Abstract
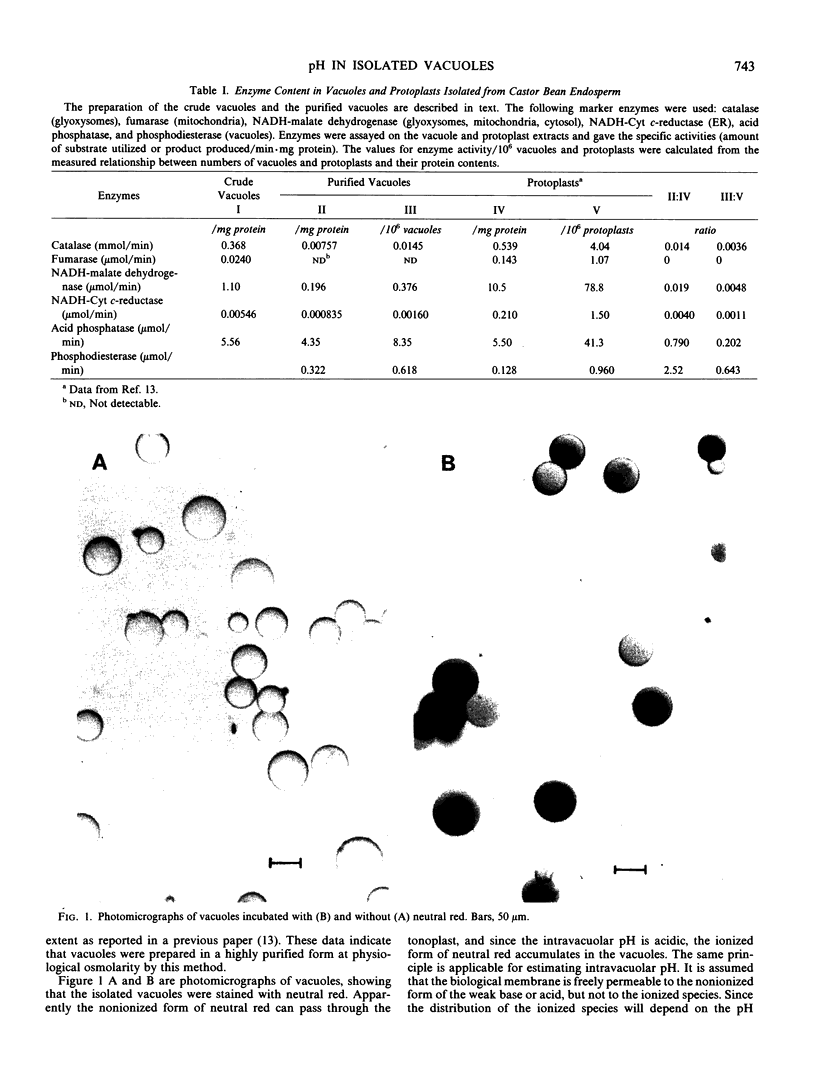
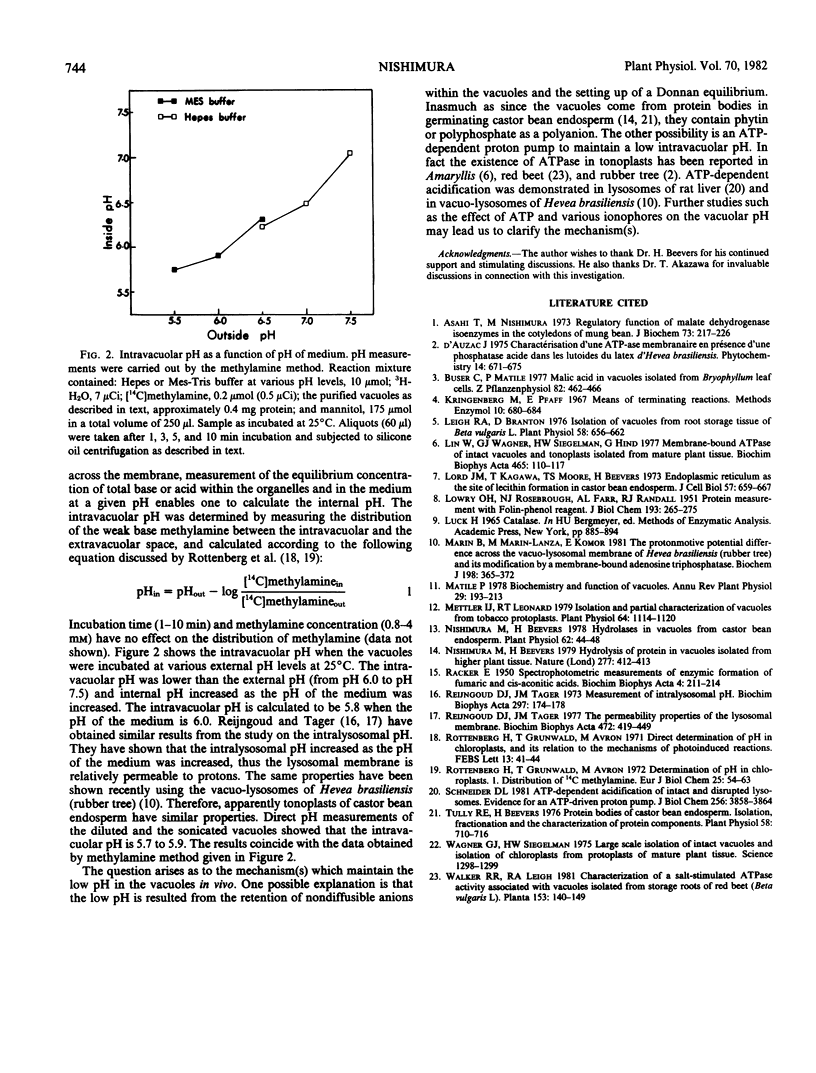
Vacuoles were prepared from germinating castor bean endosperm (Ricinus communis var Hale) and purified by filtration through a cotton layer under physiological osmolarity. The purity of vacuoles prepared by this method was comparable with that prepared by a sucrose step gradient centrifugation reported in a previous paper (Nishimura, Beevers 1978 Plant Physiol 62: 44-48). It was shown by assays of marker enzymes that the final preparation contained trace contamination of other organelles (glyoxysomes, mitochondria, and endoplasmic reticulum) and the cytosol. The isolated vacuoles were stained with neutral red, indicating that the intravacuolar pH is acidic. Intravacuolar pH of isolated vacuoles was determined by measuring the distribution of [14C]methylamine in the vacuoles and by directly measuring the pH of vacuolar extracts. The pH of isolated vacuolar extracts was 5.7 to 5.9. Similar values were obtained by the methylamine method and it was shown that intravacuolar pH increased as the pH of the medium was increased.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahi T., Nishimura M. Regulatory function of malate dehydrogenase isoenzymes in the cotyledons of mung bean. J Biochem. 1973 Feb;73(2):217–225. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B., Marin-Lanza M., Komor E. The protonmotive potential difference across the vacuo-lysosomal membrane of Hevea brasiliensis (rubber tree) and its modification by a membrane-bound adenosine