Abstract
Objective
Schizophrenia is a serious mental disease that brings not only serious burdens to patients and their families but also serious challenges to society. More research is needed to find better drugs to treat schizophrenia. This meta-analysis investigated the efficacy and safety of sodium nitroprusside in the treatment of schizophrenia.
Methods
Randomized controlled trials comparing the efficacy and safety of sodium nitroprusside in the treatment of schizophrenia were searched via English and Chinese databases. The outcomes, including the Positive and Negative Syndrome Scale (PANSS) and Brief Psychiatric Rating Scale (BPRS), were recorded. RevMan 5.3 was used for the meta-analysis.
Results
A total of six randomized controlled trials (174 patients) were included. The overall quality of the included studies was good. No statistically significant benefit of sodium nitroprusside over placebo was found when combined PANSS total and BPRS-18 (95% CI: −1.40, 0.02). Except for PANSS positive (95% CI: −1.86, −0.01), there was no significant difference in the scale score after sodium nitroprusside treatment compared with the control group in PANSS total (95% CI: −4.93, 0.23), PANSS general (95% CI: −2.53, 1.33), and PANSS negative (95% CI: −4.44, 0.89). The results of the sensitivity analysis excluding the study with clinical heterogeneity showed that sodium nitroprusside had no statistical benefit for the score of PANSS positive (95% CI: −2.19, 0.46). Moreover, there was also no significant difference in the BPRS-18 (95% CI: −3.23, −0.43).
Conclusion
We conservatively believe that sodium nitroprusside does not alleviate the symptoms of schizophrenia compared with placebo. The subjects tolerated sodium nitroprusside well. Our findings provide a new idea for researchers to explore and solve the drug treatment of schizophrenia.
Keywords: sodium nitroprusside, schizophrenia, meta-analysis, Positive and Negative Syndrome Scale, Brief Psychiatric Rating Scale
Introduction
Schizophrenia is a serious mental disease with disorders in perception, thinking, emotion, and behavior that bring not only serious burdens to patients and their families but also serious challenges to society (1). The average lifetime morbid risk of schizophrenia was 11.9 per 1,000, with a slightly higher incidence in men (2). The incidence of the disease is higher in poorer and more dispersed social environments (3). Even worse, independent of familial factors, patients with mental disorders are at increased risk for subsequent complications (4). Thus, there is an urgent need to find effective treatments to improve the symptoms of schizophrenia.
Whether it is a positive or negative symptom of schizophrenia, there are different evaluations of the efficacy of nondrug therapy, and drug therapy is still the main treatment (5). To date, available antipsychotic drugs fail to meet clinical needs in the treatment of negative symptoms and cognitive impairment, and side effects caused by long-term use are gradually being reported (6, 7). Therefore, more research is needed to find better drugs to treat schizophrenia.
Sodium nitroprusside, a nitric oxide donor, is being investigated as a potential antipsychotic drug. The occurrence of schizophrenia is related to dysfunction of the N-methyl-D-aspartate receptor, and the expression level of nitric oxide is decreased (8, 9). In addition, cyclic guanosine monophosphate, one of the intermediate metabolites, is also a key molecule in the development of schizophrenia (9). Interestingly, sodium nitroprusside is an antihypertensive drug that releases blood vessels by releasing carbon monoxide, which might have the ability to regulate N-methyl-D-aspartate and intermediate metabolites (10).
Reports on the effectiveness of sodium nitroprusside have been inconsistent, including two previous randomized controlled trials published in JAMA Psychiatry (11, 12). Given that sodium nitroprusside does have the potential to treat schizophrenia, it is necessary to look for evidence in the treatment of schizophrenia with this drug.
Methods
Eligibility criteria
We used specific methods from our previously published protocol (13). In brief, randomized controlled trials of sodium nitroprusside in patients who were diagnosed with schizophrenia by the “Diagnostic and Statistical Manual of Mental Disorders” or “International Classification of Diseases” were included. Both Chinese and English trials were included, and there were no special requirements for the race, age, or gender of the subjects.
