Abstract
Burgeoning literature demonstrates that monoamine transporters with high transport capacity but lower substrate affinity (i.e., uptake 2) contribute meaningfully to regulation of monoamine neurotransmitter signalling. However, studying behavioural influences of uptake 2 is hindered by an absence of selective inhibitors largely free of off-target, confounding effects. This contrasts with study of monoamine transporters with low transport capacity but high substrate affinity (i.e., uptake 1), for which there are many reasonably selective inhibitors. To circumvent this dearth of pharmacological tools for studying uptake 2, researchers have instead employed mice with constitutive genetic deficiency in three separate transporters. By studying baseline behavioural shifts, plus behavioural responses to environmental and pharmacological manipulations—the latter primarily targeting uptake 1—investigators have been creatively characterizing the behavioural, and often sex-specific, influences of uptake 2. This non-systematic mini review summarizes current uptake 2 behaviour literature, highlighting emphases on stress responsivity in organic cation transporter 2 (OCT2) work, psychostimulant responsivity in OCT3 and plasma membrane monoamine transporter (PMAT) investigations, and antidepressant responsivity in all three. Collectively, this small but growing body of work reiterates the necessity for development of selective uptake 2-inhibiting drugs, with reviewed studies suggesting that these might advance personalized treatment approaches.
Keywords: organic cation transporters, plasma membrane monoamine transporter, psychoactive drugs, sex differences, stress
1 |. INTRODUCTION
Transporters of monoamine neurotransmitters serve multiple concurrent functions. Transporter-mediated uptake of monoamine neurotransmitters from the extracellular space impacts both the duration and magnitude of extra-cellular signalling, plus facilitates recycling of monoamine neurotransmitters and/or intracellular signaling processes (e.g., serotonylation).1–3 Historically, the most intensely studied monoamine transporters are those that fall under the classification of uptake 1, meaning they have relatively high affinity for their substrates, but low capacity. These include the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET), so named because of their preferential transport of single substrates, for example, dopamine transport by DAT. Affinities of uptake 1 (measured as a Michaelis constant, Km) for their named substrates (e.g., DAT for dopamine) range between Km = 0.2–4 μM (see review4), with lower numbers indicating greater affinity. In contrast, uptake 2 monoamine transporters possess lower affinities for monoamine substrates (Km = 80–5450 μM, from review4), but transport monoamines at much higher capacities than uptake 1.
Uptake 2 include organic cation transporter 1, 2 and 3 (OCT1, OCT2, and OCT3, respectively), plasma membrane monoamine transporter (PMAT), and multidrug and toxin extrusion 1 and 2-K (MATE1 and MATE2-K, respectively) transporters. Transport capacity (i.e., rate of maximal transport, Vmax; higher numbers indicate faster transport) of uptake 2 versus uptake 1 is exemplified by contrasting the capacity of PMAT to transport dopamine and serotonin (18.2 and 6.5 nmol/min/mg protein, respectively5), with the capacities of DAT (0.3 nmol/min/mg protein) and SERT (0.012 nmol/min/mg protein) to transport dopamine or serotonin, respectively.6 Indeed, uptake 2 are generally polyspecific, having multiple preferred monoamine substrates, in contrast to singular monoamine substrates of uptake 1 (e.g., SERT affinity for serotonin). For example, PMAT preferentially transports dopamine and serotonin over other monoamines, whereas OCT3 preferentially transports histamine, nor-epinephrine, and epinephrine over other monoamines.7
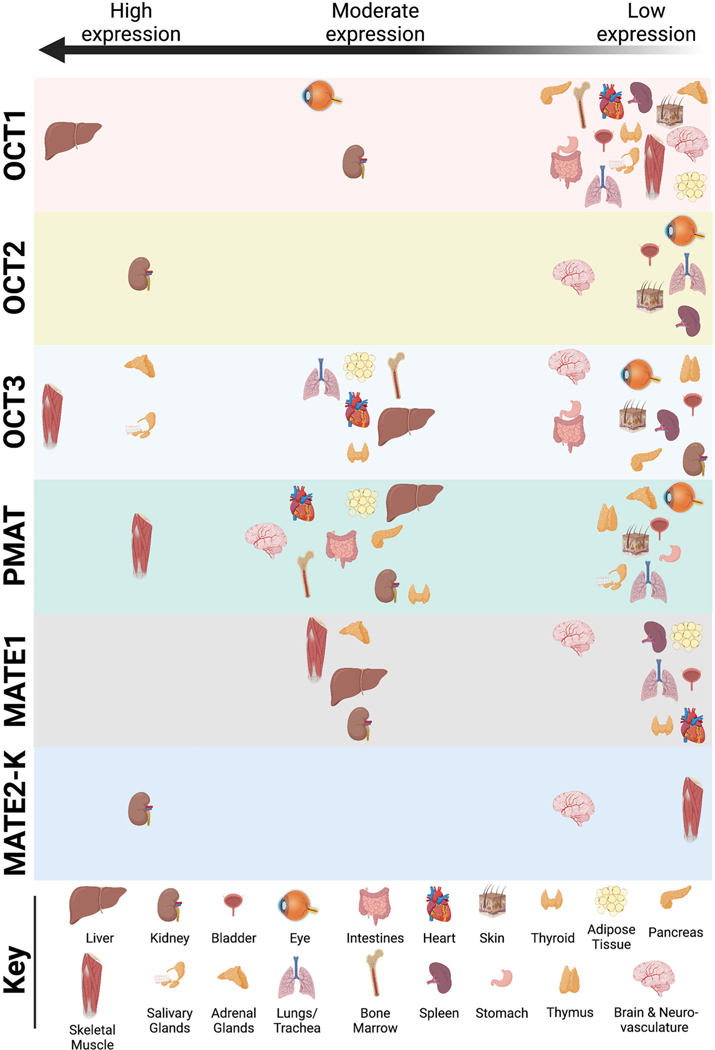
Broadly, most uptake 2 are expressed in a variety of major organs including the brain (see review8; Figure 1). Readers are referred to an excellent chapter by Prof. Hermann Koepsell that quantitatively and comprehensively compares uptake 1 and uptake 2 substrate and inhibitor affinities, plus localized expression profiles of these transporters within the brain.4 Other recent reviews provide details regarding (sub)cellular localization of uptake 2 across organs15 and specifically within brain region and brain cell types16 that are beyond the scope of this mini review. Accordingly, it is important to keep in mind that uptake 2 deficiency or pharmacological blockade within these peripheral organs might indirectly influence brain function to shift behavioural outcomes in the studies reviewed here.
FIGURE 1. Distribution of uptake 2 transporters in major organs and tissues throughout the body.
This figure is a graphical representation of the data assimilated by Prof. Hermann Koepsell in Table 6 of his 2020 review, Organic Cation Transporters in Health and Disease9 for OCT1, OCT2, OCT3, MATE1, and MATE2-K, based on >130 references therein; plus data reported for PMAT expression by numerous research labs.5,10–14 The vast majority of these distribution data is based upon mRNA expression reports, not protein expression. Tissues reported include adipose tissue, adrenal glands, bladder, bone marrow, brain and neurovasculature, eye, heart, intestines, kidney, liver, lungs/trachea, salivary glands, skeletal muscle, skin, spleen, stomach, thymus and thyroid. For biological sex-specific tissues (e.g., prostate, cervix, etc.), readers are referred to the cited text. Similarly, not all reports are consistent regarding relative levels of expression, so readers should refer to cited text for details regarding tissue collection and expression quantification methods. Relative expression levels for different uptake 2 span from “very high”9 or high expression on the left side of the graph, descending to moderate in the middle, and low/“low but significant”9 expression on the right. If uptake 2 was not detected or not reported in a particular organ/tissue, then that organ/tissue is not represented within the horizontal block for each corresponding uptake 2. A key indicating the visual marker for each organ/tissue is located at the bottom of the figure.
In comparison to uptake 1 transporters, uptake 2 are far less studied, particularly the behavioural consequences of constitutive deficiency. For example, searching Google Scholar (11 October 2022) for articles using DAT knockout (KO) mice returns 1600 results with the search “DAT knockout.” In contrast, using the same search engine to locate articles using OCT3 knockouts returns 138 results for “OCT3 knockout.” Adding “behavior” results in a greater quantitative contrast of 1460 to 71, respectively, i.e., a 21-fold difference. Study of behavioural differences resulting from constitutive uptake 2 deficiency is particularly critical considering, unlike for uptake 1, there is a dearth of selective pharmacological tools to advance study of uptake 2.
