. 2014;155:232–243. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Predictive factors for the development of persistent pain after breast cancer surgery. Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Pain. 2015;156:2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 44.Predictive factors for persistent and late radiation complications in breast cancer survivors. Fekrmandi F, Panzarella T, Dinniwell RE, Helou J, Levin W. Clin Transl Oncol. 2020;22:360–369. doi: 10.1007/s12094-019-02133-8. [DOI] [PubMed] [Google Scholar]
- 45.Ionizing radiation: the good, the bad, and the ugly. Ryan JL. J Invest Dermatol. 2012;132:985–993. doi: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Post-breast surgery pain syndrome: establishing a consensus for the definition of post-mastectomy pain syndrome to provide a standardized clinical and research approach - a review of the literature and discussion. Waltho D, Rockwell G. Can J Surg. 2016;59:342–350. doi: 10.1503/cjs.000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Postmastectomy pain: a cross-sectional study of prevalence, pain characteristics, and effects on quality of life. Beyaz SG, Ergönenç JŞ, Ergönenç T, Sönmez ÖU, Erkorkmaz Ü, Altintoprak F. Chin Med J (Engl) 2016;129:66–71. doi: 10.4103/0366-6999.172589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Re-visiting post-breast surgery pain syndrome: risk factors, peripheral nerve associations and clinical implications. Kokosis G, Chopra K, Darrach H, Dellon AL, Williams EH. Gland Surg. 2019;8:407–415. doi: 10.21037/gs.2019.07.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Post-mastectomy pain syndrome: the magnitude of the problem. Couceiro TC, Menezes TC, Valênça MM. Rev Bras Anestesiol. 2009;59:358–365. doi: 10.1590/s0034-70942009000300012. [DOI] [PubMed] [Google Scholar]
- 50.Persistent post-mastectomy pain: risk factors and current approaches to treatment. Tait RC, Zoberi K, Ferguson M, et al. J Pain. 2018;19:1367–1383. doi: 10.1016/j.jpain.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuropathic pain associated with non-surgical treatment of breast cancer. Jung BF, Herrmann D, Griggs J, Oaklander AL, Dworkin RH. Pain. 2005;118:10–14. doi: 10.1016/j.pain.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Cancer-related post-treatment pain and its impact on health-related quality of life in breast cancer patients: a cross sectional study in Palestine. Abu Farha NH, Khatib MT, Salameh H, Zyoud SH. Asia Pac Fam Med. 2017;16:7. doi: 10.1186/s12930-017-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assessing the impact of pain on the life of breast cancer survivors using the Brief Pain Inventory. Ferreira VT, Dibai-Filho AV, Kelly de Oliveira A, Gomes CA, Melo ES, Maria de Almeida A. J Phys Ther Sci. 2015;27:1361–1363. doi: 10.1589/jpts.27.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuropathic pain after breast cancer treatment and its impact on sleep quality one year after cancer diagnosis. Fontes F, Gonçalves M, Pereira S, Lunet N. Breast. 2017;33:125–131. doi: 10.1016/j.breast.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 55.Persistent post-surgical pain and its relationship to health-related quality of life in Pakistani women after breast cancer surgery. Bibi R, Maneewat K, Sangchan H, Sae-Sia W. Belitung Nurs J. 2022;8:258–265. doi: 10.33546/bnj.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Effects of chronic widespread pain on the health status and quality of life of women after breast cancer surgery. Burckhardt CS, Jones KD. Health Qual Life Outcomes. 2005;3:30. doi: 10.1186/1477-7525-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Post mastectomy pain syndrome: a systematic review of prevention modalities. Yuksel SS, Chappell AG, Jackson BT, Wescott AB, Ellis MF. JPRAS Open. 2022;31:32–49. doi: 10.1016/j.jpra.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Psychological resilience associates with pain experience in women treated for breast cancer. Liesto S, Sipilä R, Aho T, Harno H, Hietanen M, Kalso E. Scand J Pain. 2020;20:545–553. doi: 10.1515/sjpain-2019-0137. [DOI] [PubMed] [Google Scholar]
