Abstract
The behavior of Campylobacter jejuni at environmental temperatures was examined by determining the physiological activities of this human pathogen. The minimal growth temperatures were found to be 32 and 31°C for strains 104 and ATCC 33560, respectively. Both strains exhibited a sudden decrease in growth rate from the maximum to zero within a few degrees not only near the maximal growth temperature but also near the minimal growth temperature. This could be an indication that a temperature-dependent transition in the structure of a key enzyme(s) or regulatory compound(s) determines the minimal growth temperature. Oxygen consumption, catalase activity, ATP generation, and protein synthesis were observed at temperatures as low as 4°C, indicating that vital cellular processes were still functioning. PCR analysis showed that cold shock protein genes, which play a role in low-temperature adaptation in many bacteria, are not present in C. jejuni. The fact that chemotaxis and aerotaxis could be observed at all temperatures shows that the pathogen is able to move to favorable places at environmental temperatures, which may have significant implications for the survival of C. jejuni in the environment.
Campylobacter jejuni is an important cause of food-borne infections. Most often, this microorganism causes gastroenteritis (campylobacteriosis), but in rare cases, C. jejuni is associated with neurological diseases such as Guillain-Barré syndrome (29). Although campylobacteriosis is related mostly to consumption of poultry products (27), other sources, such as milk (26, 30) and water (24, 25, 33), are also important in causing this disease.
C. jejuni can be isolated from the gastrointestinal tracts of animals all over the world (3, 5, 19, 28, 32, 41) and from a wide variety of watery environmental sources (4, 6, 15). Since the minimal growth temperature reported in the literature varies between 32 and 36°C (7, 11, 13), it can be assumed that C. jejuni survives rather than grows in watery environments. At high temperatures, the microorganism transforms quickly to the nonculturable coccoid form, but the survival time of culturable spiral-shaped campylobacters under nutrient-poor and low-temperature conditions can be as long as 1 to 4 months (14, 20, 31). Therefore it is likely that although growth is not possible, natural waters play a role in maintaining the contamination cycle of this pathogen.
Growth temperature extremes are usually determined by fluidity of lipids and membrane components, resulting in changes in the transport of substrates, ions, or products; forces affecting tertiary and quaternary structures of enzymes; or temperature-dependent interactions of regulatory compounds at the enzyme, ribosome, RNA, or DNA level (39). Not much is known about the metabolism and general behavior of bacteria below their minimal growth temperatures. It has been reported, however, that many microorganisms produce cold shock proteins when they are transferred to temperatures near the minimal growth temperature (21). One of the major proteins induced at low temperature in Escherichia coli is CspA, which is thought to be involved in adaptation to low temperature. CspA is believed to act as an RNA chaperone to block the formation of secondary structures in the mRNA (40). The presence of cold shock proteins in campylobacters has not been described.
C. jejuni, being a microaerophilic organism, possesses an electron transfer chain to generate a proton motive force which is subsequently used to synthesize ATP via a membrane-bound ATPase (16). It is also a highly motile bacterium due to the presence of flagella (13). This report describes the physiological behavior of C. jejuni at growth temperatures and at temperatures below the minimal growth temperature. Respiration, ATP synthesis, and catalase activities were tested. Chemotaxis and aerotaxis assays were performed to examine the ability of C. jejuni to move under environmental conditions. Furthermore, the ability to synthesize proteins at low temperatures was investigated, and the presence of common cold shock protein genes was examined.
MATERIALS AND METHODS
C. jejuni strains and culture conditions.
C. jejuni 104 (isolated in our laboratory from sewage) and ATCC 33560 (from bovine feces) were maintained in 15 to 20% (vol/vol) glycerol in brain heart infusion broth (BHI; Difco catalog no. 0037-17-8) with glass beads at −80°C. For culture, one glass bead was inoculated in a tube with 10 ml of BHI. After 30 to 48 h, the strains were subcultured in BHI or swabbed onto Columbia agar base plates (CAB; Oxoid CM331) supplemented with 5% lysed, defibrinated horse blood or onto BHI agar (BHIA) plates. All cultures were incubated microaerobically at 37°C (unless otherwise stated) in jars or incubators flushed with a gas mixture of 5% O2, 10% CO2, and 85% N2.
Growth curves.
Cultures grown at 37°C (see above) were subcultured in 50 ml of BHI in 100-ml serum bottles with thick rubber stoppers. The headspace of these bottles was flushed with a gas mixture as mentioned above (overpressure of 500 hPa). The bottles were incubated stationary for 1 week in water baths at 4, 10, 20, 28, 30, 31, 33, 35, 37, 39, 42, 44, and 46°C. Samples for plate counts were taken at regular time intervals by piercing the rubber stoppers with a syringe. Plate counts were performed by spread plating decimal dilutions of cell suspensions on CAB. For adaptation experiments, cells precultured at 33 and 35°C were subcultured at 28 and 30°C. Samples were taken as described above.
