Abstract
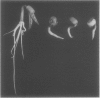
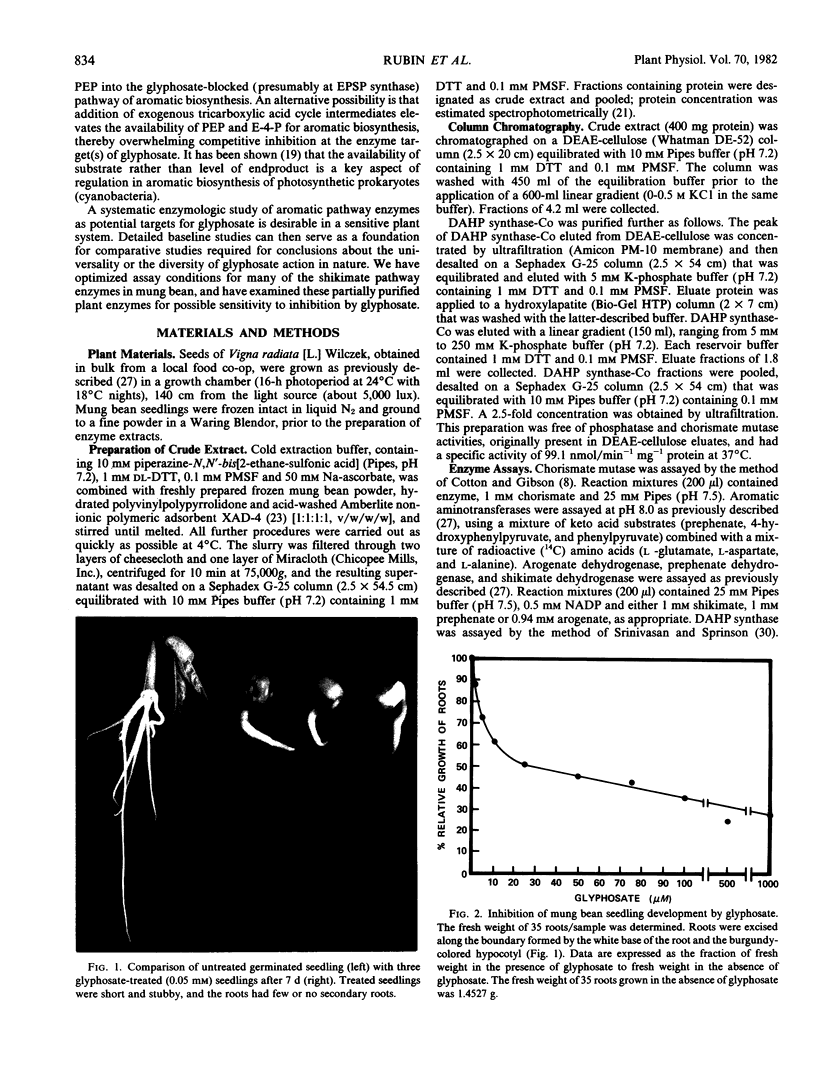
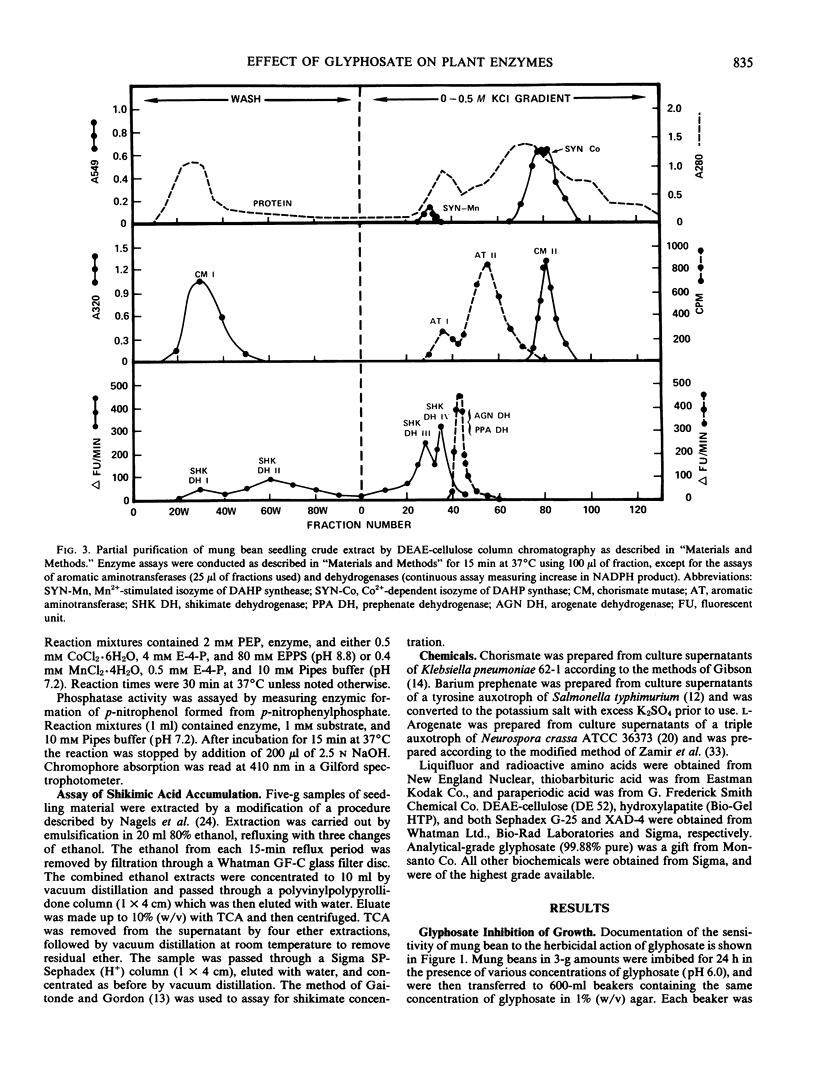
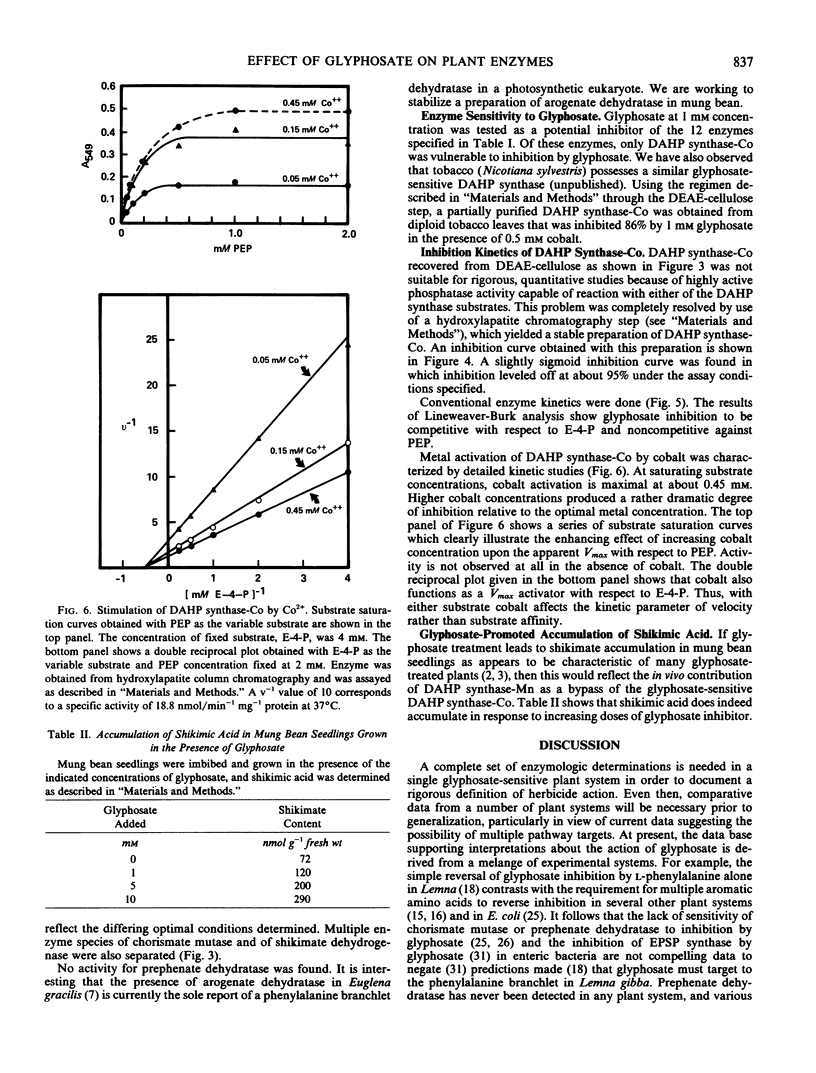
The effects of 1 millimolar glyphosate (N-[phosphonomethyl]glycine) upon the activities of enzymes of aromatic amino acid biosynthesis, partially purified by ion-exchange chromatography from mung bean seedings (Vigna radiata [L.] Wilczek), were examined. Multiple isozyme species of shikimate dehydrogenase, chorismate mutase, and aromatic aminotransferase were separated, and these were all insensitive to inhibition by glyphosate. The activities of prephenate dehydrogenase and arogenate dehydrogenase were also not sensitive to inhibition. Two molecular species of 3-deoxy-d-arabino-heptulosonate 7-phosphate (DAHP) synthase were resolved, one stimulated several-fold by Mn2+ (DAHP synthase-Mn), and the other absolutely dependent upon the presence of Co2+ for activity (DAHP synthase-Co). Whereas DAHP synthase-Mn was invulnerable to glyphosate, greater than 95% inhibition of DAHP synthase-Co was found in the presence of glyphosate. Since Co2+ is a Vmax activator with respect to both substrates, glyphosate cannot act simply by Co2+ chelation because inhibition is competitive with respect to erythrose-4-phosphate. The accumulation of shikimate found in glyphosate-treated seedlings is consistent with in vivo inhibition of both 5-enolpyruvylshikimic acid 3-phosphate synthase and one of the two DAHP synthase isozymes. Aromatic amino acids, singly or in combination, only showed a trend towards reversal of growth inhibition in 7-day seedlings of mung bean. The possibilities are raised that glyphosate may act at multiple enzyme targets in a given organism or that different plants may vary in the identity of the prime enzyme target.