STRUCTURED ABSTRACT
Objective:
Our understanding of the impact of a center’s case volume on failure to rescue (FTR) after cardiac surgery is incomplete. We hypothesized that increasing center case volume would be associated with lower FTR.
Methods:
Patients undergoing an STS index operation in a regional collaborative (2011-2021) were included. After excluding patients with missing STS predicted risk of mortality, patients were stratified by mean annual center case volume. The lowest quartile of case volume was compared to all other patients. Logistic regression analyzed the association between center case volume and FTR, adjusting for patient demographics, race, insurance, comorbidities, procedure type, and year.
Results:
A total of 43,641 patients were included across 17 centers during the study period. Of these, 5315 (12.2%) developed an FTR complication, and 735 (13.8% of those who developed an FTR complication) experienced FTR. Median annual case volume was 226, with 25th and 75th-percentile cutoffs of 136 and 284 cases, respectively. Increasing center-level case volume was associated with significantly higher center-level major complication rates, but lower mortality and FTR rates (all p-values < 0.01). Observed to expected FTR was significantly associated with case volume (p = 0.040). Increasing case volume was independently associated with decreasing FTR rate in the final multivariable model (OR 0.87 per quartile, CI 0.80 – 0.95, p = 0.001).
Conclusions:
Increasing center case volume is significantly associated with improved failure to rescue rates. Assessment of low volume centers’ FTR performance represents an opportunity for quality improvement.
Keywords: critical care, Quality, Failure to Rescue
INTRODUCTION
Increasing center-level case volume has been associated with improved outcomes following cardiac surgery, including lower resource utilization as well as lower rates of post-operative complications, mortality, and failure to rescue (FTR).[1-8] These findings provide the foundation for the ongoing debate within cardiothoracic surgery regarding the potential benefit of regionalization, and also are used as justification for increasing the use of high-fidelity simulation for both trainees and surgical teams. [9-14]
The Society of Thoracic Surgeons’ (STS) recently published their plan to incorporate FTR as a quality measure within the adult cardiac surgery database. [15] The STS defines FTR as operative mortality after one or more of the following complications: prolonged ventilation, renal failure, reoperation, or permanent stroke. While prior studies have identified a significant association between center-level case volume and FTR after cardiac surgery, many of these relied on administrative claims data. [1] Edwards et al. investigated the relationship of a similar definition of FTR among a national cohort of cardiac surgery patients from the STS ACSD – however, their analysis was limited to those undergoing isolated CABG, and also did not provide stratification of patient-level characteristics nor outcomes (beyond mortality, FTR, and composite rate of any complication[s]) by volume. [16] Given this definition will soon be employed to assess center-level quality, a more granular understanding of how STS-defined FTR is associated with center-level case volume – and the potential opportunities for improvement it may provide – is needed.
The objective of this study was to evaluate the effect of center-level case volume on failure to rescue following cardiac surgery. We hypothesized that increase center-level case volume would be independently associated with risk-adjusted odds of failure to rescue.
METHODS
Patients and Data
The Virginia Cardiac Services Quality Initiative (VCSQI) includes 17 hospitals and surgical groups in Virginia. VCSQI data include 99% of all adult cardiac surgery in the region. Clinical data [17] and cost methodology [18] have been described previously. STS data from individual centers are compiled in a central registry. This study was exempt from review by the University of Virginia’s Institutional Review Board due to the de-identified nature of the quality database (Protocol #23305).
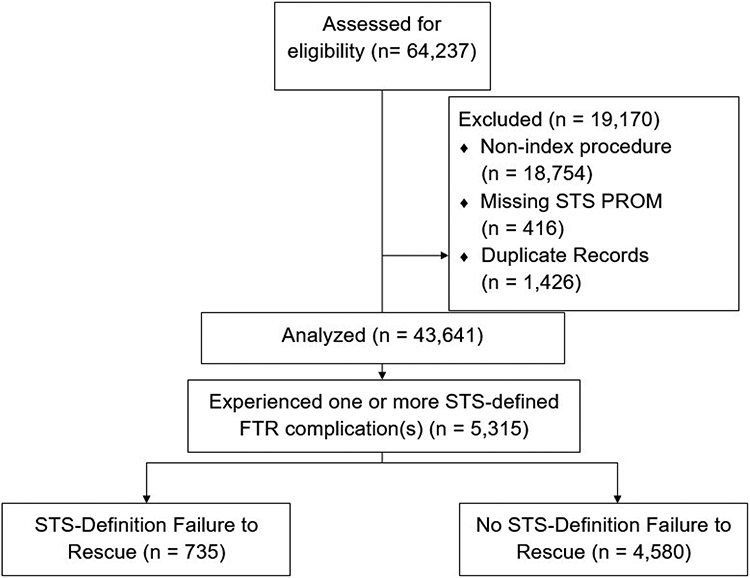
All patients undergoing a STS index operation (CABG, AVR, MVR/r) between July 2011 and July 2021 were extracted from the VCSQI database. Patients were excluded if they were missing STS predicted risk of mortality, or underwent a non-index procedure (Figure 1).
Figure 1.
CONSORT Diagram
Standard STS definitions were used for all variables [19]. Operative mortality is defined as either 30-day or in-hospital mortality. Failure to rescue is defined as operative mortality after an STS-defined FTR complication (prolonged ventilation, post-operative renal failure requiring dialysis, reoperation, and stroke)[15].
