Abstract
Background
Tislelizumab plus chemotherapy improved overall survival compared to chemotherapy alone, while maintaining an acceptable level of safety. But it’s still unclear which strategy is the most cost-effective. The objective of the study was to compare the cost-effectiveness of tislelizumab plus chemotherapy as first-line therapy for patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) versus chemotherapy alone.
Methods
A partitioned survival model with three states was constructed based on the RATIONALE-306 trial. The model’s time horizon was ten years, and its cycle was three weeks. Only direct medical costs were considered from the healthcare perspective in China. Calculations were performed on total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs). One-way sensitivity and probabilistic sensitivity analysis (PSA) were performed to determine the uncertainty regarding model parameters.
Results
Tislelizumab plus chemotherapy provided 1.35 QALYs for $26,450.77, while chemotherapy alone provided 0.89 QALY for $16,687.15. Compared to chemotherapy alone, tislelizumab had an ICER of $21,062.09/QALY. At the threshold of three times the Chinese GDP per capita ($38,253/QALY), the PSA indicated that tislelizumab had a 96.4% likelihood of being designated cost-effective. At the threshold of 1.5 times the Chinese GDP per capita ($19,126.5/QALY), the PSA indicated that tislelizumab had a probability of 48.7% of being designated cost-effective.
Conclusion
Tislelizumab plus chemotherapy as the first treatment for patients with advanced or metastatic ESCC may be a cost-effective option compared to chemotherapy alone at 3 times Chinese GDP per capita.
Keywords: esophageal squamous cell carcinoma, cost-effectiveness, partitioned survival model, tislelizumab
Introduction
Esophageal cancer (EC) is a malignancy originating from esophageal epithelial cells, recognized for its high incidence, substantial mortality, and regional predilection. Globally, its incidence is ranked seventh amongst malignant tumors, with mortality standing at sixth.1 On a histological level, EC can be subdivided into esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC), with the latter making up around 90% of all EC cases.2 China, in particular, is an ESCC high-risk region;3 data from 2020 reported 324,000 new ESCC cases, with both incidence and mortality rates surpassing the Asian and global averages.4 Often, ESCC presents insidiously, with more than half of the patients diagnosed in the mid to late stages of the disease, resulting in a poor prognosis.
The disease and economic burdens imposed by ESCC are predicted to escalate significantly. Projections indicate that by 2023, the incidence of ESCC in China will rise from 0.061% to 0.065%, with direct medical costs escalating from $33.4 billion to $76.4 billion, reflecting a substantial increase of 128.7%.5 EC treatment primarily hinges on the stage of the disease, with the Programmed Cell Death Receptor 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) inhibitors playing a crucial role in managing advanced ESCC. The use of these treatments in conjunction with chemotherapy is now considered to be conventional therapeutic practice.6–10
Tislelizumab, a novel humanized anti PD-1 monoclonal antibody, was created separately in China. Notably, it is among the first domestic PD-1 monoclonal antibodies to undergo overseas clinical trials.11–13 Tislelizumab possesses a unique binding epitope, setting it apart from other PD-1 monoclonal antibodies. Although initially costly, its price reduced by over 70% after being officially added to China’s medical insurance catalogue in March 2021. According to the RATIONALE-306 clinical trial, Tislelizumab combined with chemotherapy displayed promising efficacy and safety in treating ESCC. The trial reported a median overall survival (OS) of 17.2 months and progression-free survival (PFS) of 7.3 months for patients with unresectable, locally advanced, recurrent or metastatic ESCC.14 In May 2023, the tislelizumab plus chemotherapy was authorized by the National Drug Administration (NMPA) as a first-line treatment for ESCC,15 based on the results of the RATIONALE-306 study. According to recent cost-effectiveness analyses, treating advanced or metastatic ESCC with immunotherapeutic agents may not always be cost-effective when adding immunotherapy.16–21 With its superior efficacy and improved accessibility, this therapeutic approach promises to deliver dual benefits, both clinical and economic, to a broader patient population. In March 2021, tislelizumab was officially added to China’s National Health Insurance Drug List. Several rounds of negotiations have resulted in the Tislelizumab ‘s price being comparatively low for its class, increasing the likelihood that it will be cost-effective. Therefore, we hypothesize that tislelizumab+chemotherapy is cost-effective compared to chemotherapy alone. As a first-line treatment option for advanced or metastatic ESCC, tislelizumab in combination with chemotherapy will be assessed in this study to determine its cost-effectiveness and provide useful insights for clinical practice and policy formation from a healthcare system perspective in China.
Methods
Model Structure
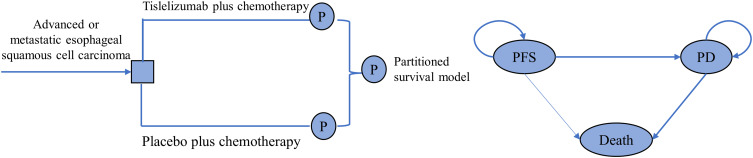
To evaluate the cost-effectiveness of tislelizumab combined with chemotherapy as the primary treatment for advanced or metastatic ESCC, we used a partitioned survival model (PSM) built in Microsoft Excel. As illustrated in Figure 1, the PSM encompassed three distinct health states: PFS, progressed disease (PD), and death. It was initially postulated that patients started in the PFS state, but they could either progress to PD, die, or remain in the PFS state.22 Transition probabilities for PFS and OS in both the tislelizumab-chemotherapy group and the placebo-chemotherapy group were sourced from the RATIONALE-306 clinical trial. These probabilities were extrapolated beyond the model’s time horizon using conventional statistical techniques as detailed by Guyot.23 To ascertain the percentage of individuals in the PD state, we employed the difference between the OS and PFS Kaplan-Meier curves. The PFS and OS curves were digitized using GetData Graph Digitizer (version 2.26) and fitted to the model using parametric survival functions: exponential, gamma, Weibull, log-logistic, and log-normal via the R programming language. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) were utilized to determine the survival functions with the best fit. (For detailed model selection methods, refer to eTable 1).