Indeed, drugs that inhibit uptake 2 are notoriously problematic. The most frequently engaged uptake 2 inhibitor is decynium-22 (D22), a compound that blocks OCT1, OCT2, OCT3, and PMAT.17,18 This action precludes the ability to determine individual transporter contributions to outcomes. Corticosterone, an endogenously produced steroid hormone, preferentially inhibits OCT3.19 However, it also acts at glucocorticoid receptors to elicit both genomic and non-genomic effects.20 Lopinavir is an antiretroviral drug that interferes with metabolic processes and contributes to cognitive dysfunction,21 but can preferentially inhibit PMAT over other uptake 2 transporters.22 Consequently, genetic manipulations to induce constitutive deficiency in individual uptake 2 transporters will remain the most targeted method for evaluating separate transporter contributions until more selective drugs are developed, and/or inducible KO models become available. Much of what researchers have gleaned regarding uptake 2 function comes from the integration of constitutive uptake 2 deficiency with uptake 1-targeting pharmacological tools.
This non-systematic mini review serves as a summary of the behavioural consequences of constitutive deficiency in each of three (OCT2, OCT3, and PMAT) individual uptake 2 transporters in mice and how these constitutive deficiencies interact with exposure to drugs that predominantly block uptake 1. Though OCT1 also transports monoamines, evidence indicates that brain expression is so low that its deletion is not anticipated to have any meaningful impact.16,23–25 Perhaps accordingly, and as suggested by others,16,26 this is why behaviours in OCT1 KO mice27 and OCT1/OCT2 double knockouts28 have not been assessed. Similarly, though MATE1 KO mice exist,29 to date, no studies have evaluated behaviours in these mice. No KO mice have been developed for MATE2-K, the active variant of MATE2.30
This mini review builds upon a terrific review published 7 years ago31 on how constitutive uptake 2 deficiency impacts responses to psychoactive drugs, plus upon thorough reviews summarizing the neurophysiological/neurochemical and behavioural influences of single uptake 2 transporters (e.g., OCT332,33; PMAT34,35). As study of uptake 2 has gained traction, more behavioural data have been reported in the past few years. Thus, the purpose of this mini review is to summarize and consolidate behavioural findings to date, reported in mice with constitutive genetic deficiency in single uptake 2 transporters (i.e., not multiple knockouts) (Table 1), including behavioural experiments where drugs were administered peripherally (Table 2). For more information on neurophysiological and neurochemical components relevant to uptake 2, a recent book edited by Prof. Lynette C. Daws is an excellent resource.47
TABLE 1.
Non-pharmacological behavioural assessments in mice with constitutive uptake 2 monoamine transporter deficiency
| Year | Sex(es) Genotypes analysed n per genotype | Background strain Age/weight | Findings versus wild-types | First author Last author DOI Ref. no. |
|---|---|---|---|---|
| OCT2 (Slc22a2) | ||||
| 2012 | Both, but sex not analysed | C57BL/6J | ≈ Locomotion | A Bacq |
| WT | 8–10 weeks | ⇓ Rotarod performance | S Gautron | |
| KO | ⇑ Time, open-field centre | 10.1038/mp.2011.87 | ||
| n = 9–30 | ⇑ Crossings, open-field centre ⇑ Time, elevated O-maze open zones ⇓ Latency, elevated O-maze open zones ⇓ Latency, novelty-suppressed feeding ⇑ Immobility, forced swim ⇑ Immobility, tail suspension |
Ref.36 | ||
| 2015 | Male | C57BL/6J | ⇑ Immobility, forced swim | T Couroussé |
| WT | 8–16 weeks | After unpredictable chronic mild stress: | S Gautron | |
| KO | ⇑ Performance decline, object location | 10.1038/mp.2014.86 | ||
|
n = 7–9 n = 6–15 |
⇓ Social interaction ⇓ Nest building ⇓ Coat condition ≈ Sucrose preference |
Ref.37 | ||
| OCT3 (Slc22a3) | ||||
| 2004 | Not stated | Not stated | ≈ Water consumption | V Vialou |
| WT | 8–12 weeks | ≈ 0.3 M NaCl consumption | S Gautron | |
| KO | After 24 h water deprivation: | 10.1523/JNEUROSCI.5147-03.2004 | ||
| n = 8 | ≈ Water consumption ⇑ 0.3M NaCl consumption |
Ref.38 | ||
| 2008 | Not stated | C57BL/6J | ≈ Locomotion | V Vialou |
| WT | 8–10 weeks | ≈ Rotarod performance | S Gautron | |
| KO | ⇓ Time, open-field centre | 10.1111/j.1471-4159.2008.05506.x | ||
| n = 7–10 | ⇓ Locomotion, two Y-maze start arms | Ref.39 | ||
| 2009 | Male | C57BL/6 | ⇑ Per cent time, elevated plus maze open arms (KO) | T Wultsch |
| WT | 27–35 g | ⇑ Per cent time, open-field centre (HT and KO) | A Reif | |
| HT | ≈ Morris water maze performance | 10.1007/S00702-009-0205-1 | ||
| KO | ≈ Resident-intruder test | Ref.40 | ||
| n = 9–11 | ||||
| PMAT (Slc29a4) | ||||
| 2018 | Both | C57BL/6J | ≈ Time, elevated plus maze open arms | TL Gilman |
| WT | ≥90 days | ≈ Time, elevated plus maze closed arms | LC Daws | |
| HT | ⇑ Latency, elevated plus maze open arm (collapsing across sexes only; HT) | 10.1111/ejn.l3968 Ref.41 | ||
| KO | ||||
| n = 9–25 | ⇓ Distance, elevated plus maze (collapsing across sexes only; HT) | |||
| ≈ Locomotion | ||||
| ⇓ Faecal boli, locomotor test (collapsing across sexes only; KO) | ||||
| ≈ Marble burying | ||||
| ≈ Immobility, forced swim | ||||
| ⇑ Swimming, forced swim (females only; KO) | ||||
| ≈ Climbing, forced swim | ||||
| ≈ Latency, forced swim first immobility | ||||
| ≈ Faecal boli, forced swim test | ||||
| 2022 | Both | C57BL/6J | ⇑ Immobility, tail suspension (males only; HT and KO) | JN Beaver |
| WT | ≥90 days | TL Gilman | ||
| HT | 10.3390/cells11121874 | |||
| KO | Ref.42 | |||
| n = 6–10 | ||||
Abbreviations: HT, heterozygote; KO, knockout; WT, wild-type.
TABLE 2.