Statistical Analysis
Center-level case volume was defined as the annual mean number of all STS index cases performed by an individual center, and was computed using data from January 1st, 2012 through December 31st, 2020. Patients were stratified by center-level case volume quartile. Center-level case volume quartile was defined at the hospital-level (i.e., quartiles are defined to contain approximately the same number of hospitals, not necessarily the same number of patients). The lowest volume quartile patients were established as a reference to which the other three quartiles of patients were compared for the multivariable model; univariate patient characteristics and outcomes were analyzed by increasing center-level case volume quartile (ordinal variable). Categorical variables are presented as counts (%) and continuous variables are presented as median (interquartile range) due to skewed distributions. Wilcoxon rank sum test was used for non-normal distributed continuous variables and the χ2 test for all categorical variables. Logistic regression modeled the association between center-level case volume quartile and failure to rescue, adjusting for patient demographics, comorbid conditions, and operative characteristics. Variables utilized in the final multivariable model were selected based on clinical importance and statistical significance in univariate analyses. All statistical analyses were carried out using SAS Version 9.4 (SAS Institute, Cary, NC) with a p-value less than 0.05 determining significance.
RESULTS
Patient Characteristics
A total of 43,641 patients were included for analysis. Overall rate of STS-defined FTR complications and mortality in this cohort were 12.2% (5,315) and 2.33% (1,017), respectively. Of the 17 centers included, median center-level case volume was 226, with 25th and 75th-percentile cutoffs of 136 and 284 cases, respectively. Minimum annual volume was 79 and maximum was 682. These data informed the creation of center-level case volume quartiles (1st quartile: low volume, 2nd quartile: medium volume, 3rd quartile: high volume, 4th quartile: very high volume). Five centers were placed into the 1st quartile, with the remaining 12 centers equally divided among the remaining three quartiles (i.e., 4 per quartile). Patient characteristics of those who developed one or more STS-defined FTR complications by center-level case volume quartile can be found in Table 1. Increasing center-level case volume quartile was significantly associated with decreasing patient age, non-white race, elective status, absence of health insurance, reoperative surgery, non-CABG procedure type, increasing cardiopulmonary bypass time, increasing intraoperative blood product use and increasing STS predicted risk of mortality (all p-values < 0.05; Table 1).
Table 1.
Patient characteristics among those who developed one or more STS-defined FTR complications, by center-level case volume quartile.
| Characteristic | Low Volume (n = 468, 8.81%, n centers = 5) |
Medium Volume (n = 857, 16.1%, n centers = 4) |
High Volume (n = 1269, 23.9%, n centers = 4) |
Very High Volume (n = 2721, 51.2%, n centers = 4) |
p-value |
|---|---|---|---|---|---|
| Age | 69 (61 – 75) | 68 (60 – 75) | 67 (60 – 73) | 67 (60 -74) | 0.050 |
| Female Sex | 161 (34.4) | 299 (34.9) | 480 (37.8) | 942 (34.6) | 0.232 |
| White Race | 370 (79.1) | 697 (81.3) | 841 (66.3) | 2045 (75.2) | <0.001 |
| ESRD | 19 (4.06) | 35 (4.08) | 105 (8.27) | 138 (5.07) | <0.001 |
| Pre-operative creatinine level | 1.02 (0.815 - 1.34) | 1.03 (0.83 – 1.32) | 1.10 (0.90 – 1.50) | 1.00 (0.80 – 1.40) | < 0.001 |
| Pre-operative Hemoglobin level | 12.6 (11.1 – 14.0) | 12.7 (11.1 – 14.0) | 12.3 (10.7 – 13.7) | 12.6 (11.0 – 13.9) | < 0.001 |
| Pre-operative white blood cell count | 8.00 (6.50 – 9.90) | 7.92 (6.40 – 10.0) | 8.10 (6.40 – 10.2) | 8.00 (6.50 – 10.0) | 0.741 |
| Pre-operative total albumin | 3.60 (3.20 – 3.80) | 3.60 (3.10 – 3.80) | 3.50 (3.10 – 3.80) | 3.80 (3.40 – 4.10) | <0.001 |
| Chronic Lung Disease | 173 (37.0) | 298 (34.8) | 556 (43.8) | 987 (36.3) | <0.001 |
| MELD | 7.47 (6.40 - 10.0) | 7.57 (7.31 - 10.3) | 8.64 (7.47 – 12.2) | 8.15 (7.31 – 11.0) | <0.001 |
| Liver Disease | 30 (6.41) | 37 (4.32) | 78 (6.15) | 177 (6.50) | 0.306 |
| Oxygen-dependent lung disease | 7 (1.50) | 5 (0.58) | 16 (1.26) | 15 (0.55) | 0.032 |
