Abstract
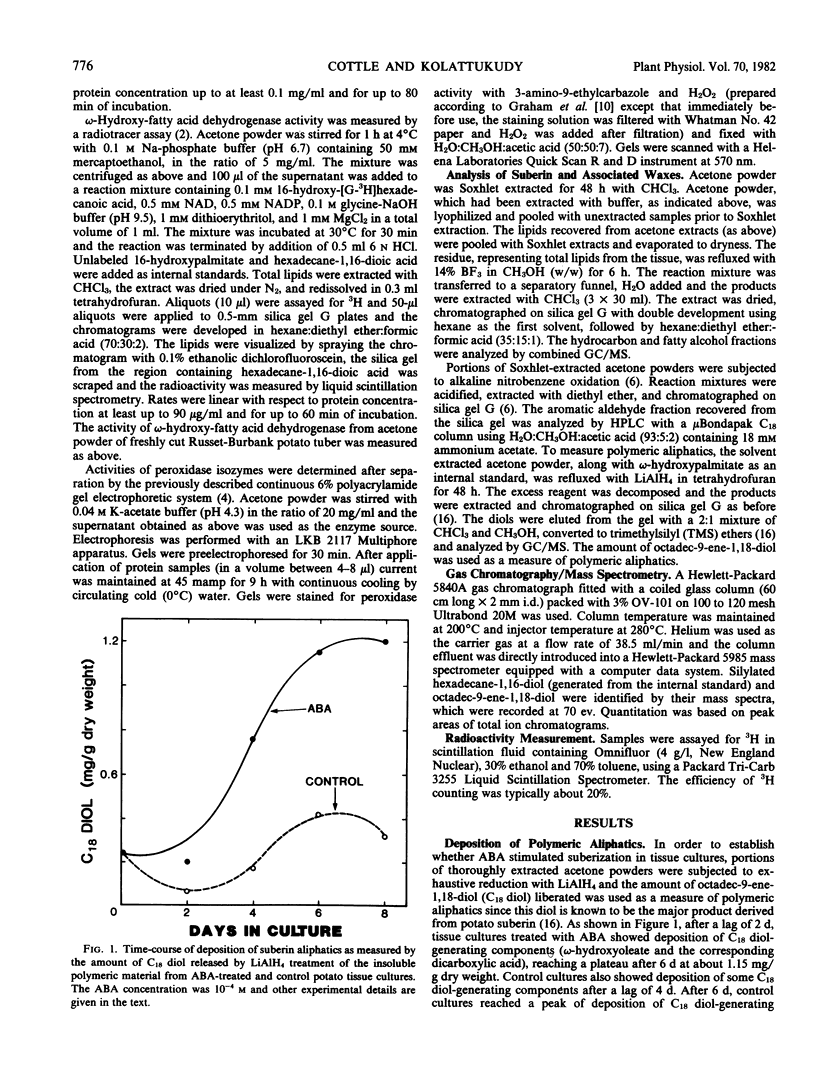
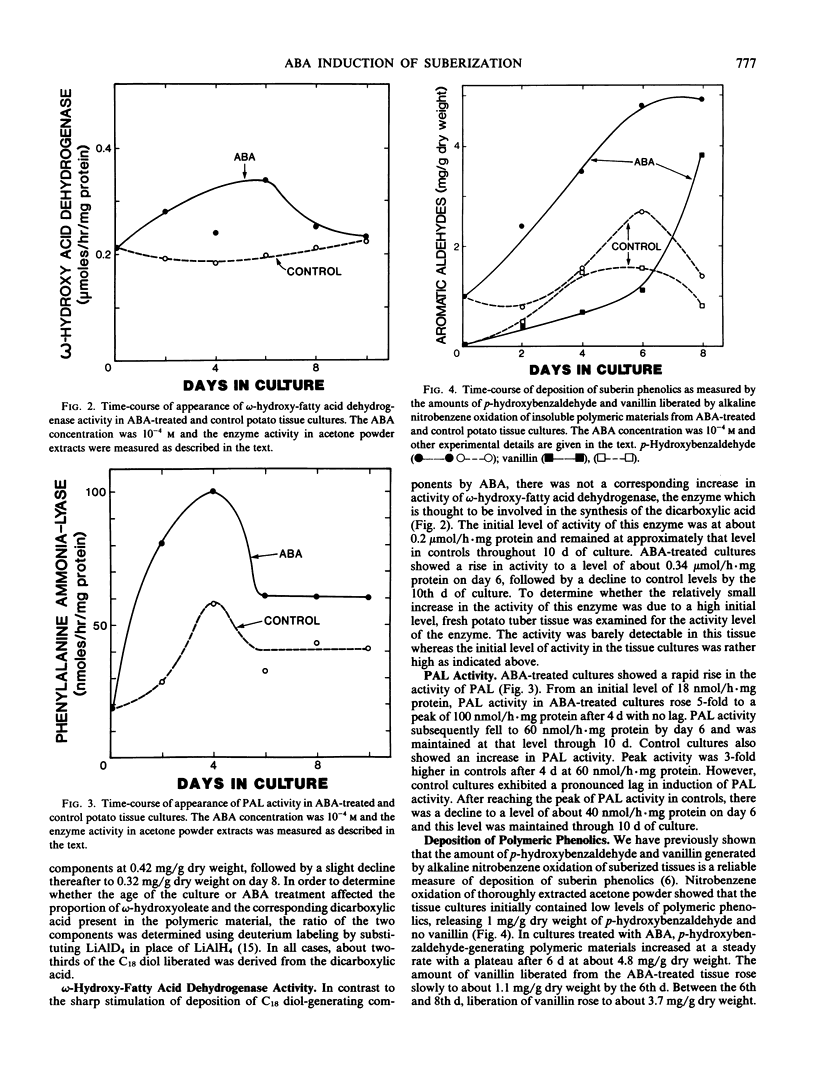
Effect of abscisic acid (ABA) on suberization of potato (Solanum tuberosum var. Russet-Burbank) tuber tissue culture was studied by measuring deposition of suberin components and the level of certain key enzymes postulated to be involved in suberization. ABA treatment resulted in a 3-fold increase in the polymeric aliphatic components of suberin and a 4-fold increase in the polymeric aromatic components. Hydrocarbons and fatty alcohols, two components characteristic of waxes associated with potato suberin, increased 9- and 5-fold, respectively, as a result of ABA treatment. Thus, the deposition of the polymeric aliphatics and aromatics as well as waxes, all of which have been postulated to be components of suberized cell walls, was markedly stimulated by ABA. ω-Hydroxy-fatty acid dehydrogenase which showed a rather high initial level of activity increased only 60% due to ABA treatment. Phenylalanine ammonia-lyase activity reached a maximum at a 5-fold level after 4 days in the ABA medium, whereas the control showed only a 3-fold increase. ABA treatment