Pharmacological behavioural assessments in mice with constitutive uptake 2 monoamine transporter deficiency
| Year | Sex(es) Genotypes analysed | Background strain Age/weight | Behavioural measure Drug (ROA, time course) Findings versus wild-types (dose) | First author Last author DOI Ref. no. |
|---|---|---|---|---|
| OCT2 (Slc22a2) | ||||
| 2012 | Both, but sex not analysed | C57BL/6J | Forced swim | A Bacq |
| 8–10 weeks | Saline (i.p., 30 min before test) | S Gautron | ||
| WT | ⇑ Per cent immobility time of control | 10.1038/mp.2011.87 | ||
| KO | (10 ml/kg) | Ref.36 | ||
| n = 6–12 | Citalopram (i.p., 30 min before test) | |||
| n = 6–14 | ≈ Per cent immobility time of control (2.5 mg/kg) ≈ Per cent immobility time of control (5 mg/kg) ≈ Per cent immobility time of control (10 mg/kg) Reboxetine (i.p., 30 min before test) ≈ Per cent immobility time of control (10 mg/kg) ⇓ Per cent immobility time of control (20 mg/kg) ≈ Per cent immobility time of control (30 mg/kg) Venlafaxine (i.p., 30 min before test) ≈ Per cent immobility time of control (8 mg/kg) ⇑ Per cent immobility time of control (16 mg/kg) ⇑ Per cent immobility time of control (32 mg/kg) Social interaction (males only) Hydroxypropyl-[β-cyclodextrin (drinking water [vehicle control], 4 weeks) ≈ Social interaction (0.45%) Corticosterone (drinking water, 4 weeks) ≈ Social interaction (35 μg/ml) Novelty-suppressed feeding (males only) Hydroxypropyl-β-cyclodextrin (drinking water [vehicle control], 4 weeks) ⇓ Latency (0.45%) Corticosterone (drinking water, 4 weeks) ≈ Latency (35 μg/ml) Coat state (males only) Hydroxypropyl-β-cyclodextrin (drinking water [vehicle control], 4 weeks) ≈ Coat state (0.45%) Corticosterone (drinking water, 4 weeks) ≈ Coat state (35 μg/ml) Corticosterone (drinking water, 7 weeks) plus venlafaxine (i.p., last 3 of 7 weeks) ⇓ Coat state (35 μg/ml + 16 mg/kg/day) |
|||
| Sucrose preference (males only) | ||||
| Hydroxypropyl-β-cyclodextrin (drinking water [vehicle control], 4 weeks) | ||||
| ≈ Sucrose consumption (0.45%) | ||||
| Corticosterone (drinking water, 4 weeks) | ||||
| ≈ Sucrose consumption (35 μg/ml) | ||||
| Corticosterone (drinking water, 7 weeks) plus venlafaxine (i.p., last 3 of 7 weeks) | ||||
| ⇓ Sucrose consumption (35 μg/ml + 16 mg/kg/day) | ||||
| Elevated O maze (males only) | ||||
| Hydroxypropyl-β-cyclodextrin (drinking water [vehicle control], 4 weeks) | ||||
| ⇑ Time, O-maze open zones (0.45%) | ||||
| Corticosterone (drinking water, 4 weeks) | ||||
| ⇑ Time, O-maze open zones (35 μg/ml) | ||||
| Corticosterone (drinking water, 7 weeks) plus venlafaxine (i.p., last 3 of 7 weeks) | ||||
| ≈ Time, O-maze open zones (35 μg/ml + 16 mg/kg/day) | ||||
| Splash/spray test (males only) | ||||
| Hydroxypropyl-β-cyclodextrin (drinking water [vehicle control], 4 weeks) | ||||
| ≈ Grooming, splash/spray test (0.45%) | ||||
| Corticosterone (drinking water, 4 weeks) | ||||
| ≈ Grooming (35 μg/ml) | ||||
| Corticosterone (drinking water, 7 weeks) plus venlafaxine (i.p., last 3 of 7 weeks) | ||||
| ⇓ Grooming (35 μg/ml + 16 mg/kg/day) | ||||
| OCT3 (Slc22a3) | ||||
| 2004 | Not stated | Not stated | Fluid consumption with sodium-depleted diet | V Vialou |
| WT | 8–12 weeks | S Gautron | ||
| KO | Saline (route not stated, 24 and 18 h before test) | 10.1523/JNEUROSCI.5147-03.2004. | ||
| n = 6–8 | ≈ Water consumption (volume not stated, 2×) ≈ 0.3 M NaCl consumption (volume not stated, 2× ) Furosemide (route not stated, 24 and 18 h before test) ≈ Water consumption (0.6 mg, 2×) ⇑ 0.3 M NaCl consumption (0.6 mg, 2×) Fluid consumption with normal sodium diet Furosemide (route not stated, 24 and 18 h before test) ≈ Water consumption (0.6 mg, 2×) ⇑ 0.3 M NaCl consumption (0.6 mg, 2×) |
5147–03.2004 Ref.38 |
||
| 2008 | Not stated | C57BL/6J | Locomotion | V Vialou |
| WT | 8–10 weeks | Saline (i.p., at test start) | S Gautron | |
| KO | ≈ Locomotion (volume not stated) | 10.1111/j.1471-4159.2008.05506.x | ||
| n = 8–14 |
Amphetamine (i.p., at test start) ≈ Locomotion (3 mg/kg) ⇑ Locomotion (10 mg/kg) Cocaine (i.p., at test start) ≈ Locomotion (10 mg/kg) ≈ Locomotion (20 mg/kg) ⇑ Locomotion (40 mg/kg) Conditioned place preference Saline (i.p., four 15 min pairings every other day for 8 days) ≈ Place preference (volume not stated) Amphetamine (i.p., four 15 min pairings every other day for 8 days) ≈ Place preference (2 mg/kg) Locomotor sensitization Amphetamine (route not stated, every 3 days for 9 injection days) ≈ Sensitization (2 mg/kg) |
Ref.39 | ||
| 2013 | Male | C57BL/6 | Tail suspension a | RE Horton |
| WT | >60 days, 25–30 g | Decynium-22 (i.p., 60 min before test) | LC Daws | |
| KO | plus saline (i.p., 30 min before test) | 10.1523/JNEUROSCI.5687-11.2013 | ||
| n = 7–11 | ≈ Per cent saline control immobility (0.1 mg/kg + 5–20 ml/kg) ≈ Per cent saline control immobility, KO only (0.32 mg/kg + 5–20 ml/kg) Saline (i.p., 60 min before test) plus fluvoxamine (i.p., 30 min before test) ≈ Per cent saline control immobility (5–20 ml/kg + 10 mg/kg) Decynium-22 (i.p., 60 min before test) plus fluvoxamine (i.p., 30 min before test) ⇑ Per cent saline control immobility (0.1 mg/kg + 10 mg/kg) ⇓ Per cent saline control immobility, KO only (0.32 mg/kg + 10 mg/kg) |
Ref.43 | ||
| 2018 | Male | C57BL/6 | Locomotion | FP Mayer |
| WT | >60 days, 25–30 g | Saline (i.p., at test start) plus saline (i.p., 60 min before test) | HH Sitte | |
| KO | 10.1038/s41386-018-0053-5 | |||
| n = 7–8 | ≈ Locomotion (10 ml/kg + 10 ml/kg) Saline (i.p., at test start) plus decynium-22 (i.p., 60 min before test) ≈ Locomotion (10 ml/kg + 0.1 mg/kg) Amphetamine (i.p., at test start) plus saline (i.p., 60 min before test) ≈ Locomotion (3.2 mg/kg + 10 ml/kg) Amphetamine (i.p., at test start) plus decynium-22 (i.p., 60 min before test) ⇑ Locomotion (3.2 mg/kg + 0.1 mg/kg) |
Ref.44 | ||
| 2021 | Both | C57BL/6 | Conditioned place preference | NJ Clauss |
| WT | 61–181 days | Amphetamine (i.p., four 30 min pairings every other day for 8 days) | LC Daws | |
| KO | 10.3390/ijms222413420 | |||
| n = 16 | plus saline (i.p., 60 min before each pairing) ≈ Place preference in females (1 mg/kg + 10 ml/kg) ⇓ Place preference in males (1 mg/kg + 10 ml/kg) Amphetamine (i.p., four 30 min pairings every other day for 8 days) plus decynium-22 (i.p., 60 min before each pairing) ⇑ Place preference in females (1 mg/kg + 0.1 mg/kg) ≈ Place preference in males (1 mg/kg + 0.1 mg/kg) Locomotor sensitization Amphetamine (i.p., every other day for 4 injection days) plus saline (i.p., 60 min before each injection) ⇓ Sensitization, both sexes (1 mg/kg + 10 ml/kg) Amphetamine (i.p., every other day for 4 injection days) plus decynium-22 (i.p., 60 min before each injection) ≈ Sensitization, both sexes (1 mg/kg + 0.1 mg/kg) |
Ref.45 | ||
| PMAT ( Slc29a4 ) | ||||
| 2020 | Male | C57BL/6 | Forced swim | MA Bowman |
| WT | 3–12 months | Saline (i.p., 60 min before test) | LC Daws | |
| KO | ≈ Immobility time (10 ml/kg) | 10.3390/ijms21207581 | ||
| n = 8–11 |
Ketamine (i.p., 60 min before test) ⇑ Immobility time (32 mg/kg) Locomotion
Saline (i.p., at test start) ≈ Locomotion (10 ml/kg) Ketamine (i.p., at test start) ≈ Locomotion (32 mg/kg) |
Ref.46 | ||
| 2021 | Both | C57BL/6 | Conditioned place preference | NJ Clauss |
| WT | 61–181 days | Amphetamine (i.p., four 30 min pairings every other day for 8 days) | LC Daws | |
| KO | 10.3390/ijms222413420 | |||
| n = 16 | plus saline (i.p., 60 min before each pairing) ≈ Place preference, both sexes (1 mg/kg + 10 ml/kg) Amphetamine (i.p., four 30 min pairings every other day for 8 days) plus decynium-22 (i.p., 60 min before each pairing) |
Ref.45 | ||
| 2022 | Both | C57BL/6J | ⇑ Place preference in females (1 mg/kg + 0.1 mg/kg) ≈ Place preference in males (1 mg/kg + 0.1 mg/kg) Locomotor sensitization Amphetamine (i.p., every other day for 4 injection days) plus saline (i.p., 60 min before each injection) ⇓ Sensitization, both sexes (1 mg/kg + 10 ml/kg) Amphetamine (i.p., every other day for 4 injection days) plus decynium-22 (i.p., 60 min before each injection) ≈ Sensitization, both sexes (1 mg/kg + 0.1 mg/kg) Tail suspension |
JN Beaver |
| WT | ≥90 days | Saline (i.p., 30 min before test) | TL Gilman | |
| HT KO n = 6–14 |