Search strategy
The literature search followed the PRISMA reporting guidelines. We searched randomized controlled trials from English (PubMed, Web of Science, Embase, and Cochrane Library) and Chinese databases (China Biology Medicine disc, VIP, WanFang Data, and China National Knowledge Internet) using the keywords “sodium nitroprusside” and “schizophrenia.” The specific search strategy was listed in our previously published protocol (13). The final search time of all databases was May 21, 2023.
Assessment of methodological quality and data synthesis
The same two investigators (XF and SW) independently extracted the data, and the third investigator (JL) participated in the discussion of conflicting data. The quality and risk of bias of the randomized controlled trials were evaluated according to the bias risk assessment tool of the Cochrane Handbook for Systematic Reviews of Interventions. The outcomes, including the Positive and Negative Syndrome Scale (PANSS) and Brief Psychiatric Rating Scale (BPRS), were synthesized by RevMan 5.3 (Cochrane Collaboration). The test level was set at two-sided α = 0.05. Depending on the heterogeneity of the studies, we used either random- or fixed-effects models. Subgroup analysis and sensitivity analysis were performed.
Results
Literature search
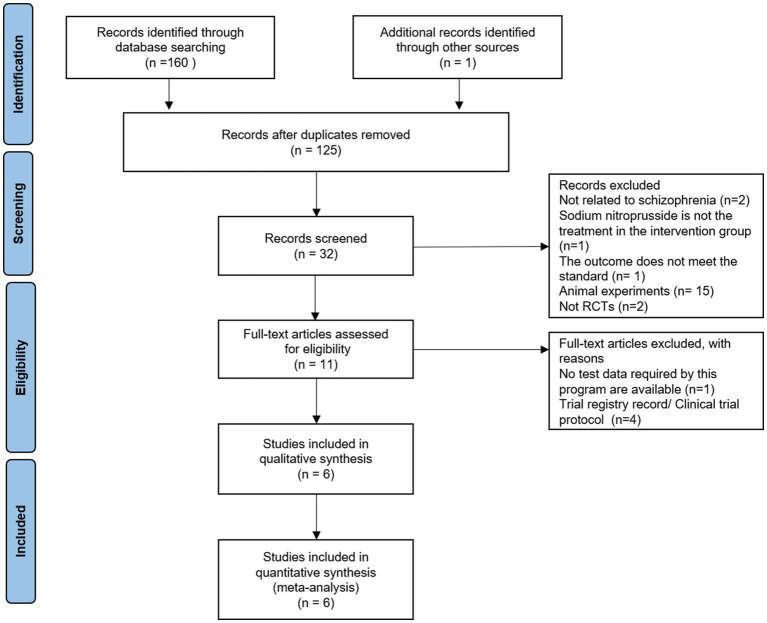
A total of 161 articles were retrieved by searching the above databases. Finally, six randomized controlled trials (n = 174) (11, 12, 14–17) were included (Figure 1). Figure 1 shows the selection process for randomized controlled trials.
Figure 1.
PRISMA flow diagram for the selection process.
