Abstract
BACKGROUND AND OBJECTIVES
The Pediatric Emergency Care Applied Research Network Fluid Therapies Under Investigation in Diabetic Ketoacidosis (DKA) (FLUID) Trial found that rapid fluid infusion does not increase the risk of cerebral injury. Concern persists, however, whether fluid rates should be adjusted for overweight or obese patients. We used the FLUID Trial database to evaluate associations between fluid infusion rate and outcomes in these patients.
METHODS
We compared children and youth who were overweight, obese, or normal weight, in regard to protocol adherence, mental status changes, time to DKA resolution, and electrolyte abnormalities. We investigated associations between outcomes and the amount of fluid received in these groups.
RESULTS
Obese children and youth were more likely to receive fluids at rates slower than dictated by protocol. Overweight and obese children and youth in the fast fluid arms, who received fluids per the study protocol based on their measured weight, had similar rates of mental status changes or clinically apparent cerebral injury as those with normal weights. Risk of hypophosphatemia was increased in those receiving larger initial bolus volumes and reduced in those receiving higher rehydration rates. No other metabolic outcomes were associated with rehydration.
CONCLUSIONS
Protocol adherence data in the FLUID Trial suggest that physicians are uncomfortable using weight-based fluid calculations for overweight or obese children. However, higher rates of fluid infusion were not associated with increased risk of mental status changes or cerebral injury, suggesting that physicians should not limit fluid resuscitation in obese children and youth with DKA.
What’s Known on This Subject:
A recent clinical trial by our group found that rapid fluid rehydration did not increase the risk of cerebral injury in children with DKA, however, there were no specific analyses of data for overweight or obese children.
What This Study Adds:
Our analysis suggests that physicians are uncomfortable with weight-based fluid rates in overweight children with DKA. However, higher infusion rates did not increase risk of cerebral injury in this group, suggesting fluid rates should not be limited based on weight.
In the past, protocols for treatment of diabetic ketoacidosis (DKA) in children and youth frequently recommended conservative rates of infusion for initial fluid resuscitation, as well as ongoing rehydration. These recommendations reflected concerns that rapid changes in osmolality might increase the risk of cerebral edema and cerebral injury.1 The Pediatric Emergency Care Applied Research Network (PECARN) Fluid Therapies Under Investigation in DKA (FLUID) Trial prospectively assessed the impact of variations in intravenous fluid protocols on mental status changes during treatment and on cognitive outcomes after recovery in children and youth with DKA.2 The PECARN FLUID Trial found no significant associations between either fluid infusion rate or fluid sodium content and risk of acute or long-term neurologic injuries.
Although the PECARN FLUID Trial results suggest that a less conservative approach to rehydration in children and youth with DKA is warranted, questions remain about specific subgroups of children with DKA. Among these subgroups are children and youth who are overweight or obese. Because obesity has become more common in children in the United States, overweight and obese children and youth with DKA are seen more frequently in emergency departments (EDs).3 Whether DKA fluid protocol recommendations should be adjusted for these children and youth is unclear.
In this secondary analysis of the PECARN FLUID study database, we assessed physicians’ practices toward rehydration in overweight or obese patients by determining whether they typically adhered to the assigned protocol for these patients or administered less than the protocol-determined fluid amount. We also compared outcomes of treatment including mental status changes as measured by decreased Glasgow Coma Scale (GCS) scores, treatment of cerebral injury, time to resolution of DKA, and electrolyte abnormalities during treatment. We hypothesized that clinicians would be hesitant to deliver the protocol-determined bolus volume and replacement fluid rate in overweight or obese children and youth. This would result in these patients receiving less total fluid per kilogram than their lean counterparts. We also hypothesized that administration of less than the protocol-determined fluid amount would result in longer duration to recovery from DKA and/or an increased frequency of complications resulting from insufficient electrolyte replacement compared with patients who received their protocol-determined fluid amounts.