Figure 1.
The model structure of the partitioned survival model.
Data on patients who underwent subsequent therapy post-disease progression was sourced from the RATIONALE-306 trial. As the specific types of subsequent therapies administered in China were not recorded, we assumed that all patients received second-line chemotherapy with paclitaxel and cisplatin, mirroring the proportion reported in the RATIONALE-306 trial. The average patient was assumed to have a body surface area of 1.72 m2 and a weight of 65 kg. Essential clinical inputs can be found in Table 1. The model adopted a three-week cycle length and a ten-year time horizon.
Table 1.
Key Model Inputs
| Variable | Base | Min | Max | Distribution | Source |
|---|---|---|---|---|---|
| PFS survival model | |||||
| Log-logistic-Tislelizumab+ chemotherapy | λ=8.07, γ=1.78 | ||||
| Lognormal-Chemotherapy alone | Meanlog=4.53, sdlog=3.20 | ||||
| OS survival model | |||||
| Log-logistic-Tislelizumab+ chemotherapy | λ=17.59, γ=1.64 | ||||
| Log-logistic-Chemotherapy alone | λ=11.75, γ=1.67 | ||||
| Cost input, $/per cycle | |||||
| Tislelizumab (200mg) | 409.92 | 327.93 | 409.92 | Gamma | Local charge |
| Paclitaxel(30mg) | 23.96 | 19.17 | 23.96 | Gamma | Local charge |
| Cisplatin(30mg) | 2.84 | 2.28 | 2.84 | Gamma | Local charge |
| Routine follow-up cost per cycle | 27.42 | 21.93 | 32.90 | Gamma | Local charge |
| Administration per cycle | 435.21 | 348.17 | 522.26 | Gamma | Local charge |
| Laboratory tests and radiological examinations/per cycle-pfs | 84.76 | 67.81 | 101.71 | Gamma | Local charge |
| Laboratory tests and radiological examinations/per cycle-pd | 10.06 | 8.05 | 12.07 | ||
| Hospitalization and daily care | 89.27 | 71.42 | 107.13 | Gamma | Local charge |
| Anemia’s treatment- | 371.98 | 297.58 | 446.37 | Gamma | Local charge |
| Decreased neutrophil count ‘s treatment | 462.19 | 369.75 | 554.62 | Gamma | Local charge |
| Decreased white blood cell count ‘s treatment | 462.19 | 369.75 | 554.62 | Gamma | Local charge |
| Body surface area (meters2) | 1.72 | 1.38 | 2.06 | Normal | [24] |
| Discount rate | 0.05 | 0.00 | 0.08 | Fixed | [24] |
| Utility input | |||||
| Utility of PFS | 0.74 | 0.59 | 0.89 | Beta | [25] |
| Utility of PD | 0.58 | 0.46 | 0.70 | Beta | [25] |
| Disutility due to AEs | |||||
| Anaemia | 0.07 | 0.06 | 0.08 | Beta | [26] |
| Decreased neutrophil count | 0.20 | 0.16 | 0.24 | Beta | [26] |
| Decreased white blood cell count | 0.20 | 0.16 | 0.24 | Beta | [26] |
| Risk for main AEs in tislelizumab plus chemotherapy group | |||||
| Anaemia | 0.15 | 0.12 | 0.18 | Beta | [14] |
| Decreased neutrophil count | 0.31 | 0.248 | 0.372 | Beta | [14] |
| Decreased white blood cell count | 0.11 | 0.088 | 0.132 | Beta | [14] |
| Risk for main AEs in chemotherapy alone group | |||||
| Anaemia | 0.13 | 0.104 | 0.156 | Beta | [14] |
| Decreased neutrophil count | 0.33 | 0.264 | 0.396 | Beta | [14] |
| Decreased white blood cell count | 0.16 | 0.128 | 0.192 | Beta | [14] |
Abbreviations: OS, overall survival; PD, progressed disease; PFS, progression-free survival; AE, adverse event.
The primary outcome measure was the incremental cost-effectiveness ratio (ICER). Costs, initially recorded in Chinese Yuan (CNY), were converted to United States dollars (USD) using the 2022 average exchange rate: 1 USD = 6.72 CNY. The willingness-to-pay (WTP) threshold was established at 3 times 2022 Chinese GDP per capita to appraise the probability of cost-effectiveness.
