Abstract
Alcohol Use Disorder (AUD) is recognized as harmful for the developing brain. Numerous studies have sought environmental and genetic risk factors that predict the development of AUD, but recently identified resilience factors have emerged as protective. This chapter reviews normal processes of brain development in adolescence and emerging adulthood, delineates disturbed growth neurotrajectories related to heavy drinking, and identifies potential endogenous, experiential, and time-linked brain markers of resilience. For example, concurrent high dorsolateral prefrontal activation serving inhibitory control and low nucleus accumbens activation serving reward functions engender positive adaptation and low alcohol use. Also discussed is the role that moderating factors have in promoting risk for or resilience to AUD. Longitudinal research on the effects of all levels of alcohol drinking on the developing brain remains crucial and should be pursued in the context of resilience, which is a promising direction for identifying protective biomarkers against developing AUDs.
Keywords: resilience, vulnerability, alcohol, youth, family history
1. Epidemiology of Alcohol Use in Adolescence
Alcohol Use Disorder (AUD) is a major public health concern being an identified cause in almost 6% of deaths and more than 200 fatal diseases (World Health Organization, 2018). Numerous studies have considered the burden of AUD and have noted behavioral consequences and brain disturbances (Bernardin, Maheut-Bosser, & Paille, 2014; Bühler & Mann, 2011; Le Berre, Fama, & Sullivan, 2017; Oscar-Berman & Marinković, 2007; Sullivan & Pfefferbaum, 2019), which in turn increase the risk of perpetuating heavy drinking (Zahr, Pfefferbaum, & Sullivan, 2017). In the last decade, efforts using prospective longitudinal designs have accelerated investigations of the potential vulnerabilities and consequences of alcohol use in adolescents and young adults. Indeed, excessive alcohol use is prevalent in youth, and epidemiological surveys have found that the younger adolescents initiate drinking, the more likely they are to develop AUD (Enoch, 2006; Grant et al., 2015).
Adolescence is a critical period of development when biological, social, cognitive, and affective changes shape adult behavior. It is during this developmental stage that experimenting with risky behaviors, such as alcohol use, is initiated. The National Institute on Alcohol Abuse and Alcoholism (NIAAA, 2018) reports that by age 18, 58% of youth have already drunk alcohol, while about 60% of college students have drunk in the last month, and 20% meet criteria for an AUD diagnosis. Unlike adult drinking patterns, which usually entail multiple, moderate drinking episodes during the week, underage drinking is typically done on weekends and often at binge levels. Binge drinking denotes consumption of high quantities of alcohol in a single occasion, corresponding to a Blood Alcohol Concentration level of 0.08g/dl or greater. Other patterns of alcohol misuse are also reported in youth studies (details are described in Table 1).
Table 1.
Alcohol misuse patterns described in youth and definitions of binge drinking according to countries.
| Quantity | Time frame | Frequency | Country and alcohol definition | |
|---|---|---|---|---|
|
| ||||
| HAZARDOUS DRINKING | ||||
|
| ||||
| AUDIT | AUDIT ≥ 8 | / | / | International 8 (UK), 10 (Europe), or 14 (USA) grams of pure ethanol in one alcohol drink |
| AUDIT-C | AUDIT-C ≥ 3 (female) AUDIT-C ≥ 4 (males) |
|||
|
| ||||
| HEAVY DRINKING | ||||
|
| ||||
| Cahalan inventory | 3–4 drinks on average | On one occasion | At least twice a month | USA |
|
| ||||
| BINGE DRINKING | ||||
| NIAAA | 4+ (female) 5+ (male) BAC level: .08g/dL |
Within less than 2 hours | / | USA |
|
| ||||
| SAMSHA | 4+ (female) 5+ (male) |
On one occasion | At least one day in the past month | USA |
|
| ||||
| WHO | 6+ | On one occasion | At least once | International |
Notes. NIAAA is the National Institute on Alcohol Abuse and Alcoholism. SAMHSA is the Substance Abuse and Mental Health Services Administration. WHO is the World Health Organization.
2. Overview of Factors Associated with Alcohol Misuse in Adolescence
Several factors have been identified that contribute to persistent alcohol misuse during adolescence, including low socioeconomic status (SES), early age of alcohol use onset, binge drinking, and family history of AUDs (Brown & Tapert, 2004). Binge drinking has been identified as a predictor of a future AUD (Bonomo, Bowes, Coffey, Carlin, & Patton, 2004; Gowin, Sloan, Stangl, Vatsalya, & Ramchandani, 2017; Jones, Steele, & Nagel, 2017). In particular, repeated alternations between drunkenness and abstinence characterizing binge drinking is purported to induce cognitive impairments and brain disturbances (Stephens & Duka, 2008) similar to those observed following repeated withdrawal episodes in adulthood (Becker, 1998; Duka, Townshend, Collier, & Stephens, 2003). Binge drinking could thus impair neuroplasticity and favor the development of a future AUD (Loheswaran et al., 2016). Cross-sectional studies have now established that binge drinking in youth is related to central nervous system (CNS) disturbances (Cservenka & Brumback, 2017), cognitive dysfunction (Carbia, López-Caneda, Corral, & Cadaveira, 2018), and emotional difficulties (Lannoy, Duka, et al., 2021). Such episodes are thus thought to increase the risk of uncontrolled and problematic drinking in youth (e.g., Bell et al., 2013). Nevertheless, longitudinal studies focusing on adolescents before drinking onset are necessary to confirm these results and establish causality.
Genetic predisposition is another mechanism contributing to hazardous drinking, given that binge drinking and drunkenness rates in the transition from adolescence to adulthood have been linked to a GABRA2 polymorphism (Dick et al., 2014). The hypothesis is that excessive drinking in adolescence favors the expression of gene variants associated with AUD vulnerability (Agrawal et al., 2009). Thus, youth with a genetic vulnerability, often assumed from a positive family history (FHP) of AUD, are at a higher risk for binge drinking and alcohol misuse relative to family history negative (FHN) youth (Gowin et al., 2017).
Family history of AUD is established when an individual has biological relatives (most often biological parents) diagnosed with AUD, generally across multiple generations (Alterman, 1988; Schuckit, 1985;1995). Family history of AUD has been associated with heightened risk of alcohol-related problems (e.g., social and academic difficulties, higher rates of hangover; Elliott, Carey, & Bonafide, 2012). This linkage has been supported by a population-based cohort study showing that offspring from high-density families (i.e., multiple relatives with AUD) have a heightened risk for alcohol misuse and AUD. High-density AUD families are also associated with low socio-economic status and high risk of psychiatric disorders (Kendler, Ohlsson, Bacanu, Sundquist, & Sundquist, 2020). Critically, these social and psychiatric risks are attenuated by achieving high levels of school attainment, promoting resilience, which has been measured as the ability to cope with stressful life events (Kendler et al., 2020). Compared with their peers, FHP youth also exhibited impairments in executive and affective processing and disrupted functional brain activations even before the onset of an AUD (Cservenka, 2016).
Youth from AUD parents were found to be less reactive to alcohol effects, as observed with electrophysiological measures (e.g., Schuckit et al., 1988). Electrophysiological studies also indicated a developmental delay in FHP youth, revealed at the P300 level (Hill et al., 1999; Porjesz et al., 1998; Rangaswamy et al., 2007), a component related to cognitive and decisional processes (Polich, 2007). The proposal is that genetic variations modulate event-related oscillations and contribute to AUD vulnerability (Porjesz et al., 2002; Chen et al., 2009). In addition, brain volume differences were observed between FHP and FHN youth during adolescence in the amygdala, orbitofrontal cortex, and cerebellum (Hill et al., 2001; Hill et al., 2007; O’Brien and Hill, 2017). These differential brain volumes have been associated with P300 developmental delay (Hill et al., 2001) and predicted substance use in young adulthood (O’Brien and Hill, 2017). Longitudinal studies further indicated brain changes related to substance use in FHP youth compared to controls, notably, greater activation of the nucleus accumbens during reward anticipation (Cope et al., 2019). Nevertheless, potentially consistent with previous observations (Kendler et al., 2020), some studies targeting FHP adolescents showed that they did not differ from their peers (Bjork, Knutson, & Hommer, 2008; Müller et al., 2015). In these studies, striatal activation (nucleus accumbens) during reward elicitation was similar between matched FHP and FHN adolescents. Clearly then, genetic makeup can heighten an individual’s risk but does not determine the ultimate engagement in excessive drinking or the development of an AUD (Jones et al., 2017). Therefore, protective factors of resilience that account for avoiding alcohol misuse despite all odds need to be identified.
Next, we describe the presumed effects of alcohol use on the developing brain and recognize that some effects might be pre-existing. Then, we approach the role of resilience and focus on brain measures to identify which markers may protect from alcohol misuse development. Finally, we review moderators found to be involved in the pathway toward AUD.