| Obstructive sleep apnea | 73 (15.6) | 133 (15.5) | 261 (20.6) | 491 (18.0) | 0.011 |
| Current Tobacco Use | 118 (25.2) | 211 (24.6) | 304 (24.0) | 504 (18.5) | <0.001 |
| Cerebrovascular Disease | 150 (32.1) | 238 (27.8) | 340 (26.8) | 661 (24.3) | < 0.001 |
| Prior Stroke | 72 (15.4) | 104 (12.1) | 170 (13.4) | 342 (12.6) | 0.316 |
| Hypertension | 419 (89.5) | 747 (87.2) | 1151 (90.7) | 2325 (85.5) | <0.001 |
| Peripheral Arterial Disease | 90 (19.2) | 155 (18.1) | 239 (18.8) | 458 (16.8) | 0.339 |
| Diabetes Mellitus | 240 (51.3) | 391 (45.6) | 660 (52.0) | 1273 (46.8) | 0.004 |
| Immunocompromise | 30 (6.41) | 38 (4.43) | 79 (6.23) | 125 (4.59) | 0.066 |
| Pre-operative Arrhythmia | 61 (13.0) | 121 (14.1) | 215 (16.9) | 427 (15.7) | 0.037 |
| CHF | 229 (48.9) | 274 (32.0) | 710 (56.0) | 1333 (49.0) | <0.001 |
| Prior MI | 295 (63.0) | 453 (52.9) | 688 (54.2) | 1390 (51.1) | <0.001 |
| Body Surface Area | 2.87 (2.71 – 2.98) | 2.87 (2.72 – 3.00) | 2.83 (2.71 – 2.97) | 2.87 (2.72 – 2.97) | 0.126 |
| Ejection Fraction | 55.0 (40.0 – 60.0) | 55.0 (43.0 - 60.0) | 50.0 (35.0 – 60.0) | 55.0 (40.0 – 60.0) | <0.001 |
| Prior PCI | 133 (28.4) | 238 (27.8) | 340 (26.8) | 676 (24.8) | 0.166 |
| Prior CABG | 13 (2.78) | 24 (2.80) | 46 (3.62) | 140 (5.15) | 0.003 |
| Prior Valve | 8 (1.71) | 21 (2.45) | 60 (4.73) | 158 (5.81) | <0.001 |
| Health Insurance | 441 (94.2) | 802 (93.6) | 1064 (83.9) | 2562 (94.2) | < 0.001 |
| First Cardiac Surgery | 453 (96.8) | 821 (95.8) | 1181 (93.1) | 2447 (89.9) | <0.001 |
| Status | <0.001 | ||||
| Elective | 139 (29.7) | 308 (35.9) | 407 (32.1) | 1048 (38.5) | |
| Urgent | 271 (57.9) | 486 (56.7) | 761 (60.0) | 1382 (50.8) | |
| Emergent | 57 (12.2) | 55 (6.42) | 87 (6.86) | 264 (9.70) | |
| Emergent Salvage | 1 (0.21) | 8 (0.93) | 14 (1.10) | 27 (0.99) | |
| Pre-operative Intraaortic balloon pump | 132 (28.2) | 204 (23.8) | 325 (25.6) | 644 (23.7) | 0.136 |
| Procedure Type | <0.001 | ||||
| Isolated CABG | 333 (71.2) | 566 (66.0) | 788 (62.1) | 1547 (56.9) | |
| Isolated AVR | 34 (7.26) | 74 (8.63) | 114 (8.98) | 295 (10.8) | |
| AVR + CABG | 39 (8.33) | 98 (11.4) | 115 (9.06) | 269 (9.89) | |
| MV Repair | 15 (3.21) | 21 (2.45) | 47 (3.70) | 140 (5.15) | |
| MV Repair + CABG | 17 (3.63) | 25 (2.92) | 64 (5.04) | 120 (4.41) | |
| MV Replacement + CABG | 16 (3.42) | 36 (4.20) | 44 (3.47) | 84 (3.09) | |
| MV Replacement | 14 (2.99) | 37 (4.32) | 97 (7.64) | 266 (9.78) | |
| Aortic Stenosis | 82 (17.5) | 188 (21.9) | 239 (18.8) | 543 (20.0) | 0.188 |
| Severe Aortic Regurgitation | 11 (2.35) | 28 (3.27) | 32 (2.68) | 94 (3.45) | 0.035 |
| Mitral Stenosis | 11 (2.35) | 29 (3.38) | 53 (4.18) | 150 (5.51) | 0.003 |
| Severe Mitral Regurgitation | 54 (11.5) | 92 (10.7) | 187 (14.7) | 439 (16.1) | <0.001 |
| Tricuspid Stenosis | 2 (0.43) | 2 (0.23) | 0 (0.00) | 9 (0.33) | 0.205 |
| Severe Tricuspid Regurgitation | 6 (1.28) | 10 (1.17) | 34 (2.68) | 73 (2.68) | <0.001 |
| Cross clamp time | 75.0 (57.0 - 103) | 86.0 (67.0 - 116) | 83.0 (71.0 – 115) | 78.0 (61.0 – 104) | <0.001 |
| Cardiopulmonary bypass time | 103 (80.5 – 153) | 120 (95.0 - 159) | 120 (92.0 - 165) | 112 (86.0 – 151) | < 0.001 |
| Intraoperative blood product | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) | <0.001 |
| STS Predicted Risk of Mortality | 2.45 (1.13 – 5.98) | 2.44 (1.22-5.08) | 3.19 (1.45 – 7.43) | 2.81 (1.31 – 5.91) | <0.001 |
Q1: Centers with annual median case volume ranging from 79-136 cases, Q2: >136 - 226 cases, Q3: >226-284 cases, Q4: >284 -682 cases.
Among patients with one or more STS-defined FTR complications, 13.8% (735) experienced failure to rescue. Patient characteristics of those who developed one or more STS-defined FTR complications by failure to rescue status can be found in Table 2. Failure to rescue was significantly associated with increasing STS predicted risk of mortality (5.08% vs. 2.56%, p < 0.001), cardiopulmonary bypass time (126 minutes vs. 114 minutes, p < 0.001), intraoperative blood product use (1 unit vs 0 units, p < 0.001), patient age (71 years vs. 67 years, p < 0.001) as well as increased incidence of pre-operative intraaortic balloon pump use (34.8% vs. 22.9%, p < 0.001), non-elective status (p < 0.001), and female sex (42.0% vs. 34.3%, p < 0.001).
Table 2.