The characteristics and quality of the included studies
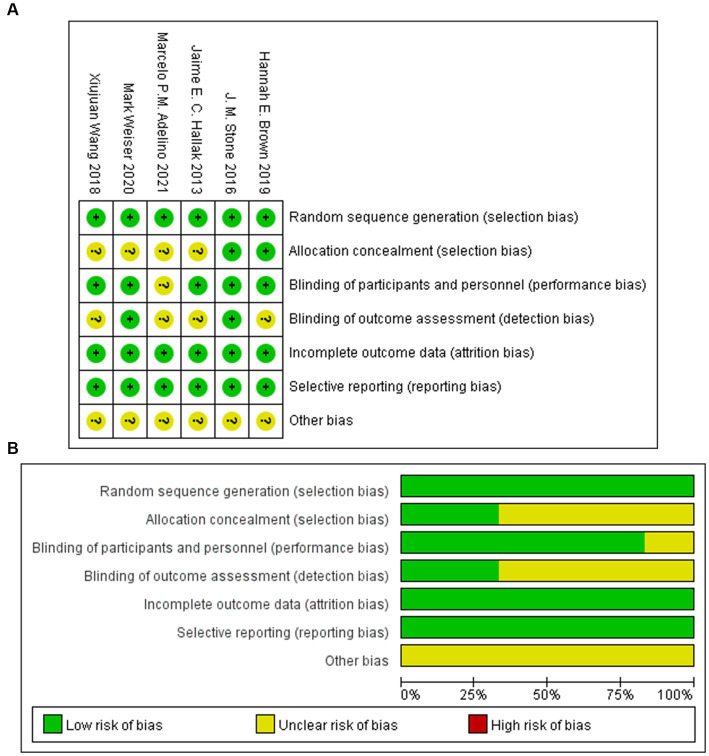
The basic characteristics of the included studies are shown in Table 1. Six randomized controlled trials have compared the efficacy and safety of intravenous sodium nitroprusside with placebo for the treatment of schizophrenia. In all randomized controlled trials, the dosage of sodium nitroprusside was 0.5 μg/(kg·min). All studies selected the PANSS as the primary outcome, and three studies selected the BPRS-18 as a secondary outcome. The six included trials were all double-blind studies, and the overall quality of the included studies was good (Figure 2).
Table 1.
Sodium nitroprusside for schizophrenia: Summary of studies included in this meta-analysis.
| Study | Year | Country | Participants | Female sex | Age | Intravenous infusion [μg/(kg·min)] | Follow-up (weeks) | Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention/ Control | Sodium nitroprusside | Placebo | |||||||
| Hallak et al. | 2013 | Canada | 10/10 | 3/3 | 25.5 ± 6.7/25.6 ± 3.9 | 0.5 | 0.5 | 4 | PANSS; BPRS-18 |
| Stone et al. | 2016 | Britain | 10/10 | 3/2 | 34 ± 9/40 ± 10 | 0.5 | 0.5 | 4 | PANSS; BPRS-18 |
| Wang et al. | 2018 | China | 21/21 | 10/9 | 30.5 ± 7.3/29.4 ± 7.5 | 0.5 | 0.5 | 4 | PANSS |
| Brown et al. | 2019 | United States | 18/34 | 4/8 | 47.1 ± 10.5/43.0 ± 11.78 | 0.5 | 0.5 | 2 | PANSS |
| Weiser et al. | 2020 | Moldova & Romania | 10/10 | 3/7 | 36.1 ± 5.3/34.5 ± 3.6 | 0.5 | 0.5 | 4 | PANSS |
| Adelino et al. | 2021 | Brazil | 10/10 | 11a | 43.4 ± 8.9a | 0.5 | 0.5 | 4 | PANSS; BPRS-18 |
Gender and age in each group were not specified.
Figure 2.
Summary (A) and graph (B) of the risk of bias.
PANSS total and BPRS-18
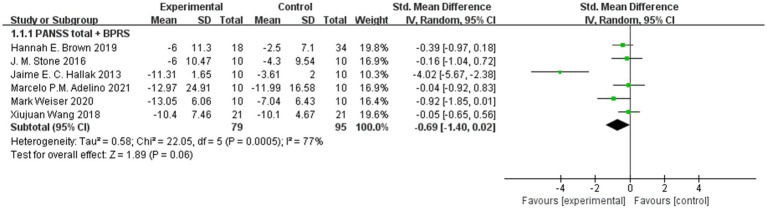
We combined the PANSS total and BPRS-18 scores in 6 trials, and we did not find that sodium nitroprusside had a statistically significant benefit over placebo (95% CI: −1.40, 0.02) (Figure 3). However, we also found that this synthesis led to high heterogeneity (I2 = 77%), so further subgroup analysis and sensitivity analysis were carried out.
Figure 3.
PANSS total and BPRS-18 scores in the comparison of sodium nitroprusside vs. placebo.