3. Alcohol Use during Adolescence: Its Effect on Brain Structure and Function
The normal developmental trajectory of brain neurobiology is characterized by a decrease in cortical (Giedd, 2008; Gur et al., 2019; Pfefferbaum et al., 2018; Raznahan et al., 2014) and cerebellar (Giedd, 2008; Sullivan et al., 2020) gray matter volume, often interpreted as evidence for neuronal pruning and restructuring in response to environmental interactions occurring postnatally through adolescence (Campbell & Feinberg, 2009; Feinberg, Higgins, Khaw, & Campbell, 2006). An increase in white matter volume throughout adolescence and emerging adulthood likely represents increased structural and functional connectivity, again in response to an individual’s experience and typical developmental processes (Giedd, 2008; Pfefferbaum et al., 2018; Raznahan et al., 2014; Sullivan et al., 2020). Brain development is influenced by myriad factors, notably biological (physiological, genetic) and psychosocial (external and internal milieu), which can alter brain structure, function, and behavior (Walker et al., 2011). These changes affect functioning and can have long-lasting consequences even into later adulthood, thus implicating adolescence as a time of vulnerability for developing psychiatric disorders (Meyer & Lee, 2019), including addiction (e.g., Bell et al., 2013; Silveri & Spear, 2002; Spear, 2010; Stephens & Duka, 2008; Witt, 1994). While adolescence is a vulnerable period for the development of neuropsychiatric pathology, it also constitutes a critical period when protective factors can exert a mitigating effect (Figure 1). For example, positive rearing environment and care-giver interactions promote problem-solving and behavioral regulation, which can concomitantly favor brain and behavioral development (Walker et al., 2011). Nevertheless, even apparently ideal rearing conditions are not a guarantee to thwart risk-taking or thrill-seeking behavior.
Figure 1.
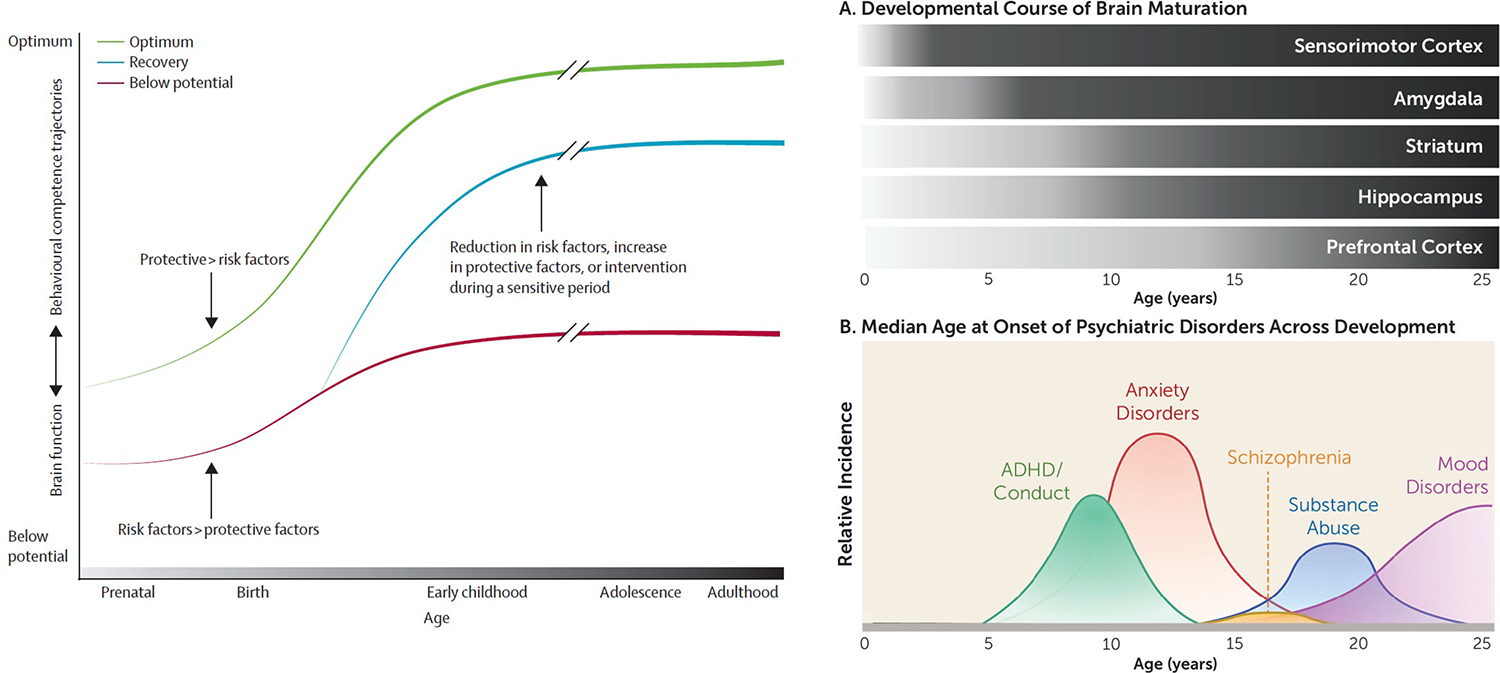
LEFT: Differing trajectories of brain and behavioural development as a function of exposure to risk and protective factors. The cumulative effect is illustrated by the progressive strengthening (darker lines) of the trajectories over time. Figure and caption reprinted with permission from Walker et al. (2011).
RIGHT, TOP: Comparison of different developmental courses of selective frontal and subcortical structures relevant to addiction.
RIGHT, BOTTOM: Distributions of median ages of the incidence of onest of psychiatric disorders occurring from pre-adolescence to emerging adulthood. Figures reprinted with permission from Meyer et al. (2019).
The development of brain structures and circuitry during adolescence are characterized by heterochronicity in maturation and a possible source of vulnerability. For instance, the limbic brain system matures quickly during early adolescence (Meruelo, Castro, Cota, & Tapert, 2017; Morgane, Galler, & Mokler, 2005; Steinberg, 2008), while the prefrontal cortex matures gradually over the course of adolescence (Bava & Tapert, 2010; Brown & Tapert, 2004; Giedd, 2008; Pfefferbaum et al., 2018; Raznahan et al., 2014; Steinberg, 2008). As a hypothetical example, asynchronous brain systems development may result in enhanced reward sensitivity linked to early striatal development and immature executive skills linked to later prefrontal development, rendering adolescents susceptible to risk-taking behavior (Jadhav & Boutrel, 2019), such as engagement in alcohol drinking. In addition, excessive alcohol use is a particular concern in adolescence because the young brain is highly susceptible to alcohol-related consequences. Ample evidence is available from animal studies, showing that adolescents are more sensitive to various alcohol effects (Nixon & McClain, 2010; Obernier, White, Swartzwelder, & Crews, 2002; Spear, 2014). Notably, intermittent ethanol exposure (which can model binge drinking in human) impacts adult behavior with long-term effects on alcohol drinking, emotional symptoms, memory, and executive functions (Crews, 2008; Crews et al., 2019; Hauser et al., 2019; Varlinskaya, Hosová, Towner, Werner, & Spear, 2020). Prospective human studies support the deleterious effects of adolescent heavy drinking on brain volume and integrity (Jernigan et al., 2016; Pfefferbaum et al., 2018; Sullivan et al., 2020; Zhao et al., 2019). Adolescence is thus defined as a developmental window of vulnerability for the onset of excessive and potentially problematic alcohol use (e.g., Bell et al., 2013; Spear, 2018; Varlinskaya & Spear, 2015). Studies showing an association between alcohol use and neuroradiologically-determined neuropathology in adolescent human samples are described next.
3.1. Brain structural disturbance
Neurobiological measures from young drinkers provide objective and prospective evidence that alcohol disturbs neuromaturation. Both alcohol use initiation and binge drinking led to cortical thinning in frontal, anterior cingulate, and posterior cingulate cortices in adolescents and young adults (Luciana, Collins, Muetzel, & Lim, 2013; Mashhoon et al., 2014). Regionally thin cortices were also related to greater drinking quantity and frequency and to recent alcohol use (Mashhoon et al., 2014). Research focusing on substance-naive adolescents at baseline corroborates that thinner dorsolateral prefrontal cortex and inferior frontal cortex predicted binge drinking and externalizing symptoms in late adolescence, especially in youth who were FHP (Brumback et al., 2016).
In addition, smaller gray matter volumes in frontal, striatal, and limbic regions were found in adolescents (Heikkinen et al., 2017) considered hazardous drinkers (Table 1). This result observed in young adults, was supported, with evidence for accelerated decline of gray matter volumes in the hippocampus and para-hippocampus over 2 years of binge drinking; functionally, poorer memory and more memory blackouts were associated with greater volumetric decline in the hippocampus (Meda et al., 2018). Longitudinally, heavy drinking (Table 1) was found to disrupt normal brain development. In particular, adolescents who became heavy drinkers showed accelerated volume loss in frontal (Pfefferbaum et al., 2018) and cerebellar (Sullivan et al., 2020) gray matter; decreased frontal volumes were also related to the maximum number of drinks consumed on one occasion in the past year (Pfefferbaum et al., 2018).
Regarding white matter, a longitudinal study provided evidence for attenuated white matter volume growth in drinking adolescents and young adults (Squeglia et al., 2015). Results from diffusion tensor imaging, which assesses the microstructure of fiber tracks, showed poorer white matter integrity in hazardous and binge drinkers compared with controls (Pohl et al., 2016). Lower fractional anisotropy (an index of fiber organization), greater radial diffusivity (an index of edematous tissue or myelin disturbance), and greater axial diffusivity (an index of abnormally thin axonal caliber) occurred in several brain regions of heavy-drinking adolescents and young adults relative to no-to-low drinking youth (Pohl et al., 2016; Shen et al., 2019; Smith et al., 2017; Zhao et al., 2020). In young adults, low fractional anisotropy was related to binge-drinking intensity and poorer working memory (Smith et al., 2017). Of particular relevance is the longitudinal analysis of Zhao et al. (2020) conducted prospectively on 451 adolescents over a 5-year interval that presented seminal findings supporting excessive drinking in young adolescents as likely causative of accelerated decline in and deviation from the no-to-low drinking group in the trajectories of white matter microstructural development in selective frontal system.