Clinical Data Inputs
The clinical data inputs for our model are aligned with those of the RATIONALE-306 clinical trial. This trial was designed to evaluate the clinical efficacy and safety of a specified intervention. Executed using a randomized, double-blind, parallel-arm, placebo-controlled approach, the study had a global reach with patient recruitment spanning 162 medical facilities across Asia, Europe, North America, and Oceania. Eligible participants were individuals aged 18 or older, possessing an ECOG performance status of 1 or less, and having a pathological (histological) diagnosis of ESCC. Furthermore, participants either were initially identified with stage IV unresectable ESCC or had unresectable, locally advanced recurrent, or metastatic disease according to the AJCC 7th edition. A precondition for those who underwent prior platinum-based neoadjuvant/adjuvant chemotherapy was a treatment-free period of at least 6 months. Patients were excluded if they had received palliative radiotherapy for ESCC within 4 weeks prior to the study’s onset, had previous systemic treatments for unresectable, locally advanced recurrent, or metastatic ESCC, manifested uncontrolled effusions necessitating frequent interventions, demonstrated inoperable complete esophageal obstruction, received prior therapies targeting PD-1, PD-L1, or PD-L2, or displayed unintentional weight loss of ≥5% within the month leading up to randomization or had a Nutritional Risk Index (NRI) lower than 83.5.
Tislelizumab is administered at a dose of 200 mg intravenously (IV) on the first day of each 21-day cycle, alongside one of three dual-chemotherapy regimens. Depending on specific criteria or standard protocols, the platinum compound chosen can be either cisplatin or oxaliplatin, except in areas such as mainland China, Taiwan, Japan, and certain countries where oxaliplatin is not approved. The outlined regimens are as follows: 1) cisplatin (60–80 mg/m2) or oxaliplatin (130 mg/m2) IV on day 1, repeated every three weeks (Q3W), in conjunction with 5-FU (750–800 mg/m2/day) IV, administered as a continuous 24-hour infusion from days 1–5, Q3W; 2) the aforementioned platinum dosages paired with capecitabine (1000 mg/m2 orally, bi-daily) from days 1–14, Q3W; and 3) the indicated platinum dose combined with paclitaxel (175 mg/m2 IV) on day 1, Q3W. It’s crucial to highlight that cisplatin’s dosage can be divided over days 1–3, but the total dosage per cycle must remain within the range of 60–80 mg/m2. Simultaneously, an equivalent placebo is given on the first day of each 21-day cycle, paired with one of the three chemotherapy options. Within our model, we posit that the primary co-administered chemotherapy regimen is the combination of cisplatin and paclitaxel, which is also utilized in follow-up treatments after disease progression is detected.
Cost and Utility Inputs
We focused exclusively on direct medical costs. These encompassed the expenses for medications, management of adverse events (AEs) of grade 3 or higher that had a frequency of 10% and manifested solely in the initial cycle. Costs also factored in laboratory tests, radiological assessments, routine follow-up, administration, and hospital care. Tests considered in these costs included routine blood screenings, biochemical assays, coagulation evaluations, urinalysis, and a 12-lead ECG. Pricing data was sourced from the Fujian Cancer Hospital.
Absent critical quality-of-life data from the RATIONALE-306 study, utility values for PFS and PD were extrapolated from prior research.25 A disutility metric was integrated to quantify the influence of AEs of grade 3 or higher, with occurrences surpassing 10%, underscoring their detrimental effects on patient quality of life. Costs associated with medications, utility values, AE risks, and other relevant parameters are detailed in Table 1.
The ICER was calculated by dividing the difference in costs by the difference in quality-adjusted life-years (QALYs) acquired between the tislelizumab-plus-chemotherapy cohort and the standalone chemotherapy group. If the ICER was below the pre-established willingness-to-pay benchmark ($38,253/QALY), the combined intervention (tislelizumab and chemotherapy) was considered cost-effective as per the recommendation. Both costs and QALYs were discounted at a 5% annual rate.
Sensitivity Analysis
To comprehensively address parameter uncertainty in our study, we undertook a one-way sensitivity analysis. By selecting a 20% variation range, we factored in uncertainties pertaining to utilities, AE incidence, body surface area, and associated financial costs. This range was especially pertinent when considering drug costs: pharmaceutical pricing in China is under tight regulatory scrutiny, limiting drastic price alterations. Consequently, we adjusted the drug cost parameters to range from 80% to 100% of the originally set value. To further ensure a holistic analysis, the annual discount rate was systematically varied from 0% to 8%. A tornado diagram, instrumental in such analyses, was designed to visually represent and identify the most influential parameters on the study outcomes. Further reinforcing our analytical rigor, we employed the Monte Carlo simulation method, involving 1000 iterations, for the probabilistic sensitivity analysis. This method allowed us to assign a unique distribution to each parameter, as explicitly cataloged in Table 1. The outcomes of this intricate analysis were then communicated via an ICER scatterplot and the creation of cost-effectiveness acceptability curves, offering a nuanced understanding of cost versus efficacy.
Subgroup Analysis
Recognizing the heterogeneity within patient populations, our research further engaged in subgroup analyses. These were performed on the complete patient dataset. The analyses were bolstered by integrating subgroup-specific hazard ratios which were sourced directly from the RATIONALE-306 trial. Our primary intent was twofold: firstly, to probe into the treatment’s effect across different subgroups, and secondly, to dissect the outcomes across patient subgroups that were differentiated by distinct clinical or demographic characteristics. This granular analysis facilitates a deeper understanding of treatment efficacy and offers potential insights for personalized therapeutic approaches.