PANSS
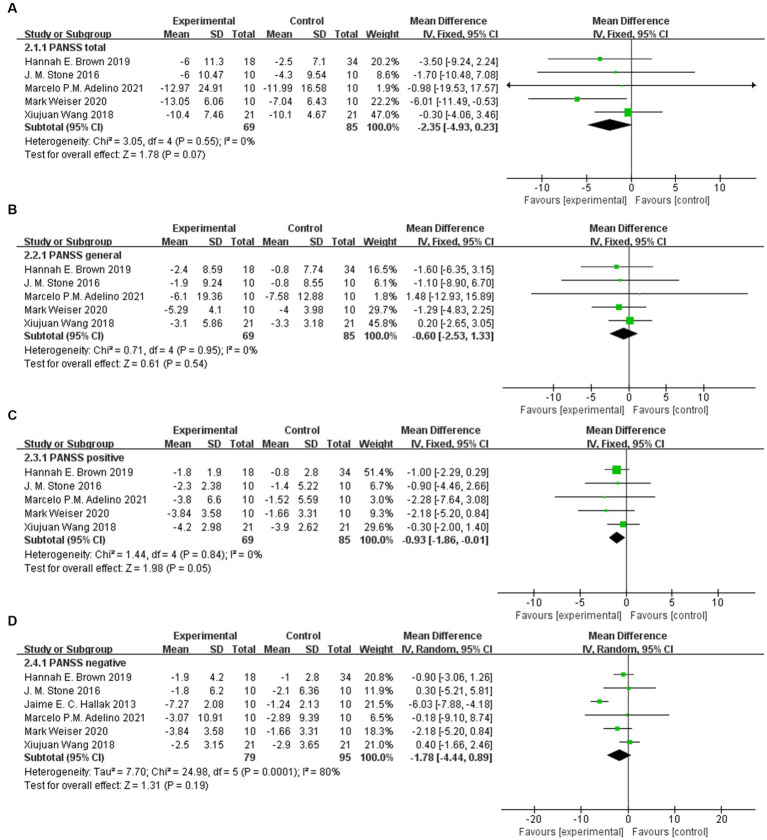
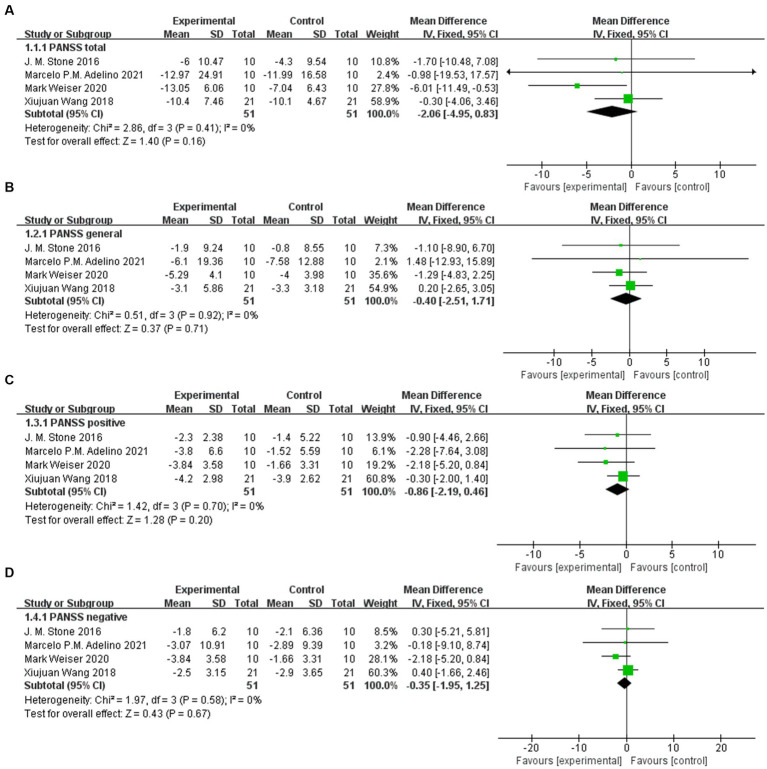
Except for PANSS positive (95% CI: −1.86, −0.01), there was no significant difference in the scale score after sodium nitroprusside treatment compared with the control group in PANSS total (95% CI: −4.93, 0.23), PANSS general (95% CI: −2.53, 1.33), and PANSS negative (95% CI: −4.44, 0.89) (Figures 4A–D).