Methods
Overview of PECARN FLUID Trial
In the current study, we analyzed data collected during the PECARN FLUID Trial.2 Detailed methods4 and results2 from this trial were previously published. Relevant features of the trial are outlined here. The trial involved 13 PECARN-affiliated EDs, located in urban centers in the United States. Trial participants were randomized to 1 of 4 intravenous fluid treatment protocols using a 2 × 2 factorial study design. After an initial fluid bolus, the rehydration treatment protocols used either 0.45% or 0.9% sodium chloride solutions infused at either a more rapid or slower rate. The rapid rate prescribed a 20 mL/kg bolus followed by estimated deficit of 10% measured body weight, half of which was replaced over the first 12 hours, plus maintenance fluids based on measured body weight. The slow rate prescribed a 10 mL/kg bolus followed by 5% of body weight (deficit) replaced over 48 hours, plus maintenance fluids based on measured body weight. Initial fluid boluses were subtracted from the fluid deficit that was used to calculate the deficit replacement rate.2 The maximum amount of bolus and rate of rehydration was based on a weight of 100 kg.
Children and youth enrolled in the trial had ongoing monitoring of mental status during DKA treatment using hourly GCS score assessments and tests of short-term memory (digit span recall) every 4 hours during waking hours. Glucose levels were measured hourly and electrolyte concentrations every 2 to 4 hours.
Study Participants
DKA visits were included in the PECARN FLUID Trial if the participants were aged 0 to 18 years and were diagnosed with DKA (blood glucose >300 mg/dL/16.6 mmol/L, venous pH <7.25 or serum bicarbonate <15 mmol/L, and positive urine or blood test for ketones). Visits were excluded from the study if there were underlying disorders that could alter mental status testing or cognition (including alcohol or drug intoxication, head trauma, or neurologic diagnoses affecting mental status or cognition), if they had substantial treatment of DKA before arrival at the study site, if they were known to be pregnant, if they presented with low GCS scores (≤11), or other clinical scenarios for which treating physicians felt specific fluid and electrolyte therapy was required.
For the current analysis, patients were classified as obese, overweight, or normal on the basis of BMI calculated using their DKA visit discharge weight, or their weight 12 hours after treatment initiation if the discharge weight was not known. BMI for children and youth aged >48 months was calculated using the weight documented at treatment initiation and height abstracted from the medical record using the 2000 Centers for Disease Control and Prevention growth charts.5 Specifically, patient visits were classified as obese if visit BMI was above the 95th percentile, overweight if BMI was between 85th and 95th percentile (inclusive), and normal if BMI was below the 85th percentile at time of enrollment. Children aged <48 months were excluded from the current analyses because none were classified as overweight or obese. Inclusion of these children might have caused confounding because of differences in outcomes or protocol adherence in this age group. Patients missing height information (within 6 months of the ED visit) were also excluded because of inability to calculate BMI (n = 28). Children and youth enrolled in the FLUID Trial and later found to have type 2 diabetes (n = 44) were also excluded.
Outcome Measures and Comparisons
Protocol Compliance
Protocol compliance measures were calculated with reference to the study protocol assigned at randomization. Measures included the percentage of fluid dictated by the study protocol that was received by the patient over the first 12 hours and over the first 24 hours, total fluid received in milliliter per kilogram, the amount of intravenous fluid bolus received, whether the child or youth received more or less fluid bolus volume than dictated by the protocol, the percentage of nonbolus fluid received that matched the concentration dictated by the protocol over 12 hours, and the average fluid rate in milliliter per kilogram per hour over the first 12 hours and between 12 and 24 hours, excluding the initial bolus.
Outcome Measures
Outcomes assessed between and within subgroups included: Time from randomization to DKA resolution, hyperchloremic acidosis, hypoglycemia, hypophosphatemia, changes in mental status, and cerebral injury. DKA resolution was defined as the time of transition to subcutaneous insulin. Mental status changes were defined by GCS scores <14 that were confirmed on repeat assessment 15 minutes later. Hyperchloremic acidosis was defined using age-based standards as presence of anion gap ≤12 along with: 4 to <6 years, bicarbonate <19 mmol/L; 6 to <8 years, bicarbonate <20 mmol/L; 8 years and older, bicarbonate <21 mmol/L; or hyperchloremic acidosis reported as an adverse event for any age. Hypoglycemia was defined as glucose <70 mg/dL, hypokalemia as potassium <3.0 mmol/L, and hypophosphatemia as phosphate below age-adjusted ranges: <5 years, <4.3 mg/dL; 5 to <14 years, <3.7 mg/dL; 14 to <16 years, <3.5 mg/dL; and 16 to <18 years, <3.1 mg/dL. Clinically apparent cerebral injury was determined by an independent adjudication panel using published criteria in a review of records of children and youth treated with either mannitol, hypertonic saline, or intubation, or those who died of a cerebral event.6
Statistical Analyses
We compared patient demographics, diabetes history, and presenting clinical characteristics by BMI category using counts and relative frequencies for categorical characteristics and means and SDs for continuous measures. We tested for differences between groups using χ 2 tests for categorical characteristics and Kruskal-Wallis tests for continuous characteristics.