3.2. Brain functional impairments
Numerous studies using functional MRI brain measures indicate modified brain activity among young drinkers relative to their no-to-low drinking counterparts or even to themselves before initiating drinking. Young adult binge drinkers exhibited greater frontoparietal activation during working memory than no-to-low drinkers with no detectable hippocampal activity (Schweinsburg, McQueeny, Nagel, Eyler, & Tapert, 2010). When attempting to inhibit negative emotions elicited by viewing unpleasant images, binge drinkers showed lower frontal and striatal activations than non-bingers, and greater binge drinking frequency was predictive of its extent (Cohen-Gilbert et al., 2017).
Longitudinal studies indicated that youth who reported a history of heavy or binge drinking (Table 1) within the previous three years exhibited reduced activation in frontal and striatal networks when correctly inhibiting an automatic response at baseline compared to youth who remained no-to-low drinkers (Norman et al., 2011; Wetherill, Squeglia, Yang, & Tapert, 2013). A three-year follow-up revealed that binge drinkers had greater frontoparietal and cerebellar brain activation, whereas controls who remained no-to-low drinkers showed a decrease in these brain regions (Wetherill and colleagues, 2013), suggesting that alcohol misuse disrupts the developmental trajectory of executive control. Furthermore, longitudinal studies found that before engaging in binge drinking, adolescents who later went on to binge drink had abnormally low frontoparietal activation during working memory (Squeglia et al., 2012) and decision-making (Jones, Cservenka, & Nagel, 2016) processing, which are two different components of frontoparietal functioning. After initiation of binge drinking, attenuation of dorsal striatal activation was observed during decision-making and was negatively correlated with the total number of binge-drinking episodes in the past 90 days (Jones et al., 2016). Increased activation in the reward circuitry (nucleus accumbens, precuneus) and in the insula during decision-making, under risk, also predicted the onset of binge drinking in adolescents (Morales, Jones, Ehlers, Lavine, & Nagel, 2018; Xiao et al., 2013), suggesting that increased striatal and limbic activation in a risk/reward context is related to higher risk-taking behavior during adolescence.
Neuroimaging studies inform us about brain responsivity to affective stimuli and alcohol-related cues. Young adults who binge drink exhibit different activation patterns from non-bingers when processing emotional stimuli (Lannoy, Dricot, et al., 2021; Maurage, Bestelmeyer, Rouger, Charest, & Belin, 2013; Rae et al., 2020). For example, high frontal activation in the face of low temporal activation noted in young bingers was hypothesized to reflect functional compensation of possible alcohol-related disruption (Maurage et al., 2013). A focus on implicit brain responses to various emotional contents showed that binge drinkers, relative to controls, had low insular activation when processing happiness, low frontal and cingulate activation when viewing sadness, high cingulate activation when viewing fear, and high cerebellar activation when viewing anger (Lannoy, Dricot, et al., 2021). In response to pain-related pictures, binge drinkers also had higher activation in the fusiform and inferior temporal gyrus than non-binge drinkers (Rae et al., 2020). Distinct CNS responses are elicited during emotional and social processing, and these are assumed to be involved in alcohol misuse and its perpetuation; however, longitudinal studies are needed to delineate and confirm these results. In an alcohol-related context, longitudinal studies revealed that moderately drinking adolescents (Nguyen-Louie, Courtney, et al., 2018) and young adults (Courtney, Infante, Bordyug, Simmons, & Tapert, 2020; Dager et al., 2014), who later transitioned to heavy or binge drinking, showed elevated frontolimbic and frontostriatal activity in response to alcohol cues at baseline testing.
Finally, functional connectivity analysis has revealed the relevance of specific neurocircuitry that contributes to, or is altered by, binge drinking. A longitudinal study indicated increased functional connectivity in the Default Mode Network (DMN) in binge drinkers over two years, whereas controls showed decreased functional DMN connectivity over the same duration (Correas et al., 2016). A six-year study further showed that an earlier age-of-onset of weekly drinking was associated with higher context-dependent functional connectivity between the bilateral posterior cingulate and cortical (right frontal, parietal, and left lingual areas) and subcortical (right cingulate precuneus) areas, this being related to poor attentional abilities (Nguyen-Louie, Simmons, et al., 2018). In young adults, stronger correlations between reduced frontal activation during inhibition and increased striatal activation during monetary reward distinguished binge drinkers from controls (Weafer, Crane, Gorka, Phan, & de Wit, 2019).
Short conclusion:
This subsection delineated cross-sectional and longitudinal evidence that structural and functional brain regional and neurocircuit disturbances appear to be attributable to the initiation of alcohol misuse in adolescents and young adults. First, studies highlighted predisposing brain vulnerabilities by indicating that pre-existing differences in brain functional responses in frontal and striatal networks can be observed in future drinkers (Figure 2), supporting the role of higher reward-seeking and lower executive control in the engagement of excessive drinking. Some studies also reported functional changes in the cerebellum among drinkers, but additional longitudinal research is needed to determine its role in alcohol use onset and maintenance. Second, evidence supports functional consequences related to alcohol misuse by showing that youth who initiated hazardous or binge drinking exhibited anomalous brain changes in frontolimbic and frontostriatal areas and functional aberrations in DMN connectivity. Alcohol-related consequences on neurostructural integrity were evident from disturbed developmental trajectories of gray matter and white matter volume and cortical thickness (Figure 3). Overall, these observations are consistent with addiction models (Sullivan & Pfefferbaum, 2019; Wise & Koob, 2014) emphasizing the conjoint role of executive control and reward-processing, and their associated neural circuits, as a vulnerability factor predisposing youth to engage in alcohol abuse and as a consequence of having engaged in drinking alcohol to excess.
Figure 2.

(A) Brain activation during receipt of monetary reward (Doors task: Win>Loss) in left VS. Significant peak activation was observed at the [−6 18 −2] MNI coordinate after correcting for family wise error (pFWE < 0.05) within the reward mask. (B) Brain activation during successful response inhibition (StopInh>Go) within the frontal-insular-subcortical (FIS) mask. Significant (pFWE < 0.05) peak activation was observed in a large right prefrontal cluster encompassing precentral, middle, and inferior gyri and anterior insula. The top scatter plot presents the negative relationship between extracted BOLD signal from a 10 mm radius spherical region centered at the [−6 18 −2] peak in the left VS (A) during receipt of monetary reward and extracted BOLD signal from a 10 mm radius spherical region centered at the [42 4 32] peak in the right IFG during response inhibition (B). The bottom scatter plot presents the negative relationship between extracted BOLD signal from the left VS (A) during receipt of monetary reward and extracted BOLD signal from a 10 mm radius spherical region centered at the [28 2 50] peak in the right MFG during response inhibition (C) The negative relationships indicate that less right frontal activation during response inhibition was associated with greater left VS activation during reward. Figure and caption taken with permission from Weafer et al. (2019).
Figure 3.

Top left: The plots connect values at baseline and 1- and 2-year follow-ups for 356 adolescents who continued to meet no/low criteria (gray) and heavy drinkers (green) as a function of baseline age; the linear mixed-effects model (lmer) fits with one and two standard deviations, computed separately for no/low and heavy drinkers, are also plotted.
Top right: The figure shows lateral and medial views of the left hemisphere. Regions showing faster declines in cortical volumes in the heavy drinkers relative to the no/low drinkers, displayed in bright orange, are false discovery rate corrected (p<,0.025).
Top figures and captions taken and modified with permission from Pfefferbaum et al. (2018).
Bottom left: Trajectories (i.e., regression lines) of individual heavy drinkers (green). The lmer fits with 61 and 2 SD separately computed for no/low drinkers (gray) and heavy drinkers (green) are also plotted.
Bottom right: Jitterplots of slopes (expressed as % change per year) by tissue type for the 328 no/low drinkers (gray) and all 220 youth who initiated heavy drinking (black). Red asterisks mark differences from the no/low drinking group in slopes meeting correction for multiple comparisons. Black asterisks note differences with p ≤ .05. The cerebellar images to the right of the jitterplots display in gray the lobules showing group differences (p ≤ .05) in slopes.
Bottom figures and captions taken and modified with permission from Sullivan et al. (2020).
4. Resilience and Brain Mechanisms
Parsing resilience into its component processes is central for recognizing factors that can be independently affected positively, negatively, or indifferently during adaptations to stressful life events or unfavorable environments (Alim et al., 2012). The many studies using subjective measures have shown that self-reported resilience mitigated risks for developing an AUD (Kennedy et al., 2019; Long et al., 2017). Beyond this psychological evidence, neuroscience studies indicated that compensation and reorganization of brain functions may offset vulnerability and thus constitute resilience markers (Cicchetti & Blender, 2006; Cousijn, Luijten, & Feldstein Ewing, 2018). This proposal of brain resilience received support in findings from a large European consortium (Burt et al., 2016), reporting that larger gray matter volumes in the right middle and superior frontal gyri were shown in adolescents (12–17 years old) who had experienced adversity (stressful live events assessed through the following domains: family, accident/illness, sexuality, autonomy, deviance, relocation, and distress) and exhibited positive adaptation (good rule-abiding conduct, social skills, relationships, academics, and absence of internalizing problems). Whether such evidence for resilience to trauma generalizes to resistance to drinking remains to be established. Some indications suggest possible resilience brain markers in youth who, despite a genetic vulnerability (family history of AUD), did not develop alcohol misuse.