Figure 4.
PANSS scores in the comparison of sodium nitroprusside vs. placebo. (A) PANSS total. (B) PANSS general. (C) PANSS positive. (D) PANSS negative.
Sensitivity analysis of PANSS
The study of Hallak et al. (12) led to statistical heterogeneity after data consolidation, while the study of Brown et al. (11) had clinical heterogeneity (this randomized controlled trial assessed patients at 2 weeks). Therefore, we excluded these two studies from the additional analysis. The results of sensitivity analysis showed that there was still no significant difference in scale score between the two groups in PANSS total (95% CI: −4.95, 0.83), PANSS general (95% CI: −2.51, 1.71), PANSS positive (95% CI: −2.19, 0.46), and PANSS negative (95% CI: −1.95, 1.25) (Figures 5A–D).
Figure 5.
Sensitivity analysis of PANSS in the comparison of sodium nitroprusside vs. placebo. (A) PANSS total. (B) PANSS general. (C) PANSS positive. (D) PANSS negative.
BPRS-18
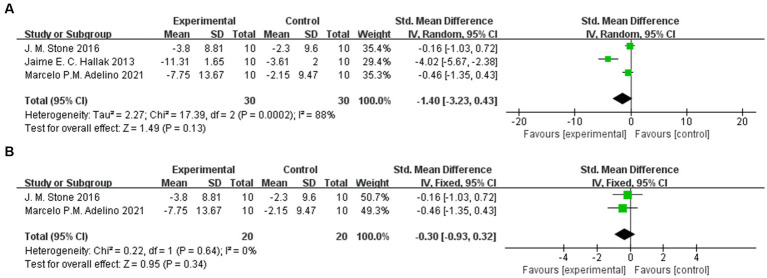
Three trials involving 60 patients were included, with 30 patients in the nitroprusside group and 30 patients in the placebo group. The combined results showed that there was no significant difference in BPRS-18 (95% CI: −3.23, −0.43) (Figure 6A). In the sensitivity analysis, we further eliminated one study that led to increased heterogeneity and finally included 2 studies for meta-analysis. Even so, there was no significant difference in BPRS-18 scores between the two groups (95% CI: −0.93, −0.32) (Figure 6B).
Figure 6.
BPRS-18 scores in the comparison of sodium nitroprusside vs. placebo. (A) BPRS-18. (B) Sensitivity analysis of BPRS-18.
Safety
In terms of safety, no serious adverse events were reported except for muscle spasms, asymptomatic hypotension, and venipuncture pain in some subjects. Most of the adverse events were relieved spontaneously during the intervention. Only one patient withdrew from the trial because of hypotension, and this patient’s data were not included in the final statistics (11).
Discussion
Schizophrenia is an important public health problem, and finding drugs to treat its symptoms, especially negative symptoms, would be very beneficial to improve the quality of life of patients and reduce the burden on society. Schizophrenia is difficult to cure and requires long-term medication. The efficacy, safety, and tolerability of drugs are important factors in drug treatment. Sodium nitroprusside is a rapid, reliable, and easy-to-adjust antihypertensive drug that releases nitric oxide to directly relax arterioles and veins and is safe in clinical practice.
Sodium nitroprusside can eliminate the behavioral effect of phencyclidine and theoretically prevent the progression of mental symptoms (18). A previous review summarized the related mechanisms of sodium nitroprusside in the treatment of schizophrenia, such as the regulation of the NMDA-nNOS-cGMP signaling pathway, the relief of cerebral perfusion deficiency, the normalization of glutamate and dopaminergic neurotransmission function, and the strong antioxidant properties of the drug itself (19). Sodium nitroprusside is useful in treating drug-induced or spontaneous psychiatric symptoms in various preclinical studies. For example, ketamine-induced hyperkinesia was reduced after sodium nitroprusside intervention in rats (20). In addition, Diana et al. (21) found that early sodium nitroprusside treatment prevented behavioral abnormalities such as deficits in situational fear conditioning, hyperactivity, and social isolation in spontaneously hypertensive rats.