We compared protocol compliance measures between BMI groups using counts and relative frequencies for categorical measures and means and SDs for continuous measures. We tested for differences between groups using multiple logistic regression models for categorical measures and multiple linear regression models for continuous measures. Regression models included age, new-onset (versus previously diagnosed) diabetes, and DKA severity as covariates. DKA severity was defined by initial pH (severe if pH <7.10, moderate if pH 7.10–<7.20, mild if pH ≥7.20) or initial bicarbonate if pH was unknown (severe if bicarbonate <5, moderate if bicarbonate 5–<10, mild if bicarbonate ≥10).
We investigated the frequency of mental status changes and clinically apparent cerebral injury among patients assigned to the fast treatment arm who received fluids per protocol, defined as receipt of 80% to 125% of the expected amount of fluid over 12 hours.
We compared metabolic measures between BMI groups using Fisher’s exact tests. We further investigated the relationship between outcome measures, including metabolic outcomes and time to DKA resolution, and the amount of initial bolus and nonbolus fluid received among overweight or obese children and youth. We fit multiple linear and logistic regression models adjusted for DKA severity and calculated adjusted linear regression coefficients and odds ratios with 95% confidence intervals (CIs). All analyses were performed using SAS/STAT Software (Version 9.4, SAS Institute Inc, Cary, NC, USA).
Results
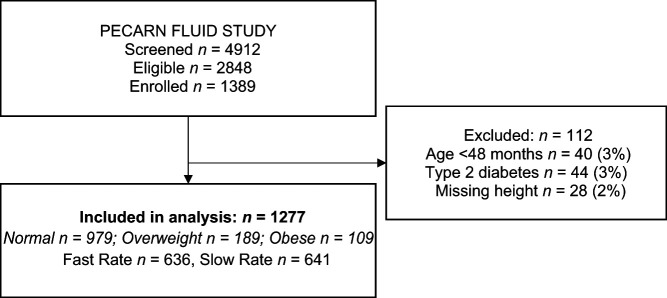
There were 1389 DKA episodes (visits) included in the PECARN FLUID Trial. Of those, 1277 visits were involved in these analyses, after excluding visits with missing height information (n = 28, 2%), children aged <48 months (n = 40, 3%), and children or youth later found to have type 2 diabetes (n = 44, 3%) (Fig 1). This study included 7 youth weighing >100 kg.
FIGURE 1.
Study flow diagram.
The overweight and obese groups were older and more likely to be previously diagnosed with diabetes (Table 1). DKA severity (determined by pH) was somewhat greater in obese and overweight patients; however, there were no significant differences in baseline GCS scores between groups.
TABLE 1.