Scenario Analysis
In the ever-evolving landscape of drug pricing and economic evaluations, simulating various scenarios that might impact the cost-effectiveness of tislelizumab became imperative. Our scenario analyses explored the implications of applying different price discounts to the drug’s cost. Central to our evaluation was the pivotal role tislelizumab’s pricing plays in determining the ICER. By adjusting its price discount, we could extrapolate and gauge its wider impact on the overarching cost-effectiveness framework. This consideration is particularly relevant in the Chinese context, where negotiations on medical insurance pricing can result in reduced drug prices, especially when new therapeutic indications are introduced. Another crucial element of our scenario analysis was establishing the WTP threshold. We strategically aligned this with China’s economic benchmarks, setting it at 1 and 1.5 times the 2022 Chinese GDP per capita. This equates to a financial range of $12,751 to $19,126.5 per QALY, enabling stakeholders to evaluate the economic viability of tislelizumab within these parameters.
Results
Base-Case Results
In the base-case scenario, the combined regimen of tislelizumab and chemotherapy yielded 1.35 QALYs at a cost of $26,450.77, whereas chemotherapy monotherapy resulted in 0.89 QALYs for $16,687.15. This indicates that tislelizumab in combination with chemotherapy conferred an incremental benefit of 0.46 QALYs relative to chemotherapy alone, with an associated incremental cost of $9763.62. Consequently, the ICER was computed at $21,062.09 per QALY gained. Given a WTP threshold set at 3 times the 2022 Chinese GDP per capita (equivalent to $38,253/QALY), the combination of tislelizumab and chemotherapy emerges as a cost-effective intervention, as detailed in Table 2.
Table 2.
Summary of Cost and Outcome Results in the Base-Case Analysis
| Strategy | Cost | QALY | Incr Cost | Incr QALY | ICER |
|---|---|---|---|---|---|
| Tislelizumab + chemotherapy | $26,450.77 | 1.35 | $9763.62 | 0.46 | 21,062.09 |
| Chemotherapy alone | $16,687.15 | 0.89 | – | – |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; Incr, incremental.
Sensitivity Analysis
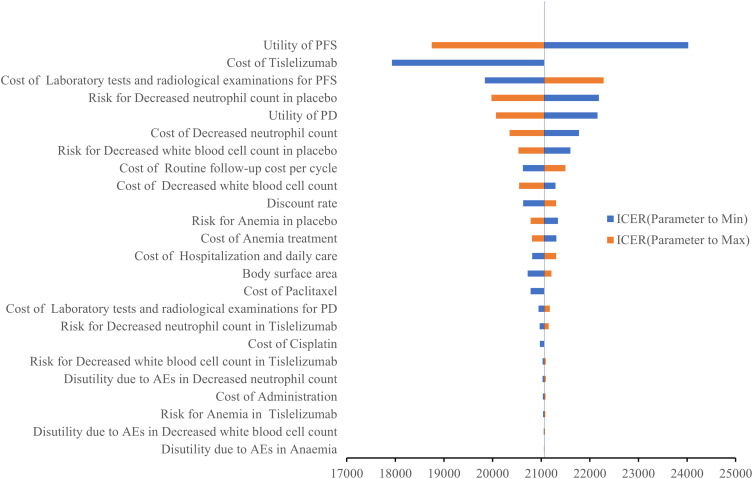
The tornado diagram (Figure 2) illustrated the parameters that exerted the most significant impact on ICER. These variables encompassed the utility value of PFS, the cost of tislelizumab, the cost of laboratory tests and radiological examinations for PFS, the incidence of AEs of decreased neutrophil count, the utility value of PD, etc. Despite these parameters’ values varying, the ICER stayed below the predetermined WTP threshold.
Figure 2.
Tornado diagram of one-way sensitivity analyses of tislelizumab plus chemotherapy versus placebo plus chemotherapy in order of magnitude of the association.
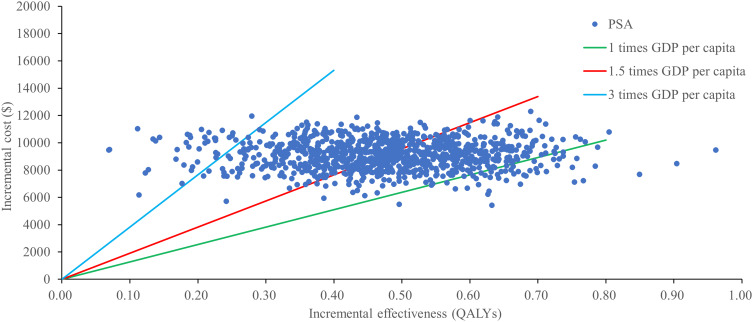
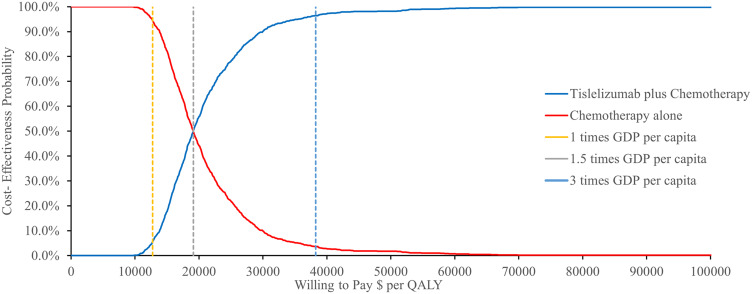
A probabilistic sensitivity analysis with monte carlo simulations revealed that combination of tislelizumab and chemotherapy had a 96.4% chance of being cost-effective in China considering a WTP threshold of $38,253/QALY (Figure 3). The cost-effectiveness acceptability presented in Figure 4, further supported this finding.
Figure 3.
ICER scatter plot of tislelizumab plus chemotherapy vs placebo plus chemotherapy.
Figure 4.