Patient characteristics by failure to rescue
| Characteristic | No failure to rescue (n = 4580, 86.2%) |
Failure to rescue (n= =735, 13.8%) |
p-value |
|---|---|---|---|
| Age | 67.0 (59.0 – 74.0) | 71.0 (63.0 – 78.0) | <0.001 |
| Female Sex | 1537 (34.3) | 309 (42.0) | <0.001 |
| White Race | 3389 (74.0) | 564 (76.7) | 0.290 |
| ESRD | 234 (5.11) | 63 (8.57) | <0.001 |
| Pre-operative creatinine level | 1.03 (0.81 – 1.40) | 1.12 (0.90 - 1.50) | <0.001 |
| Pre-operative Hemoglobin level | 12.6 (11.0 - 13.9) | 11.9 (10.3 – 13.4) | <0.001 |
| Pre-operative white blood cell count | 8.00 (6.50 – 9.90) | 8.40 (6.50 – 11.1) | <0.001 |
| Pre-operative total albumin | 3.70 (3.30 – 4.00) | 3.60 (3.00 – 3.90) | <0.001 |
| Chronic Lung Disease | 1712 (37.4) | 302 (41.1) | 0.054 |
| MELD | 7.94 (7.31 – 10.7) | 9.22 (7.47 – 13.3) | < 0.001 |
| Liver Disease | 261 (5.70) | 61 (8.30) | 0.021 |
| Oxygen-dependent lung disease | 32 (0.70) | 11 (1.50) | 0.025 |
| Obstructive sleep apnea | 843 (18.4) | 115 (15.7) | 0.071 |
| Current Tobacco Use | 1001 (21.9) | 136 (18.5) | 0.012 |
| Cerebrovascular Disease | 1160 (25.3) | 229 (31.2) | < 0.001 |
| Prior Stroke | 578 (12.6) | 110 (15.0) | 0.079 |
| Hypertension | 3987 (87.0) | 655 (89.1) | 0.118 |
| Peripheral Arterial Disease | 750 (16.4) | 192 (26.1) | <0.001 |
| Diabetes Mellitus | 2205 (48.1) | 359 (48.8) | 0.725 |
| Immunocompromise | 214 (4.67) | 58 (7.89) | <0.001 |
| Pre-operative Arrhythmia | 676 (14.7) | 148 (20.1) | <0.001 |
| CHF | 2137 (46.7) | 409 (55.7) | <0.001 |
| Prior MI | 2385 (52.1) | 441 (60.0) | <0.001 |
| Body Surface Area | 2.87 (2.71 – 2.97) | 2.83 (2.67 - 2.97) | <0.001 |
| Ejection Fraction | 55.0 (40.0 – 60.0) | 53.0 (35.0 – 60.0) | 0.029 |
| Prior PCI | 1172 (25.6) | 215 (29.3) | 0.036 |
| Prior CABG | 178 (3.89) | 45 (6.12) | 0.005 |
| Prior Valve | 208 (4.54) | 39 (5.31) | 0.361 |
| Health Insurance | 4190 (91.5) | 679 (92.4) | 0.416 |
| First Cardiac Surgery | 4231 (92.4) | 671 (91.3) | 0.112 |
| Status | <0.001 | ||
| Elective | 1682 (36.7) | 220 (29.9) | |
| Urgent | 2493 (54.4) | 407 (55.4) | |
| Emergent | 371 (8.10) | 92 (12.5) | |
| Emergent Salvage | 34 (0.74) | 16 (2.18) | |
| Pre-operative Intraaortic balloon pump | 1049 (22.9) | 256 (34.8) | <0.001 |
| Procedure Type | 0.007 | ||
| Isolated CABG | 2812 (61.4) | 422 (57.4) | |
| Isolated AVR | 460 (10.0) | 57 (7.76) | |
| AVR + CABG | 443 (9.67) | 78 (10.6) | |
| MV Repair | 190 (4.15) | 33 (5.17) | |
| MV Repair + CABG | 188 (4.10) | 38 (5.17) | |
| MV Replacement + CABG | 143 (3.12) | 37 (5.03) | |
| MV Replacement | 344 (7.51) | 70 (9.52) | |
| Aortic Stenosis | 893 (19.5) | 159 (21.6) | 0.178 |
| Severe Aortic Regurgitation | 146 (3.19) | 21 (2.86) | 0.642 |
| Mitral Stenosis | 189 (4.13) | 54 (7.35) | <0.001 |
| Severe Mitral Regurgitation | 643 (14.0) | 129 (17.6) | <0.001 |
| Tricuspid Stenosis | 12 (0.26) | 1 (0.14) | 0.521 |
| Tricuspid Regurgitation | 92 (2.01) | 31 (4.22) | <0.001 |
| Cross clamp time | 80.0 (63.0 – 106) | 84.0 (66.0 – 119) | 0.001 |
| Cardiopulmonary bypass time | 114 (88.0 - 153) | 126 (95.0 – 178) | <0.001 |
| Intraoperative blood product | 0 (0 – 2.00) | 1 (0 – 3.00) | <0.001 |
| STS Predicted Risk of Mortality | 2.56 (1.24 – 5.43) | 5.08 (2.22 – 12.4) | <0.001 |
Univariate Outcomes by Center-Level Case Volume
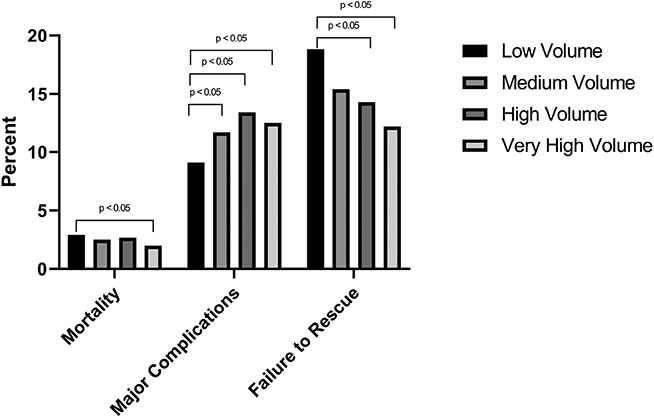
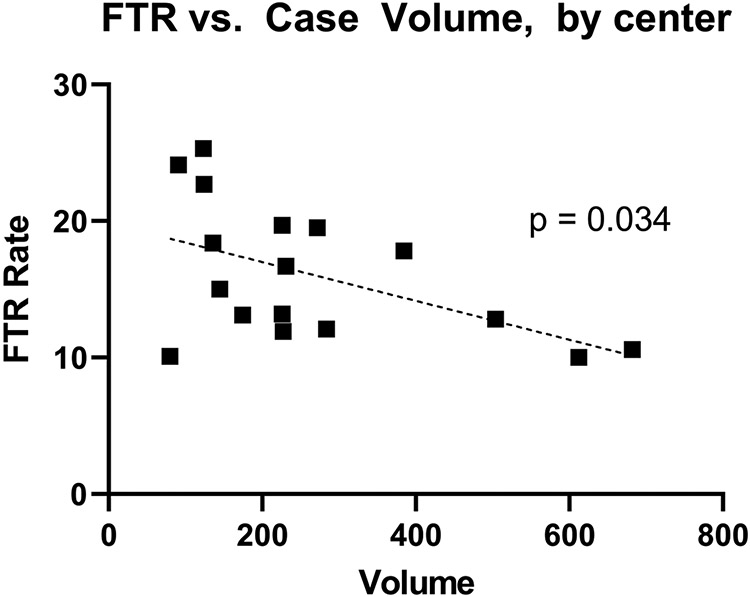