4.1. Resilience Brain Markers
Neuroimaging research supports the existence of specific brain correlates associated with resilience in youth with a family history of AUD. Comparing resilient (non-drinkers) to vulnerable (hazardous drinkers, Table 1) emerging adults (18–25 year-olds) of AUD parents, cross-sectional results showed that the vulnerable group reported emotional distress, risky substance use, and greater activity in the middle frontal gyrus when rating negative emotional pictures compared with their resilient counterparts and showed higher activation of the posterior cingulate cortex during a working memory challenge (Brown-Rice et al., 2018). In another cross-sectional study, blunted nucleus accumbens responsivity was observed during decision-making in FHP participants who did not present alcohol use problems, suggesting that this attenuated activity is protective (Yau et al., 2012).
A longitudinal study assessed self-reported psychological resilience in early adolescence (12–15 years old) and related it to measures taken in late adolescence (Weiland et al., 2012). Greater resilience was predictive of lower substance use, fewer alcohol-related problems, and better executive performance. Associations also emerged between resilience and subthalamic nucleus and pallidum activation detected with fMRI during a working memory task. Moreover, compared with a low resilience group, youth with high resilience had stronger functional connectivity between the subthalamic nucleus and median cingulate cortex (Weiland et al., 2012). In the same cohort, psychological resilience in adolescents with a family history of AUD and carriers of the G allele (GABRA2) was associated with lower activation in the inferior parietal cortex during emotional processing and less severe externalizing behaviors (Trucco, Cope, Burmeister, Zucker, & Heitzeg, 2018); however, which resilience factor exerted the greatest influence remains unknown.
Several studies also directly compared resilient and vulnerable youth with prospective evaluations, for which follow-up was conducted after the onset of hazardous drinking or substance use in vulnerable youth. The relevance of functional connectivity between frontal and striatal brain networks (dorsolateral prefrontal cortex and posterior cingulate) was found, such that resilient youth (11–16 years old) had greater functional connectivity between the left dorsolateral prefrontal cortex and the left posterior cingulate cortex than vulnerable youth before the onset of excessive drinking (Martz et al., 2019). These two networks were also explored separately by evaluating brain responses during executive control and reward processing (adolescents from 12 to 14 years old at baseline). Greater activation in the right dorsolateral prefrontal cortex during correct inhibition was a significant predictor of resilience, whereas neural activation in the ventral striatum (nucleus accumbens) was not a significant predictor of resilience (Martz, Zucker, Schulenberg, & Heitzeg, 2018). Functional brain responses were also evaluated during passive viewing of emotional words (positive, negative, or neutral). Resilient adolescents (11–17 years old) had greater activation in bilateral orbital frontal gyrus and left insula in response to emotional stimuli, whereas vulnerable ones showed higher activation of the dorsomedial prefrontal cortex and lower activation of the ventral striatum and the amygdala bilaterally. The vulnerable group also had more externalizing symptoms, such as aggressive and delinquent behaviors, than the resilient group (Heitzeg, Nigg, Yau, Zubieta, & Zucker, 2008).
Short conclusion:
Specific patterns of brain responsivity may serve as potential neurophysiological resilience markers and are observed principally in prefrontal and striatal systems (Figure 4). To wit, greater activation of the dorsolateral prefrontal cortex occurs during executive control in resilient versus vulnerable youth. The dorsolateral prefrontal cortex is considered a primary hub of the executive network, involved in goal-directed behaviors, and has a role in excessive drinking regulation (Abernathy, Chandler, & Woodward, 2010). It has also been related to impaired adaptation in adult AUD patients (Beylergil et al., 2017), supporting its role as a neurophysiological resilience marker. Functional connectivity between the dorsolateral prefrontal and posterior cingulate cortices and the overall conjoint role of frontal and limbic brain regions has also been supported as related to resilience. In striatal regions, lower activation of the nucleus accumbens during reward processing is associated with resilience. Indeed, the nucleus accumbens is considered a fundamental driver of reinforcement components of alcohol-related behaviors (Casey & Jones, 2010; Koob et al., 2014), and its lower activation may act as a resilience marker by reducing incentive salience, alcohol cue-reactivity, and further consumption.
Figure 4.

Extracted task-related functional connectivity change with NAcc during Incentive>Neutral anticipatory processing by family history group. L, left; R, right; NAcc, nucleus accumbens; SSMA, supplementary sensoriomotor area; PoG, postcentral gyrus; SMA, supplementary motor area; OCC, occipital. Figure and caption taken with permission from Weiland et al. (2013).
5. Moderators of the pathway to AUD
The study of both risk and protective resilience factors should be pursued together to identify patterns of brain structural, functional, and behavioral factors that distinguish vulnerable from resilient youth as it pertains to drinking. As noted by Brown and Tapert (2004), other factors may be involved in gateways towards alcohol misuse and AUD such as earlier age of alcohol use onset and lower SES. Given the complexity and heterogeneity of factors forming the constellation of different outcomes, data-driven machine-learning analysis of large and complex datasets is providing new leads beyond detection with traditional univariate analysis toward identifying specific and robust risk and resilience factors. Findings support the role of early drinking onset, stressful life events, and neural activation related to language and social processing (Gowin, Manza, Ramchandani, & Volkow, 2020; Ruan et al., 2019; Squeglia et al., 2017) and will require replication with prospective, hypothesis-directed studies.
Most of the studies described in this review compared groups of similar age, SES, or childhood history, which can statistically parse the contributions of each variable to the overall variance of a target outcome but preclude understanding the role of specific factors in isolation. What was determined, however, was that early drinking onset (~14 years old) predicted dependence symptoms and the development of AUD (Conde, Peltzer, Gimenez, & Cremonte, 2020; Dawson, Goldstein, Patricia Chou, June Ruan, & Grant, 2008), such that differences in brain functioning were salient between youth who started to drink early versus their peers who refrained from drinking (Baker et al., 2019; Zhao et al., 2020). Numerous studies have also identified stressful life events such as childhood trauma history or poor SES as predictors of alcohol misuse. Longitudinal studies showed that childhood trauma predicted peri-adolescent binge drinking (De Bellis et al., 2020) and alcohol-related problems (Hagborg, Thorvaldsson, & Fahlke, 2020). Disturbed brain functional connectivity (Silveira et al., 2020) and neural oscillations (Meyers et al., 2019) have also been related to trauma history and have predicted AUD in adolescents. Similarly, longitudinal studies showed that low SES and financial difficulties influenced drinking in adolescents (Nooner, De Bellis, Clark, Thompson, & Brumback, 2020; Poonawalla, Kendzor, Owen, & Caughy, 2014) and interacted with genetic factors to predict alcohol-related problems (Barr, Silberg, Dick, & Maes, 2018; Davis & Slutske, 2018). Taken together, these negative factors increased the likelihood of developing AUD. Conversely, some support has been given for the role of resilience in explaining low substance use in youth with trauma history (Wingo, Ressler, & Bradley, 2014). Critically, specific neuroanatomical and neurophysiological markers of reliably-detected resilience factors have yet to be identified. Further, under what conditions do aversive factors such as trauma become sources of resilience need delineation.
6. Trajectories of risk and resilience: Integration of findings
Research to date may guide our understanding of pathways to AUD and alternative routes to avert AUD, as summarized in Figure 5.
Figure 5.

This model depicts the role of different factors involved in the pathway toward AUD: (1) Risk factors for drinking in pink: poor control abilities (frontal network), high reward sensitivity (striatal network), as hypothesized as predisposing factors; emotional processes (limbic network) and executive and emotional processes (cerebellar network) to be investigated; and gray and white matter compromise as consequence of binge/hazardous drinking, increasing the risk of AUD. (2) Family and environmental factors as influencing binge/hazardous drinking. (3) Resilience-related brain activations, potentially moderating the risk associated with family and environmental factors and the pathway toward or away from AUD.
Pathways to AUD can be represented by the complex interaction of multiple factors, each contributing a specific weight to the overall risk. A comprehensive evaluation of family and environmental factors (e.g., family history of AUD, stressful life events) may help to determine alcohol behaviors in adolescence but also explain later risk of developing AUD. Alcohol use itself can modify specific brain physiology that engender AUD and perpetuate alcohol use. This review noted predisposing differences in frontal and striatal functional responses between future drinkers and non-drinkers. As highlighted in addiction models (Wise & Koob, 2014), these brain regions may be related to behavioral outcomes favoring excessive drinking such as high incentive salience (alcohol desire) and low executive function (inability to control alcohol desire). Differences in limbic functional activation have also been explored in drinkers, although further controlled longitudinal studies are needed. Current findings from prospective research have identified specific drinking consequences, showing that the onset of alcohol misuse affects gray and white matter brain volumes and integrity, especially at frontal sites and frontally-based circuitry, which in turn, may reinforce the risk of uncontrolled drinking.
