Abstract
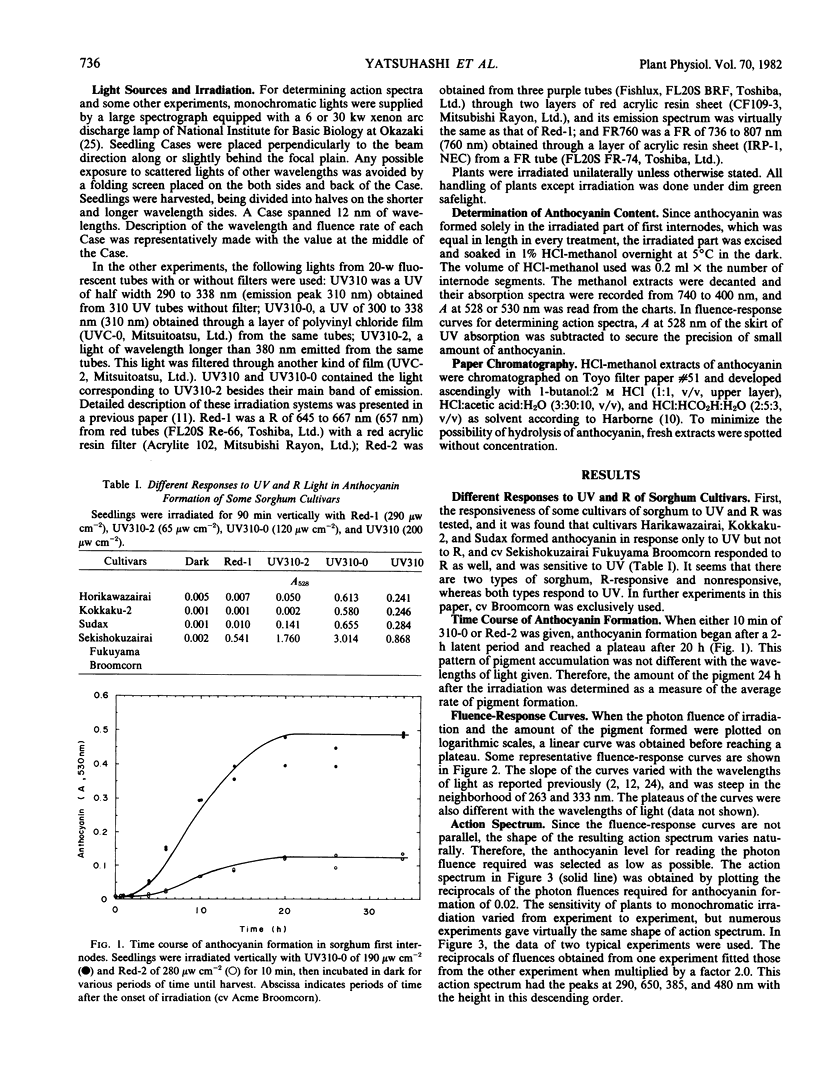
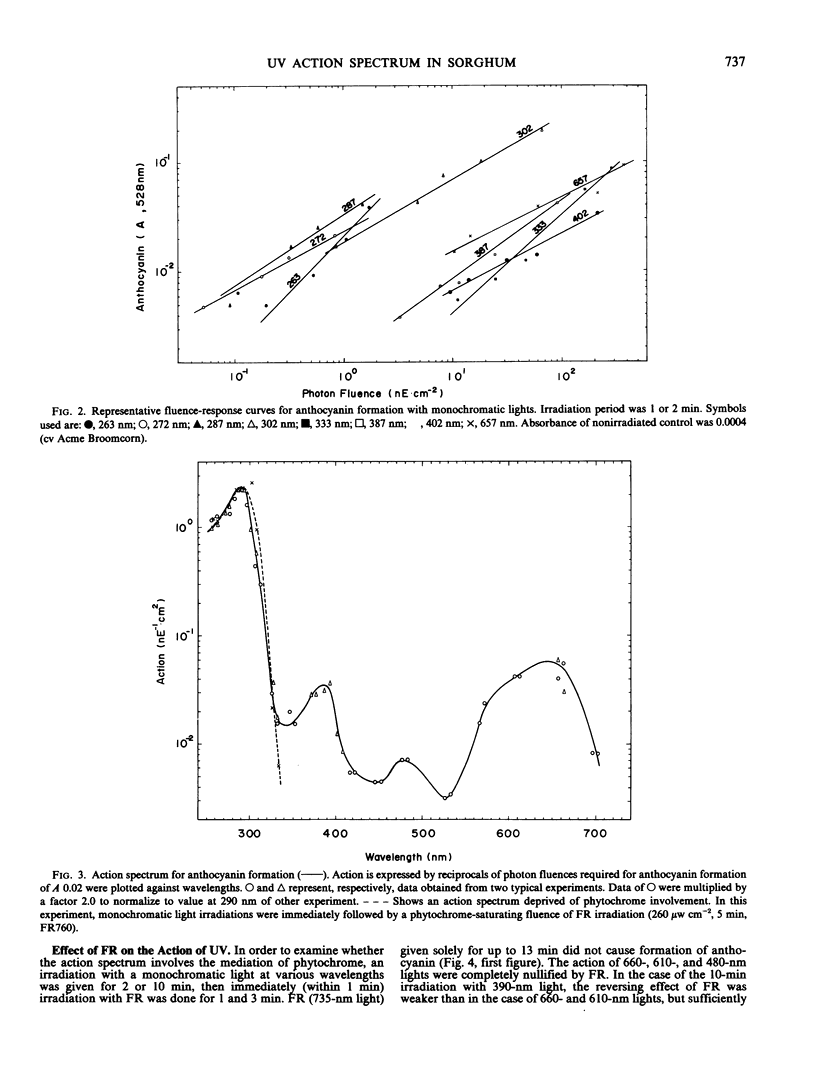
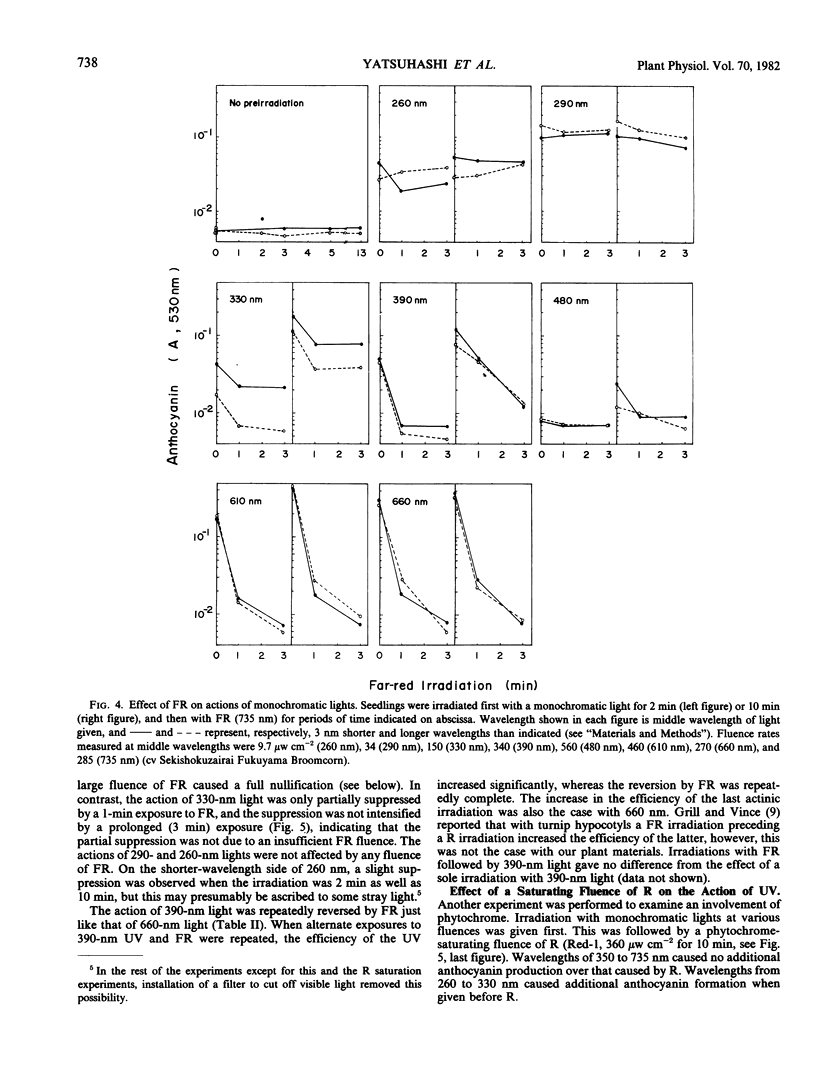
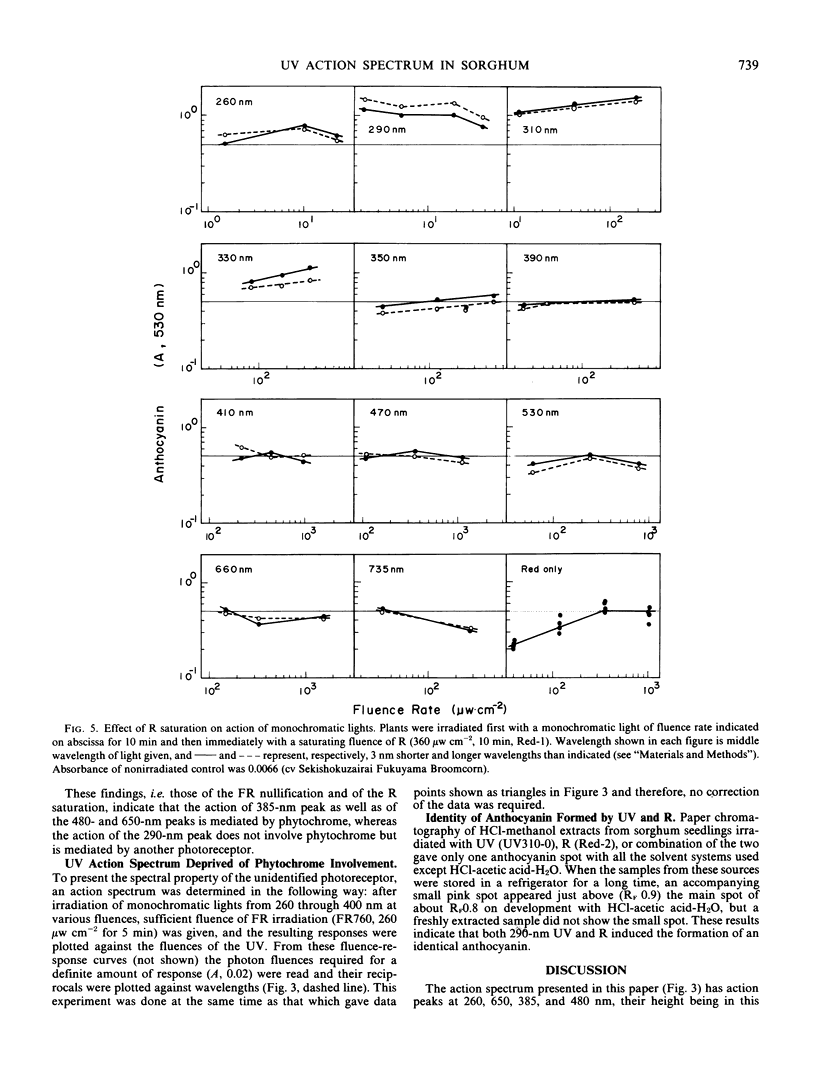
An action spectrum for anthocyanin formation in dark-grown broom sorghum (Sorghum bicolor Moench, cv Acme Broomcorn and cv Sekishokuzairai Fukuyama Broomcorn) seedlings was determined over the wavelength range from 260 to 735 nanometers. The action peaks were at 290, 650, 385, and 480 nanometers in descending order of height. The action of the 290-nanometer peak was not affected by subsequently given far red light, whereas those of the other three action peaks were nullified completely. The nullification of the 385-nanometer peak action by far red light was reversible. When an irradiation at these action peaks was followed by a phytochrome-saturating fluence of red light irradiation, the action of the 290-nanometer peak remained, whereas that of the 385-nanometer peak as well as those of the 650- and 480-nanometer peaks was masked by the action of the second irradiation. These findings suggested that the 