More importantly, some clinical studies have reported its efficacy in the treatment of schizophrenia, which is the main reason for our meta-analysis (22, 23). The original research by Hallak et al. (12) found that sodium nitroprusside was able to alleviate schizophrenia symptoms, but subsequent studies were unable to replicate this result. In our study, the initial synthetic results show that sodium nitroprusside is effective in the treatment of positive symptoms in patients with schizophrenia. This result is not consistent with the results of previous studies (17). More importantly, no study has reported that sodium nitroprusside can alleviate the positive symptoms of schizophrenia. Therefore, it is necessary to further analyze the characteristics of the included studies. We found that there was a clinical heterogeneity in the study published by Brown et al. (11) compared with other included studies, so we excluded this trial for additional analysis. Finally, we conservatively believe that both the primary outcome (PANSS) and secondary outcome (BPRS-18) did not change significantly after treatment with sodium nitroprusside.
At present, there are many scales for the evaluation of mental illness worldwide, but there are certain requirements for the selection of scales because of the complex symptoms of schizophrenia (24). The PANSS can evaluate the positive and negative symptoms and general conditions of patients with schizophrenia, which makes up for the shortcomings of poor sensitivity and accuracy of previous rating scales (25). The PANSS includes 7 items of the positive symptom scale, 7 items of the negative symptom scale, and 16 items of the General Psychopathology Scale. The scale assesses the presence and severity of psychiatric symptoms by the score of high or low, and the higher the score, the more serious the symptoms (26–28). Moreover, the BPRS-18 is widely used in clinical practice because of its few evaluation items and simple evaluation (29). Thus, to ensure the accuracy of outcome indicators and wide clinical applicability, the outcomes selected in our study were the PANSS, which has a more comprehensive evaluation range, and the BPRS-18 scale, which is commonly used in clinical practice. Of note, because the PANSS and BPRS-18 are highly correlated, we also combined the PANSS total and BPRS-18 findings before combining those for each scale separately.
Although we obtained a negative result in our study, this is more common in single-center studies than in multicenter trials. In the process of drug development, the probability of positive results in small single-center trials is higher than that in large-scale trials conducted in multiple centers (30). Slightly greater intervention effects have also been reported in single-center trials with continuous outcomes than in multicenter trials (31). The reasons for these results are manifold. First, single-center trials often have difficulty ensuring that enough samples are collected in a short period, which affects the follow-up statistical analysis to a certain extent. Second, some studies assume that the data are normally distributed. Due to the small sample size, it is difficult to ensure that the data completely conform to the normal distribution. Finally, the risk of bias is higher in single-center trials, which tend to include more homogeneous populations and may have more comprehensive research teams than multicenter trials (30, 32). These are important factors that cannot be repeated in the subsequent study, but we must admit that the initial study of Hallak et al. (12) has a great impact on the changes in psychopathology and promotes the development of the whole discipline.
There are some limitations in our study. Some studies did not introduce random and distribution hiding methods, which may have the possibility of bias. In addition, the included studies were only indexed journal articles that had been published and could be retrieved, which may have resulted in publication bias.