⇓ Latency to first immobility bout, female KO (10 ml/kg) Escitalopram (i.p., 30 min before test) ≈ Per cent saline control immobility, both sexes (1 mg/kg) ≈ Per cent saline control immobility, all females (2 mg/kg) ⇑ Per cent saline control immobility, male KO (2 mg/kg) ⇑ Per cent saline control immobility latency, female KO (1 mg/kg) ⇑ Per cent saline control immobility latency, female KO (2 mg/kg) ≈ Per cent saline control immobility latency, all males (1 mg/kg) ≈ Per cent saline control immobility latency, all males (2 mg/kg) Bupropion (i.p., 30 min before test) Per cent saline control immobility, both sexes (4 mg/kg) ≈ Per cent saline control immobility, both sexes (8 mg/kg) ≈ Per cent saline control immobility latency, female KO (4 mg/kg) ⇑ Per cent saline control immobility latency, female KO (8 mg/kg) ≈ Per cent saline control immobility latency, all males (4 mg/kg) ≈ Per cent saline control immobility latency, all males (8 mg/kg) Locomotion Saline (i.p., 30 min before test) ≈ Locomotion, both sexes (10 ml/kg) Escitalopram (i.p., 30 min before test) |
10.3390/cells11121874 Ref.42 |
||
| ⇓ Per cent saline control locomotion, female HT and KO (1 mg/kg) | ||||
| ⇓ Per cent saline control locomotion, female HT (2 mg/kg) | ||||
| ≈ Per cent saline control locomotion, all males (1 mg/kg) | ||||
| ≈ Per cent saline control locomotion, all males (2 mg/kg) | ||||
| Bupropion (i.p., 30 min before test) | ||||
| ≈ Per cent saline control locomotion, all females (4 mg/kg) | ||||
| ⇓ Per cent saline control locomotion, females HT and KO (8 mg/kg) | ||||
| ⇑ Per cent saline control locomotion, male HT (4 mg/kg) | ||||
| ⇑ Per cent saline control locomotion, male HT (8 mg/kg) | ||||
| Cocaine (i.p., cumulative dosing over 40 min) | ||||
| ≈ Locomotion, both sexes (40 mg/kg cumulative) | ||||
| Amphetamine (i.p., cumulative dosing over 40 min) | ||||
| ≈ Locomotion, both sexes (4.62 mg/kg cumulative) | ||||
| Locomotor sensitization | ||||
| Cocaine (i.p., every day for 5 injection days) | ||||
| ⇓ Sensitization, female HT (40 mg/kg cumulative, 5th injection day) | ||||
| Amphetamine (i.p., every 3 days for 5 injection days) | ||||
| ⇓ Locomotion, female HT (4.62 mg/kg cumulative, 3rd injection day) |
Abbreviations: HT, heterozygote; ip., intraperitoneal; KO, knockout; WT, wild-type.
Genotypes not statistically compared; indications of genotype “differences” here are based on within-genotype findings that do not align between genotypes.
2 |. OCT2 (Slc22a2)
Presently, there exist constitutive knockouts of three uptake 2 monoamine transporters. Of these three genetic mouse models, publications on OCT2-deficient mice are least numerous. Nonetheless, the behavioural and pharmacological investigations using these mice have been extensive, assessing general locomotion, anxiety- and coping-related measures, social interaction, and reward-related behaviours. The two published behavioural papers using OCT2 mice further evaluated responses to unpredictable chronic mild stress (UCMS; in male mice only; Table 1) and a pharmacological analogue in which male mice were administered corticosterone (the predominant stress hormone in rodents) in their drinking water for several weeks (Table 2).
2.1 |. Baseline behaviour
Though no differences in locomotor activity were observed in OCT2 KO mice relative to control wild-types (WTs), KO mice did exhibit reduced performance on the rotarod (Table 1), a measure of motor balance/coordination.36 Generally, these OCT2 KO mice displayed reduced anxiety-related behaviours that were consistent across open field, elevated O maze, and novelty-suppressed feeding tests.36 Mice without functional OCT2 were more immobile during forced swim36,37 and tail suspension tests,36 indicative of more passive coping behaviours (Table 1). Of note, for these baseline behaviour tests, the authors of one paper stated that “similar effects of genotype were observed for male and female mice.”36 But, it is unclear how many mice of each sex were used for behaviour tests and what the outcomes were of any statistical tests used to evaluate potential sex differences. The other paper used exclusively male mice.37
2.2 |. Pharmacological findings
Expanding upon forced swim findings, investigators gave WT and KO mice (presumably both sexes) injections of saline, citalopram, reboxetine, or venlafaxine 30 min prior to testing.36 Saline-injected KOs mirrored the increased immobility observed in injection-naïve KOs, relative to respective WT controls (Table 2). Venlafaxine injections at three different doses normalized KO immobility to resemble those of WTs, with WT mice only exhibiting attenuated immobility after the highest (32 mg/kg) venlafaxine dose. In other words, OCT2 KO mice exhibited a leftward shift in their dose–response curve to venlafaxine.36 A similar leftward shift was observed in the behavioural responses of OCT2 KOs to three separate doses of reboxetine, whereas immobility in WT mice was significantly reduced only by the two highest reboxetine doses.36 Though OCT2 KO mice also experienced a reduction in immobility in response to three increasing citalopram doses, the pattern was different from responses to venlafaxine and reboxetine, in that the two highest citalopram doses reduced WT immobility but maintained the observed genotype difference seen in saline-treated animals36 (Table 2). Combined, these data indicate that OCT2 KO mice behaviourally respond more to drugs that block uptake 1-mediated transport of norepinephrine (reboxetine), or both norepinephrine and serotonin (venlafaxine), in comparison to just serotonin (citalopram). This aligns with evidence that OCT2 transports norepinephrine nearly three times faster than serotonin.7
In addition to examining how a single drug injection affected behavioural responses to an acute stressor, the same investigators also explored how chronic increases in corticosterone levels affected behaviour in OCT2 KO mice and their responses to chronic venlafaxine administration (Table 2). Using singly housed males for these studies, mice received corticosterone (35 μg/ml) or vehicle (0.45% hydroxypropyl-β-cyclodextrin) in their drinking water for 4 weeks. Then, behavioural tests of social interaction, novelty-suppressed feeding, sucrose preference, and elevated O maze were performed.36 Investigators also evaluated overall coat state and performed a splash test (also known as a spray test) to evaluate subsequent grooming behaviour. As observed in untreated mice, vehicle-treated male KOs had a lower latency to feed in the novelty-suppressed feeding test, plus spent more time in the open areas of the elevated O maze.36 Treatment with corticosterone for 4 weeks did not affect the genotype difference in the O maze, but did abolish the genotype difference in the novelty-suppressed feeding test (Table 2). Similarly, increased grooming in the splash/spray test noted in male KOs was abolished by 4 weeks of corticosterone administration.36 The absence of genotype differences in social interaction, sucrose preference, and coat state observed in vehicle-treated males persisted in corticosterone-treated males. Thus, though corticosterone treatment did shift overall behaviour in both WTs and KOs, there were relatively few KO-specific changes induced after the 4 week period.
A similar series of experiments performed by the same group assessed how UCMS affected several of these same measures (social interaction, coat condition and sucrose preference), plus some different parameters (object location test, nest building and forced swim).37 The UCMS protocol involved exposure of male mice to nine different types of stressors over the course of 8 weeks, though the order, repetitions, and duration of each stressor exposure are unclear save for one detail (sounds of predators for 15 min).37 Unlike with mice chronically consuming corticosterone in the drinking water,36 OCT2 KO mice undergoing UCMS exhibited consistently worse coat states from weeks 2 through 8 of the UCMS manipulation37 (Table 1). Also unlike the drinking water study, UCMS impaired social interaction selectively in OCT2 KO mice at weeks 3 and 4.37 Sucrose preference, at least, remained consistently without a genotype difference across studies.36,37 Over the course of the UCMS manipulation, male OCT2 KO mice displayed accelerated impairments in object location testing.37 After 5 weeks of UCMS, nest building was impaired in male OCT2 KO mice relative to WTs, whereas the enhanced immobility in the forced swim test that male OCT2 KO mice exhibit36,37 was obscured by UCMS elevating immobility in WTs to resemble that of UCMS-exposed OCT2 KO mice37 (Table 1). Combined, the UCMS and corticosterone in drinking water studies help highlight how elevated corticosterone levels alone are insufficient to mimic a chronic stress state that involves physically/psychologically adverse experiences.