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Deus B., Gehrke P., Steinrücken H. C. The Site of the Inhibition of the Shikimate Pathway by Glyphosate: II. INTERFERENCE OF GLYPHOSATE WITH CHORISMATE FORMATION IN VIVO AND IN VITRO. Plant Physiol. 1980 Nov;66(5):830–834. doi: 10.1104/pp.66.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byng G. S., Kane J. F., Jensen R. A. Diversity in the routing and regulation of complex biochemical pathways as indicators of microbial relatedness. Crit Rev Microbiol. 1982 May;9(4):227–252. doi: 10.3109/10408418209104491. [DOI] [PubMed] [Google Scholar]
- Byng G. S., Whitaker R. J., Shapiro C. L., Jensen R. A. The aromatic amino acid pathway branches at L-arogenate in Euglena gracilis. Mol Cell Biol. 1981 May;1(5):426–438. doi: 10.1128/mcb.1.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Duke S. O., Hoagland R. E., Elmore C. D. Effects of Glyphosate on Metabolism of Phenolic Compounds: V. l-alpha-AMINOOXY-beta-PHENYLPROPIONIC ACID AND GLYPHOSATE EFFECTS ON PHENYLALANINE AMMONIA-LYASE IN SOYBEAN SEEDLINGS. Plant Physiol. 1980 Jan;65(1):17–21. doi: 10.1104/pp.65.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITONDE M. K., GORDON M. W. A microchemical method for the detection and determination of shikimic acid. J Biol Chem. 1958 Feb;230(2):1043–1050. [PubMed] [Google Scholar]