Biological oxygen monitoring.
Oxygen consumption was measured with a biological oxygen monitor (BOM; Yellow Springs Instrument model 5300) as specified by the manufacturer. Cells were cultured on CAB (24 h), harvested, and washed three times in 50 mM potassium phosphate buffer containing 0.85% (wt/vol) NaCl (PBS; pH 7) and suspended to a final optical density at 620 nm of 20 to 30. Concentrated cell suspensions were kept on ice until the measurements took place. Cells (100 μl) were added to PBS in the sample chamber (total volume, 4 ml). After flushing with air to saturate the suspension with oxygen, a substrate (malate or formate; end concentration, 15 mM) was added and respiration was measured. Cells (100 μl) were stored at −20°C and used for determination of total cellular protein by the method of Sedmak and Grossberg (35).
ATP measurements.
Concentrated cell suspensions were used as described above. Cells (100 μl) were added to 3.9 ml of PBS which was kept at different temperatures in a BOM. Samples (100 μl) were taken at time zero and 5 min later and kept on ice. Substrate (malate or formate) was added immediately after the second sample was taken. Samples were then taken after 20 s and 1, 2, 3, 5, and 10 min and kept on ice. After that, ATP concentrations in the samples were analyzed in a Biocounter M2010 (Lumac, Landgraaf, The Netherlands) as specified by the manufacturer, with the NRB/LUMIT-PM kit (Lumac catalog no. 9268-7). To calculate the actual ATP concentrations from the units measured in the Biocounter, a standard curve was constructed with ATP (Sigma catalog no. 5394). Cells (100 μl) were stored at −20°C for determination of total cellular protein.
Catalase activity measurements.
Concentrated cell suspensions were prepared as described earlier for the respiration experiments, except that the cells were precultured on both CAB and BHIA to determine the effect of preculture conditions. For determination of catalase activity, H2O2 (0.003% final concentration) was used as a substrate in a BOM, and oxygen production was measured.
Chemotaxis assays.
Studies on chemotaxis were carried out essentially by the method of Hugdahl et al. (17), except that filter discs were used instead of hard-agar plugs. In short, concentrated cell suspensions were prepared as described earlier and mixed (500 μl of cells) with 12 ml of PBS-agar (0.4% agar) at 45°C. This mixture was poured into 9-cm-diameter petri dishes. Stock solutions (1 M) of sodium malate, sodium formate, or sodium pyruvate (pH 7) were applied (30 μl) to sterile filter discs (0.25-in. sterile blanks; Difco catalog no. 1599-33). These wetted discs were placed on the cell-agar mixture in the middle of the petri dishes. Plates were incubated aerobically and microaerobically at 4, 20, or 40°C and viewed after 0.5, 1, 2, 3, 4, and 16 h.
Aerotaxis assays.
Cell suspensions (50 μl) in PBS-agar (5 ml) were prepared as described for the chemotaxis assays. Aerotaxis was assayed in PBS-agar or in PBS-agar supplemented with sodium pyruvate or sodium formate (50 mM final concentration). Mixtures were poured into small glass tubes (10-mm diameter) and viewed after overnight incubation in air at 4, 20, or 40°C.
Protein synthesis.
De novo protein synthesis was studied by using the incorporation of [35S]methionine. Cells were precultured at 37°C in 100 ml of Campylobacter defined medium as described by Tenover et al. (37). After centrifugation of 30-ml portions, cells were resuspended in 1 ml of Campylobacter defined medium without methionine. Portions were supplied with 10 μl of [35S]methionine (100 μCi; Tran35S-label, ICN Pharmaceuticals, Inc.) and incubated at 37°C for 15 min and at 4 or 20°C for 60 min. Incorporation of [35S]methionine was stopped by addition of cold methionine (8 mM final concentration). Cells were disrupted with zirconium glass beads (four times for 1.5 min each time at 1-min intervals on ice) in a mini Bead Beater (Biospec Products, Bartlesville, Okla.), after which proteins were precipitated with 50% trichloroacetic acid (TCA) on ice (10 min). After centrifugation, the pellet was washed successively with 20% TCA and 10% TCA. Finally, the pellet was washed with acetone (−20°C) and allowed to dry in air. The pellet was resuspended in 100 μl of water, and radioactivity was determined by liquid scintillation spectrometry (Beckman LS 7500). For electrophoresis, the sample was mixed with sample buffer at a 1:1 ratio. After 10 min of incubation at 95°C, 15 μl was loaded onto a Tricine-sodium dodecyl sulfate–16% polyacrylamide gel as described by Schägger and von Jagow (34). The gel was run for 150 min at 65 mA. After drying (in a Bio-Rad model 534 gel dryer), the gel was exposed to X-ray film (Kodak Scientific Imaging Film Biomax MR) for 2 days at −80°C.
