Abstract
The effect of several polyamines (putrescine, spermidine, and spermine), their precursors (l-arginine and l-ornithine), and some analogs and metabolic inhibitors (l-canavanine, l-canaline, and methylglyoxal-bis [guanylhydrazone]) on root formation have been studied in mung bean (Vigna radiata [L.] Wilczek) hypocotyl cuttings.
Exogenously applied polyamines did not promote adventitious root formation. Rooting was inhibited by l-canavanine and l-canaline, and this inhibition was reversed by the corresponding amino acids l-arginine and l-ornithine. Methylglyoxal-bis (guanylhydrazone), an inhibitor of S-adenosylmethionine decarboxylase and polyamine biosynthesis, was also found to inhibit root formation. All compounds at concentrations of >10−4 molarity completely inhibited natural root formation, whereas at <10−5 molarity only the indole-butyric acid-induced root formation was inhibited.
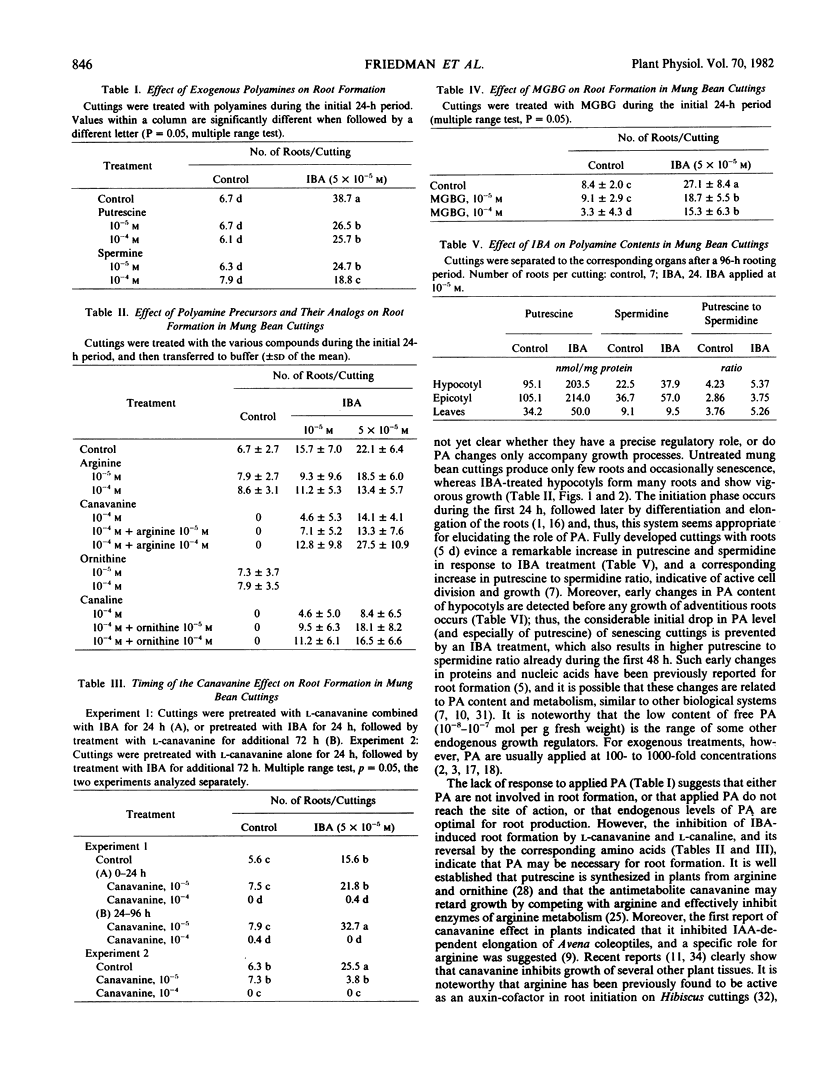
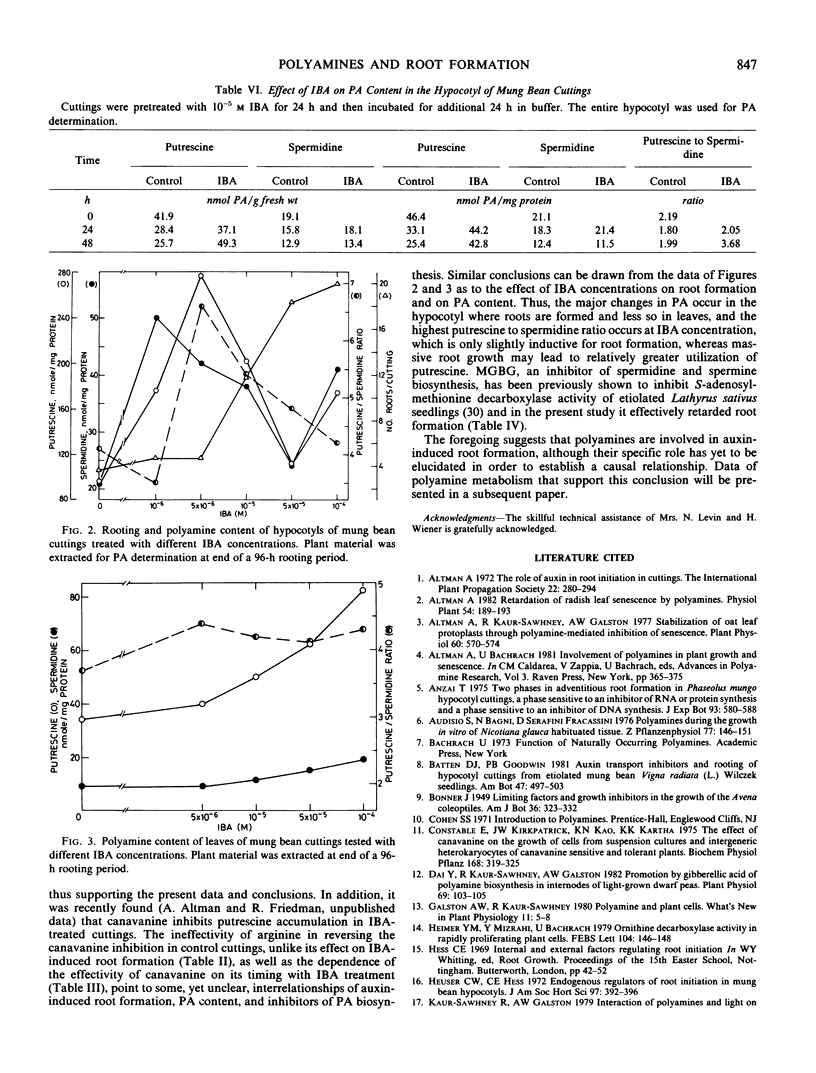
Indole-butyric acid-induced root formation was accompanied by a considerable increase in polyamine levels, more than 2-fold of the control. Whereas senescing (unrooted) cuttings evinced a rapid decline in polyamine content during 48 hours, indole-butyric acid treatment resulted in elevated levels of putrescine and increased putrescine to spermidine ratio. The changes in polyamines were dependent on indole-butyric acid concentration and were organ specific.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. R., Kaur-Sawhney R., Galston A. W. Promotion by gibberellic Acid of polyamine biosynthesis in internodes of light-grown dwarf peas. Plant Physiol. 