Univariate analysis of outcomes by center-level case volume quartile are found in Table 3. All four STS-defined FTR complications (post-operative renal failure, permanent stroke, prolonged ventilation, and reoperation) were significantly associated with center-level case volume quartile, although a clear trend of incidence of these complications by center-level case volume quartile was not present (all p-values < 0.05). STS-defined failure to rescue was significantly associated with center-level case volume quartile (p < 0.001) and clearly decreased as center-level case volume quartile increased (Low volume: 18.8% FTR; Very High Volume: 12.2% FTR). Figure 2 illustrates the rate of mortality, STS-defined FTR complications and failure to rescue, by center-level case volume quartile. FTR rates were significantly lower among high and very high volume centers, relative to low volume centers (p < 0.05). Centers’ individual FTR rates and case volume were plotted against one another in Figure 3, which demonstrates a significant, inverse relationship between increasing case volume and FTR rate (p = 0.034).
Table 3.
Rate of complications and failure to rescue by center-level case volume quartile, among patients with one or more STS-defined FTR complication
| Complication | Low Volume (n = 468, 8.81%) |
Medium Volume (n = 857, 16.1%) |
High Volume (n = 1269, 23.9%) |
Very High Volume (n = 2721, 51.2%) |
p-value |
|---|---|---|---|---|---|
| Post-operative dialysis requirement | 107 (22.9) | 205 (23.9) | 301 (23.7) | 552 (20.3) | 0.031 |
| Permanent Stroke | 51 (10.9) | 115 (13.4) | 115 (9.06) | 289 (10.6) | 0.017 |
| Prolonged Ventilation | 329 (70.3) | 625 (72.9) | 983 (77.5) | 2061 (75.7) | 0.006 |
| Reoperation | 117 (25.0) | 180 (21.0) | 231 (18.2) | 647 (23.8) | <0.001 |
| STS-Defined Failure to Rescue | 88 (18.8) | 132 (15.4) | 182 (14.3) | 333 (12.2) | <0.001 |
| Readmission | 59 (12.6) | 92 (10.7) | 151 (11.9) | 279 (10.3) | 0.274 |
| Atrial Fibrillation | 184 (39.3) | 367 (42.8) | 470 (37.0) | 996 (36.6) | 0.009 |
| Cardiac Arrest | 88 (18.8) | 130 (15.2) | 152 (12.0) | 277 (10.2) | <0.001 |
| Pneumonia | 72 (15.4) | 161 (18.8) | 145 (11.4) | 367 (13.5) | <0.001 |
| Home Discharge | 182 (38.9) | 342 (39.9) | 522 (41.1) | 1251 (46.0) | <0.001 |
| DVT/PE | 20 (4.27) | 25 (2.92) | 34 (2.68) | 213 (7.83) | <0.001 |
| Surgical Site Infection | 15 (3.21) | 28 (3.27) | 31 (2.44) | 56 (2.06) | 0.153 |
| ICU Length of Stay, Hours | 154 (94 – 281) | 165 (93 – 301) | 167 (96 – 317) | 161 (94 – 309) | 0.062 |
| Post-surgical Length of Stay, Days | 10 (7-17) | 11 (8 – 19) | 13 (8 -21) | 13 (8-21) | <0.001 |
Figure 2.
Rate of Mortality, Major Complications, and Failure to Rescue, by Center-Level Case Volume Quartile
Figure 3.
Rate of Failure to Rescue by Center-level Case Volume. Dotted Line corresponds to single linear regression. Each square corresponds to a single center.
Table 4 demonstrates observed to expected STS-defined FTR by center-level case volume quartile. A significant trend (p = 0.04) between increasing case volume quartile and decrease O:E FTR was observed.
Table 4.
Observed to expected FTR by center case volume quartile
| Center Case Volume Quartile |
Observed : Expected FTR | p-trend |
|---|---|---|
| Low Volume | 7.61 (18.8/2.5) | 0.04 |
| Medium Volume | 6.31 (15.4/2.4) | |
| High Volume | 4.48 (14.3/3.2) | |
| Very High Volume | 4.34 (12.2/2.8) |
Multivariable Logistic Regression
The full results of our multivariable logistic regression can be found in Supplemental Table 1. After adjustment, increasing center-level case volume remained significantly associated with decreased odds of FTR (OR 0.87, CI 0.799-0.946, p = 0.001; Figure 4). Other significant co-variates in the final multivariable model included age (OR 1.04, CI 1.03 – 1.05, p < 0.001), female sex (OR 1.29, CI 1.02 – 1.63, p = 0.034), and cardiopulmonary bypass time (OR 1.007, CI 1.005 – 1.010, p < 0.001). An identical multivariable logistic regression with center-level case volume modeled in its natural, continuous form yielded qualitatively similar results.