PCR assay.
To examine the presence of common cold shock protein genes in C. jejuni, a PCR assay was carried out as described by Kuipers et al. (23). The primers used (CSPU5 and CSPU3) were based on the homologous regions of several cold shock protein genes (9). As template DNAs, the total DNAs of C. jejuni 104 and ATCC 33560 were used. The PCR was performed for 25 cycles with an annealing temperature of 40°C, and PCR products were separated on a 2% agarose gel. As a positive control, Escherichia coli K-12 was included in the assay.
RESULTS
Growth curves.
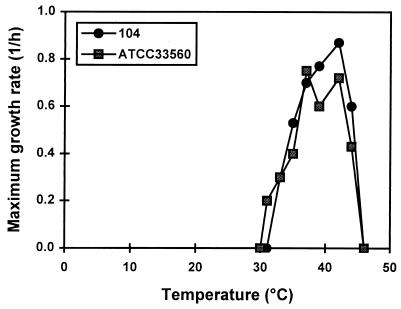
Growth experiments were carried out for 1 week to include slow-growing cells. However, when growth was not initiated within 1 day, no growth was observed during the rest of the week. From the growth curves (data not shown), the maximum growth rate during the exponential phase was calculated (Fig. 1). Similar growth characteristics were observed for the two strains tested; the minimal growth temperatures established were 32 and 31°C for strains 104 and ATCC 33560, respectively. Strikingly, growth rates decreased abruptly from the maximum to zero within a few degrees of the minimal growth temperature. A sudden growth rate decrease near the maximal growth temperature was also apparent.
FIG. 1.
Maximum growth rates of C. jejuni 104 and ATCC 33560 as a function of temperature. Bacteria were grown in BHI, and maximum growth rates during the exponential phase were calculated.
Additionally, cells precultured at 33 and 35°C were inoculated into medium and incubated at 28 and 30°C. However, no growth occurred at these temperatures, indicating that the minimal growth temperature for these suboptimal-temperature-adapted cells was not shifted to lower values.
Respiration.
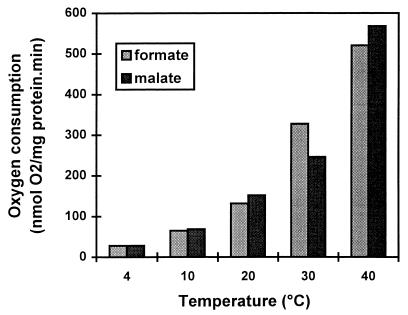
The oxygen consumption rates of C. jejuni 104 grown at 37°C were 28, 65, 132, 328, and 521 nmol of O2/mg of protein · min with formate at 4, 10, 20, 30, and 40°C, respectively (Fig. 2). Respiration of the cells with malate showed similar results (Fig. 2). Remarkably, even below the minimal growth temperature, considerable electron transfer chain activity could be detected. Respiration activities of 25, 12, and 5% compared to the activities at 40°C were observed at 20, 10, and 4°C, respectively. Strain ATCC 33560 showed similar activities (data not shown).
FIG. 2.
Respiration rates of C. jejuni 104 with formate and malate at different temperatures. A concentrated cell suspension was incubated in PBS in a BOM, and oxygen consumption was measured after addition of formate and malate (15 mM).
ATP production.
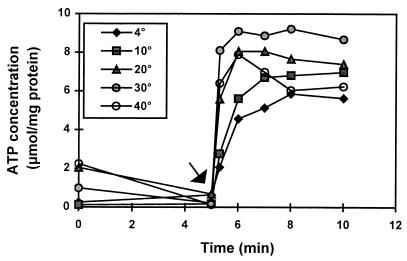
To determine whether C. jejuni is able to synthesize ATP during respiration at low temperatures as well, ATP concentrations were measured at different temperatures before and after substrate addition. As can be observed in Fig. 3, ATP production could be detected immediately after substrate addition at all of the temperatures tested, although rates and maximum ATP levels were somewhat lower at 4 and 10°C. Similar results were obtained for strain 104 (data not shown).
FIG. 3.
Effect of temperature on the ATP production of C. jejuni ATCC 33560. Cells were incubated in PBS, and the amount of ATP was determined. The arrow indicates the moment of formate addition.