Characteristics of the Study Sample by BMI
| Normal (BMI <85th Percentile) (N = 979) | Overweight (BMI 85th–95th Percentile) (N = 189) | Obese (BMI >95th Percentile) (N = 109) | P | |
|---|---|---|---|---|
| Age at screening (y) | 11.6 (3.7) | 12.6 (3.6) | 11.9 (4.0) | .002 |
| Sex | .08 | |||
| Male | 456 (46.6%) | 76 (40.2%) | 58 (53.2%) | |
| Female | 523 (53.4%) | 113 (59.8%) | 51 (46.8%) | |
| Baseline glucose (mg/dL) | 524 (158) | 504 (157) | 512 (151) | .18 |
| Baseline BUN (mg/dL) | 17.4 (7.5) | 16.2 (6.9) | 16.4 (7.4) | .16 |
| Baseline bicarbonate (mEq/L) | 9.0 (3.2) | 8.8 (3.5) | 8.3 (2.8) | .07 |
| Baseline pH | 7.16 (0.10) | 7.15 (0.11) | 7.14 (0.09) | .02 |
| Baseline pCO2 (mm Hg) | 26.4 (7.4) | 26.1 (7.2) | 25.5 (6.9) | .48 |
| Baseline glucose-corrected sodium (mEq/L) | 140.9 (5.2) | 141.8 (5.0) | 141.2 (5.7) | .17 |
| Baseline chloride (mEq/L) | 98.2 (6.1) | 98.9 (5.5) | 98.8 (6.2) | .31 |
| Baseline creatinine (z-score adjusted for age) | 1.1 (1.5) | 1.3 (1.3) | 1.4 (1.5) | .004 |
| Dehydration severitya | .24 | |||
| Mild (<5%) | 378 (38.6%) | 86 (45.5%) | 48 (44.0%) | |
| Moderate (5–<10%) | 315 (32.2%) | 55 (29.1%) | 36 (33.0%) | |
| Severe (≥10%) | 101 (10.3%) | 16 (8.5%) | 4 (3.7%) | |
| Unknown | 185 (18.9%) | 32 (16.9%) | 21 (19.3%) | |
| DKA severityb | .15 | |||
| Mild (pH ≥7.2) | 435 (44.4%) | 76 (40.2%) | 35 (32.1%) | |
| Moderate (7.10≤ pH <7.2) | 332 (33.9%) | 69 (36.5%) | 44 (40.4%) | |
| Severe (pH <7.10) | 212 (21.7%) | 44 (23.3%) | 30 (27.5%) | |
| Baseline GCS score | .58 | |||
| <14 | 17 (1.7%) | 4 (2.1%) | 3 (2.8%) | |
| 14 | 65 (6.6%) | 18 (9.5%) | 9 (8.3%) | |
| 15 | 897 (91.6%) | 167 (88.4%) | 97 (89.0%) | |
| Previously diagnosed with diabetes | <.001 | |||
| No | 488 (49.8%) | 61 (32.3%) | 43 (39.4%) | |
| Yes | 491 (50.2%) | 128 (67.7%) | 66 (60.6%) | |
| 12-mo weighted mean HbA1c result: Mean (SD) | 10.7 (1.9) | 10.7 (1.9) | 10.4 (1.9) | .58 |
Continuous characteristics are summarized with mean (SD), P value is from a Kruskal-Wallis test; categorical characteristics are summarized with N (%), P value is from a χ 2 test. HbA1c, hemoglobin A1c.
Dehydration is calculated as 100% x (discharge weight–initial weight)/discharge weight.
If pH was unknown, bicarbonate was used: Severe if 0 to <5, moderate if 5 to <10, and mild if ≥10.
Patients in the obese group received significantly less than the total protocol-determined fluid amounts when compared with the overweight or normal BMI groups at both 12 and 24 hours (Table 2). Obese and overweight children and youth also received fluid rehydration at rates slower than the normal BMI group for the first 12 hours (excluding initial bolus) and during the 12 to 24-hour period (excluding those in whom DKA had resolved before 24 hours). These differences persisted when adjusted for age, new-onset versus previously diagnosed diabetes, and DKA severity (Table 2).
TABLE 2.
Compliance With Protocol by BMI
| Normal (BMI <85th Percentile) (N = 979) | Overweight (BMI 85th–95th Percentile) (N = 189) | Obese (BMI >95th Percentile) (N = 109) | Adjusted P a | |
|---|---|---|---|---|
| Percentage of expected fluid that was observed (up to 12 h) | 99.7 (15.7) | 100.6 (13.9) | 94.6 (15.9) | <.001 |
| Fast treatment assigned | 95.4 (11.1) | 95.8 (10.8) | 90.9 (11.5) | .005 |
| Slow treatment assigned | 104.0 (18.2) | 104.9 (15.0) | 98.6 (19.0) | .04 |