1982 Jan;69(1):103–106. doi: 10.1104/pp.69.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y., Bachrach U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979 Aug 1;104(1):146–148. doi: 10.1016/0014-5793(79)81102-3. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Shih L. M., Galston A. W. Relation of polyamine biosynthesis to the initiation of sprouting in potato tubers. Plant Physiol. 1982 Feb;69(2):411–415. doi: 10.1104/pp.69.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefford N. P. Effect of hormone antagonist on the rooting of shoot cuttings. Plant Physiol. 1973 Jan;51(1):214–216. doi: 10.1104/pp.51.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Montague M. J., Armstrong T. A., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: II. Changes in Arginine Decarboxylase Activity. Plant Physiol. 1979 Feb;63(2):341–345. doi: 10.1104/pp.63.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G. A. The biological and biochemical properties of L-canaline, a naturally occurring structural analogue of L-ornithine. Life Sci. 1978 Jul 10;23(2):93–98. doi: 10.1016/0024-3205(78)90255-2. [DOI] [PubMed] [Google Scholar]
- Suresh M. R., Adiga P. R. Putrescine-sensitive (artifactual) and insensitive (biosynthetic) S-adenosyl-L-methionine decarboxylase activities of Lathyrus sativus seedlings. Eur J Biochem. 1977 Oct 3;79(2):511–518. doi: 10.1111/j.1432-1033.1977.tb11835.x. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]