- Gibson F. Chorismic acid: purification and some chemical and physical studies. Biochem J. 1964 Feb;90(2):256–261. doi: 10.1042/bj0900256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haderlie L. C., Widholm J. M., Slife F. W. Effect of glyphosate on carrot and tobacco cells. Plant Physiol. 1977 Jul;60(1):40–43. doi: 10.1104/pp.60.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killmer J., Widholm J., Slife F. Reversal of glyphosate inhibition of carrot cell culture growth by glycolytic intermediates and organic and amino acids. Plant Physiol. 1981 Dec;68(6):1299–1302. doi: 10.1104/pp.68.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisch U., Lingens F. Effect of the herbicide N-phosphonomethylglycine on the biosynthesis of aromatic amino acids. Angew Chem Int Ed Engl. 1974 Jun;13(6):400–400. doi: 10.1002/anie.197404001. [DOI] [PubMed] [Google Scholar]
- Roisch U., Lingens F. Zum Wirkungsmechanismus des Herbizids N-(Phosphonomethyl)glycin. Einfluss von N-(Phosphonomethyl)glycin auf das Wachstum und auf die Enzyme der Aromatenbiosynthese von Escherichia coli. Hoppe Seylers Z Physiol Chem. 1980 Jul;361(7):1049–1058. [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Enzymology of l-Tyrosine Biosynthesis in Mung Bean (Vigna radiata [L.] Wilczek). Plant Physiol. 1979 Nov;64(5):727–734. doi: 10.1104/pp.64.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., SPRINSON D. B. 2-Keto-3-deoxy-D-arabo-heptonic acid 7-phosphate synthetase. J Biol Chem. 1959 Apr;234(4):716–722. [PubMed] [Google Scholar]
- Steinrücken H. C., Amrhein N. The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1207–1212. doi: 10.1016/0006-291x(80)90547-1. [DOI] [PubMed] [Google Scholar]