Catalase activity.
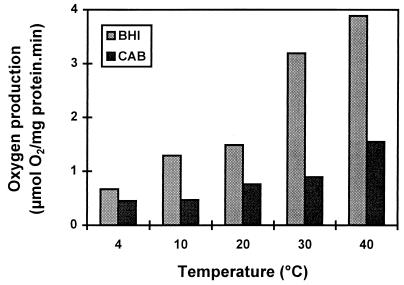
To determine whether C. jejuni is able to counteract the effects of hydrogen peroxide, a toxic by-product of oxygen metabolism, catalase activity was measured at different temperatures. Catalase activity varied from 3.89 to 0.67 μmol of O2/mg of protein · min at 40 to 4°C (Fig. 4). The activity at 4°C was 15 to 30% of the activity observed at 40°C for cells cultured on BHIA and CAB, respectively. The catalase activity of cells precultured on BHIA was always higher than that of cells precultured on CAB. Similar results were obtained for strain ATCC 33560.
FIG. 4.
Catalase activity of C. jejuni 104 at different temperatures. Cells harvested from CAB or BHIA were incubated in PBS in a BOM. Catalase activity was determined by measuring oxygen production after addition of hydrogen peroxide (0.003%).
Chemotaxis assay.
Both C. jejuni 104 and ATCC 33560 showed chemotaxis toward formate at 4, 20, and 40°C under microaerobic conditions. Turbid zones in plates incubated at 4°C were less dense than in those incubated at 20 and 40°C, but the diameters of the turbid rings were similar at all temperatures (4 cm after 4 h of incubation). Plate counts of samples taken from the agar plates showed that the turbid zones contained 10 to 100 times the number of CFU as the clear areas outside the turbid zones, which indicates that migration of cells toward the substrate took place. Similar behavior toward malate as a substrate was observed and aerobic culture conditions were similar to microaerobic culture conditions. When pyruvate was applied as a substrate, only a faint ring was observed. To exclude the possibility of growth, a control experiment was carried out in which C. jejuni was incubated microaerobically at 40°C for 16 h in PBS supplemented with each of the substrates. No growth occurred during these incubations (data not shown). Another indication that chemotaxis and not growth was the explanation for the appearance of turbid zones was the observation that these zones appeared within 30 min of incubation of the soft-agar plates at all temperatures. The filter disc method proved to be an easy and quick method for determining chemotaxis and will be useful as a tool for determining motility in general as well, since results are known within a few hours.
Aerotaxis assay.
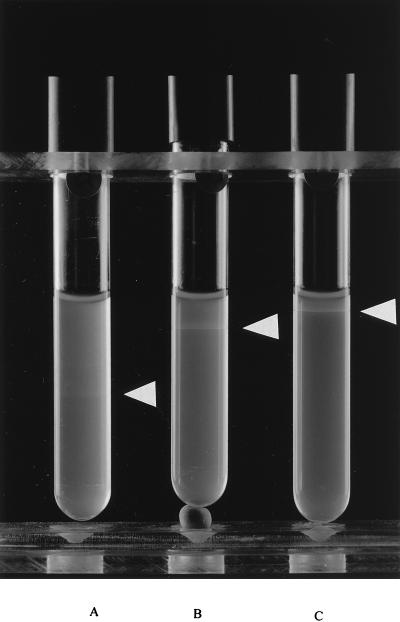
The aerotaxis assay is based on the fact that oxygen diffuses into a tube of soft agar. The oxygen concentration at the top of the agar will be near atmospheric concentrations, whereas at the bottom of the tube, the oxygen concentration will be very low. Movement toward a favorable concentration of oxygen at 20°C was observed as the formation of a ring of bacteria in each tube (Fig. 5). The position of the ring was dependent on substrate addition. When no substrate was added, a turbid ring appeared some centimeters below the agar surface, whereas with the addition of pyruvate and formate, distinct rings were found at 1 and 0.5 cm, respectively, below the surface. Rings were best visible at 20°C, whereas at 4 and 40°C, fainter rings occurred which appeared, however, at positions in the agar tubes similar to those of rings found at 20°C (data not shown). Similar results were obtained for strain ATCC 33560.
FIG. 5.
Aerotaxis of C. jejuni 104 in PBS agar (A) and in PBS agar supplemented with pyruvate (B) or formate (C) after incubation at 20°C for 16 h. The white arrowheads indicate the concentration of cells as a result of aerotaxis.
Protein synthesis.