- 59.Symptom distress and coping in young Korean breast cancer survivors: the mediating effects of social support and resilience. Lee JH, Kim HY. J Korean Acad Nurs. 2018;48:241–253. doi: 10.4040/jkan.2018.48.2.241. [DOI] [PubMed] [Google Scholar]
- 60.Towards automated risk prediction of persistent pain: exploring psychosocial factors from electronic health records after breast cancer surgery. Liukas T, Rosio R, Peltonen LM. Nurs Open. 2023;10:3399–3405. doi: 10.1002/nop2.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Targeted muscle reinnervation following breast surgery: a novel technique. O'Brien AL, Kraft CT, Valerio IL, Rendon JL, Spitz JA, Skoracki RJ. Plast Reconstr Surg Glob Open. 2020;8:0. doi: 10.1097/GOX.0000000000002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Targeted breast re-innervation (TBR) for post-mastectomy pain. Gatherwright J, Knackstedt R. J Plast Reconstr Aesthet Surg. 2019;72:1700–1738. doi: 10.1016/j.bjps.2019.06.040. [DOI] [PubMed] [Google Scholar]
- 63.A randomised controlled trial of pectoral nerve-2 (PECS 2) block vs. serratus plane block for chronic pain after mastectomy. Fujii T, Shibata Y, Akane A, et al. Anaesthesia. 2019;74:1558–1562. doi: 10.1111/anae.14856. [DOI] [PubMed] [Google Scholar]
- 64.Role of physiotherapy in supporting recovery from breast cancer treatment: a qualitative study embedded within the UK PROSPER trial. Rees S, Mazuquin B, Richmond H, Williamson E, Bruce J. BMJ Open. 2021;11:0. doi: 10.1136/bmjopen-2020-040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perioperative analgesia to prevent chronic postmastectomy pain. Kehlet H. Anesth Analg. 2006;103:494–495. doi: 10.1213/01.ANE.0000227067.35946.A2. [DOI] [PubMed] [Google Scholar]
- 66.Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Anesthesiology. 2013;119:1215–1221. doi: 10.1097/ALN.0b013e3182a9a896. [DOI] [PubMed] [Google Scholar]
- 67.Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Fassoulaki A, Triga A, Melemeni A, Sarantopoulos C. Anesth Analg. 2005;101:1427–1432. doi: 10.1213/01.ANE.0000180200.11626.8E. [DOI] [PubMed] [Google Scholar]
- 68.Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Amr YM, Yousef AA. Clin J Pain. 2010;26:381–385. doi: 10.1097/AJP.0b013e3181cb406e. [DOI] [PubMed] [Google Scholar]
- 69.Memantine before mastectomy prevents post-surgery pain: a randomized, blinded clinical trial in surgical patients. Morel V, Joly D, Villatte C, et al. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0152741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Preventive analgesic efficacy of nefopam in acute and chronic pain after breast cancer surgery: a prospective, double-blind, and randomized trial. Na HS, Oh AY, Koo BW, Lim DJ, Ryu JH, Han JW. Medicine (Baltimore) 2016;95:0. doi: 10.1097/MD.0000000000003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.A comprehensive review of the diagnosis, treatment, and management of postmastectomy pain syndrome. Capuco A, Urits I, Orhurhu V, et al. Curr Pain Headache Rep. 2020;24:41. doi: 10.1007/s11916-020-00876-6. [DOI] [PubMed] [Google Scholar]
- 72.Acceptance- and mindfulness-based interventions for the treatment of chronic pain: a meta-analytic review. Veehof MM, Trompetter HR, Bohlmeijer ET, Schreurs KM. Cogn Behav Ther. 2016;45:5–31. doi: 10.1080/16506073.2015.1098724. [DOI] [PubMed] [Google Scholar]
- 73.Psychological interventions for women with non-metastatic breast cancer. Jassim GA, Doherty S, Whitford DL, Khashan AS. Cochrane Database Syst Rev. 2023;1:0. doi: 10.1002/14651858.CD008729.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mindfulness-based interventions for women with breast cancer: an updated systematic review and meta-analysis. Haller H, Winkler MM, Klose P, Dobos G, Kümmel S, Cramer H. Acta Oncol. 2017;56:1665–1676. doi: 10.1080/0284186X.2017.1342862. [DOI] [PubMed] [Google Scholar]