| Percentage of expected fluid that was observed (up to 24 h) | 99.9 (16.2) | 100.2 (12.5) | 96.2 (13.9) | .02 |
| Fast treatment assigned | 96.3 (10.6) | 96.5 (11.2) | 92.5 (11.0) | .01 |
| Slow treatment assigned | 103.6 (19.7) | 103.5 (12.8) | 100.2 (15.6) | .29 |
| Total fluid received (mL/kg) | 73.1 (34.5) | 69.1 (42.4) | 66.3 (28.8) | .03 |
| Fast treatment assigned | 87.2 (30.9) | 88.5 (50.9) | 81.2 (26.9) | .03 |
| Slow treatment assigned | 59.1 (32.2) | 51.9 (21.8) | 49.9 (20.9) | .03 |
| Amount of IV bolus fluid received (mL/kg) | 16.4 (5.8) | 16.5 (6.1) | 15.8 (5.5) | .51 |
| Fast treatment assigned | 19.7 (3.1) | 20.5 (4.2) | 19.7 (2.7) | .13 |
| Slow treatment assigned | 13.0 (5.9) | 13.0 (5.2) | 11.6 (4.5) | .09 |
| IV bolus amount at treatment initiation | ||||
| Less than dictated by protocol | 43 (4.4%) | 8 (4.2%) | 5 (4.6%) | >.99 |
| Amount dictated by protocol | 781 (79.8%) | 144 (76.2%) | 90 (82.6%) | |
| More than dictated by protocol | 155 (15.8%) | 37 (19.6%) | 14 (12.8%) | .27 |
| Fast treatment assigned | ||||
| Less than dictated by protocol | 38 (7.8%) | 6 (6.7%) | 3 (5.3%) | .72 |
| Amount dictated by protocol | 433 (88.4%) | 77 (86.5%) | 52 (91.2%) | |
| More than dictated by protocol | 19 (3.9%) | 6 (6.7%) | 2 (3.5%) | .43 |
| Slow treatment assigned | ||||
| Less than dictated by protocol | 5 (1.0%) | 2 (2.0%) | 2 (3.8%) | .41 |
| Amount dictated by protocol | 348 (71.2%) | 67 (67.0%) | 38 (73.1%) | |
| More than dictated by protocol | 136 (27.8%) | 31 (31.0%) | 12 (23.1%) | .34 |
| Percentage of nonbolus fluid that matched the assigned Na concentration (up to 12 h) | [N = 970] 94.8 (15.9) | [N = 189] 95.5 (13.1) | [N = 108] 94.0 (18.8) | .82 |
| 0.45% NaCl treatment assigned | [N = 484] 91.6 (19.7) | [N = 96] 94.6 (13.0) | [N = 47] 90.6 (20.7) | .34 |
| 0.9% NaCl treatment assigned | [N = 486] 97.9 (10.0) | [N = 93] 96.5 (13.3) | [N = 61] 96.6 (16.8) | .49 |
| Average 12-h fluid rate in mL/kg per h (excluding initial bolus) | [N = 978] 3.9 (1.3) | [N = 189] 3.5 (1.2) | [N = 107] 3.3 (1.3) | <.001 |
| Fast treatment assigned | [N = 490] 4.8 (1.1) | [N = 89] 4.5 (0.9) | [N = 56] 4.2 (0.9) | <.001 |
| Slow treatment assigned | [N = 488] 2.9 (0.8) | [N = 100] 2.6 (0.5) | [N = 51] 2.3 (0.8) | <.001 |
| Average fluid rate between 12 and 24 h in mL/kg per h (excluding initial bolus) | [N = 617] 3.7 (1.56) | [N = 118] 3.1 (1.03) | [N = 76] 3.3 (1.12) | <.001 |
| Fast treatment assigned | [N = 307] 4.3 (1.59) | [N = 54] 3.7 (1.03) | [N = 42] 3.8 (1.13) | .03 |
| Slow treatment assigned | [N = 310] 3.2 (1.31) | [N = 64] 2.5 (0.63) | [N = 34] 2.6 (0.65) | .01 |
N (%) shown for the categorical outcomes related to pretreatment intravenous bolus; mean (SD) shown for all other outcomes. N is shown for continuous outcomes when the outcome is unknown or not applicable for some DKA visits; for example, if there was 0 nonbolus fluid, if all non-bolus fluid was different from either half-normal or normal saline, or if DKA resolved before 12 hours. IV, intravenous; Na, sodium; NaCl, sodium chloride.
P values are from multivariable logistic (pretreatment intravenous bolus) or linear (all other outcomes) regression models adjusted for age, new onset versus previous diagnosis, and DKA severity defined by baseline pH and bicarbonate values. In the case of pretreatment intravenous bolus, 2 separate multiple logistic models were fit comparing less and more than the amount dictated by protocol versus all other visits.