Returning to the study involving 4 weeks of corticosterone in the drinking water,36 these same male mice continued consuming corticosterone in their drinking water, but for the next 3 weeks also received daily injections of venlafaxine (16 mg/kg/day, ip.; Table 2). It is unclear what control/vehicle injection was given (if any) for venlafaxine treatment during these last 3 weeks of corticosterone in drinking water. This question arises given that behaviour in mice receiving corticosterone without venlafaxine exhibited some shifts between the 4 and 7 week time-points.36 However, these shifts could also be attributable, at least in part, to reexposure of the same mice to the same tests. In particular, the genotype difference in open area Time in the O maze disappears at 7 weeks in control (i.e., corticosterone only, no venlafaxine treatment) mice. Coat state also worsened across genotypes, as anticipated with continued corticosterone consumption. The reductions in sucrose consumption, grooming in the splash/spray test, overall coat state, and O maze open zone time that were induced in both OCT2 genotypes after 4 weeks of corticosterone administration were selectively ameliorated by a further 3 weeks of concurrent venlafaxine treatment in OCT2 WT, but not in OCT KO, mice36 (Table 2). Though unclear why, it appears that social interaction and novelty-suppressed feeding were not tested again at the 7 week time-point in these mice.
Interestingly, these findings contradict observations with antidepressant treatment in the acute forced swim test, where OCT2 KO mice exhibited leftward-shifted dose–response curves (Table 2). However, this apparent contradiction can be remedied by remembering that forced swim tests are far from perfect for screening putative antidepressants,48–50 and further highlights how OCT2 deficiency could reflect a contribution to the notoriously poor effectiveness of antidepressant drugs as treatments, recognized in the past decade.51–53 Alternatively, it could be the combination of continued corticosterone treatment in conjunction with OCT2 deficiency that impeded the effectiveness of venlafaxine treatment, given that at least in some situations, treatment with antidepressants might help alleviate endogenous corticosterone (or cortisol, in humans) levels that would feed forward to improve behaviour. Of course, the behavioural improvements in response to venlafaxine in WT mice still consuming corticosterone argue against this interpretation. Overall, these studies suggest that intact OCT2 function dampens anxiolytic processes, but simultaneously promotes active coping behaviours and facilitates effectiveness of antidepressant treatments.
3 |. OCT3 (Slc22a3)
Articles using OCT3-deficient mice are more numerous than those using OCT2-deficient mice. Nonetheless, investigations with OCT3-deficient mice have characterized relatively few baseline behaviours (Table 1), with the predominant focus placed upon how constitutively reduced OCT3 function influences responses to psychostimulant drugs (Table 2).
3.1 |. Baseline behaviour
Overall locomotion and motor coordination in OCT3 KO mice appear unaffected,39 though activity in familiar arms of the Y maze indicated a drop in locomotor activity (Table 1). Whether this study used one or both sexes is unclear.39 While these investigators reported reduced time in the centre of the open field,39 indicative of elevated anxiety-related behaviour, another group of researchers found that their male OCT3-deficient mice spent more per cent time in the open arms of the elevated plus maze and in the centre of the open field,40 suggesting reduced anxiety-related behaviour (Table 1). The latter investigators further reported that constitutive OCT3 deficiency had no impact on performance of male mice in the Morris water maze or in the resident–intruder test.40 Thus, behavioural shifts from OCT3 deficiency appear relatively minor, and the evidence regarding anxiety-related behaviour remains conflicting.
Interestingly, one group of researchers explored how OCT3 KO impacted consumption of water and salt (NaCl) solution during conditions of satiety and water deprivation.38 They observed no genotype effects under satiated conditions, but enhanced salt solution consumption after 24 h of water deprivation38 (Table 1). In combination with the other behavioural findings summarized above, and the pharmacological studies reviewed below, the influence of OCT3 (and PMAT, covered in the next section) might be more evident under conditions of substantial (neuro)physiological perturbation.
3.2 |. Pharmacological findings
Researchers expanded upon their findings regarding how OCT3 KO affects fluid consumption by introducing injections of saline or furosemide, a diuretic compound (routes not stated), in conjunction with diets replete with, or depleted of, sodium.38 They found that OCT3 KO mice (sex(es) not stated) consumed equivalent amounts of fluid as WT when on a sodium-depleted diet and injected with saline, but that KO consumption of salt solutions were increased relative to WT when furosemide treatment was given in conjunction with sodium-depleted diet38 (Table 2). In fact, even when mice had a replete sodium diet and received furosemide, OCT3 KO mice still consumed more salt solution than their WT counterparts.38 In other words, intact OCT3 function appears instrumental in diminishing an appetite for salt under conditions of dehydration. Whether this is specific to salt in solution, or might generalize to salt in food, remains unexplored.
One study investigated how OCT3 deficiency affected coping behaviour in the tail suspension test (TST) in response to the antidepressant fluvoxamine, uptake 2 blocker D22, or their combination43 (Table 2). Though OCT3 WT and KO mice were not directly statistically compared, the researchers observed that D22 (0.1 mg/kg) or fluvoxamine (10 mg/kg) alone did not impact TST immobility in either OCT3 WT or KO mice.43 Co-administering these two drugs enhanced active coping (i.e., decreased immobility) in OCT3 WT, but not KO, mice (Table 2), suggesting that OCT3 expression significantly contributes to the combinatorial effect of these drugs. However, increasing D22 to 0.32 mg/kg and co-administering it with the same fluvoxamine dose did reduce immobility in OCT3 KO mice,43 suggesting that OCT3 expression is not necessary for this active coping effect. This latter drug combination was not tested in OCT3 WT mice nor was the higher D22 dose, though the higher D22 dose alone was without effect in OCT3 KOs.43
Three articles explored how constitutive OCT3 deficiency influences behavioural responses to psychostimulants, particularly amphetamine (Table 2). Two examine locomotor activity after acute administration of cocaine39 or amphetamine.39,44 While one group reported no differences between male WT and KO mice receiving 1, 3.2, or 10 mg/kg of amphetamine,44 the other group found that KO mice (sex(es) not stated) displayed increased locomotor activity to the highest doses of amphetamine (10 mg/kg) and cocaine (40 mg/kg) given39 (Table 2). Because the latter group did not specify the sex or sexes of the mice they used, these genotype differences might have been driven by females, given the absence of differences in males given 10 mg/kg.44 When D22 was given to OCT3 WT males, the amphetamine-induced locomotor response was attenuated—an effect lost in OCT3 KO males (Table 2), indicating that OCT3 function contributes to this behavioural consequence of amphetamine.44
Two separate groups of researchers consistently found that OCT3 deficiency does not affect amphetamine-induced locomotor sensitization.39,45 Findings are less consistent regarding conditioned place preference (CPP), as one group reported no differences (sex(es) not stated39), whereas another found that OCT3 KO males—but not females—failed to develop CPP to amphetamine.45 This may be a dose–response effect, given the former group used 2 mg/kg doses,47 and the latter used 1 mg/kg doses,49 both with four pairings across 8 days (Table 2). The more recent study further investigated amphetamine-induced locomotor sensitization and CPP by incorporating D22 pretreatments with both paradigms.49 While amphetamine-induced locomotor sensitization remained unaffected by genotype or D22 pretreatment, CPP to amphetamine was attenuated by D22 pretreatment specifically in female OCT3 KO mice45 (Table 2). This suggests that in females, an uptake 2 other than OCT3, possibly due to increased compensatory expression, contributes to development of amphetamine-mediated CPP.
Though OCT3’s influence on baseline behaviour has been relatively underexplored (Table 1), considerable evidence supports OCT3 as contributing to the behavioural effects elicited by amphetamine (Table 2). Specifically, in males, OCT3 is likely important for the locomotor-stimulating effects of amphetamine, as well as reward-related effects of low doses of amphetamine measured using CPP. Further investigations are necessary to determine how OCT3 function influences stereotypic behaviour that emerges with higher doses of cocaine and amphetamine. Additionally, studies exploring how constitutive OCT3 deficiency affects self-administration of reinforcing compounds could help delineate OCT3’s role(s) in the locomotor-stimulating versus reward-related components of psychostimulants.