Conclusion
In summary, our findings provide a new idea for researchers to explore and solve the drug treatment of schizophrenia. We conservatively believe that sodium nitroprusside does not alleviate the symptoms of schizophrenia compared with placebo. Additionally, the subjects tolerated sodium nitroprusside well. Necessarily, we hope that follow-up studies can provide more and higher quality clinical evidence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XF: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. JL: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SW: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JW: Project administration, Resources, Writing – original draft, Writing – review & editing. CG: Writing – review & editing. RQ: Writing – review & editing. YG: Conceptualization, Project administration, Supervision, Writing – review & editing. YH: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Innovative Entrepreneurship training Program for College students of Southwest Medical University (grant number S2022106 32190 and S2022106 32209).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24. doi: 10.1016/s2215-0366(18)30511-x, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet (London, England). (2022) 399:473–86. doi: 10.1016/s0140-6736(21)01730-x, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Jacinto RP, Ding T, Stafford J, Baio G, Kirkbride JB. The incidence of psychotic disorders in the Republic of Ireland: a systematic review. Ir J Psychol Med. (2023) 31:1–13. doi: 10.1017/ipm.2023.35, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Shen Q, Mikkelsen DH, Luitva LB, Song H, Kasela S, Aspelund T, et al. Psychiatric disorders and subsequent risk of cardiovascular disease: a longitudinal matched cohort study across three countries. EClinicalMedicine. (2023) 61:102063. doi: 10.1016/j.eclinm.2023.102063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fei X, Wang S, Zheng X, Liu K, Liang X. Global research on cognitive behavioural therapy for schizophrenia from 2000 to 2019: a bibliometric analysis via CiteSpace. Gen Psychiatr. (2021) 34:e100327. doi: 10.1136/gpsych-2020-100327, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. (2018) 20:104. doi: 10.1007/s11920-018-0960-5, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Chang CY, Luo DZ, Pei JC, Kuo MC, Hsieh YC, Lai WS. Not just a bystander: the emerging role of astrocytes and research tools in studying cognitive dysfunctions in schizophrenia. Int J Mol Sci. (2021) 22:5343. doi: 10.3390/ijms22105343, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camp CR, Traynelis SF. Additional depth to the NMDA receptor hypofunction and Parvalbumin cell dysfunction hypotheses of schizophrenia. Biol Psychiatry. (2023) 94:283–4. doi: 10.1016/j.biopsych.2023.06.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadeghi MA, Nassireslami E, Yousefi Zoshk M, Hosseini Y, Abbasian K, Chamanara M. Phosphodiesterase inhibitors in psychiatric disorders. Psychopharmacology. (2023) 240, 6:1201–19. doi: 10.1007/s00213-023-06361-3, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Issy AC, Pedrazzi JF, Yoneyama BH, Del-Bel EA. Critical role of nitric oxide in the modulation of prepulse inhibition in Swiss mice. Psychopharmacology. (2014) 231:663–72. doi: 10.1007/s00213-013-3277-4, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Brown HE, Freudenreich O, Fan X, Heard SO, Goff D, Petrides G, et al. Efficacy and tolerability of adjunctive intravenous sodium nitroprusside treatment for outpatients with schizophrenia: a randomized clinical trial. JAMA Psychiatry. (2019) 76:691–9. doi: 10.1001/jamapsychiatry.2019.0151, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallak JE, Maia-de-Oliveira JP, Abrao J, Evora PR, Zuardi AW, Crippa JA, et al. Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: a randomized, double-blind, placebo-controlled trial. JAMA Psychiatry. (2013) 70:668–76. doi: 10.1001/jamapsychiatry.2013.1292, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Fei X, Wang S, Li J, Wang J, Gao Y, Hu Y. The efficacy and safety of sodium nitroprusside in the treatment of schizophrenia: protocol for an updated systematic review and meta-analysis. PLoS One. (2023) 18:e0283185. doi: 10.1371/journal.pone.0283185, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhao J, Hu Y, Jiao Z, Lu Y, Ding M, et al. Sodium nitroprusside treatment for psychotic symptoms and cognitive deficits of schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychiatry Res. (2018) 269:271–7. doi: 10.1016/j.psychres.2018.08.079, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Stone JM, Morrison PD, Koychev I, Gao F, Reilly TJ, Kolanko M, et al. The effect of sodium nitroprusside on psychotic symptoms and spatial working memory in patients with schizophrenia: a randomized, double-blind, placebo-controlled trial. Psychol Med. (2016) 46:3443–50. doi: 10.1017/s0033291716002245, PMID: [DOI] [PubMed] [Google Scholar]