Cost-effectiveness acceptability curves for tislelizumab plus chemotherapy vs placebo plus chemotherapy.
Subgroup Analysis
Across all analyzed subgroups, the ICER for tislelizumab in comparison to placebo consistently remained beneath the WTP threshold of $38,253/QALY. Notably, there were variations in the ICER among the subgroups, spanning from $13,036.34/QALY (exhibiting a 100% probability of cost-effectiveness) in patients with locally advanced disease status at study entry to $23,222.93/QALY (possessing a 94.2% probability of cost-effectiveness) in those who have never smoked. When adjusting the WTP to 1.5 times the Chinese GDP per capita, the ICER for the tislelizumab-chemotherapy combination remained below the WTP threshold of $19,126.5/QALY for the majority of the subgroups. This ranged from $13,036.34/QALY (with a 99.1% probability of cost-effectiveness) in female patients to $19,004.34/QALY (exhibiting a 63.5% probability of cost-effectiveness) in patients who had not undergone prior definitive therapy. However, in specific subgroups — those aged under 65 years, male, never smokers, selected by investigators to receive platinum with paclitaxel chemotherapy, with an ECOG status of 0, hailing from the Asian region (excluding Japan), of Asian or other racial backgrounds, and having metastatic disease status at study commencement — the ICER for the tislelizumab and chemotherapy combination exceeded the WTP threshold of $19,126.5/QALY. These findings are comprehensively detailed in Table 3.
Table 3.
Results of Subgroup Analyses Under the WTP Set as 1.5 Times and 3 Times Chinese GDP per Capita
| Subgroup | HR for OS | ICER, US$/QALY | Cost-Effectiveness Probability (%) | |
|---|---|---|---|---|
| 3 Times GDP per Capita | 1.5 Times GDP per Capita | |||
| Age | ||||
| <65 years | 0.73 | 21,126.44 | 97.0% | 48.2% |
| ≥65 years | 0.62 | 16,988.55 | 99.9% | 80.2% |
| Sex | ||||
| Male | 0.72 | 20,662.67 | 99.8% | 51.5% |
| Female | 0.46 | 13,368.01 | 100% | 98.4% |
| Smoking status | ||||
| Current or former smoker | 0.65 | 17,935.24 | 99.5% | 74.3% |
| Never | 0.77 | 23,222.93 | 94.2% | 37.3% |
| Investigator-chosen chemotherapy* | ||||
| Platinum with fluoropyrimidine | 0.66 | 18,276.94 | 99.4% | 70.8% |
| Platinum with paclitaxel | 0.69 | 19,391.89 | 98.8% | 61.9% |
| ECOG | ||||
| 0 | 0.72 | 20,662.67 | 99.8% | 51.5% |
| 1 | 0.66 | 18,276.94 | 99.4% | 70.8% |
| Geographical region analysis 1 | ||||
| Asia | 0.67 | 18,633.01 | 99.3% | 70.0% |
| Other regions | 0.66 | 18,276.94 | 99.4% | 70.8% |
| Geographical region analysis 2 | ||||
| Asia (excluding Japan) | 0.70 | 19,796.68 | 98.6% | 60.1% |
| Japan | 0.49 | 13,906.40 | 100% | 97.4% |
| Other regions | 0.66 | 18,276.94 | 99.4% | 70.8% |
| Race | ||||
| Asian and other races† | 0.69 | 19,391.89 | 98.8% | 61.9% |
| White | 0.61 | 16,696.79 | 100% | 83.5% |
| Disease status at study entry | ||||
| Metastatic | 0.72 | 20,662.67 | 98.0% | 51.5% |
| Locally advanced | 0.44 | 13,036.34 | 100% | 99.1% |
| Previous definitive therapy* | ||||
| Yes | 0.67 | 18,633.01 | 99.3% | 70.0% |
| No | 0.68 | 19,004.34 | 99.2% | 63.5% |
Notes: *Per case report form. †Other races include American Indian, Alaska Native, not reported, and unknown.
Abbreviations: HR, hazard ratio; ECOG, Eastern Cooperative Oncology Group; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Scenario Analysis
As depicted in Table 4, our scenario analysis revealed that a 55% reduction in the cost of chemotherapy led to a corresponding decline in the ICER to $12,445.71 per QALY. Intriguingly, this value is marginally below the threshold of 1 times the Chinese GDP per capita, which stands at $12,751. Under this revised cost structure, the combination of tislelizumab and chemotherapy demonstrated a 100% likelihood of being deemed cost-effective. Upon adjusting the WTP to 1.5 times the Chinese GDP per capita, the combination treatment’s probability of cost-effectiveness in China stood at 48.7%. However, when the WTP was further reduced to align with 1 times the Chinese GDP per capita, this probability dwindled to a mere 5%.
Table 4.