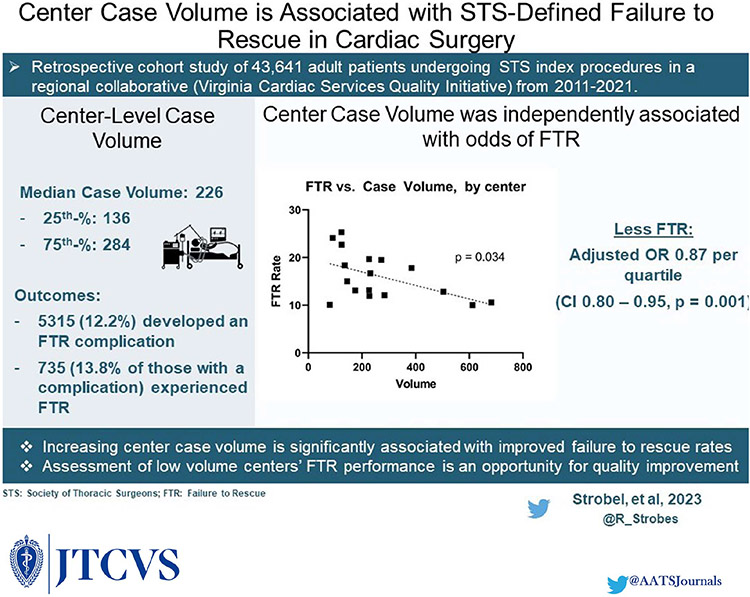
Figure 4.
Graphical Abstract
Effect Modification Analysis
Based on the adjusted association of female sex with increased odds of FTR observed in our final multivariable model, we also undertook an effect modification analysis investigating whether patient sex modifies the relationship of center-level case volume with FTR (Female Sex * Center-level Case Volume). Significant effect modification was observed (estimate: 0.085; p = 0.033), with the magnitude and directionality of the effect modification substantially reducing the beneficial impact of increasing center-level case volume among female patients.
DISCUSSION
In this regional prospective cohort study, increasing center-level case volume was significantly associated with lower failure to rescue rates after cardiac surgery. Prior to adjustment, increasing center-level case volume was associated with significantly greater major complication rates, but significantly lower operative mortality and failure to rescue rates. After adjustment, increasing case volume was independently associated with decreasing odds of FTR. Observed to expected FTR was significantly associated with center-level case volume.
FTR is a well-recognized predictor of interhospital variability in mortality. Previous studies have demonstrated inconsistent associations between hospital volume and major complication rates after high-risk procedures.[1-4] However, there is a growing body of evidence indicating that increasing center case volume is significantly associated with lower FTR and mortality rates. [4-8] The primary objective of this study was to assess the relationship between hospital volume and FTR after cardiac surgery, with the relationship between hospital volume and post-operative complication rates as a secondary endpoint. We found that increasing center case volume is significantly associated with higher center-level complication rates, but lower mortality and FTR rates. The ratio of observed to expected FTR for hospitals in the lowest volume quartile was 1.69 times that of hospitals in the highest volume quartile (p-trend = 0.04).
In their foundational analysis, Gonzalez and colleagues examined the underlying mechanisms of the volume-outcome relationship in cardiac surgery by analyzing the outcomes of 119,434 Medicare beneficiaries undergoing coronary artery bypass grafting, aortic valve repair, or abdominal aortic aneurysm repair between 2005 and 2006.[1] Similarly, our study included patients undergoing one of three operations (CABG, AVR, MVR/r), with the caveat that they experienced an STS-defined FTR complication (prolonged ventilation, post-operative renal failure, reoperation, and/or stroke). Interestingly, our analysis found that increasing center-level case volume was associated with significantly higher rates of major complications. This is in contrast to Gonazalez’s finding of no statistical difference in complication rate among CABG patients by volume quintile, and significantly lower rates of complications among AVR patients operated on at the highest volume quintile, relative to the lowest volume quintile. Our finding of significantly higher complication rates among high volume centers may be because these centers are operating on patients whose risk of major morbidity and death is higher than those at lower volume centers, but for reasons which are not adequately captured in the current iteration of the STS ACSD (i.e., frailty). Nevertheless, our findings are uniquely positioned to build upon previous studies, as our analysis included patients of all ages who recently underwent an STS-defined index operation between 2011-2021.
Our findings have important implications for the field of cardiovascular surgery, as they (i) validate STS-defined FTR as a significant driver of higher mortality at low volume hospitals, and (ii) provide insight into potential levers for decreasing STS-defined failure to rescue rates. While preventing post-operative complications is exceedingly important, perhaps even more important is reducing mortality and increasing the success rate of live-saving interventions. This may be supported by our study’s finding that higher volume centers had significantly worse rates of post-operative complications but nevertheless significantly lower rates of FTR. Though there are many hospital-level features associated with a lower volume center that may be difficult to remediate, there exist potential immediate avenues for reducing composite and individual hospital failure to rescue rates. First, patients identified as high-risk can be directed in a standardized process toward hospitals where resources and characteristics are most appropriate for the level of care needed. Second, more attention can be devoted to preparing systems at low-volume centers to respond quickly and efficaciously to adverse events (ex., simulation curricula for ICU teams, protocols for post-complication care, development of rapid response teams, consideration of transfer to quaternary care facility) to prevent avoidable failure to rescue and mortality. It is possible that there exist some lower volume centers which, despite best efforts to implement the above two points, may lack the critical resources (i.e., cushion of additional ancillary and medical/surgical staff available for surge capacity and concurrent emergencies) to lower their FTR rate. If unable to obtain these additional critical resources, such programs may need to step back and evaluate the relative benefit of access their provision of care provides, weighed against their higher rate of FTR.