4 |. PMAT (Slc29a4)
Studies of the behavioural consequences of constitutive PMAT deficiency have emerged later than those of OCT2 and OCT3, given that a PMAT KO mouse was not successfully created until 2013.54 PMAT also belongs to a different gene class than OCT2 and OCT3, as it was originally identified as equilibrative nucleoside transporter 4 (ENT4), and only later was its capability to transport monoamines discovered.5,35 Also unlike OCT2 and OCT3, which preferentially transport the monoamines histamine, norepinephrine, and epinephrine, PMAT preferentially transports dopamine and serotonin. Despite its relatively recent arrival in the scientific world, there are already four articles that have evaluated behaviour in PMAT-deficient mice.
4.1 |. Baseline behaviour
Largely, PMAT-deficient mice do not exhibit prominent behavioural differences (Table 1), similar to OCT3-deficient mice. When PMAT-deficient mice of both sexes were assessed across all three genotypes (WT, heterozygote [HT] and KO), main effects of genotype were detected in elevated plus maze latency to first enter open arm and distance travelled in same, differences that appeared driven by HTs.41 However, no differences across genotypes in either sex were found for time in open or closed arms of the elevated plus maze, nor in locomotor activity, marble burying, or measures of immobility, climbing, latency to first immobility bout, or faecal boli in the forced swim test.41 A main effect of genotype was noted across sexes for faecal boli after a 4 h locomotor test, an effect that appeared driven by KOs (Table 1). The most prominent genotype effect was observed in female KOs, which exhibited increased swimming behaviour in the forced swim test.41 Interestingly, in the TST, non-injected male PMAT HT and KO specifically exhibited increased immobility,42 despite displaying no behavioural differences in the forced swim test.41 Together, these findings highlight that constitutive PMAT deficiency appears detectable predominantly under conditions of (neuro)physiological perturbations.
4.2 |. Pharmacological findings
The first pharmacological study involving PMAT-deficient mice (Table 2) found that male KOs fail to exhibit a reduction in immobility in the forced swim test after ketamine administration, unlike their male WT counterparts.46 This unresponsiveness to ketamine did not appear confounded by any changes in locomotor activity, suggesting that ketamine acts, in part, by interfering with PMAT function to attenuate immobility in the forced swim test.46 When assessing responses to anti-depressant drugs in the TST, an unexpected reduction in latency to first immobility bout was observed in female KOs injected with saline.42 Given this sex- and genotype-specific difference in saline controls, all mice given drugs were evaluated as ≈ Per cent change from their same-sex and same-genotype saline-injected counterparts. In analysing the data in this way, escitalopram (1 and 2 mg/kg) and bupropion (8 mg/kg) emerged as significantly increasing the latency to first immobility bout specifically in female KOs.42 In contrast, males exhibited no genotype-specific responses to either drug (Table 2). However, male PMAT KOs failed to exhibit the immobility-reducing effects of escitalopram (2 mg/kg), whereas no effects of genotype were observed in females to either escitalopram or bupropion in terms of immobility time.42 Largely, these effects were not attributable to locomotor confounds, as escitalopram reduced locomotor activity (relative to same-sex/genotype saline-injected controls) in female HTs (both doses) and KOs (1 mg/kg).42 Likewise, bupropion reduced locomotor activity in female HTs and KOs (8 mg/kg), but increased locomotor activity specifically in male HTs (4 and 8 mg/kg), which did not exhibit any altered responses to these drugs in the TST.42 In conjunction with the findings for ketamine (Table 2), evaluations of these antidepressant drugs in PMAT-deficient mice suggest that PMAT function contributes to the effectiveness of escitalopram in males, but might undermine the effectiveness of these drugs in females.
Shifting to psychostimulants, two studies have assessed amphetamine-induced locomotor sensitization, while one study each has looked at either cocaine-induced locomotor sensitization or amphetamine-mediated CPP (Table 2). First, when evaluating amphetamine-induced locomotor sensitization occurring during CPP conditioning, one group found that in both sexes, PMAT KO mice exhibited reduced locomotor sensitization, an observation unaffected by D22 pretreatment.45 Another group that assessed amphetamine-induced locomotor sensitization using a different cumulative dosing paradigm across 13 days, with injection gaps of 3 days, found a significant reduction in amphetamine-induced locomotor sensitization only on day 7 (i.e., the third injection day of five total) and only in female HTs.42 The same group similarly found that only female HTs exhibited attenuated cocaine-induced locomotor sensitization and only on the fifth of five consecutive injection days (Table 2). Initial (i.e., injection day 1) responses to both cocaine and amphetamine were unaffected in either sex across PMAT genotypes,42 unlike OCT3 KO mice that exhibited enhanced locomotion in response to the highest studied doses of the same drugs.39 Assessment of amphetamine-mediated CPP in PMAT KO and WT mice revealed no impact of PMAT deficiency, whereas pretreatment with D22 abrogated CPP in males of both genotypes, but in females only impeded CPP in WTs45 (Table 2). This indicates that D22’s ability to block amphetamine-mediated CPP in females is likely, at least in part, through inhibition of PMAT. Combined with the same article’s findings regarding CPP in OCT3 KO mice with and without D22 pretreatment, these outcomes indicate that in males, OCT3 is more involved in CPP, whereas in females, PMAT is a key contributor. Investigation of CPP in female PMAT HTs, considering what was observed regarding attenuated psychostimulant-induced locomotor sensitization, could be interesting considering there are common polymorphisms identified in humans that attenuate (but do not completely ablate) PMAT function.55–57
Consistent across PMAT studies is evidence that this uptake 2 transporter’s function, like OCT3’s, is most evident under conditions of disrupted (neuro)physiological homeostasis, whether through environmental stress (forced swim and tail suspension; Table 1) or drug administration (antidepressants and psychostimulants; Table 2). In three of the four studies, evidence supports sex-specific influences of constitutive PMAT deficiency upon behaviour,41,42,45 an observation also found with cardiovascular measures.58 A recent report indicates that PMAT function may be reduced through an estradiol-mediated signalling mechanism.59 This might help explain why HT females exhibited more prominent PMAT genotype effects in psychostimulant-induced locomotor sensitization paradigms,42 but it remains unclear why some behavioural differences are observed in female PMAT KOs but not male PMAT KOs, or vice versa, given there are no functional PMAT to down-regulate in these mice. Future studies into sex-specific effects in PMAT HT mice will advance understanding of estradiol’s influences on this transporter, and as with studies in OCT3-deficient mice, use of self-administration paradigms could provide helpful information regarding PMAT’s influence in responses to reinforcing stimuli.
5 |. LOOKING FORWARD
Contrary to some perceptions of uptake 2 monoamine transporters merely serving as redundancies to uptake 1 monoamine transporters, the articles covered in this mini review demonstrate that the former class of transporters does contribute meaningfully to monoamine signalling under baseline conditions (Table 1). Moreover, behavioural shifts are largely consistent within, but not across, transporters. This is surprising regarding OCT2 and OCT3, given their similarities in preferred substrates.4 But this makes more sense considering their sequence homology is only ~47–48%,60 and the expression profile of OCT2 is greater in limbic and stress-responsive areas36,37 versus OCT3 expression being greater in dopaminergic and motor-related brain regions.39 Of the three knocked out genes, OCT3 remains least characterized for baseline behavioural changes (Table 1), but most thoroughly assessed regarding the cellular mechanism of action of amphetamine.44 Both OCT2 and PMAT KO mouse models have undergone more baseline behavioural evaluations (Table 1), and all uptake 2 mice have had assessments of acute behavioural responses to antidepressant compounds (Table 2). Where the research using OCT and PMAT transporter models diverges is on their responsivity to different manipulations. In OCT2 KO mice, research has focused upon stress and stress hormone responsivity, whereas research in PMAT-deficient mice has focused more upon psychostimulant responsivity. Consequently, much knowledge awaits discovery through continued baseline (OCT3), psychostimulant (OCT2), and stress (hormone) responsivity (PMAT) behavioural assessments in these different constitutively deficient mouse lines.
While constitutive genetic deficiency in transporter function can provide only some information regarding the roles of intact uptake 2, this information is nonetheless useful. Considering that polymorphisms with functional consequences exist in human genes for OCT2 and OCT3 (see excellent review61) and PMAT,55–57 HT mouse models may be particularly useful for understanding the neurobehavioural consequences of these polymorphisms. Surprisingly, few studies have included HTs in their analyses.40–42 Nonetheless, all three of these studies observed shifts in behaviour of HTs in at least one measure utilized. In addition to studying HT mice, behavioural studies of humans with these polymorphisms would provide further insight into influences of uptake 2 on behaviour, possibly with direct clinical relevance.