A model that emerges from the constellation of findings reviewed on risk and resilience brain markers entails a see-saw pattern of functional brain activation that is dependent on and modified with environmental, genetic, and developmental influences. Risk and resilience factors may be ubiquitously present but depending on the strength vs. weakness of relevant factors and developmental timing drinking or non-drinking will be manifest. Thus, regarding resilience, protective factors would enable positive adaption and low alcohol use despite unavoidable high vulnerabilities such as family history and genetic factors. Recognition of the continuing development of frontally-based functional and structural systems throughout adolescence provides temporal markers of heightened vulnerability. Indeed, resilience—as described by high dorsolateral prefrontal and low striatal functional activations—may reduce the chances of engaging in excessive and binge drinking during adolescence and have the consequence of averting the onset of later AUD.
Future Directions
Additional research is needed to delineate the individual and interactive effects of risk and resilience factors affecting the initiation of youthful drinking and AUD. To improve our knowledge, we have identified three points that deserve serious research focus. A hope is that identification of behavioral and brain biological markers of risk and resilience will enable recognition of at-risk youth and offer avenues for diverting attraction to drinking.
The role of the cerebellum as a site of disruption and compensation.
Functions of the cerebellum are traditionally linked to the long-term adverse consequences of AUD on gait and balance (Fitzpatrick, Jackson, & Crowe, 2008; Sullivan, Rose, & Pfefferbaum, 2006). It is now clear, however, that the cerebellum is also involved in executive, language, and affective processes, modeled as the “Cognitive and Affective Syndrome” (Schmahmann, 2019; Schmahmann & Sherman, 1998). Selective relations between cognitive and emotional difficulties and regional cerebellar structure or function in adults recovering from AUD (Pitel, Chanraud, Müller-Oehring, Pfefferbaum, & Sullivan, 2013; Sawyer et al., 2016; Sullivan, Zahr, Saranathan, Pohl, & Pfefferbaum, 2019) provide a foundation for pursuing such relations in heavily drinking youth. Indeed, exploration of such vulnerabilities and drinking-related changes in the cerebellum of young drinkers remain a critical yet under-appreciated research focus. The few such extant studies reported differences in functional cerebellar responses during executive (Wetherill et al., 2013) and emotional (Lannoy, Dricot, et al., 2021) processing that distinguished young drinkers from low or no drinkers. A recent structural MRI study from the NCANDA multi-site consortium found accelerated cerebellar volume decline in youth who initiated heavy drinking compared with youth who remained no-to-low drinkers over 4-years of longitudinal study (Sullivan et al., 2020). These findings offer ample justification for expansion of the cerebellar research avenue in youth, especially given functional relevance of frontocerebellar circuitry in adult AUD with respect to its disruption (Sawyer et al., 2016; Sullivan & Pfefferbaum, 2005; Zahr et al. 2017) and as a coordinated system of compensatory functional repair with sobriety (Chanraud, Pitel, Müller-Oehring, Pfefferbaum, & Sullivan, 2013; Chanraud & Sullivan, 2014).
The role of drinking characteristics in AUD risk.
Animal models strongly support the role of intermittent alcohol exposure in inducing and enhancing voluntary drinking in adolescent mice and rats (e.g., Bell et al., 2013; Crews et al., 2019). Whether intermittent drinking style accelerates or is a causative antecedent to human AUD, however, remains unknown. Consistent with the animal models is the proposal that adolescents who present binge-drinking features (e.g., hangover, blackout, unconsciousness) early in life are particularly vulnerable to exhibit cognitive impairments and disturbed brain functional responses (Min et al., 2019; Nguyen-Louie et al., 2017; Nguyen-Louie, Simmons, et al., 2018). Age at first alcohol-related unconsciousness episode as well as repetition and duration of these episodes are also identified as salient predictors of cognitive and brain alterations (Min et al., 2019); however, prospective evaluations are needed to support this proposal. Similarly, cross-sectional results suggest that altered cortical gyrification was specifically related to hangover and binge-related symptoms (Hua et al., 2020). Longitudinal evaluation of overall alcohol use in adult drinkers has offered promising avenues by showing the associations between unconsciousness episodes and risk for dementia (Kivimäki et al., 2020). Future studies should pursue this question longitudinally by quantitating youthful drinking characteristics. Although human studies do not have the same control level as animal model, the implementation of prospective evaluation offers a well-controlled human natural experiment.
The role of resilience brain markers in adverting AUD.
We identified several neurophysiological resilience markers that enhance resistance to initiating heavy drinking, but much work remains to be done to delineate the contexts that makes them viable in contrast to contexts where the same markers may be detrimental to resilience or inoperative as a resilience resource. A further question involves identifying biological and psychological mechanisms of resilience that are protective against unfavorable environments, experiences of trauma, or genetic risk. Additional considerations entail discovering factors that moderate resilience abilities, for example, sleep habits (Hairston et al., 2016). Prospective longitudinal studies will provide the most compelling answers to these questions and bring further insights into the risk and resilience factors involved in the dynamic pathways toward AUD and those resistant to AUD.
Acknowledgment:
This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism (AA010723, AA017923, AA017347, AA021697). Dr. Lannoy received salary support from the Belgian American Educational Foundation.
Role of funding sources
This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism (AA010723, AA017923, AA021697) and Dr. Lannoy receives salary support from the Belgian American Educational Foundation. These funds did not exert any editorial direction or censorship on any part of this article.
Abbreviations
- AUD
Alcohol Use Disorder
- CNS
Central Nervous System
- DMN
Default Mode Network
- FHP
Family History (of alcohol use disorder) Positive
- FHN
Family History (of alcohol use disorder) Negative
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- SES
Socioeconomic status
Footnotes
Conflict of Interest
Nothing to declare.
References
- Abernathy K, Chandler LJ, & Woodward JJ (2010). Alcohol and the prefrontal cortex. In International Review of Neurobiology (Vol. 91, pp. 289–320). Elsevier. doi: 10.1016/S0074-7742(10)91009-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, … Heath AC (2009). Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism: Clinical and Experimental Research, 33(12), 2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim TN, Lawson WB, Feder A, Lacoviello BM, Saxena S, Balley CR, … Neumeister A (2012). Resilience to meet the challenge of addiction psychobiology and clinical considerations. Alcohol Research: Current Reviews, 10, 506–515. [PMC free article] [PubMed] [Google Scholar]
- Baker TE, Castellanos-Ryan N, Schumann G, Cattrell A, Flor H, Nees F, … the IMAGEN consortium. (2019). Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychological Medicine, 49(5), 801–810. doi: 10.1017/S0033291718001459 [DOI] [PubMed] [Google Scholar]
- Barr PB, Silberg J, Dick DM, & Maes HH (2018). Childhood socioeconomic status and longitudinal patterns of alcohol problems: Variation across etiological pathways in genetic risk. Social Science & Medicine, 209, 51–58. doi: 10.1016/j.socscimed.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, & Tapert SF (2010). Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review, 20(4), 398–413. doi: 10.1007/s11065-010-9146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (1998). Kindling in alcohol withdrawal. Research World, 22(1), 9. [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, … Bloom FE (1998). Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section, 108(3), 244–250. doi: 10.1016/S0168-5597(98)00002-1 [DOI] [PubMed] [Google Scholar]
- Bell RL, Franklin KM, Hauser S, & Engleman EA (2013). Next stop dependence – Binge drinking on the road to alcoholism : Preclinical findings on its neurobiology from rat animal models. In Harris SB (Ed.). Binge eating and binge drinking. Psychological, social, and medical implications. New York: Nova Publishers. [Google Scholar]
- Bernardin F, Maheut-Bosser A, & Paille F (2014). Cognitive impairments in alcohol-dependent subjects. Frontiers in Psychiatry, 5. doi: 10.3389/fpsyt.2014.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylergil SB, Beck A, Deserno L, Lorenz RC, Rapp MA, Schlagenhauf F, … Obermayer K (2017). Dorsolateral prefrontal cortex contributes to the impaired behavioral adaptation in alcohol dependence. NeuroImage: Clinical, 15, 80–94. doi: 10.1016/j.nicl.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, & Hommer DW (2008). Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction, 103(8), 1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, & Patton GC (2004). Teenage drinking and the onset of alcohol dependence: A cohort study over seven years. Addiction, 99(12), 1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x [DOI] [PubMed] [Google Scholar]
- Brown SA, & Tapert SF (2004). Adolescence and the trajectory of alcohol use: Basic to clinical studies. Annals of the New York Academy of Sciences, 1021(1), 234–244. doi: 10.1196/annals.1308.028 [DOI] [PubMed] [Google Scholar]
- Brown-Rice KA, Scholl JL, Fercho KA, Pearson K, Kallsen NA, Davies GE, … Forster GL (2018). Neural and psychological characteristics of college students with alcoholic parents differ depending on current alcohol use. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 284–296. doi: 10.1016/j.pnpbp.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback T, Worley M, Nguyen-Louie TT, Squeglia LM, Jacobus J, & Tapert SF (2016). Neural predictors of alcohol use and psychopathology symptoms in adolescents. Development and Psychopathology, 28(4pt1), 1209–1216. doi: 10.1017/S0954579416000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler M, & Mann K (2011). Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcoholism: Clinical and Experimental Research, 35(10), 1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x [DOI] [PubMed] [Google Scholar]
