Abstract
The brown algae Desmarestia ligulata var. ligulata (Lightf.) Lamour., and D. viridis (Mull.) Lamour., accumulate H2SO4 until their average internal pH is 0.5 to 0.8. A related species, D. aculeata (L.) Lamour., does not accumulate acid. The H2SO4 accumulation is accompanied by a reduction in the K+ and Cl− content, presumedly to maintain osmotic balance. Measurements of the membrane potential and H+ and SO42− concentrations indicate that both ions are accumulated in the vacuole against their electrochemical potential gradients.
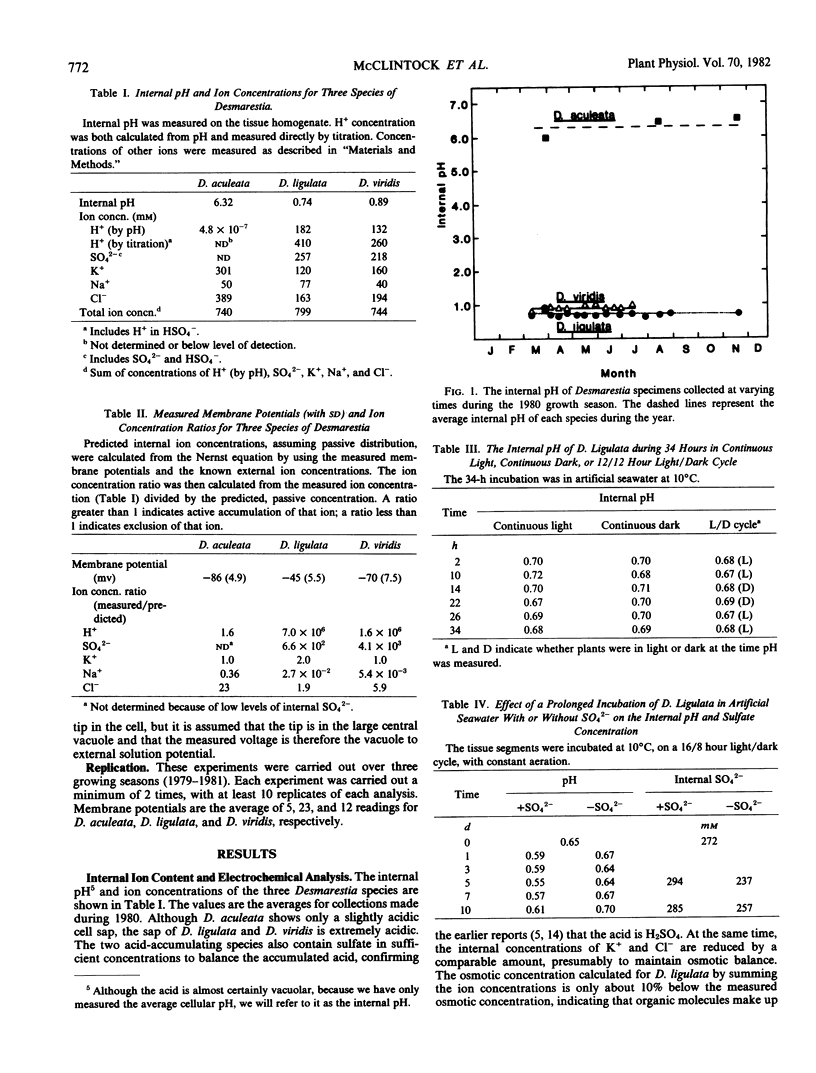
The internal pH remains constant in all three species over the growing season, despite striking changes in the algal morphology. The pH is not affected by periods of darkness of up to 34 hours. Sulfate accumulated in the vacuoles appears to be trapped there since incubation of D. ligulata for up to 10 days in sulfate-free medium resulted in little loss of either vacuolar sulfate or H+. Although the uptake of H2SO4 into the vacuole must require energy, the maintenance of the vacuolar H2SO4 may be due to the impermeability of the tonoplast, with little necessity for continued expenditure of energy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuppoletti J., Segel I. H. Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry. 1975 Oct 21;14(21):4712–4718. doi: 10.1021/bi00692a023. [DOI] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. L., Smith I. K. Sulfate Transport in Cultured Tobacco Cells : EFFECTS OF CALCIUM AND SULFATE CONCENTRATION. Plant Physiol. 1981 Mar;67(3):445–448. doi: 10.1104/pp.67.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEEUSE B. J. Free sulfuric acid in the brown alga, Desmarestia. Biochim Biophys Acta. 1956 Feb;19(2):372–374. doi: 10.1016/0006-3002(56)90442-5. [DOI] [PubMed] [Google Scholar]
- Reenstra W. W., Forte J. G. H+/ATP stoichiometry for the gastric (K+ + H+)-ATPase. J Membr Biol. 1981;61(1):55–60. doi: 10.1007/BF01870752. [DOI] [PubMed] [Google Scholar]
- Roomans G. M., Kuypers G. A., Theuvenet A. P., Borst-Pauwels G. W. Kinetics of sulfate uptake by yeast. Biochim Biophys Acta. 1979 Feb 20;551(1):197–206. doi: 10.1016/0005-2736(79)90365-1. [DOI] [PubMed] [Google Scholar]
- Sachs G., Spenney J. G., Lewin M. H+ transport: regulation and mechanism in gastric mucosa and membrane vesicles. Physiol Rev. 1978 Jan;58(1):106–173. doi: 10.1152/physrev.1978.58.1.106. [DOI] [PubMed] [Google Scholar]