ICER for Tislelizumab Price Reduction in Scenario Analyses
| Price Reduction | ICER ($/QALY) |
|---|---|
| 10% | 19,495.48 |
| 20% | 17,928.86 |
| 30% | 16,362.25 |
| 40% | 14,795.63 |
| 50% | 13,229.02 |
| 55% | 12,445.71 |
| 60% | 11,662.40 |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
Discussion
Our team’s research explores the cost-effectiveness of tislelizumab in combination with chemotherapy from a healthcare system perspective based on the RATIONALE-306 clinical trial. The combination of PD-1 inhibitor into the EC treatment paradigm represents a significant breakthrough.17,27–32 Efficacy and safety have been notably corroborated through international multicenter clinical trials evaluating PD-1 combined with chemotherapy first-line treatment for this disease. Despite these advancements, the drug’s accessibility and cost-effectiveness still pose considerable challenges. Health economics researchers have underscored that pembrolizumab, serplulimab, camrelizumab and toripalimab when combined with chemotherapy, does not represent a cost-effective strategy for treating late-stage ESCC in contrast to chemotherapy alone.16–21 The long-term financial implications of immunotherapy could inflict substantial strain on families with average income levels. Our research indicates that the ICER of tislelizumab combined with chemotherapy, compared to chemotherapy alone, stands at $21,062.09/QALY. This is significantly lower than three times the average GDP per capita in China ($38,253/QALY) and marginally surpasses 1.5 times this value ($19,126.5/QALY). Probability sensitivity analysis suggests that at a WTP threshold of $ 38,253/QALY, tislelizumab combined with chemotherapy exhibits a 96.4% probability of being cost-effective compared to chemotherapy alone. At a WTP threshold of $ 19,126.5/QALY, this probability is 48.7%. In a parallel vein, the ORIENT-15 study,33 spearheaded by Chinese scholars, examined the efficacy of sintilimab in combination with chemotherapy versus chemotherapy alone for first-line treatment of advanced ESCC. This combination therapy improved median survival time by 4.2 months, with a HR of 0.63. Following this study, certain researchers published pharmacoeconomic results indicating sintilimab, combined with chemotherapy, as a cost-effective treatment strategy compared to chemotherapy alone at a WTP threshold of three times China’s average GDP per capita.34,35
The cost-effectiveness of tislelizumab in combination with chemotherapy is largely due to China’s drug pricing policies. After tislelizumab was officially incorporated into China’s National Health Insurance Drug List in March 2021, its price reduced from $1590.48/100mg to $204.96/100mg, marking a 32% decrease from its previous price.36 The competitive pricing of tislelizumab was corroborated through our scenario analysis and univariate sensitivity analysis. Significant influence on the model results was noted with fluctuations in the price of tislelizumab. As per our scenario analysis, when the cost of tislelizumab dipped below $327.93/cycle, the ICER was found to be lower than the per capita GDP ($12,751), making it more advantageous for patients with ESCC. Moreover, the lower price point of tislelizumab not only renders its combination with chemotherapy as a cost-effective first-line treatment for ESCC, but it also bolsters the cost-effectiveness of tislelizumab in treating a diverse range of cancers. Some investigators posit that tislelizumab is a cost-effective treatment strategy for the second-line treatment of advanced ESCC,37 first- and second-line treatment of advanced non-small cell lung cancer (NSCLC),38–42 and the first-line treatment of inoperable hepatocellular carcinoma (HCC).43 Consistently, sensitivity analyses from these studies have underscored that the price advantage of tislelizumab is a key determinant of its cost-effectiveness.
Our research delves into an extensive analysis of cost-effectiveness across distinct subgroups. At a WTP threshold equating to 1.5 times Chinese GDP per capita, the combination of tislelizumab and chemotherapy not only holds a greater than 50% probability of being cost-effective compared to chemotherapy alone in subgroups such as patients over 65, female patients, smokers, recipients of either platinum with fluoropyrimidine or platinum with paclitaxel, patients with an ECOG performance status of 1, patients with locally advanced disease status, and patients with previous definitive therapy but also exhibits cost-effectiveness within the aforementioned WTP threshold across specific subgroups. These subgroups remain unaffected by race, choice between platinum and fluorouracil or platinum and paclitaxel chemotherapy regimens, and geographical location, encompassing regions such as Asia, Europe, North Africa, and Oceania. It’s worth emphasizing that the cost-effectiveness of tislelizumab in combination with chemotherapy as a first-line treatment for EC is unparalleled amongst other domestic PD-1 inhibitors because of different races or regions. This trial demonstrates more extensive representation compared to the ORIENT-15,44 JUPITER-06,45 and ESCORT-1st9 studies, in which the vast majority or entirety of the patient population were from China. Patients of varying geographical origin and ethnicity can potentially achieve clinical and economic benefits from the treatment regimen combining tislelizumab with chemotherapy. Furthermore, the RATIONALE-306 study14 incorporated chemotherapy regimens reflecting the established medication practices of various nations and regions, a feature absent in the KEYNOTE 590,46 ORIENT-15,44 JUPITER-06,45 and ESCORT-1st9 studies regardless of the chosen chemotherapy regimen, the tislelizumab combination treatment consistently exhibits clinical and economic benefits. This constitutes a clinically effective approach for patients from diverse regions and ethnicities in the world, aligning with traditional chemotherapy practices across varied nations and regions, and presents a cost-effective treatment solution. This novel evidence-based medical value has the potential to guide treatment options and economic benefits for foreign populations residing, working, or studying in China. Moreover, it provides valuable references for foreign patients considering treatment for ESCC in China, thereby fostering greater hope for patients worldwide.