- 16.Adelino MPM, Nunes MV, Nunes MFQ, Costa ER, Jr, Ajub E, Mitrovitch MPB, et al. Treatment-resistant schizophrenia - a RCT on the effectiveness of repeated-dose sodium nitroprusside. Schizophr Res. (2021) 231:70–2. doi: 10.1016/j.schres.2021.03.005, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Weiser M, Zamora D, Levi L, Matei V, Gonen I, Radu P, et al. Sodium nitroprusside infusion for the treatment of schizophrenia. Schizophr Bull. (2020) 1:sgaa047. doi: 10.1093/schizbullopen/sgaa047 [DOI] [Google Scholar]
- 18.Smith JB, Ogonowski AA. Behavioral effects of NMDA receptor agonists and antagonists in combination with nitric oxide-related compounds. Eur J Pharmacol. (2003) 471:121–8. doi: 10.1016/s0014-2999(03)01820-x, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Zoupa E, Pitsikas N. The nitric oxide (NO) donor sodium nitroprusside (SNP) and its potential for the schizophrenia therapy: lights and shadows. Molecules (Basel, Switzerland). (2021) 26:3196. doi: 10.3390/molecules26113196, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandratavicius L, Balista PA, Wolf DC, Abrao J, Evora PR, Rodrigues AJ, et al. Effects of nitric oxide-related compounds in the acute ketamine animal model of schizophrenia. BMC Neurosci. (2015) 16:9. doi: 10.1186/s12868-015-0149-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diana MC, Peres FF, Justi V, Bressan RA, Lacerda ALT, Crippa JA, et al. Sodium nitroprusside is effective in preventing and/or reversing the development of schizophrenia-related behaviors in an animal model: the SHR strain. CNS Neurosci Ther. (2018) 24:624–32. doi: 10.1111/cns.12852, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maia-de-Oliveira JP, Belmonte-de-Abreu P, Bressan RA, Cachoeira C, Baker GB, Dursun SM, et al. Sodium nitroprusside treatment of clozapine-refractory schizophrenia. J Clin Psychopharmacol. (2014) 34:761–3. doi: 10.1097/jcp.0000000000000217, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Maia-de-Oliveira JP, Baker GB, Dursun SM, Hallak JE. Letter to the editor: sodium nitroprusside for schizophrenia: could methodological variables account for the different results obtained? Psychol Med. (2017) 47:981–2. doi: 10.1017/s003329171600307x, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Kirkpatrick B, Luther L, Strauss GP. Negative symptoms in the clinic: we treat what we can describe. Br J Psychiatry. (2023) 223:271–2. doi: 10.1192/bjp.2023.68, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang MW, Gibson RC, Jayaram MB, Caroff SN. Antipsychotics for schizophrenia spectrum disorders with catatonic symptoms. Cochrane Database Syst Rev. (2022) 7:Cd013100. doi: 10.1002/14651858.CD013100, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleischhacker W, Galderisi S, Laszlovszky I, Szatmári B, Barabássy Á, Acsai K, et al. The efficacy of cariprazine in negative symptoms of schizophrenia: post hoc analyses of PANSS individual items and PANSS-derived factors. European psychiatry. Eur Psychiatry. (2019) 58:1–9. doi: 10.1016/j.eurpsy.2019.01.015, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr Res. (2005) 79:231–8. doi: 10.1016/j.schres.2005.04.008, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Shafer A, Dazzi F. Meta-analytic exploration of the joint factors of the brief psychiatric rating scale - expanded (BPRS-E) and the positive and negative symptoms scales (PANSS). J Psychiatr Res. (2021) 138:519–27. doi: 10.1016/j.jpsychires.2021.04.016, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Unverzagt S, Prondzinsky R, Peinemann F. Single-center trials tend to provide larger treatment effects than multicenter trials: a systematic review. J Clin Epidemiol. (2013) 66:1271–80. doi: 10.1016/j.jclinepi.2013.05.016, PMID: [DOI] [PubMed] [Google Scholar]
- 31.Bafeta A, Dechartres A, Trinquart L, Yavchitz A, Boutron I, Ravaud P. Impact of single Centre status on estimates of intervention effects in trials with continuous outcomes: meta-epidemiological study. BMJ. (2012) 344:e813. doi: 10.1136/bmj.e813, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. (2011) 155:39–51. doi: 10.7326/0003-4819-155-1-201107050-00006, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.