- 75.An investigation of monoamine receptors involved in antinociceptive effects of antidepressants. Yokogawa F, Kiuchi Y, Ishikawa Y, Otsuka N, Masuda Y, Oguchi K, Hosoyamada A. Anesth Analg. 2002;95:163–168. doi: 10.1097/00000539-200207000-00029. [DOI] [PubMed] [Google Scholar]
- 76.Venlafaxine in neuropathic pain following treatment of breast cancer. Tasmuth T, Härtel B, Kalso E. Eur J Pain. 2002;6:17–24. doi: 10.1053/eujp.2001.0266. [DOI] [PubMed] [Google Scholar]
- 77.A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. Clin Pharmacokinet. 2010;49:661–669. doi: 10.2165/11536200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.Postmastectomy pain syndrome in our region: characteristics, treatment, and experience with gabapentin [Article in Spanish] de Miguel-Jimeno JM, Forner-Cordero I, Zabalza-Azparren M, Matute-Tobias B. https://neurologia.com/articulo/2015464. Rev Neurol. 2016;16:258–266. [PubMed] [Google Scholar]
- 79.Pregabalin in the treatment of postmastectomy chronic pain: results of an open label, single-arm clinical study. Kaur N, Kumar A, Saxena AK, Grover RK. Breast J. 2019;25:465–468. doi: 10.1111/tbj.13242. [DOI] [PubMed] [Google Scholar]
- 80.Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Anand P, Bley K. Br J Anaesth. 2011;107:490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peripheral neuropathic pain following breast cancer: effectiveness and tolerability of high-concentration capsaicin patch. Dupoiron D, Jubier-Hamon S, Seegers V, et al. J Pain Res. 2022;15:241–255. doi: 10.2147/JPR.S341378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Capsaicin in the treatment of refractory neuropathic pain after mastectomy surgery: a case report. Flöther L, Avila-Castillo D, Burgdorff AM, Benndorf R. Case Rep Oncol. 2020;13:997–1001. doi: 10.1159/000508948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Efficacy and safety of topical morphine: a narrative review. Nosek K, Leppert W, Puchała Ł, Łoń K. Pharmaceutics. 2022;14:1499. doi: 10.3390/pharmaceutics14071499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Effect of topical morphine on acute and chronic postmastectomy pain: what is the optimum dose? Mohamed SA, Abdel-Ghaffar HS, Kamal SM, Fares KM, Hamza HM. Reg Anesth Pain Med. 2016;41:704–710. doi: 10.1097/AAP.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 85.Ultrasound guided intercostobrachial nerve blockade in patients with persistent pain after breast cancer surgery: a pilot study. Wijayasinghe N, Duriaud HM, Kehlet H, Andersen KG. https://www.painphysicianjournal.com/linkout?issn=&vol=19&page=E309. Pain Physician. 2016;19:309–318. [PubMed] [Google Scholar]
- 86.Analgesic and sensory effects of the pecs local anesthetic block in patients with persistent pain after breast cancer surgery: a pilot study. Wijayasinghe N, Andersen KG, Kehlet H. Pain Pract. 2017;17:185–191. doi: 10.1111/papr.12423. [DOI] [PubMed] [Google Scholar]
- 87.Serratus plane block for persistent pain after partial mastectomy and axillary node dissection. Takimoto K, Nishijima K, Ono M. https://www.painphysicianjournal.com/linkout?issn=&vol=19&page=E481. Pain Physician. 2016;19:481–486. [PubMed] [Google Scholar]
- 88.Dorsal root ganglion stimulation for the treatment of persistent post-mastectomy pain: case report. Morgalla MH. Neuromodulation. 2019;22:117–118. doi: 10.1111/ner.12894. [DOI] [PubMed] [Google Scholar]
- 89.Pulsed radiofrequency on thoracic dorsal root ganglion versus thoracic paravertebral nerve for chronic postmastectomy pain, a randomized trial: 6-month results. Hetta DF, Mohamed SA, Mohamed KH, Mahmoud TA, Eltyb HA. https://www.painphysicianjournal.com/linkout?issn=&vol=23&page=23. Pain Physician. 2020;23:23–35. [PubMed] [Google Scholar]