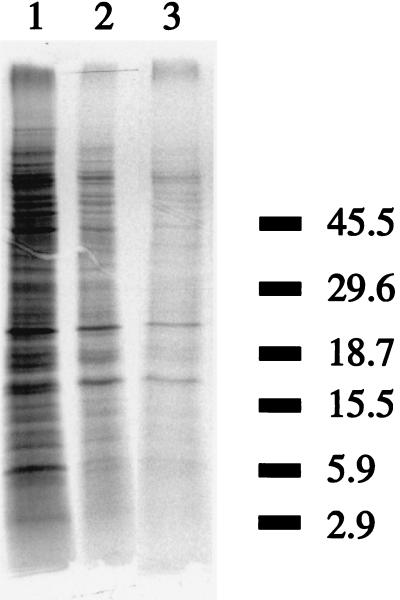
De novo protein synthesis was observed at 4, 20, and 37°C (Fig. 6). C. jejuni is even able to synthesize proteins far below its minimal growth temperature. However, it can be seen that the amounts of protein produced at 4 and 20°C were lower than the amount of protein produced at 37°C. This was confirmed in the scintillation counts of the samples when samples incubated at 4 and 20°C exhibited counts of 9 and 16%, respectively, of that of the sample incubated at 37°C. The total numbers of protein bands produced seemed to be similar for all temperatures, and no cold induction of specific proteins was observed. When we used this protein gel, which is specific for separation of proteins in the low-molecular-weight region, we observed no induced bands around 7 kDa, the expected size for CspA homologues.
FIG. 6.
Autoradiogram of protein profiles of C. jejuni ATCC 33560 after pulse labeling with [35S]methionine for 15, 60, and 60 min at 37, 20, and 4°C, respectively (lanes 1, 2, and 3, respectively). Molecular mass standards (in kilodaltons) are indicated on the right.
PCR assay.
Common cold shock genes were not detected in C. jejuni 104 and ATCC 33560. For the positive control, E. coli, a clear band of the expected size of 200 bp was observed (data not shown).
DISCUSSION
C. jejuni has a quite narrow growth temperature range. The minimal growth temperature determined in our experiments (31 to 32°C) is similar to that reported by Doyle and Roman (7) and Grant et al. (13) and somewhat lower than that reported by Gill and Harris (34 to 36°C) (12). The sudden growth rate decline at temperatures of around 30°C is remarkable, since most microorganisms show a more gradual growth rate decrease near the minimal growth temperature in such a way that the growth rate is linearly proportional to the square root of the growth temperature minus the minimum growth temperature (43). Close to the maximum growth temperature, the growth rate also suddenly declined, but this is generally observed for other microorganisms as well. No adaptation to growth at lower temperatures was observed when C. jejuni was precultured at temperatures just above the minimal growth temperature.
The question of whether Campylobacter is physiologically active at temperatures below the minimal growth temperature arose. The respiratory rates with formate, determined at 40°C, are of the same order of magnitude as those reported by Hoffman and Goodman (16), who measured them at 37°C. The rates on malate, however, are much higher then those found by those authors. Apparently, the use of different substrates by the bacteria is strain dependent. At temperatures below the minimal growth temperatures, respiration was also observed, the rate being proportional to the temperature. C. jejuni was even able to perform respiration at 4°C, which shows that substrate transport and the electron transfer chain are still active far below the minimal growth temperature.
When oxygen is used as a terminal electron acceptor during respiration, hydrogen peroxide can be formed due to the activity of superoxide dismutase. Hydrogen peroxide is toxic to cells due to the fact that it oxidizes SH groups. To overcome this toxic effect, Campylobacter possesses the enzyme catalase, which catalyzes the formation of water and oxygen from hydrogen peroxide. Although the catalase activities were proportional to temperature, high activities of this enzyme were still observed at low temperatures, indicating that the detoxification of oxygen by-products is efficient at very low temperatures. The catalase activity is so high (0.5 μmol of oxygen/mg of protein · min at 4°C) that it can be assumed that it can easily compensate for the formation of hydrogen peroxide as a result of respiration (30 nmol of oxygen/mg of protein · min at 4°C). The fact that catalase activity was much higher in cells precultured on BHIA than in those cultured on CAB can be explained by the fact that CAB contains blood, which partly protects the cells from the toxic effects of oxygen by-products (18). On BHIA, however, cells are more exposed to oxygen and need higher catalase activity. Apparently, the cells are able to regulate their catalase activity, depending on the growth medium.