Two patients in the overweight/obese group (n = 298) developed clinically apparent cerebral injury; 1 in a fast fluid infusion arm and 1 in a slow infusion arm. Both were treated with mannitol, and 1 was treated with hypertonic saline. Neither received any other intervention for cerebral injury. Both survived without apparent neurologic deficits. Nine additional patients in the overweight/obese group had mental status changes (GCS scores <14) during DKA treatment; 4 in fast infusion arms and 5 in slow infusion arms.
To determine whether overweight/obese children or youth treated with fluid infusion rates calculated according to body weight were at increased risk of cerebral injury, we compared rates of clinically apparent CI and mental status declines between those who were overweight or obese and those who were normal weight, who were randomized to the fast infusion arms, and who were treated with the full fluid amount dictated by protocol (n = 585). Among overweight or obese children in this group, 1 of 130 developed clinically apparent CI (0.8%), a rate similar to that observed in the normal-weight group (2 of 455 [0.4%], P = .53). Rates of mental status declines during DKA treatment were also similar in the overweight/obese group (2.3%) compared with normal-weight children (3.1%, P = .78). These rates were also similar to those observed overall in the FLUID Trial (0.9% developed clinically apparent cerebral injury and 3.5% had declines in GCS scores).
Higher rates of hypophosphatemia were observed in the obese group compared with the normal BMI group (67.0% vs 54.2%, P = .02); however, no significant differences were observed between groups in hyperchloremic acidosis, hypoglycemia, or hypokalemia.
Table 3 describes outcomes and frequency of adverse events for the combined overweight and obese group, as well as the results of multiple linear and logistic regression models describing the association between metabolic and length of stay outcomes and fluid bolus, and infusion rates within groups. Increased fluid infusion rate was associated with a reduced risk of developing hypophosphatemia, and a higher fluid bolus amount was associated with greater risk of hypophosphatemia. Neither the amount of bolus fluid administration nor the rate of fluid administration were significantly associated with any other outcome.
TABLE 3.
Clinical Outcomes According to Fluid Bolus Amount and Fluid Rate Received Among Overweight and Obese Children and Youth (n = 298)
| Outcomes | Rate (%) or Mean (SD) | Effect of +1 mL/kg Per h of Nonbolus Fluid | Effect of +10 mL/kg Bolus Fluid Amount at Treatment Initiation |
|---|---|---|---|
| Time from randomization to DKA resolution (h) | 15.5 (7.9) | Coefficient: −0.0 (−0.7 to 0.7) | Coefficient: 1.0 (−0.5 to 2.5) |
| Hyperchloremic acidosis | 92 (30.9%) | aOR: 1.2 (1.0–1.5) | aOR: 1.2 (0.7–2.0) |
| Hypoglycemia | 78 (26.2%) | aOR: 0.9 (0.7–1.1) | aOR: 1.1 (0.7–1.7) |
| Hypokalemia | 54 (18.1%) | aOR: 1.2 (0.9–1.5) | aOR: 1.3 (0.7–2.4) |
| Hypophosphatemia | 168 (56.4%) | aOR: 0.7 (0.6–0.9) | aOR: 2.0 (1.2–3.2) |
Multivariable linear and logistic regression results are shown as adjusted coefficients and 95% CIs (time from randomization to DKA resolution), and adjusted odds ratios and 95% CIs (all other outcomes). Outcomes are listed in column 1. Independent variables were the amount of nonbolus fluid and bolus fluid at treatment initiation, as well as DKA severity defined by baseline pH and bicarbonate values (results not shown). Two patients were excluded from regression models because of unknown nonbolus fluid infusion rate. aOR, adjusted odds ratio.