- Burt KB, Whelan R, Conrod PJ, Banaschewski T, Barker GJ, Bokde ALW, … the IMAGEN Consortium. (2016). Structural brain correlates of adolescent resilience. Journal of Child Psychology and Psychiatry, 57(11), 1287–1296. doi: 10.1111/jcpp.12552 [DOI] [PubMed] [Google Scholar]
- Campbell IG, & Feinberg I (2009). Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proceedings of the National Academy of Sciences, 106(13), 5177–5180. doi: 10.1073/pnas.0812947106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbia C, López-Caneda E, Corral M, & Cadaveira F (2018). A systematic review of neuropsychological studies involving young binge drinkers. Neuroscience & Biobehavioral Reviews, 90, 332–349. doi: 10.1016/j.neubiorev.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Casey BJ, & Jones RM (2010). Neurobiology of the adolescent brain and behavior: Implications for substance use disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 49(12), 1189–1201. doi: 10.1097/00004583-201012000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel A-L., Müller-Oehring EM., Pfefferbaum A., & Sullivan EV. (2013). Remapping the brain to compensate for impairment in recovering alcoholics. Cerebral Cortex, 23, 97–104. doi: 10.1093/cercor/bhr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, & Sullivan EV (2014). Compensatory recruitment of neural resources in chronic alcoholism. In Sullivan EV & Pfefferbaum A (Eds.). Handbook of Clinical Neurology, Vol. 125. Alcohol and the Nervous System. Elsevier. [DOI] [PubMed] [Google Scholar]
- Chen ACH, Tang Y, Rangaswamy M, Wang JC, Almasy L, Foroud T, … Porjesz B (2009). Association of single nucleotide polymorphisms in a glutamate receptor gene (GRM8) with theta power of event-related oscillations and alcohol dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(3), 359–368. doi: 10.1002/ajmg.b.30818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Blender JA (2006). A multiple-levels-of-analysis perspective on resilience: Implications for the developing brain, neural plasticity, and preventive interventions. Annals of the New York Academy of Sciences, 1094(1), 248–258. doi: 10.1196/annals.1376.029 [DOI] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Nickerson LD, Sneider JT, Oot EN, Seraikas AM, Rohan ML, & Silveri MM (2017). College binge drinking associated with decreased frontal activation to negative emotional distractors during inhibitory control. Frontiers in Psychology, 8, 1650. doi: 10.3389/fpsyg.2017.01650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde K, Peltzer RI, Gimenez PV, & Cremonte M (2020). The association between early drinking and dependence varies by drinking context. Frontiers in Behavioral Neuroscience, 14, 17. doi: 10.3389/fnbeh.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope LM, Martz ME, Hardee JE, Zucker RA, Heitzeg MM (2019) Reward activation in childhood predicts adolescent substance use initiation in a high-risk sample. Drug and Alcohol Dependence, 194, 318–325. 10.1016/j.drugalcdep.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas A, Cuesta P, López-Caneda E, Rodríguez Holguín S, García-Moreno LM, Pineda-Pardo JA, … Maestú F (2016). Functional and structural brain connectivity of young binge drinkers: A follow-up study. Scientific Reports, 6(1), 31293. doi: 10.1038/srep31293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Infante MA, Bordyug M, Simmons AN, & Tapert SF (2020). Prospective associations between BOLD markers of response Inhibition and the transition to frequent binge drinking. Alcoholism: Clinical and Experimental Research, 44(2), 463–469. doi: 10.1111/acer.14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Luijten M, & Feldstein Ewing SW (2018). Adolescent resilience to addiction: A social plasticity hypothesis. The Lancet Child & Adolescent Health, 2(1), 69–78. doi: 10.1016/S2352-4642(17)30148–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT (2008). Alcoholrelated neurodegeneration and recovery. Alcohol Research and Health, 31(4), 12. [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Robinson DL, Chandler LJ, Ehlers CL, Mulholland PJ, Pandey SC, … Vetreno RP (2019). Mechanisms of persistent neurobiological changes following adolescent alcohol exposure: NADIA consortium findings. Alcoholism: Clinical and Experimental Research, 43(9), 1806–1822. doi: 10.1111/acer.14154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A (2016). Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence, 158, 8–21. doi: 10.1016/j.drugalcdep.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, & Brumback T (2017). The burden of binge and heavy drinking on the brain: Effects on Adolescent and Young Adult Neural Structure and Function. Frontiers in Psychology, 8, 1111. doi: 10.3389/fpsyg.2017.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, Anderson BM, Rosen R, Khadka S, Sawyer B, Jiantonio-Kelly RE, … Pearlson GD (2014). Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students: FMRI and subsequent drinking. Addiction, 109(4), 585–595. doi: 10.1111/add.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CN, & Slutske WS (2018). Socioeconomic status and adolescent alcohol involvement: Evidence for a gene–environment interaction. Journal of Studies on Alcohol and Drugs, 79(5), 725–732. doi: 10.15288/jsad.2018.79.725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Patricia Chou S, June Ruan W, & Grant BF (2008). Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research, 32(12), 2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Nooner KB, Brumback T, Clark DB, Tapert SF, & Brown SA (2020). Posttraumatic stress symptoms predict transition to future adolescent and young adult moderate to heavy drinking in the NCANDA Sample. Current Addiction Reports, 7(2), 99–107. doi: 10.1007/s40429-020-00303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Cho SB, Latendresse SJ, Aliev F, Nurnberger JI, Edenberg HJ, … Kuperman S (2014). Genetic influences on alcohol use across stages of development: GABRA2 and longitudinal trajectories of drunkenness from adolescence to young adulthood: GABRA2 & trajectories of risk. Addiction Biology, 19(6), 1055–1064. doi: 10.1111/adb.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, & Stephens DN (2003). Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients: Alcoholism: Clinical & Experimental Research, 27(10), 1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7 [DOI] [PubMed] [Google Scholar]
- Elliott JC, Carey KB, & Bonafide KE (2012). Does family history of alcohol problems influence college and university drinking or substance use? A meta-analytical review: Family history and college substance use. Addiction, 107(10), 1774–1785. doi: 10.1111/j.1360-0443.2012.03903.x [DOI] [PubMed] [Google Scholar]
- Enoch M-A (2006). Genetic and environmental Influences on the development of acoholism: Resilience vs. risk. Annals of the New York Academy of Sciences, 1094(1), 193–201. doi: 10.1196/annals.1376.019 [DOI] [PubMed] [Google Scholar]
- Feinberg I, Higgins LM, Khaw WY, & Campbell IG (2006). The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 291(6), R1724–R1729. doi: 10.1152/ajpregu.00293.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LE, Jackson M, & Crowe SF (2008). The relationship between alcoholic cerebellar degeneration and cognitive and emotional functioning. Neuroscience & Biobehavioral Reviews, 32(3), 466–485. doi: 10.1016/j.neubiorev.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Giedd JN (2008). The teen brain: Insights from neuroimaging. Journal of Adolescent Health, 42(4), 335–343. doi: 10.1016/j.jadohealth.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Gowin JL, Manza P, Ramchandani VA, & Volkow ND (2020). Neuropsychosocial markers of binge drinking in young adults. Molecular Psychiatry. doi: 10.1038/s41380-020-0771-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, & Ramchandani VA (2017). Vulnerability for alcohol use disorder and rate of alcohol consumption. American Journal of Psychiatry, 174(11), 1094–1101. doi: 10.1176/appi.ajp.2017.16101180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry, 72(8), 757. doi: 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Moore TM, Rosen AFG, Barzilay R, Roalf DR, Calkins ME, … Gur RC (2019). Burden of environmental adversity associated with psychopathology, maturation, and brain behavior parameters in youths. JAMA Psychiatry, 76(9), 966–975. doi: 10.1001/jamapsychiatry.2019.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagborg JM, Thorvaldsson V, & Fahlke C (2020). Child maltreatment and substance-use-related negative consequences: Longitudinal trajectories from early to mid adolescence. Addictive Behaviors, 106, 106365. doi: 10.1016/j.addbeh.2020.106365 [DOI] [PubMed] [Google Scholar]
- Hairston IS, Conroy DA, Heitzeg MM, Akbar NZ, Brower KJ, & Zucker RA (2016). Sleep mediates the link between resiliency and behavioural problems in children at high and low risk for alcoholism. Journal of Sleep Research, 25(3), 341–349. doi: 10.1111/jsr.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Knight CP, Truitt WA, Waeiss RA, Holt IS, Carvajal GB, … Rodd ZA (2019). Adolescent intermittent ethanol increases the sensitivity to the reinforcing properties of Eethanol and the expression of select cholinergic and dopaminergic genes within the posterior ventral tegmental area. Alcoholism: Clinical and Experimental Research, 43(9), 1937–1948. doi: 10.1111/acer.14150 [DOI] [PubMed] [Google Scholar]
- Heikkinen N, Niskanen E, Könönen M, Tolmunen T, Kekkonen V, Kivimäki P, … Vanninen R (2017). Alcohol consumption during adolescence is associated with reduced grey matter volumes: Adolescent alcohol use and grey matter. Addiction, 112(4), 604–613. doi: 10.1111/add.13697 [DOI] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau W-YW, Zubieta J-K, & Zucker RA (2008). Affective circuitry and risk for alcoholism in late adolescence: Differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcoholism: Clinical and Experimental Research, 32(3), 414–426. doi: 10.1111/j.1530-0277.2007.00605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, De Bellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T (2001). Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry, 49, 894–905. 10.1016/S0006-3223(01)01088-5 [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M (2007). Cerebellar volume in offspring from multiplex alcohol dependence families. Biol. Psychiatry 61:41–47. 10.1016/j.biopsych.2006.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke J, Steinhauer SR, Konicky C, Lowers L, Connolly J (1999). Developmental delay in P300 production in children at high risk for developing alcohol-related disorders. Biological Psychiatry, 46, 970–981. 10.1016/S0006-3223(99)00032-3 [DOI] [PubMed] [Google Scholar]