C. jejuni was able to produce ATP very well at all of the temperatures tested. Even at low temperatures, the ATP concentration in the cell suspensions was approximately half of the ATP concentration of cells incubated at 40°C. This is remarkable when one takes into account the fact that the respiration rate at 4°C is only 5% of the rate at 40°C but can be explained by the fact that in the ATP measurements, the net amount of ATP i.e., the sum of ATP production and consumption, is measured. Both production and consumption of ATP is highest at high temperatures, due to higher metabolic activities, and the overall ATP concentration appears to remain relatively low. From these results, it can be concluded that the electron transfer chain is active and a proton motive force is generated which can be used to synthesize ATP.
Bacterial chemotaxis is a complex signal transduction system by which bacteria are able to sense environmental stimuli and respond to them by flagellar rotation (8). For campylobacters, the exact mechanism of chemotaxis is not known, but motility seems to be important for virulence (22). The chemotaxis experiments in this study showed taxis toward malate, formate, and, to a lesser extent, pyruvate at all of the temperatures and in all of the atmospheres tested. Hugdahl et al. (17) reported chemotaxis toward malate and pyruvate at 42°C in a microaerobic atmosphere which was confirmed by our tests. Formate was not tested in their experiments but was included in our study since it is likely to be present in the environment due to the fact that it is an important product of microbial fermentations.
Motility seemed to be comparable at all of the temperatures tested. This was concluded because chemotaxis rings occurred after the same lapse of time (already after 30 min) at different temperatures. The fact that no clear differences in motility were observed stands in contrast to the finding that C. coli, which is closely related to C. jejuni, was shown to have higher motility at 42°C than at 37°C (1). Although the exact mechanism of Campylobacter taxis is not known, our data indicate that C. jejuni is able to move toward substrates under environmental conditions, even at temperatures far below the minimal growth temperature of the microorganism.
Aerotaxis, which is chemotaxis with air or oxygen as a stimulus, is seen in many different microorganisms, such as the facultative anaerobes Salmonella typhimurium and E. coli (36), the microaerophilic bacterium Azospirillum brasilense (42), and the phototrophic bacterium Rhodobacter sphaeroides (10). In our study, C. jejuni showed aerotactic bands in soft-agar tubes incubated in air. Enhanced taxis after substrate addition was observed since bands were best visible with the addition of pyruvate or formate, even though a faint band could be observed when no substrate was added. When no substrate was added, a band appeared a few centimeters below the agar surface, whereas addition of substrates produced bands nearer to the surface. This can be explained by the fact that the cells will be concentrated close to the surface if a substrate is oxidized at a high rate. In these cases, respiration takes place, oxygen is used, and the cells will move up the tube to the most favorable oxygen concentration. Similar results were observed at the different temperatures tested, but the bands were best visible at 20°C. Therefore, it is likely that C. jejuni, in addition to substrate taxis, is able to move to favorable oxygen concentrations in the environment.
In conclusion, our study indicates that many important physiological activities take place under conditions in which growth of C. jejuni is not possible. Since C. jejuni is able to move and to perform respiration with generation of ATP, which are both membrane-bound reactions, it is not likely that lack of membrane fluidity or inhibition of substrate transport is the reason for the inability of C. jejuni to grow at low temperatures. Furthermore, this study shows that protein synthesis still takes place at low temperatures, even at 4°C. In addition, no major induced proteins could be observed at low temperatures. Since cold shock protein (cspA) homologues have been reported in many organisms (40), we tried to identify the genes encoding these proteins with a PCR strategy. By using universal primers, by which csp genes in many bacteria were detected (9), we were not able to identify csp homologues in C. jejuni. From the genomic sequence of the closely related bacterium Helicobacter pylori, it was learned that this bacterium also contains no cspA homologues (38, 40). The absence of cold shock proteins in C. jejuni might be an explanation for the fact that the pathogen is not able to grow at temperatures below 30°C. On the other hand, transitions in the structure of a key enzyme(s) or regulatory compound(s) may also play an important role in this respect, which is confirmed by the observation that the growth rate rapidly decreases from the maximum to zero near the minimal growth temperature. If the minimal growth temperature is determined by only one enzyme, for example, it is conceivable that mutants could occur that grow at low temperatures. These mutants would not be recognized as C. jejuni, since in the conventional isolation and determination procedure, one identification criterion is the fact that the organism should not grow at 25°C (2).
Regardless of the reason for the inability to grow at low temperatures, the fact that C. jejuni is able to move to favorable places (substrates) in an environment plays a role in its survival in that environment.