Pharmacological tools remain important in advancing understanding of protein function. Unfortunately, there are no commercially available drugs that selectively inhibit individual uptake 2 without also producing confounding off-target physiological effects. As the relatively recent history of studying SERT and selective serotonin reuptake inhibitors has shown,62–64 pharmacological inhibition of a transporter can have drastically different behavioural effects than constitutive genetic KO of the same transporter. One indirect goal of this mini review is to highlight the dire need for appropriate pharmacological tools to advance study of uptake 2 in whole organisms. Simultaneously, the findings summarized here are intended to provide an impetus for development of such drugs.
Studies reviewed here that have integrated constitutive genetic deficiency of uptake 2 with pharmacological disruption of at least one uptake 1 (Table 2) suggest three key points. First, studies in uptake 2 mice indicate that function of these transporters might help explain the ineffectiveness of many antidepressant drugs in alleviating depression symptoms in humans. Second, studies in OCT3-and PMAT-deficient mice suggest that function of these transporters influences behavioural responses to drugs that can be abused, including cocaine and amphetamine. Though additional work is needed to determine if these transporters affect reinforcing or other experiential aspects of drugs that promote continued drug (ab)use, there may be therapeutic potential through targeted inhibition of uptake 2. Third, consistent evidence across labs studying OCT3 and PMAT demonstrates sex-specific outcomes of reduced or ablated transporter function. When this information is integrated with the first and second key points above, this means drugs targeting different uptake 2 could be implemented for personalized medicine/treatment approaches. These three key points remain, in the end, hypothetical. How much could become reality necessitates that selective inhibitors of the individual uptake 2 be identified and made commercially available for broad investigative purposes. Until that becomes an option, continued diligent study using constitutive genetic deficiency mouse models will be one of this field’s best approaches for uncovering further behavioural influences of uptake 2.
ACKNOWLEDGEMENTS
This work was supported by a 2017 NARSAD Young Investigator Grant (26249) from the Brain & Behavior Research Foundation and Vital Projects Fund, Inc. to TLG, by a National Institute of Mental Health grant (R15 MH118705) to TLG, by Kent State University, and by the Applied Psychology Center in the Department of Psychological Sciences at Kent State University. Figure 1 was created using Biorender.com (Toronto, ON).
Funding information
Brain & Behavior Research Foundation and Vital Projects Fund, Inc., Grant/Award Number: 26249; National Institute of Mental Health, Grant/Award Number: R15 MH118705; Kent State University; Applied Psychology Center in the Department of Psychological Sciences at Kent State University
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose. The funders of this work were not involved in the preparation of this manuscript, the interpretations of the articles reviewed, nor the decision to submit this manuscript for publication.
REFERENCES
- 1.Gainetdinov RR, Caron MG. Monoamine transporters: from genes to behavior. Pharmacol Toxicol. 2003;43(1):261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309 [DOI] [PubMed] [Google Scholar]
- 2.Muma NA, Mi Z. Serotonylation and transamidation of other monoamines. ACS Chem Nerosci. 2015;6(7):961–969. doi: 10.1021/cn500329r [DOI] [PubMed] [Google Scholar]
- 3.Bockaert J, Bécamel C, Chaumont-Dubel S, Claeysen S, Vandermoere F, Marin P. Novel and atypical pathways for serotonin signaling. Fac Rev. 2021;10:52. doi: 10.12703/r/10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koepsell H General Overview of Organic Cation Transporters in Brain. In: Daws LC. ed. Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology. Vol. 266. Springer, Cham; 2021:1–39. doi: 10.1007/164_2021_449 [DOI] [PubMed] [Google Scholar]
- 5.Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279(48):50042–50049. doi: 10.1074/jbc.m407913200 [DOI] [PubMed] [Google Scholar]
- 6.Gu H, Wall SC, Rudnick G. Stable expression of biogenic amine transporters reveals differences in inhibitor sensitivity, kinetics, and ion dependence. J Biol Chem. 1994;269(10):7124–7130. doi: 10.1016/s0021-9258(17)37256-3 [DOI] [PubMed] [Google Scholar]
- 7.Duan H, Wang J Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335(3):743–753. doi: 10.1124/jpet.110.170142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X, Giacomini KM. Transporters involved in metformin pharmacokinetics and treatment response. J Pharm Sci. 2017; 106(9):2245–2250. doi: 10.1016/j.xphs.2017.04.078 [DOI] [PubMed] [Google Scholar]
- 9.Koepsell H Organic cation transporters in health and disease. Pharmacol Rev. 2020;72(1):253–319. doi: 10.1124/pr.118.015578 [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35(10):1956–1962. doi: 10.1124/dmd.107.015495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleasby K, Castle JC, Roberts CJ, et al. Expression profiles of 50 xenobiotic transporter genes in humans and pre-clinical species: a resource for investigations into drug disposition. Xenobiotica. 2006;36(10–11):963–988. doi: 10.1080/00498250600861751 [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Duan H, Hebert MF, Liang CJ, Rice KM, Wang J. Taste of a pill. J Biol Chem. 2014;289(39):27055–27064. doi: 10.1074/jbc.m114.570564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arner P, Kulyté A, Batchelor K, Laurencikiene J, Livingston J, Rydén M. Mapping of biguanide transporters in human fat cells and their impact on lipolysis. Diabetes Obes Metab. 2018; 20(10):2416–2425. doi: 10.1111/dom.13395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes K, Dobrzynski H, Foppolo S, et al. Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res. 2006;99(5):510–519. doi: 10.1161/01.res.0000238359.18495.42 [DOI] [PubMed] [Google Scholar]
- 15.Koepsell H Update on drug-drug interaction at organic cation transporters: mechanisms, clinical impact, and proposal for advanced in vitro testing. Expert Opin Drug Met. 2021;17(6): 635–653. doi: 10.1080/17425255.2021.1915284 [DOI] [PubMed] [Google Scholar]
- 16.Sweet DH. Organic Cation Transporter Expression and Function in the CNS. In: Daws LC. ed. Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology. Vol. 266; Springer, Cham; 2021:41–80. doi: 10.1007/164_2021_463 [DOI] [PubMed] [Google Scholar]
- 17.Fraser-Spears R, Krause-Heuer AM, Basiouny M, et al. Comparative analysis of novel decynium-22 analogs to inhibit transport by the low-affinity, high-capacity monoamine transporters, organic cation transporters 2 and 3, and plasma membrane monoamine transporter. Eur J Pharmacol. 2019;842:351–364. doi: 10.1016/j.ejphar.2018.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schömig E, Babin-Ebell J, Russ H. 1,1′-Diethyl-2,2′-cyanine (decynium22) potently inhibits the renal transport of organic cations. Naunyn-schmiedeberg’s Archives Pharmacol. 1993; 347(4):379–383. doi: 10.1007/bf00165387 [DOI] [PubMed] [Google Scholar]
- 19.Gasser PJ, Lowry CA, Orchinik M. Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci. 2006;26(34):8758–8766. doi: 10.1523/jneurosci.0570-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Kloet ER, Karst H, Joëls M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol. 2008;29(2):268–272. doi: 10.1016/j.yfrne.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Pistell PJ, Gupta S, Knight AG, et al. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antiviral Res. 2010;88(3):334–342. doi: 10.1016/j.antiviral.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan H, Hu T, Foti RS, Pan Y, Swaan PW, Wang J. Potent and selective inhibition of plasma membrane monoamine transporter by HIV protease inhibitors. Drug Metab Dispos. 2015;43(11):1773–1780. doi: 10.1124/dmd.115.064824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–881. doi: 10.1089/dna.1997.16.871 [DOI] [PubMed] [Google Scholar]
- 24.Gründemann D, Schechinger B, Rappold G, Schömig E. Molecular identification of the corticosterone-sensitive extra-neuronal catecholamine transporter. Nat Neurosci. 1998;1(5): 349–351. doi: 10.1038/1557 [DOI] [PubMed] [Google Scholar]