also resulted in a dramatic (7-fold) increase in an isozyme of peroxidase which has been specifically associated with suberization. Thus, ABA appears to induce certain key enzymes which are most probably involved in suberization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal V. P., Kolattukudy P. E. Biochemistry of Suberization: omega-Hydroxyacid Oxidation in Enzyme Preparations from Suberizing Potato Tuber Disks. Plant Physiol. 1977 Apr;59(4):667–672. doi: 10.1104/pp.59.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal V. P., Kolattukudy P. E. Purification and characterization of a wound-induced omega-hydroxyfatty acid:NADP oxidoreductase from potato tuber disks (Solanum tuberosum L.). Arch Biochem Biophys. 1978 Dec;191(2):452–465. doi: 10.1016/0003-9861(78)90384-3. [DOI] [PubMed] [Google Scholar]
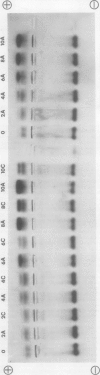
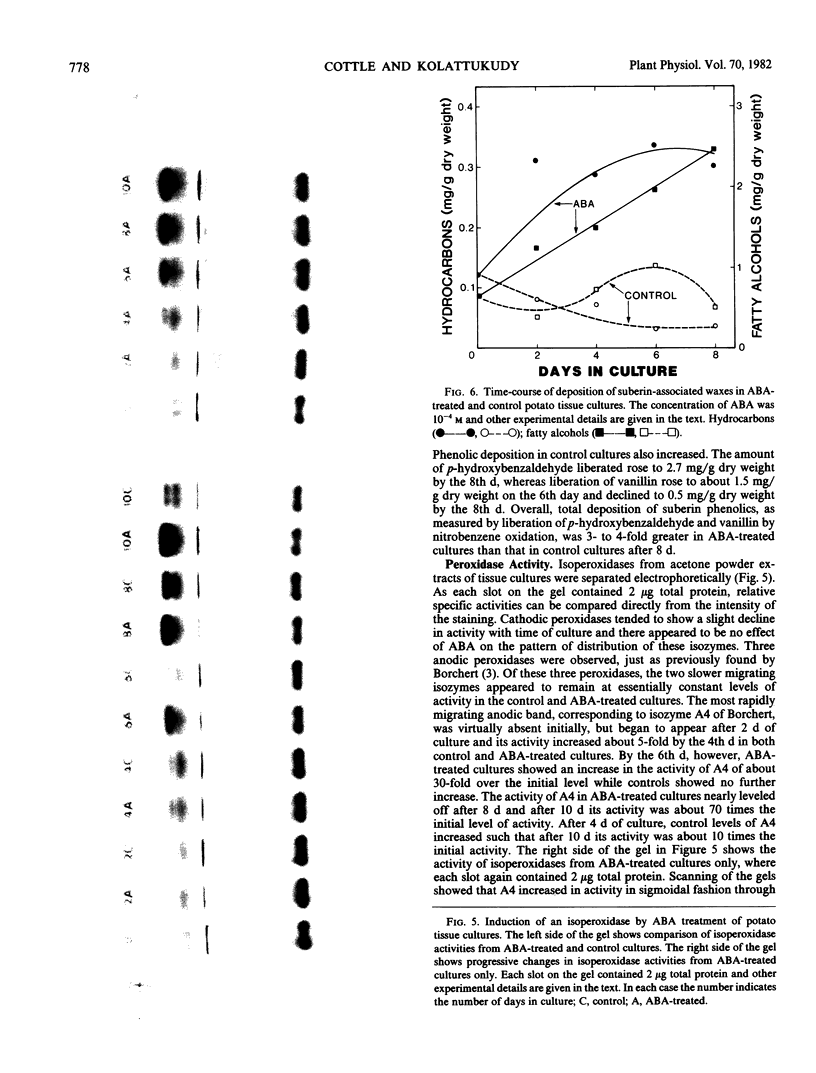
- Borchert R. Simultaneous separation of acidic and basic isoperoxidases in wounded potato tissue by acrylamide gel electrophoresis. Plant Physiol. 1978 Nov;62(5):794–797. doi: 10.1104/pp.62.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchert R. Time course and spatial distribution of phenylalanine ammonia-lyase and peroxidase activity in wounded potato tuber tissue. Plant Physiol. 1978 Nov;62(5):789–793. doi: 10.1104/pp.62.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Dean B. B. Structure, gas chromatographic measurement, and function of suberin synthesized by potato tuber tissue slices. Plant Physiol. 1974 Jul;54(1):116–121. doi: 10.1104/pp.54.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. G., Kolattukudy P. E. Evidence for Covalently Attached p-Coumaric Acid and Ferulic Acid in Cutins and Suberins. Plant Physiol. 1975 Nov;56(5):650–654. doi: 10.1104/pp.56.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliday C. L., Dean B. B., Kolattukudy P. E. Suberization: inhibition by washing and stimulation by abscisic Acid in potato disks and tissue culture. Plant Physiol. 1978 Feb;61(2):170–174. doi: 10.1104/pp.61.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Effects of Abscisin II on Phenylalanine Ammonia-Lyase Activity in Excised Bean Axes. Plant Physiol. 1968 Mar;43(3):467–469. doi: 10.1104/pp.43.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]