REFERENCES
- 1.Alm R A, Guerry P, Trust T J. The Campylobacter ς54flaB flagellin promoter is subject to environmental regulation. J Bacteriol. 1993;175:4448–4455. doi: 10.1128/jb.175.14.4448-4455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Microbiology of food and animal feeding stuffs—horizontal method for detection of thermotolerant Campylobacter. International Standard ISO 10272; 1995. [Google Scholar]
- 3.Bauwens L, De Meurichy W. The occurrence of thermophilic campylobacters in zoo animals. Acta Zool Pathol Antverp. 1981;76:181–189. [Google Scholar]
- 4.Bolton F J, Coates D, Hutchinson D N, Godfree A F. A study of thermophilic campylobacters in a river system. J Appl Bacteriol. 1987;62:167–176. doi: 10.1111/j.1365-2672.1987.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 5.Cabrita J, Rodrigues J, Bragança F, Morgado C, Pires I, Penha Gonçalves A. Prevalence, biotypes, plasmid profile and antimicrobial resistance of Campylobacter isolated from wild and domestic animals from Northeast Portugal. J Appl Bacteriol. 1992;73:279–285. doi: 10.1111/j.1365-2672.1992.tb04978.x. [DOI] [PubMed] [Google Scholar]
- 6.Carter A M, Pacha R E, Clark G W, Williams E A. Seasonal occurrence of Campylobacter spp. in surface waters and their correlation with standard indicator bacteria. Appl Environ Microbiol. 1987;53:523–526. doi: 10.1128/aem.53.3.523-526.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle M P, Roman D J. Growth and survival of Campylobacter fetus subsp. jejuni as a function of temperature and pH. J Food Prot. 1981;44:596–601. doi: 10.4315/0362-028X-44.8.596. [DOI] [PubMed] [Google Scholar]
- 8.Eisenbach M. Control of bacterial chemotaxis. Mol Microbiol. 1996;20:903–910. doi: 10.1111/j.1365-2958.1996.tb02531.x. [DOI] [PubMed] [Google Scholar]
- 9.Francis K P, Stewart G S A B. Detection and speciation of bacteria through PCR using universal major cold-shock protein primer oligomers. J Ind Microbiol Biotechnol. 1997;19:286–293. doi: 10.1038/sj.jim.2900463. [DOI] [PubMed] [Google Scholar]
- 10.Gauden D E, Armitage J P. Electron transport-dependent taxis in Rhodobacter sphaeroides. J Bacteriol. 1995;177:5853–5859. doi: 10.1128/jb.177.20.5853-5859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gill C O, Harris L M. Survival and growth of Campylobacter fetus subsp. jejuni on meat and in cooked foods. Appl Environ Microbiol. 1982;44:259–263. doi: 10.1128/aem.44.2.259-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill C O, Harris L M. Limiting conditions of temperature and pH for growth of “thermophilic” campylobacters on solid media. J Food Prot. 1983;46:767–768. doi: 10.4315/0362-028X-46.9.767. [DOI] [PubMed] [Google Scholar]
- 13.Grant C C R, Konkel M E, Cieplak W, Jr, Tompkins L S. Role of flagella in adherence, internalization, and translocation of Campylobacter jejuni in nonpolarized and polarized epithelial cell cultures. Infect Immun. 1993;61:1764–1771. doi: 10.1128/iai.61.5.1764-1771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeleger W C, Janse J D, Koenraad P M F J, Beumer R R, Rombouts F M, Abee T. Temperature-dependent membrane fatty acid and cell physiology changes in coccoid forms of Campylobacter jejuni. Appl Environ Microbiol. 1995;61:2713–2719. doi: 10.1128/aem.61.7.2713-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill G A, Grimes D J. Seasonal study of a freshwater lake and migratory fowl for Campylobacter jejuni. Can J Microbiol. 1984;30:845–849. doi: 10.1139/m84-130. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman P S, Goodman T G. Respiratory physiology and energy conservation efficiency of Campylobacter jejuni. J Bacteriol. 1982;150:319–326. doi: 10.1128/jb.150.1.319-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugdahl M B, Beery J T, Doyle M P. Chemotactic behavior of Campylobacter jejuni. Infect Immun. 1988;56:1560–1566. doi: 10.1128/iai.56.6.1560-1566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphrey T J. The synergistic inhibition of Campylobacter jejuni by rifampicin and hydrogen peroxide. Lett Appl Microbiol. 1990;10:97–100. [Google Scholar]
- 19.Ito K, Kubokura Y, Kaneko K, Totake Y, Ogawa M. Occurrence of Campylobacter jejuni in free-living wild birds from Jpn. J Wildl Dis. 1988;24:467–470. doi: 10.7589/0090-3558-24.3.467. [DOI] [PubMed] [Google Scholar]