We also observed female sex to be significantly associated with increased risk-adjusted odds of FTR. Worryingly, effect modification analysis demonstrated that the beneficial impact of increasing center-level case volume is only felt among male patients. [20-23] The underlying cause of the observed gender disparity in FTR is likely multifactorial. As described by Cho et al., women with CAD are typically older, have greater burden of comorbidities relative to men, smaller coronaries which may increase technical difficulty of CABG, and often experienced delayed diagnosis, treatment conservatism, and referral bias which results in more frequent urgent or emergent presentation for revascularization. [23] All of these factors could play into the development of complications which ultimately lead to FTR. These findings add to the literature describing gender inequities in cardiac surgical outcomes, and are another reminder of the urgent need for equitable progress in improving outcomes for patients undergoing cardiac surgery
Several limitations to this study must be acknowledged. First, it is possible that there are confounding variables driving the significant association between increasing hospital volume, higher center-level complication rates, and decreasing FTR and mortality rates. Sheetz and colleagues found that teaching status, high hospital technology, increasing nurse-to-patient ratio, and presence of >20 ICU beds were significantly associated with improved FTR rates for a range of procedures across medical specialties.[24] Hospital volume may be capturing these and other hospital characteristics, including surgeon and allied health professional experience. As a result of this manuscript, we are undertaking a mixed methods analysis which aims to collect additional granular detail on ICU staffing (level of expertise, 24/7 coverage or less, etc.) and other protocolized processes of post-operative care. Our findings in this study may also not be generalizable to other non-cardiac high-risk operations. However, previous studies have illustrated relative equivalence of variables impacting FTR across operations. Additional studies may be required to ensure that this is true of hospital volume. The STS ACSD does not include phase of care mortality analysis data or timing of specific complications (i.e., did a complication occur in the ICU, step-down unit, on POD1 or on POD 15), which somewhat limits the granularity of our inference. Finally, though we cannot assert with certainty that we are able to extrapolate our results outside the region studied, the population centers included in the study are geographically diverse and differences in demographic variables were adjusted accordingly.
CONCLUSION
In summary, we report a significant, positive association between increasing hospital case volume and decreasing FTR rate in our final multivariable analysis. Future studies should examine how high-volume hospitals more consistently succeed at rescuing cardiac surgery patients following major post-operative complications, and seek to identify low performing, low volume centers for quality improvement.
Supplementary Material
Central Picture Legend.
Rate of FTR by Center-level Case Volume. Dotted Line = single linear regression.
Central Message.
Increasing center-level case volume, as defined by a center’s mean annual STS index case volume, is significantly associated with improved STS-defined FTR.
Perspective Statement.
The STS has recently announced plans to incorporate FTR formally into the ACSD. We found that increasing center-level case volume is independently associated with improved risk-adjusted odds of STS-defined FTR. Low performing, low volume centers may improve their FTR rates via quality improvement efforts incorporating feedback and best practices from higher performing centers.
ACKNOWLEDGEMENTS AND DISCLOSURES
Sources of Funding: This work was funded in part by a grant from the Cardiothoracic Surgery Trials Network of the National Institutes of Health Clinical Research and Implementation Skills Program under Award Number 2UM HL088925, as well as by the National Heart, Lung, and Blood Institute (grant T32 HL007849).
Conflict of Interest Statement and Separate Funding Statement:
The authors had full control of the design of the study, methods used, results, data analysis and production of the written manuscript. Research reported in this publication/presentation/work was supported in part by the National Heart, Lung, and Blood Institute (grant T32 HL007849), as well as by a grant under Award Number 2UM HL088925.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary of Abbreviations
- FTR
Failure to Rescue
- STS
Society of Thoracic Surgeons
- VCSQI
Virginia Cardiac Services Quality Initiative
- ACSD
Adult Cardiac Surgery Database
- CABG
Coronary artery bypass grafting
- AVR
Aortic Valve Replacement
- MVR
Mitral Valve Replacement
- MVr
Mitral Valve Repair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting: Oral Presentation, STS Annual Meeting, San Diego, CA, January 21st – 23rd, 2023.
Institutional Review Board (IRB) Approval: This study was exempt from review by the University of Virginia’s Institutional Review Board due to the de-identified nature of the quality database (Protocol #23305).