Discussion
Despite the FLUID Trial results demonstrating that neither larger fluid bolus volume nor more-rapid fluid infusion rates increase the frequency of mental status changes or clinically apparent CI in children with DKA, concerns have persisted that high weight-based fluid rates in overweight or obese children and youth might be associated with risk of cerebral injury. Some clinicians have preferred to base fluid calculations for overweight or obese children and youth on ideal body weight, rather than actual body weight, because of lack of evidence about fluid protocol safety that is specific to these subgroups. Notably, during the FLUID Trial, patients who were overweight or obese, on average, received less fluid than prescribed by the protocol for their randomization group. The current study is the first to specifically address concerns about fluid protocol safety in overweight and obese children and youth with DKA. We demonstrated that more-rapid fluid infusion, using rates calculated according to body weight, is not associated with higher risk of mental status changes or clinical diagnoses of cerebral injury in children and youth who are overweight or obese. These results are consistent with the FLUID Trial results and recent theoretical constructs on the pathophysiology of DKA-related cerebral injury.2,7,8 Recent data suggest that DKA-related cerebral injury is not because of rapid fluid infusion and resultant osmotic changes, but rather it is associated with alterations in DKA-related cerebral perfusion and neuroinflammation.8–10
We hypothesized that slower rates of infusion may lead to increased electrolyte abnormalities and increased time to resolution of DKA. In patients who were overweight or obese, we found that variations in fluid infusion altered the risk of hypophosphatemia. Larger fluid bolus amounts increased this risk and higher fluid infusion rates decreased this risk. These findings likely reflect the absence of phosphate in bolus fluids and provision of phosphate in subsequent fluid replacement. Although severe hypophosphatemia is infrequent in DKA, it can have serious consequences including rhabdomyolysis, hemolytic anemia, encephalopathy, and arrythmias. Provision of fluid replacement at recommended rates after administration of bolus fluids may help to diminish this risk of hypophosphatemia in overweight or obese children and youth.
The current study has some limitations. This was a subanalysis of data collected during the PECARN FLUID Trial, and sample size requirements for the trial were not based on the specific comparisons included in the current analyses. The group of obese children and youth was relatively small and the study therefore lacked sufficient statistical power to detect differences of smaller magnitude, particularly those related to time to DKA resolution. Children and youth who are more severely ill also tend to require more fluid boluses and higher infusion rates, confounding the associations between fluid infusion variations and time to DKA resolution. In addition, the number of outcomes in our analysis was insufficient to perform multivariable analyses on mental status change and CI outcomes. Finally, we did not query clinicians about the rationale for deviations from the study protocol, and therefore cannot verify the reasons for reductions in fluid infusion rates in some overweight or obese children and youth compared with what was dictated by the protocol.
Conclusions
In summary, the current study demonstrates that clinicians administered intravenous fluids at slower rates in overweight or obese children and youth with DKA compared with standard weight-based calculations at the time of the FLUID Trial. However, we found no increases in risk of mental status changes or diagnoses of cerebral injury when fluids were administered according to weight-based calculations. Furthermore, the risk of hypophosphatemia was increased in children and youth who received fluid infusions at lower rates. These data suggest that physicians should continue to follow weight-based rehydration strategies for obese children and youth with DKA.
Acknowledgments
Contributions of PECARN committees and individuals to the study: Participating coinvestigators of the PECARN DKA FLUID Study Group at the time of the design and initiation of the study in addition to the authors include Clinton S. Perry III, PhD, and James P. Marcin, MD, MPH (UC Davis Health, University of California, Davis); Mary Murray, MD, Jared Henricksen, MD, Brad Poss, MD, and J. Michael Dean, MD, MB (Primary Children’s Medical Center, University of Utah); Bema Bonsu, MD, Tensing Maa, MD, and Justin Indyk, MD, PhD (Nationwide Children’s Hospital, Ohio State University); Marian Rewers, MD, PhD, and Peter Mourani, MD (Children’s Hospital Colorado, University of Colorado); Jake A. Kushner, MD, and Laura L. Loftis, MD (Texas Children’s Hospital, Baylor University); Monika Goyal, MD, MSCE, Rakesh Mistry, MD, MS, Vijay Srinivasan, MD, Andrew Palladino, MD, and Colin Hawkes, MD (Children’s Hospital of Philadelphia, University of Pennsylvania); Joseph I. Wolfsdorf, MD, and Michael S. Agus, MD (Boston Children’s Hospital, Harvard University); Linda Snelling, MD (Rhode Island Hospital, Brown University); Charlotte Boney, MD, MS (University of Massachusetts Medical School, Baystate and Rhode Island Hospital, Brown University); Fran R. Cogen, MD, CDE, and Sonali Basu, MD (Children’s National Medical Center, George Washington University); Neil H. White, MD, CDE, and Nikoleta S. Kolovos, MD (St Louis Children’s Hospital, Washington University in St Louis); Donald Zimmerman, MD, and Denise Goodman, MD, MS (Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University); Andrew D. DePiero, MD, Daniel A. Doyle, MD, and Meg A. Frizzola, MD (A. I. DuPont Hospital for Children, Thomas Jefferson University); Scott Baird, MD, and David Schnadower, MD (New York Presbyterian Morgan Stanley Children’s Hospital, Columbia University). We honor the memory and contributions of our former colleague Aris Garro, MD (Departments of Emergency Medicine and Pediatrics, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence).:
Glossary
- CI
confidence interval
- DKA
diabetic ketoacidosis
- ED
emergency department
- FLUID
Fluid Therapies Under Investigation in DKA
- GCS
Glasgow Coma Scale
- PECARN
Pediatric Emergency Care Applied Research Network
Footnotes
Dr Brown conceptualized and designed this subanalysis, drafted the initial manuscript, contributed to data analysis, and critically reviewed and revised the manuscript; Drs Glaser and Kuppermann conceived and designed the original study and this subanalysis, contributed to the design of this subanalysis, obtained grant funding, supervised training of study personnel, supervised patient enrollment and data abstraction, contributed to data analysis, helped draft the initial manuscript and edit the final manuscript; Mr Olsen had full access to the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis, conducted data analyses, drafted tables and figures for the final manuscript, and revised the final manuscript; Dr Casper had full access to the study data and takes responsibility for the integrity of the data and the accuracy of the data analysis, supervised data analyses and drafting of tables and figures for the final manuscript, and revised the final manuscript; Dr Ghetti conceived and designed the original study, supervised training of study personnel and neurocognitive data collection, contributed to data analysis, and reviewed the final manuscript; Drs McManemy, DePiero, Nigrovic, Quayle, Stoner, Schunk, Trainor, Tzimenatos, Rewers, and Kwok supervised patient enrollment and data abstraction, contributed to study design, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant U01HD062417) and the Emergency Medical Services for Children Network Development Demonstration Program of the Maternal and Child Health Bureau, Health Resources and Services Administration, under cooperative agreement (awards U03MC00008, U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC22684, and U03MC22685). The content and conclusions of this article are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the Health Resources and Services Administration, the Department of Health and Human Services, or the US government. The funders had no role in the design or conduct of this study. No honorarium, grant, or other form of payment was given to any of the authors to produce the manuscript.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest relevant to this article to disclose.
References
- 1. Harris GD, Fiordalisi I, Finberg L. Safe management of diabetic ketoacidemia. J Pediatr. 1988;113(1 Pt 1):65–68 [DOI] [PubMed] [Google Scholar]
- 2. Kuppermann N, Ghetti S, Schunk JE, et al. ; PECARN DKA FLUID Study Group. Clinical trial of fluid infusion rates for pediatric diabetic ketoacidosis. N Engl J Med. 2018;378(24):2275–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–265 [DOI] [PubMed] [Google Scholar]
- 4. Glaser NS, Ghetti S, Casper TC, Dean JM, Kuppermann N. Pediatric Emergency Care Applied Research Network (PECARN) DKA FLUID Study Group. Pediatric diabetic ketoacidosis, fluid therapy, and cerebral injury: the design of a factorial randomized controlled trial. Pediatr Diabetes. 2013;14(6):435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190 [PubMed] [Google Scholar]
- 6. Muir AB, Quisling RG, Yang MC, Rosenbloom AL. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004;27(7):1541–1546 [DOI] [PubMed] [Google Scholar]
- 7. Glaser N, Kuppermann N. Fluid treatment for children with diabetic ketoacidosis: How do the results of the pediatric emergency care applied research network Fluid Therapies Under Investigation in Diabetic Ketoacidosis (FLUID) Trial change our perspective?. Pediatr Diabetes . 2019;20(1):10–14 [DOI] [PubMed] [Google Scholar]
- 8. Glaser N, Chu S, Hung B, et al. Acute and chronic neuroinflammation is triggered by diabetic ketoacidosis in a rat model. BMJ Open Diabetes Res Care. 2020;8(2):e001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lo W, O’Donnell M, Tancredi D, Orgain M, Glaser N. Diabetic ketoacidosis in juvenile rats is associated with reactive gliosis and activation of microglia in the hippocampus. Pediatr Diabetes. 2016;17(2):127–139 [DOI] [PubMed] [Google Scholar]
- 10. Yuen N, Anderson SE, Glaser N, Tancredi DJ, O’Donnell ME. Cerebral blood flow and cerebral edema in rats with diabetic ketoacidosis. Diabetes. 2008;57(10):2588–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]