This study had a few restrictions. Firstly, without long-term survival data, we extrapolated the survival curve beyond the observed time horizon using a common survival distribution to fit the data, which may not accurately represent real-world conditions.47 Secondly, the cost information obtained from hospitals and reference materials may vary marginally between hospitals. Thirdly, we use utility values from available literature because Rationale-306 clinical trials did not report them. To simplify the model, we assumed that all chemotherapies used cisplatin and paclitaxel and that subsequent therapies all used chemotherapy, as there was no way to obtain the exact proportion from the literature which may not reflect current clinical practice. We only considered adverse events of grade 3 or higher with probabilities over 10%. The sensitivity analysis revealed that the economic outcomes were unaffected by SAE-related and utility values. We did not consider the cost of best supportive care and end-of-life care. Therefore, considering the method limitations in the current study, more head-to-head clinical and economic real-world data are needed. Whereas several drugs have shown cost-effectiveness in the treatment of ESCC, there is currently a lack of direct comparisons to determine which drug is the most cost-effective. Future studies should be conducted to compare these drugs and establish the optimal treatment, which would provide valuable references for clinical practice and health insurance reimbursement.
Conclusion
The findings of this study demonstrated that tislelizumab in conjunction with chemotherapy is cost-effective compared to chemotherapy alone for patients with advanced or metastatic ESCC from the perspective of the Chinese healthcare system. As a result, tislelizumab exhibits potential for future recommendation as a suitable pharmacological intervention for advanced or metastatic ESCC patients in China.
Acknowledgments
We thank all the people who have contributed to this paper.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 72074123).
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author Xin Li, upon reasonable request.
Ethics Approval
As this study did not involve patient participation, it did not require a review by the institutional review committee or exemption or approval by the ethics committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel R, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Li L, Jiang D, Zhang Q, et al. Integrative proteogenomic characterization of early esophageal cancer. Nat Commun. 2023;14(1):1666. doi: 10.1038/s41467-023-37440-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Liu Y, Peng J, Sun C, Rang W. Trends of esophageal cancer incidence and mortality and its influencing factors in China. Risk Manag Healthc Policy. 2021;14:4809–4821. doi: 10.2147/rmhp.S312790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Z, Li Z, Zhang Y, et al. Interpretation of the 2020 global cancer statistical report. Elec J Elec Compr Canc Therapy. 2021;7(02):1–14. [Google Scholar]
- 5.Li Y, Xu J, Gu Y, Sun X, Dong H, Chen C. The disease and economic burdens of esophageal cancer in China from 2013 to 2030: dynamic cohort modeling study. JMIR Public Health Surveillance. 2022;8(3):e33191. doi: 10.2196/33191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang F, Yu S. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surgery. 2018;41(3):210–215. doi: 10.1016/j.asjsur.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Ma S. History and current situation of neoadjuvant treatment for locally advanced esophageal cancer. Thoracic Cancer. 2021;12(17):2293–2299. doi: 10.1111/1759-7714.14069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harada K, Rogers J, Iwatsuki M, Yamashita K, Baba H, Ajani J. Recent advances in treating oesophageal cancer. F1000Res. 2020;9:1189. doi: 10.12688/f1000research.22926.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon H, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 Phase 3 randomized clinical trials. JAMA Oncol. 2022;8(10):1456–1465. doi: 10.1001/jamaoncol.2022.3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Z, Dai P, Han S, Qiu E, Wang Y, Li Z. Complete remission in a patient with metastatic gastric cancer receiving tislelizumab combined with chemotherapy: a case report. Front Oncol. 2023;13:1147636. doi: 10.3389/fonc.2023.1147636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuss-Patience P, Stein A. Immunotherapy in squamous cell cancer of the Esophagus. Curr Oncol. 2022;29(4):2461–2471. doi: 10.3390/curroncol29040200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Qian J, Wang H, et al. Cisplatin plus anti-PD-1 antibody enhanced treatment efficacy in advanced esophageal squamous cell carcinoma. Am J Cancer Res. 2022;12(2):451–468. [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Kato K, Raymond E, et al. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first-line treatment for advanced or metastatic oesophageal squamous cell carcinoma (RATIONALE-306): a global, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2023;24(5):483–495. doi: 10.1016/s1470-2045(23)00108-0 [DOI] [PubMed] [Google Scholar]
- 15.National Medical Products Administration. Drug approval certificate document delivery information release. Available from: https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20230523155059194.html. Accessed July 25, 2023.