REFERENCES
- 1.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg. 2014. Feb;149(2):119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowger JA, Stulak JM, Shah P, Dardas TF, Pagani FD, Dunlay SM, et al. Impact of Center Left Ventricular Assist Device Volume on Outcomes After Implantation: An INTERMACS Analysis. JACC Heart Fail 2017;5:691–9. 10.1016/j.jchf.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel NJ, Iyengar A, Kelly JJ, Han JJ, Brown CR, Desai ND. Volume of frail patients predicts outcome in frail patients after cardiac surgery. J Thorac Cardiovasc Surg 2022;163:151–60.e6. 10.1016/j.jtcvs.2020.04.097. [DOI] [PubMed] [Google Scholar]
- 4.Alkhouli M, Alqahtani F, Cook CC. Association between surgical volume and clinical outcomes following coronary artery bypass grafting in contemporary practice. J Card Surg 2019;34:1049–54. 10.1111/jocs.14205. [DOI] [PubMed] [Google Scholar]
- 5.Hadaya J, Sanaiha Y, Hernandez R, Tran Z, Shemin RJ, Benharash P. Impact of hospital volume on resource use after elective cardiac surgery: A contemporary analysis. Surgery 2021;170:682–8. 10.1016/j.surg.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Shang M, Mori M, Gan G, Deng Y, Brooks C 2nd, Weininger G, et al. Widening volume and persistent outcome disparity in valve operations: New York statewide analysis, 2005-2016. J Thorac Cardiovasc Surg 2022;164:1796–803.e5. 10.1016/j.jtcvs.2020.11.098. [DOI] [PubMed] [Google Scholar]
- 7.Shuhaiber J, Isaacs AJ, Sedrakyan A. The Effect of Center Volume on In-Hospital Mortality After Aortic and Mitral Valve Surgical Procedures: A Population-Based Study. Ann Thorac Surg 2015;100:1340–6. 10.1016/j.athoracsur.2015.03.098. [DOI] [PubMed] [Google Scholar]
- 8.Dewey TM, Herbert MA, Prince SL, Bowers BS. Influence of surgical volume on outcomes in low-risk patients undergoing isolated surgical aortic valve replacement. J Thorac Cardiovasc Surg 2022;163:2046–52.e2. 10.1016/j.jtcvs.2020.06.144. [DOI] [PubMed] [Google Scholar]
- 9.Karamlou T, Johnston DR, Backer CL, Roselli EE, Welke KF, Caldarone CA, et al. Access or excess? Examining the argument for regionalized cardiac care. J Thorac Cardiovasc Surg 2020;160:813–9. 10.1016/j.jtcvs.2019.12.125. [DOI] [PubMed] [Google Scholar]
- 10.Goldstone AB, Chiu P, Baiocchi M, Lingala B, Lee J, Rigdon J, et al. Interfacility Transfer of Medicare Beneficiaries With Acute Type A Aortic Dissection and Regionalization of Care in the United States. Circulation 2019;140:1239–50. 10.1161/CIRCULATIONAHA.118.038867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chhabra KR, Dimick JB. Strategies for Improving Surgical Care: When Is Regionalization the Right Choice? JAMA Surg 2016;151:1001–2. 10.1001/jamasurg.2016.1059. [DOI] [PubMed] [Google Scholar]
- 12.Luu HY, Nguyen TC. Commentary: 10,000 hours or 10,000 cases? An argument for regionalization of coronary and cardiac valve surgery in the new era. J Thorac Cardiovasc Surg 2020. [DOI] [PubMed] [Google Scholar]
- 13.Trehan K, Kemp CD, Yang SC. Simulation in cardiothoracic surgical training: where do we stand? J Thorac Cardiovasc Surg 2014;147:18–24.e2. 10.1016/j.jtcvs.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Emani SS, Allan CK, Forster T, Fisk AC, Lagrasta C, Zheleva B, et al. Simulation training improves team dynamics and performance in a low-resource cardiac intensive care unit. Ann Pediatr Cardiol 2018;11:130–6. 10.4103/apc.APC_117_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurlansky PA, O’Brien SM, Vassileva CM, Lobdell KW, Edwards FH, Jacobs JP, et al. Failure to Rescue: A New Society of Thoracic Surgeons Quality Metric for Cardiac Surgery. Ann Thorac Surg 2021. 10.1016/j.athoracsur.2021.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Edwards FH, Ferraris VA, Kurlansky PA, Lobdell KW, He X, O’Brien SM, et al. Failure to Rescue Rates After Coronary Artery Bypass Grafting: An Analysis From The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2016;102:458–64. 10.1016/j.athoracsur.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins RB, Downs EA, Johnston LE, et al. Impact of transcatheter technology on surgical aortic valve replacement volume, outcomes, and cost. Ann Thorac Surg. 2017;103:1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osnabrugge RLJ, Speir AM, Head SJ, et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg. 2013; 96:500–506. [DOI] [PubMed] [Google Scholar]
- 19.Adult Cardiac Surgery Database. The Society of Thoracic Surgeons. Accessed March 1, 2022. https://www.sts.org/registries-research-center/sts-nationaldatabase/adult-cardiac-surgery-database/data-collection [Google Scholar]
- 20.Enumah ZO, Canner JK, Alejo D, Warren DS, Zhou X, Yenokyan G, et al. Persistent Racial and Sex Disparities in Outcomes After Coronary Artery Bypass Surgery: A Retrospective Clinical Registry Review in the Drug-eluting Stent Era. Ann Surg 2020;272:660–7. 10.1097/SLA.0000000000004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston A, Mesana TG, Lee DS, Eddeen AB, Sun LY. Sex Differences in Long-Term Survival After Major Cardiac Surgery: A Population-Based Cohort Study. J Am Heart Assoc 2019;8:e013260. 10.1161/JAHA.119.013260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho L, Kibbe MR, Bakaeen F, Aggarwal NR, Davis MB, Karmalou T, et al. Cardiac Surgery in Women in the Current Era: What Are the Gaps in Care? Circulation 2021;144:1172–85. 10.1161/CIRCULATIONAHA.121.056025. [DOI] [PubMed] [Google Scholar]
- 23.Newell P, Asokan S, Zogg C, Prasanna A, Hirji S, Harloff M, et al. Contemporary socioeconomic-based disparities in cardiac surgery: Are we closing the disparities gap? J Thorac Cardiovasc Surg 2022. 10.1016/j.jtcvs.2022.02.061. [DOI] [PubMed] [Google Scholar]
- 24.Sheetz KH, Dimick JB, Ghaferi AA. Impact of Hospital Characteristics on Failure to Rescue Following Major Surgery. Ann Surg. 2016. Apr;263(4):692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.