- 90.Scrambler therapy for the treatment of chronic post-mastectomy pain (cPMP) Smith T, Cheville AL, Loprinzi CL, Longo-Schoberlein D. Cureus. 2017;9:0. doi: 10.7759/cureus.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. De Groef A, Van Kampen M, Dieltjens E, Christiaens MR, Neven P, Geraerts I, Devoogdt N. Arch Phys Med Rehabil. 2015;96:1140–1153. doi: 10.1016/j.apmr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 92.Effective treatment of chronic mastectomy pain with intercostal sensory neurectomy. Hart SE, Agarwal S, Hamill JB, Brown DL. Plast Reconstr Surg. 2022;149:876–880. doi: 10.1097/PRS.0000000000008975. [DOI] [PubMed] [Google Scholar]
- 93.Pain after breast surgery: etiology, diagnosis, and definitive management. Broyles JM, Tuffaha SH, Williams EH, Glickman L, George TA, Lee Dellon A. Microsurgery. 2016;36:535–538. doi: 10.1002/micr.30055. [DOI] [PubMed] [Google Scholar]
- 94.Fat grafting for alleviating persistent pain after breast cancer treatment: A randomized controlled trial. Juhl AA, Karlsson P, Damsgaard TE. J Plast Reconstr Aesthet Surg. 2016;69:1192–1202. doi: 10.1016/j.bjps.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 95.Autologous fat graft in postmastectomy pain syndrome following breast conservative surgery and radiotherapy. Maione L, Vinci V, Caviggioli F, et al. Aesthetic Plast Surg. 2014;38:528–532. doi: 10.1007/s00266-014-0311-9. [DOI] [PubMed] [Google Scholar]
- 96.Autologous fat graft in postmastectomy pain syndrome. Caviggioli F, Maione L, Forcellini D, Klinger F, Klinger M. Plast Reconstr Surg. 2011;128:349–352. doi: 10.1097/PRS.0b013e31821e70e7. [DOI] [PubMed] [Google Scholar]
- 97.Autologous fat grafting as treatment of postmastectomy pain syndrome: a randomized controlled trial. Sollie M, Toyserkani NM, Bille C, Thomsen JB, Sørensen JA. Plast Reconstr Surg. 2022;149:295–305. doi: 10.1097/PRS.0000000000008705. [DOI] [PubMed] [Google Scholar]
- 98.The beneficial effects of high-intensity laser therapy and co-interventions on musculoskeletal pain management: a systematic review. Ezzati K, Laakso EL, Salari A, Hasannejad A, Fekrazad R, Aris A. J Lasers Med Sci. 2020;11:81–90. doi: 10.15171/jlms.2020.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Long-term effect of pulsed high-intensity laser therapy in the treatment of post-mastectomy pain syndrome: a double blind, placebo-control, randomized study. Ebid AA, El-Sodany AM. Lasers Med Sci. 2015;30:1747–1755. doi: 10.1007/s10103-015-1780-z. [DOI] [PubMed] [Google Scholar]
- 100.Combined infrared laser and LED for postmastectomy pain and discomfort: a case report. Trelles MA, Calderhead RG. https://www.researchgate.net/publication/265155870_Combined_Infrared_Laser_and_Led_Therapy_for_Postmastectomy_Pain_and_Discomfort_A_Case_Report Laser Ther. 2005;14:41–45. [Google Scholar]
- 101.Acute pain management pearls: a focused review for the hospital clinician. Hyland SJ, Wetshtein AM, Grable SJ, Jackson MP. https://www.mdpi.com/2227-9032/11/1/34. Healthcare (Basel) 2022;11:34. doi: 10.3390/healthcare11010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Transition from acute to chronic pain after surgery. Glare P, Aubrey KR, Myles PS. Lancet. 2019;393:1537–1546. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 103.The acute to chronic pain transition: can chronic pain be prevented? Pozek JP, Beausang D, Baratta JL, Viscusi ER. Med Clin North Am. 2016;100:17–30. doi: 10.1016/j.mcna.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Chronification of pain: mechanisms, current understanding, and clinical implications. Pak DJ, Yong RJ, Kaye AD, Urman RD. Curr Pain Headache Rep. 2018;22:9. doi: 10.1007/s11916-018-0666-8. [DOI] [PubMed] [Google Scholar]
- 105.Poorly controlled postoperative pain: prevalence, consequences, and prevention. Gan TJ. J Pain Res. 2017;10:2287–2298. doi: 10.2147/JPR.S144066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.An interventional pain algorithm for the treatment of postmastectomy pain syndrome: a single-center retrospective review. Yang A, Nadav D, Legler A, Chen GH, Hingula L, Puttanniah V, Gulati A. Pain Med. 2021;22:677–686. doi: 10.1093/pm/pnaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]