- 20.Jones M, Sutcliffe E M, Curry A. Recovery of viable but non-culturable Campylobacter jejuni. J Gen Microbiol. 1991;137:2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- 21.Jones P G, VanBogelen R A, Neidhardt F C. Induction of proteins in response to low temperature in Escherichia coli. J Bacteriol. 1987;169:2092–2095. doi: 10.1128/jb.169.5.2092-2095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 23.Kuipers O P, Boot H J, de Vos W M. Improved site-directed mutagenesis method using PCR. Nucleic Acids Res. 1991;19:4558. doi: 10.1093/nar/19.16.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melby K, Gondrosen B, Gregusson S, Ribe H, Dahl P P. Waterborne campylobacteriosis in northern Norway. Int J Food Microbiol. 1991;12:151–156. doi: 10.1016/0168-1605(91)90064-v. [DOI] [PubMed] [Google Scholar]
- 25.Millson M, Bokhout M, Carlson J, Spielberg L, Akdusm R, Borczyk A, Lior H. An outbreak of Campylobacter jejuni gastro-enteritis linked to meltwater contamination of a municipal well. Can J Public Health. 1991;82:27–31. [PubMed] [Google Scholar]
- 26.Morgan D, Gunneberg C, Gunnell D, Healing T D, Lamerton S, Soltanpoor N, Lewis D A, White D G. An outbreak of Campylobacter infection associated with the consumption of unpasteurised milk at a large festival in England. Eur J Epidemiol. 1994;10:581–585. doi: 10.1007/BF01719576. [DOI] [PubMed] [Google Scholar]
- 27.Notermans S, Hoogenboom-Verdegaal A. Existing and emerging foodborne diseases. Int J Food Microbiol. 1992;15:197–205. doi: 10.1016/0168-1605(92)90049-9. [DOI] [PubMed] [Google Scholar]
- 28.Pearson A D, Greenwood M H, Feltham R K A, Healing T D, Donaldson J, Jones D M, Colwell R R. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl Environ Microbiol. 1996;62:4614–4620. doi: 10.1128/aem.62.12.4614-4620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees J H, Gregson N A, Griffiths P L, Hughes R A C. Campylobacter jejuni and Guillain-Barré syndrome. Q J Med. 1993;86:623–634. doi: 10.1093/qjmed/86.10.623. [DOI] [PubMed] [Google Scholar]
- 30.Riordan T, Humphrey T J, Fowles A. A point source outbreak of Campylobacter infection related to bird-pecked milk. Epidemiol Infect. 1993;110:261–265. doi: 10.1017/s0950268800068187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol. 1986;52:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosef O, Gondrosen B, Kapperud G, Underdal B. Isolation and characterization of Campylobacter jejuni and Campylobacter coli from domestic and wild mammals in Norway. Appl Environ Microbiol. 1983;46:855–859. doi: 10.1128/aem.46.4.855-859.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacks J J, Lieb S, Baldy L M, Berta S, Patton C M, White M C, Bigler W J, White J J. Epidemic campylobacteriosis associated with a community water supply. Am J Public Health. 1986;76:424–429. doi: 10.2105/ajph.76.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 35.Sedmak J J, Grossberg S E. A rapid, sensitive and versatile assay for protein using Coomassie brilliant blue G 250. Anal Biochem. 1977;79:544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- 36.Shioi J, Dang C V, Taylor B L. Oxygen as attractant and repellent in bacterial chemotaxis. J Bacteriol. 1987;169:3118–3123. doi: 10.1128/jb.169.7.3118-3123.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tenover F C, Knapp J S, Patton C, Plorde J J. Use of auxotyping for epidemiological studies of Campylobacter jejuni and Campylobacter coli infections. Infect Immun. 1985;48:384–388. doi: 10.1128/iai.48.2.384-388.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomb J-F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 39.Wiegel J. Temperature spans for growth: hypothesis and discussion. FEMS Microbiol Rev. 1990;75:155–170. [Google Scholar]
- 40.Yamanaka K, Fang L, Inouye M. The CspA family in Escherichia coli: multiple gene duplication for stress adaptation. Mol Microbiol. 1998;27:247–255. doi: 10.1046/j.1365-2958.1998.00683.x. [DOI] [PubMed] [Google Scholar]
- 41.Yogasundram K, Shane S M, Harrington K S. Prevalence of Campylobacter jejuni in selected domestic and wild birds in Louisiana. Avian Dis. 1989;33:664–667. [PubMed] [Google Scholar]
- 42.Zhulin I B, Bespalov V A, Johnson M S, Taylor B L. Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol. 1996;178:5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zwietering M H, de Koos J T, Hasenack B E, de Wit J C, van ’t Riet K. Modeling of bacterial growth as a function of temperature. Appl Environ Microbiol. 1991;57:1094–1101. doi: 10.1128/aem.57.4.1094-1101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]