triphosphatase. Biochem J. 1981 Aug 15;198(2):365–372. doi: 10.1042/bj1980365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Leonard R. T. Isolation and partial characterization of vacuoles from tobacco protoplasts. Plant Physiol. 1979 Dec;64(6):1114–1120. doi: 10.1104/pp.64.6.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. The permeability properties of the lysosomal membrane. Biochim Biophys Acta. 1977 Nov 14;472(3-4):419–449. doi: 10.1016/0304-4157(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Riejngoud D. J., Tager J. M. Measurement of intralysosomal pH. Biochim Biophys Acta. 1973 Jan 24;297(1):174–178. doi: 10.1016/0304-4165(73)90061-5. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Determination of pH in chloroplasts. I. Distribution of ( 14 C) methylamine. Eur J Biochem. 1972 Jan 31;25(1):54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Grunwald T., Avron M. Direct determination of DeltapH in chloroplasts, and its relation to the mechanisms of photoinduced reactions. FEBS Lett. 1971 Feb 12;13(1):41–44. doi: 10.1016/0014-5793(71)80659-2. [DOI] [PubMed] [Google Scholar]
- Schneider D. L. ATP-dependent acidification of intact and disrupted lysosomes. Evidence for an ATP-driven proton pump. J Biol Chem. 1981 Apr 25;256(8):3858–3864. [PubMed] [Google Scholar]
- Tully R. E., Beevers H. Protein bodies of castor bean endosperm: isolation, fractionation, and the characterization of protein components. Plant Physiol. 1976 Dec;58(6):710–716. doi: 10.1104/pp.58.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]