290- and 385-nanometer action peaks involved different photoreceptors, the latter being phytochrome. The blue light-absorbing photoreceptor as reported to be a prerequisite for phytochrome action in milo sorghum was not found to exist in the broom sorghums.
The action spectrum deprived of the involvement of phytochrome was determined in the ultraviolet region by irradiating with far red light following monochromatic ultraviolet light. The spectrum had a single intense peak at 290 nanometers and no action at all at wavelengths longer than 350 nanometers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs C. J., Holmes M. G., Jabben M., Schäfer E. Action Spectra for the Inhibition of Hypocotyl Growth by Continuous Irradiation in Light and Dark-Grown Sinapis alba L. Seedlings. Plant Physiol. 1980 Oct;66(4):615–618. doi: 10.1104/pp.66.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. J., Siegelman H. W. Photocontrol of Anthocyanin Synthesis in Milo Seedlings. Plant Physiol. 1963 Jan;38(1):25–30. doi: 10.1104/pp.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindoo S. J., Caldwell M. M. Ultraviolet-B Radiation-induced Inhibition of Leaf Expansion and Promotion of Anthocyanin Production: Lack of Involvement of the Low Irradiance Phytochrome System. Plant Physiol. 1978 Feb;61(2):278–282. doi: 10.1104/pp.61.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG Y. L., THIMANN K. V., GORDON S. A. THE BIOGENESIS OF ANTHOCYANINS. X. THE ACTION SPECTRUM FOR ANTHOCYANIN FORMATION IN SPIRODELA OLIGORRHIZA. Arch Biochem Biophys. 1964 Sep;107:550–558. doi: 10.1016/0003-9861(64)90315-7. [DOI] [PubMed] [Google Scholar]
- Pratt L. H., Butler W. L. Phytochrome conversion by ultraviolet light. Photochem Photobiol. 1970 Jun;11(6):503–509. doi: 10.1111/j.1751-1097.1970.tb06021.x. [DOI] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Anthocyanin Formation in Turnip and Red Cabbage Seedlings. Plant Physiol. 1957 Sep;32(5):393–398. doi: 10.1104/pp.32.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Photocontrol of Anthocyanin Synthesis in Apple Skin. Plant Physiol. 1958 May;33(3):185–190. doi: 10.1104/pp.33.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