- 25.Amphoux A, Vialou V, Drescher E, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006; 50(8):941–952. doi: 10.1016/j.neuropharm.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 26.Daws LC. Organic Cation Transporters in Psychiatric Disorders. In: Daws LC. ed. Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology. Vol.266; 2021:215–239. doi: 10.1007/164_2021_473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonker JW, Wagenaar E, Mol CAAM, et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21(16):5471–5477. doi: 10.1128/mcb.21.16.5471-5477.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonker JW, Wagenaar E, van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23(21):7902–7908. doi: 10.1128/mcb.23.21.7902-7908.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuda M, Terada T, Mizuno T, et al. Targeted disruption of the multidrug and toxin extrusion 1 (Mate1) gene in mice reduces renal secretion of metformin. Mol Pharmacol. 2009;75(6):1280–1286. doi: 10.1124/mol.109.056242 [DOI] [PubMed] [Google Scholar]
- 30.Masuda S, Terada T, Yonezawa A, et al. Identification and functional characterization of a new human kidney–specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J am Soc Nephrol. 2006;17(8):2127–2135. doi: 10.1681/asn.2006030205 [DOI] [PubMed] [Google Scholar]
- 31.Couroussé T, Gautron S. Role of organic cation transporters (OCTs) in the brain. Pharmacol Therapeut. 2015;146:94–103. doi: 10.1016/j.pharmthera.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 32.Gasser PJ. Roles for the uptake2 transporter OCT3 in regulation of dopaminergic neurotransmission and behavior. Neurochem Int. 2018;123(Neuropharmacology 50 2006):46–49. doi: 10.1016/j.neuint.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasser PJ, Lowry CA. Organic cation transporter 3: a cellular mechanism underlying rapid, non-genomic glucocorticoid regulation of monoaminergic neurotransmission, physiology, and behavior. Horm Behav. 2018;104(Trends Endocrinol. Metab. 9 1998):173–182. doi: 10.1016/j.yhbeh.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vieira LS, Wang J. Brain Plasma Membrane Monoamine Transporter in Health and Disease. In: Daws LC. ed. Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology; Vol. 266. Springer, Cham; 2021:253–280. doi: 10.1007/164_2021_446 [DOI] [PubMed] [Google Scholar]
- 35.Wang J The plasma membrane monoamine transporter (PMAT): structure, function, and role in organic cation disposition. Clin Pharmacol Ther. 2016;100(5):489–499. doi: 10.1002/cpt.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacq A, Balasse L, Biala G, et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2012;17(9):926–939. doi: 10.1038/mp.2011.87 [DOI] [PubMed] [Google Scholar]
- 37.Couroussé T, Bacq A, Belzung C, et al. Brain organic cation transporter 2 controls response and vulnerability to stress and GSK3β signaling. Mol Psychiatry. 2015;20(7):889–900. doi: 10.1038/mp.2014.86 [DOI] [PubMed] [Google Scholar]
- 38.Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci. 2004;24(11):2846–2851. doi: 10.1523/jneurosci.5147-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vialou V, Balasse L, Callebert J, Launay J, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 2008;106(3): 1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x [DOI] [PubMed] [Google Scholar]
- 40.Wultsch T, Grimberg G, Schmitt A, et al. Decreased anxiety in mice lacking the organic cation transporter 3. J Neural Transm. 2009;116(6):689–697. doi: 10.1007/s00702-009-0205-1 [DOI] [PubMed] [Google Scholar]
- 41.Gilman TL, George CM, Vitela M, et al. Constitutive plasma membrane monoamine transporter (PMAT, Slc29a4) deficiency subtly affects anxiety-like and coping behaviours. Eur J Neurosci. 2018;48(1):1706–1716. doi: 10.1111/ejn.13968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaver JN, Weber BL, Ford MT, Anello AE, Kassis SK, Gilman TL. Uncovering functional contributions of PMAT (Slc29a4) to monoamine clearance using pharmacobehavioral tools. Cell. 2022;11(12):1874. doi: 10.3390/cells11121874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horton RE, Apple DM, Owens WA, et al. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J Neurosci. 2013; 33(25):10534–10543. doi: 10.1523/jneurosci.5687-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayer FP, Schmid D, Owens WA, et al. An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology. 2018;43(12):1–10. doi: 10.1038/s41386-018-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clauss NJ, Koek W, Daws LC. Role of organic cation transporter 3 and plasma membrane monoamine transporter in the rewarding properties and locomotor sensitizing effects of amphetamine in male and female mice. Int J Mol Sci. 2021; 22(24):13420. doi: 10.3390/ijms222413420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowman MA, Vitela M, Clarke KM, Koek W, Daws LC. Serotonin transporter and plasma membrane monoamine transporter are necessary for the antidepressant-like effects of ketamine in mice. Int J Mol Sci. 2020;21(20):7581. doi: 10.3390/ijms21207581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daws LC (Ed). Organic Cation Transporters in the Central Nervous System. 1stEd. Springer Cham; 2021. doi: 10.1007/978-3-030-82984-1 [DOI] [Google Scholar]
- 48.Kara NZ, Stukalin Y, Einat H. Revisiting the validity of the mouse forced swim test: systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci Biobehav Rev. 2018;84:1–11. doi: 10.1016/j.neubiorev.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 49.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Nerosci. 2017;8(5):955–960. doi: 10.1021/acschemneuro.7b00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trunnell ER, Carvalho CDPO. The forced swim test has poor accuracy for identifying novel antidepressants. Drug Discov Today. 2021;26(12):2898–2904. doi: 10.1016/j.drudis.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 51.Meerman JJ, Janzing JGE, ter Hark SE, Coenen MJH. The potential of polygenic risk scores to predict antidepressant treatment response in major depression: a systematic review. J Affect Disord. 2022;304:1–11. doi: 10.1016/j.jad.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 52.Kovacs D, Gonda X, Petschner P, et al. Antidepressant treatment response is modulated by genetic and environmental factors and their interactions. Ann Gen Psychiatry. 2014;13(1):17. doi: 10.1186/1744-859x-13-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacQueen G, Santaguida P, Keshavarz H, et al. Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry. 2017; 62(1):11–23. doi: 10.1177/0706743716664885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem. 2013;288(5):3535–3544. doi: 10.1074/jbc.m112.436972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christensen MMH, Brasch-Andersen C, Green H, et al. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21(12):837–850. doi: 10.1097/fpc.0b013e32834c0010 [DOI] [PubMed] [Google Scholar]
- 56.Moeez S, Khalid S, Shaeen S, et al. Clinically significant findings of high-risk mutations in human SLC29A4 gene associated with diabetes mellitus type 2 in Pakistani population. J Biomol Struct Dyn. 2021;1–14. doi: 10.1080/07391102.2021.1975561 [DOI] [PubMed] [Google Scholar]
- 57.Dawed AY, Zhou K, van Leeuwen N, et al. Variation in the plasma membrane monoamine transporter (PMAT, encoded in SLC29A4) and organic cation transporter 1 (OCT1, encoded in SLC22A1) and gastrointestinal intolerance to metformin in type 2 diabetes: an IMI DIRECT study. Diabetes Care. 2019; 42(6):dc182182. doi: 10.2337/dc18-2182 [DOI] [PubMed] [Google Scholar]
- 58.Wei R, Gust SL, Tandio D, et al. Deletion of murine slc29a4 modifies vascular responses to adenosine and 5-hydroxytryptamine in a sexually dimorphic manner. Physiol Rep. 2020;8(5):e14395. doi: 10.14814/phy2.14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gu Y, Zhang N, Zhu S, Lu S, Jiang H, Zhou H. Estradiol reduced 5-HT reuptake by downregulating the gene expression of plasma membrane monoamine transporter (PMAT, Slc29a4) through estrogen receptor β and the MAPK/ERK sig-naling pathway. Eur J Pharmacol. 2022;924:174939. doi: 10.1016/j.ejphar.2022.174939 [DOI] [PubMed] [Google Scholar]
- 60.Maier J, Niello M, Rudin D, Daws LC, Sitte HH. The Interaction of Organic Cation Transporters 1–3 and PMAT with Psychoactive Substances. In: Daws LC. ed. Organic Cation Transporters in the Central Nervous System. Handbook of Experimental Pharmacology. Vol. 266. Springer, Cham; 2021: 199–214. doi: 10.1007/164_2021_469 [DOI] [PubMed] [Google Scholar]
- 61.Zazuli Z, Duin NJCB, Jansen K, Vijverberg SJH, van der Zee AHM, Masereeuw R. The impact of genetic polymorphisms in organic cation transporters on renal drug disposition. Int J Mol Sci. 2020;21(18):6627. doi: 10.3390/ijms21186627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bengel D, Murphy DL, Andrews AM, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53(4):649–655. doi: 10.1124/mol.53.4.649 [DOI] [PubMed] [Google Scholar]
- 63.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256 [DOI] [PubMed] [Google Scholar]
- 64.Haenisch B, Bönisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Therapeut. 2011;129(3):352–368. doi: 10.1016/j.pharmthera.2010.12.002 [DOI] [PubMed] [Google Scholar]