- Hua JPY, Piasecki TM, McDowell YE, Boness CL, Trela CJ, Merrill AM, … Kerns JG (2020). Alcohol use in young adults associated with cortical gyrification. Drug and Alcohol Dependence, 209, 107925. doi: 10.1016/j.drugalcdep.2020.107925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav KS, & Boutrel B (2019). Prefrontal cortex development and emergence of self-regulatory competence: The two cardinal features of adolescence disrupted in context of alcohol abuse. European Journal of Neuroscience, 50(3), 2274–2281. doi: 10.1111/ejn.14316 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Brown TT, Hagler DJ, Akshoomoff N, Bartsch H, Newman E, … Dale AM (2016). The pediatric imaging, neurocognition, and genetics (PING) data repository. NeuroImage, 124, 1149–1154. doi: 10.1016/j.neuroimage.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Cservenka A, & Nagel BJ (2016). Binge drinking impacts dorsal striatal response during decision making in adolescents. NeuroImage, 129, 378–388. doi: 10.1016/j.neuroimage.2016.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Steele JS, & Nagel BJ (2017). Binge drinking and family history of alcoholism are associated with an altered developmental trajectory of impulsive choice across adolescence: Alcohol effects on impulsive choice. Addiction, 112(7), 1184–1192. doi: 10.1111/add.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Bacanu S, Sundquist J, & Sundquist K (2020). The risk for drug abuse, alcohol use disorder, and psychosocial dysfunction in offspring from high-density pedigrees: Its moderation by personal, family, and community factors. Molecular Psychiatry, 25(8), 1777–1786. doi: 10.1038/s41380-018-0111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B, Chen R, Fang F, Valdimarsdottir U, Montgomery S, Larsson H, & Fall K (2019). Low stress resilience in late adolescence and risk of smoking, high alcohol consumption and drug use later in life. Journal of Epidemiology and Community Health, 73(6), 496–501. doi: 10.1136/jech-2018-211815 [DOI] [PubMed] [Google Scholar]
- Kivimäki M, Singh-Manoux A, Batty GD, Sabia S, Sommerlad A, Floud S, … Strandberg T (2020). Association of alcohol-Induced loss of consciousness and overall alcohol consumption with risk for dementia. JAMA Network Open, 3(9), e2016084. doi: 10.1001/jamanetworkopen.2020.16084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, … George O (2014). Addiction as a stress surfeit disorder. Neuropharmacology, 76, 370–382. doi: 10.1016/j.neuropharm.2013.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoy S, Dricot L, Benzerouk F, Portefaix C, Barrière S, Quaglino V, … Gierski F (2021). Neural responses to the implicit processing of emotional facial expressions in binge drinking. Alcohol and Alcoholism, 56(2), 166–174. doi: 10.1093/alcalc/agaa093 [DOI] [PubMed] [Google Scholar]
- Lannoy S, Duka T, Carbia C, Billieux J, Fontesse S, Dormal V, … Maurage P (2021). Emotional processes in binge drinking: A systematic review and perspective. Clinical Psychology Review, 84, 101971. doi: 10.1016/j.cpr.2021.101971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A-P, Fama R, & Sullivan EV (2017). Executive Functions, Memory, and Social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism: Clinical and Experimental Research, 41(8), 1432–1443. doi: 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loheswaran G, Barr MS, Rajji TK, Blumberger DM, Le Foll B, & Daskalakis ZJ (2016). Alcohol intoxication by binge drinking impairs neuroplasticity. Brain Stimulation, 9(1), 27–32. doi: 10.1016/j.brs.2015.08.011 [DOI] [PubMed] [Google Scholar]
- Long EC, Lönn SL, Ji J, Lichtenstein P, Sundquist J, Sundquist K, & Kendler KS (2017). Resilience and risk for alcohol use disorders: A Swedish Twin Study. Alcoholism: Clinical and Experimental Research, 41(1), 149–155. doi: 10.1111/acer.13274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, & Lim KO (2013). Effects of alcohol use initiation on brain structure in typically developing adolescents. The American Journal of Drug and Alcohol Abuse, 39(6), 345–355. doi: 10.3109/00952990.2013.837057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Cope LM, Hardee JE, Brislin SJ, Weigard A, Zucker RA, & Heitzeg MM (2019). Frontostriatal resting state functional connectivity in resilient and non-resilient adolescents with a family history of alcohol use disorder. Journal of Child and Adolescent Psychopharmacology, 29(7), 508–515. doi: 10.1089/cap.2018.0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Zucker RA, Schulenberg JE, & Heitzeg MM (2018). Psychosocial and neural indicators of resilience among youth with a family history of substance use disorder. Drug and Alcohol Dependence, 185, 198–206. doi: 10.1016/j.drugalcdep.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Czerkawski C, Crowley DJ, Cohen-Gilbert JE, Sneider JT, & Silveri MM (2014). Binge alcohol consumption in emerging adults: Anterior cingulate cortical “thinness” is associated with alcohol use patterns. Alcoholism: Clinical and Experimental Research, 38(7), 1955–1964. doi: 10.1111/acer.12475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Bestelmeyer PEG, Rouger J, Charest I, & Belin P (2013). Binge drinking influences the cerebral processing of vocal affective bursts in young adults. NeuroImage: Clinical, 3, 218–225. doi: 10.1016/j.nicl.2013.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Hawkins KA, Dager AD, Tennen H, Khadka S, Austad CS, … Pearlson GD (2018). Longitudinal effects of alcohol consumption on the hippocampus and parahippocampus in college students. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(7), 610–617. doi: 10.1016/j.bpsc.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo AD, Castro N, Cota CI, & Tapert SF (2017). Cannabis and alcohol use, and the developing brain. Behavioural Brain Research, 325, 44–50. doi: 10.1016/j.bbr.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, & Lee FS (2019). Translating developmental neuroscience to understand risk for psychiatric disorders. American Journal of Psychiatry, 176(3), 179–185. doi: 10.1176/appi.ajp.2019.19010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JL, Chorlian DB, Johnson EC, Pandey AK, Kamarajan C, Salvatore JE, … Porjesz B (2019). Association of polygenic liability for alcohol dependence and EEG connectivity in adolescence and young adulthood. Brain Sciences, 9(10), 280. doi: 10.3390/brainsci9100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min E-J, Kim S-G, Lee J-S, Seo B, Jung W-Y, Huh S-Y, … Yu HJ (2019). Difference in cognitive function by first onset age of alcohol induced blackout and its duration. Clinical Psychopharmacology and Neuroscience, 17(4), 503–508. doi: 10.9758/cpn.2019.17.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Jones SA, Ehlers A, Lavine JB, & Nagel BJ (2018). Ventral striatal response during decision making involving risk and reward is associated with future binge drinking in adolescents. Neuropsychopharmacology, 43(9), 1884–1890. doi: 10.1038/s41386-018-0087-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgane P, Galler J, & Mokler D (2005). A review of systems and networks of the limbic forebrain/limbic midbrain. Progress in Neurobiology, 75(2), 143–160. doi: 10.1016/j.pneurobio.2005.01.001 [DOI] [PubMed] [Google Scholar]
- Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, … the IMAGEN Consortium. (2015). No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls: MID and family history alcohol. Addiction Biology, 20(3), 534–545. doi: 10.1111/adb.12136 [DOI] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Courtney KE, Squeglia LM, Bagot K, Eberson S, Migliorini R, … Pulido C (2018). Prospective changes in neural alcohol cue reactivity in at-risk adolescents. Brain Imaging and Behavior, 12(4), 931–941. doi: 10.1007/s11682-017-9757-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Matt GE, Jacobus J, Li I, Cota C, Castro N, & Tapert SF (2017). Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcoholism: Clinical and Experimental Research, 41(12), 2082–2092. doi: 10.1111/acer.13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Louie TT, Simmons AN, Squeglia LM, Alejandra Infante M, Schacht JP, & Tapert SF (2018). Earlier alcohol use onset prospectively predicts changes in functional connectivity. Psychopharmacology, 235(4), 1041–1054. doi: 10.1007/s00213-017-4821-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, & McClain JA (2010). Adolescence as a critical window for developing an alcohol use disorder: Current findings in neuroscience: Current Opinion in Psychiatry, 23(3), 227–232. doi: 10.1097/YCO.0b013e32833864fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (2018). Alcohol Facts and Statistics. Report retrieved from: https://www.niaaa.nih.gov/sites/default/files/AlcoholFactsAndStats.pdf
- Nooner KB, De Bellis MD, Clark DB, Thompson WK, & Brumback T (2020). Longitudinal impact of life events on adolescent binge drinking in the national consortium on alcohol and neurodevelopment in adolescence (NCANDA). Substance Use & Misuse, 55(11), 1846–1855. doi: 10.1080/10826084.2020.1768549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, & Tapert SF (2011). Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence, 119(3), 216–223. doi: 10.1016/j.drugalcdep.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, & Crews FT (2002). Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacology Biochemistry and Behavior, 72(3), 521–532. doi: 10.1016/S0091-3057(02)00715-3 [DOI] [PubMed] [Google Scholar]