- 16.Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol. 2021;11:790373. doi: 10.3389/fonc.2021.790373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Lin J, Zhu H, Cai H. Cost-effectiveness analysis of pembrolizumab plus chemotherapy vs. chemotherapy alone as first-line treatment in patients with esophageal squamous cell carcinoma and PD-L1 CPS of 10 or more. Front Public Health. 2022;10:893387. doi: 10.3389/fpubh.2022.893387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Z, Xu Z, Wang H, et al. Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus chemotherapy as the first-line treatment for advanced esophageal cancer. Cancer Med. 2023;12(5):6182–6189. doi: 10.1002/cam4.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Liu K, Ding D, Zhou Y, Peng L. Pembrolizumab plus chemotherapy as first-line treatment for advanced esophageal cancer: a cost-effectiveness analysis. Adv Ther. 2022;39(6):2614–2629. doi: 10.1007/s12325-022-02101-9 [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Jiang N, Dou L, Li S. Cost-effectiveness analysis of serplulimab plus chemotherapy in the first-line treatment for PD-L1-positive esophageal squamous cell carcinoma in China. Front Immunol. 2023;14:1172242. doi: 10.3389/fimmu.2023.1172242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu K, Wu H, Zhou C, et al. Cost-effectiveness of toripalimab plus chemotherapy for advanced esophageal squamous cell carcinoma. Int J Clin Pharm. 2023;45(3):641–649. doi: 10.1007/s11096-023-01540-w [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Zhang H, Li L, Feng L, Liu Q. Cost-effectiveness analysis of pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer in China. Risk Manag Healthc Policy. 2023;16:1849–1857. doi: 10.2147/rmhp.S429394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyot P, Ades A, Ouwens M, Welton N. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Method. 2012;12(1):9. doi: 10.1186/1471-2288-12-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue X, Li Y, Wu J, Guo J. Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg Issues. 2021;24:1–5. doi: 10.1016/j.vhri.2020.07.580 [DOI] [PubMed] [Google Scholar]
- 25.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi: 10.1016/s0140-6736(17)31462-9 [DOI] [PubMed] [Google Scholar]
- 26.Nafees B, Lloyd A, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non–small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–e203. doi: 10.1111/ajco.12477 [DOI] [PubMed] [Google Scholar]
- 27.Doki Y, Ajani J, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 28.Mikuni H, Yamamoto S, Kato K. Nivolumab for the treatment of esophageal cancer. Expert Opin Biol Ther. 2021;21(6):697–703. doi: 10.1080/14712598.2021.1904887 [DOI] [PubMed] [Google Scholar]
- 29.Janjigian Y, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–2844. doi: 10.1200/jco.2017.76.6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, Sun Y, Lai M, Zhou Y, Qiu M. Efficacy and safety of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal cancer: a systematic review and network meta-analysis. Int Immunopharmacol. 2022;109:108790. doi: 10.1016/j.intimp.2022.108790 [DOI] [PubMed] [Google Scholar]
- 31.Xu J, Li Y, Fan Q, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label Phase 2 study (ORIENT-2). Nat Commun. 2022;13(1):857. doi: 10.1038/s41467-022-28408-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan H, Wang T, Luo Z, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Transl Med. 2021;9(22):1700. doi: 10.21037/atm-21-6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Wang L, Chen L, Ding Y, Zhang Q, Shu Y. Cost-effectiveness of sintilimab plus chemotherapy versus chemotherapy alone as first-line treatment of locally advanced or metastatic oesophageal squamous cell carcinoma. Front Immunol. 2023;14:1092385. doi: 10.3389/fimmu.2023.1092385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye Z, Xu Z, Zeng F, Tang Z, Zhou Q. Cost-effectiveness analysis of sintilimab combined with chemotherapy versus chemotherapy alone as the first-line treatment for advanced esophageal cancer. Front Pharmacol. 2022;13:934275. doi: 10.3389/fphar.2022.934275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen J, Du Y, Shao R, Jiang R. First-line sintilimab plus chemotherapy in locally advanced or metastatic esophageal squamous cell carcinoma: a cost-effectiveness analysis from China. Front Pharmacol. 2022;13:967182. doi: 10.3389/fphar.2022.967182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.New version of medical insurance drug list Available from: https://www.qztv.cn/index/News/detail/id/wq95wqbDnMKyd8KiwqzChnt0w5nChcKofcOdwoPCrMKuw53CgsOMcXA.html. Accessed July 28, 2023.
- 37.Shi F, He Z, Su H, Wang L, Han S. Economic evaluation of tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Front Pharmacol. 2022;13:961347. doi: 10.3389/fphar.2022.961347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang X, Chen X, Li H, Li Y. Tislelizumab plus chemotherapy is more cost-effective than chemotherapy alone as first-line therapy for advanced non-squamous non-small cell lung cancer. Front Public Health. 2023;11:1009920. doi: 10.3389/fpubh.2023.1009920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, Luo X, Zhou Z, et al. Cost-effectiveness analysis of tislelizumab, nivolumab and docetaxel as second- and third-line for advanced or metastatic non-small cell lung cancer in China. Front Pharmacol. 2022;13:880280. doi: 10.3389/fphar.2022.880280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong J, Su D, Shang J, et al. Cost-effectiveness of tislelizumab versus docetaxel for previously treated advanced non-small-cell lung cancer in China. Front Pharmacol. 2022;13:830380. doi: 10.3389/fphar.2022.830380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Shao T, Chi Z, Tang W. Effectiveness and cost-effectiveness analysis of 11 treatment paths, seven first-line and three second-line treatments for Chinese patients with advanced wild-type squamous non-small cell lung cancer: a sequential model. Front Public Health. 2023;11:1051484. doi: 10.3389/fpubh.2023.1051484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo X, Zhou Z, Zeng X, Liu Q. The cost-effectiveness of tislelizumab plus chemotherapy for locally advanced or metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. 2022;13:935581. doi: 10.3389/fphar.2022.935581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu K, Zhu Y, Zhu H. Immunotherapy or targeted therapy as the first-line strategies for unresectable hepatocellular carcinoma: a network meta-analysis and cost-effectiveness analysis. Front Immunol. 2022;13:1103055. doi: 10.3389/fimmu.2022.1103055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. doi: 10.1136/bmj-2021-068714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (Jupiter-06): a multi-center phase 3 trial. Cancer Cell. 2022;40(3):277–288.e3. doi: 10.1016/j.ccell.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 46.Sun J, Shen L, Shah M, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/s0140-6736(21)01234-4 [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Huang K, Sun S, Deng Y, Xie X. Cost-effectiveness analysis of gefitinib alone and combined with chemotherapy as first-line treatment for patients with advanced non-small-cell lung cancer. Risk Manag Healthc Policy. 2022;15:351–359. doi: 10.2147/rmhp.S352827 [DOI] [PMC free article] [PubMed] [Google Scholar]