- O’Brien JW, & Hill SY (2017). Neural predictors of substance use disorders in young adulthood. Psychiatry Research: Neuroimaging, 268, 22–26. 10.1016/j.pscychresns.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinković K (2007). Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review, 17(3), 239–257. doi: 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, … Sullivan EV (2018). Altered brain developmental trajectories in adolescents after initiating drinking. American Journal of Psychiatry, 175(4), 370–380. doi: 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel A-L, Chanraud., Müller-Oehring EM, Pfefferbaum A., & Sullivan EV. (2013). Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Structure and Function, 218, 683–695. doi: 10.1007/s00429-012-0421-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl KM, Sullivan EV, Rohlfing T, Chu W, Kwon D, Nichols BN, … Pfefferbaum A (2016). Harmonizing DTI measurements across scanners to examine the development of white matter microstructure in 803 adolescents of the NCANDA study. NeuroImage, 130, 194–213. doi: 10.1016/j.neuroimage.2016.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 118, 2128–2148. 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonawalla IB, Kendzor DE, Owen MT, & Caughy MO (2014). Family income trajectory during childhood is associated with adolescent cigarette smoking and alcohol use. Addictive Behaviors, 39(10), 1383–1388. doi: 10.1016/j.addbeh.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stirnus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J (1998). Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: Preliminary results from the COGA Project. Alcoholism Clinical and Experimental Reseach, 22, 1317–1323. doi: 10.1111/j.1530-0277.1998.tb03914.x [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, … Reich T (2002). Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biological Psychology, 61(1–2), 229–248. doi: 10.1016/S0301-0511(02)00060-1 [DOI] [PubMed] [Google Scholar]
- Rae CL, Gierski F, Smith KW, Nikolaou K, Davies A, Critchley HD, … Duka T (2020). Differential brain responses for perception of pain during empathic response in binge drinkers compared to non-binge drinkers. NeuroImage: Clinical, 27, 102322. doi: 10.1016/j.nicl.2020.102322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H (2007). Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. International Journal of Psychophysiology, 63, 3–15. 10.1016/j.ijpsycho.2006.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, … Giedd JN (2014). Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences, 111(4), 1592–1597. doi: 10.1073/pnas.1316911111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Zhou Y, Luo Q, Robert GH, Desrivières S, Quinlan EB, … Feng J (2019). Adolescent binge drinking disrupts normal trajectories of brain functional organization and personality maturation. NeuroImage: Clinical, 22, 101804. doi: 10.1016/j.nicl.2019.101804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Ruiz SM, Gálvez DA Makris N, Harris GJ., & Valera EM. (2016). Associations between cerebellar subregional morphometry and alcoholism history in men and women. Alcoholism Clinical and Experimental Reseach, 40, 1262–1272. doi: 10.1111/acer.13074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2019). The cerebellum and cognition. Neuroscience Letters, 688, 62–75. doi: 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, & Sherman JC (1998). The cerebellar cognitive affective syndrome. Brain, 121, 561–579 [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1985). Genetics and the risk for alcoholism. JAMA Network Open, 245(18), 2614–2617. [PubMed] [Google Scholar]
- Schuckit MA (1995). A longterm study of sons of alcoholics. Alcohol Health & Reserch World, 19(3), 172–175. [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO, Croot K, Finn P, & Polich J (1988). P300 latency after ethanol ingestion in sons of alcoholics and in controls. Biological Psychiatry, 24(3), 310–315. doi: 10.1016/0006-3223(88)90199–0 [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, & Tapert SF (2010). A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol, 44(1), 111–117. doi: 10.1016/j.alcohol.2009.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Heikkinen N, Kärkkäinen O, Gröhn H, Könönen M, Liu Y, … Vanninen R (2019). Effects of long-term adolescent alcohol consumption on white matter integrity and their correlations with metabolic alterations. Psychiatry Research: Neuroimaging, 294, 111003. doi: 10.1016/j.pscychresns.2019.111003 [DOI] [PubMed] [Google Scholar]
- Silveira S, Shah R, Nooner KB, Nagel BJ, Tapert SF, de Bellis MD, & Mishra J (2020). Impact of childhood trauma on executive function in adolescence—mediating functional brain networks and prediction of high-risk drinking. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(5), 499–509. doi: 10.1016/j.bpsc.2020.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KW, Gierski F, Andre J, Dowell NG, Cercignani M, Naassila M, & Duka T (2017). Altered white matter integrity in whole brain and segments of corpus callosum, in young social drinkers with binge drinking pattern: Alcohol and white matter. Addiction Biology, 22(2), 490–501. doi: 10.1111/adb.12332 [DOI] [PubMed] [Google Scholar]
- Spear LP (2010). The behavioral neuroscience of adolescence. New York: W.W. Norton. [Google Scholar]
- Spear LP (2014). Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicology and Teratology, 41, 51–59. doi: 10.1016/j.ntt.2013.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear Linda P. (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience, 19(4), 197–214. doi: 10.1038/nrn.2018.10. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, … Tapert SF (2017). Neural predictors of initiating alcohol use during adolescence. American Journal of Psychiatry, 174(2), 172–185. doi: 10.1176/appi.ajp.2016.15121587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, & Tapert SF (2012). Brain response to working memory over three years ofa: Influence of initiating heavy drinking. Journal of Studies on Alcohol and Drugs, 73(5), 749–760. doi: 10.15288/jsad.2012.73.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, & Pfefferbaum A (2015). Brain development in heavy-drinking adolescents. American Journal of Psychiatry, 172(6), 531–542. doi: 10.1176/appi.ajp.2015.14101249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. doi: 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, & Duka T (2008). Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3169–3179. doi: 10.1098/rstb.2008.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Brown SA, Baker FC, Colrain IM, … Pfefferbaum A (2020). Disturbed cerebellar growth trajectories in adolescents who initiate alcohol drinking. Biological Psychiatry, 87(7), 632–644. doi: 10.1016/j.biopsych.2019.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology, 180, 583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2019). Brain-behavior relations and effects of aging and common comorbidities in alcohol use disorder: A review. Neuropsychology, 33(6), 760–780. doi: 10.1037/neu0000557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, & Pfefferbaum A (2006). Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: A quantitative physiological and MRI study. Cerebral Cortex, 16(8), 1077–1086. doi: 10.1093/cercor/bhj048 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahra NM, Saranathan M, Pohl KM, & Pfefferbaum. A. (2019). Convergence of three parcellation approaches demonstrating cerebellar lobule volume deficits in Alcohol Use Disorder. NeuroImage : Clinical, 24, 101974. doi : 10.1016/j.nicl.2019.101974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco EM, Cope LM, Burmeister M, Zucker RA, & Heitzeg MM (2018). Pathways to youth behavior: The role of genetic, neural, and behavioral markers. Journal of Research on Adolescence, 28(1), 26–39. doi: 10.1111/jora.12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2015). Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiology & Behavior, 148, 145–150. doi: 10.1016/j.physbeh.2014.11.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Hosová D, Towner T, Werner DF, & Spear LP (2020). Effects of chronic intermittent ethanol exposure during early and late adolescence on anxiety-like behaviors and behavioral flexibility in adulthood. Behavioural Brain Research, 378, 112292. doi: 10.1016/j.bbr.2019.112292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, … Richter L (2011). Inequality in early childhood: Risk and protective factors for early child development. The Lancet, 378(9799), 1325–1338. doi: 10.1016/S0140-6736(11)60555–2 [DOI] [PubMed] [Google Scholar]
- Weafer J, Crane NA, Gorka SM, Phan KL, & de Wit H (2019). Neural correlates of inhibition and reward are negatively associated. NeuroImage, 196, 188–194. doi: 10.1016/j.neuroimage.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsh RC, Yau W-YW, Zubieta J-K, Zucker RA, & Heitzeg MM (2012). Resiliency in adolescents at high risk for substance abuse: flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcoholism: Clinical and Experimental Research, 36(8), 1355–1364. doi: 10.1111/j.1530-0277.2012.01741.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, & Tapert SF (2013). A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology, 230(4), 663–671. doi: 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Ressler KJ, & Bradley B (2014). Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: A cross-sectional study of 2024 inner-city men and women. Journal of Psychiatric Research, 51, 93–99. doi: 10.1016/j.jpsychires.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, & Koob GF (2014). The development and maintenance of drug addiction. Neuropsychopharmacology, 39(2), 254–262. doi: 10.1038/npp.2013.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED (1994). Mechanisms of alcohol abuse and alcoholism in adolescents : a case for developing animal models. Behavioral and Neural Biology, 62, 168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2018). Global status report on alcohol and health 2018. (ISBN 978–92-4–156563-9). Geneva: World Health Organization. [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, … Johnson CA (2013). Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychology of Addictive Behaviors, 27(2), 443–454. doi: 10.1037/a0027892 [DOI] [PubMed] [Google Scholar]
- Yau W-YW, Zubieta J-K, Weiland BJ, Samudra PG, Zucker RA, & Heitzeg MM (2012). Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime Alcohol Use. Journal of Neuroscience, 32(7), 2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pfefferbaum A, & Sullivan EV (2017). Perspectives on fronto-fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology, 122, 189–200. doi: 10.1016/j.neuropharm.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Sullivan EV, Honnorat N, Adeli E, Podhajsky S, De Bellis MD, Voyvodic J, Nooner KB, Baker FC, Colrain IM, Tapert SF, Brown SA, Thompson WK, Nagel BJ, Clark DB, Pfefferbaum A, & Pohl KM (2020). Young teens who initiate heavy drinking risk deviant fiber tract development in frontal brain systems. American Journal of Psychiatry, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


