Abstract
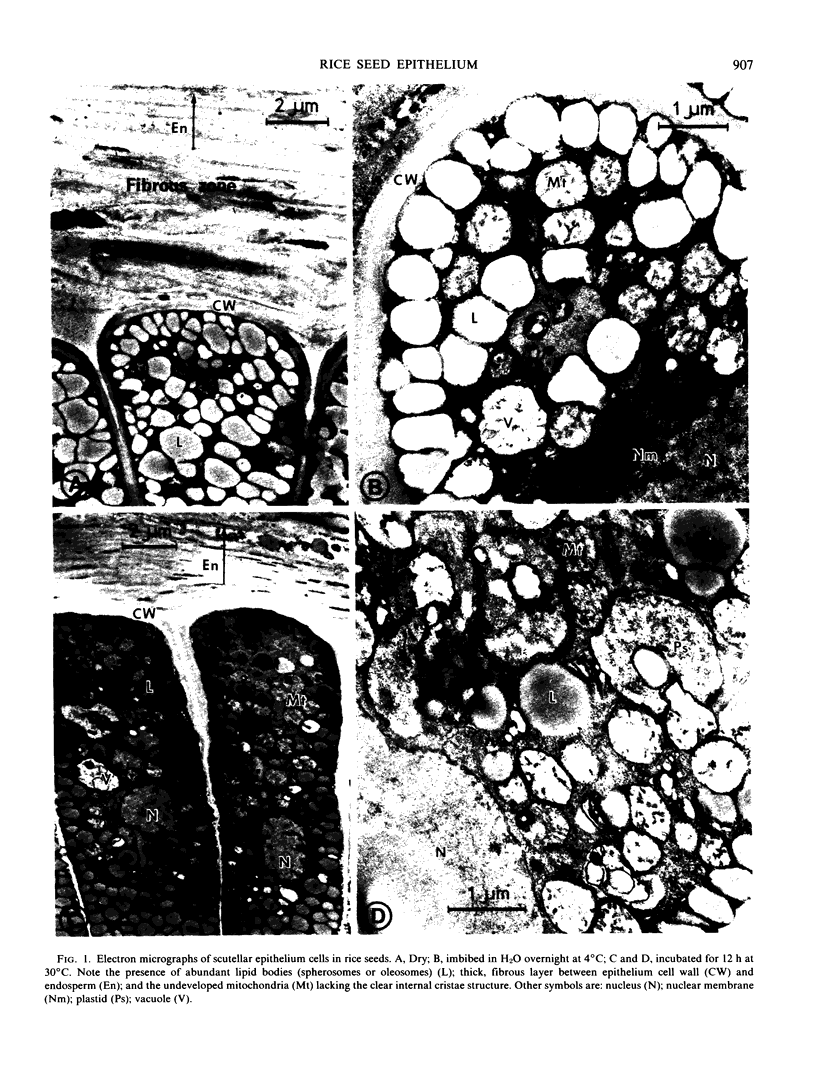
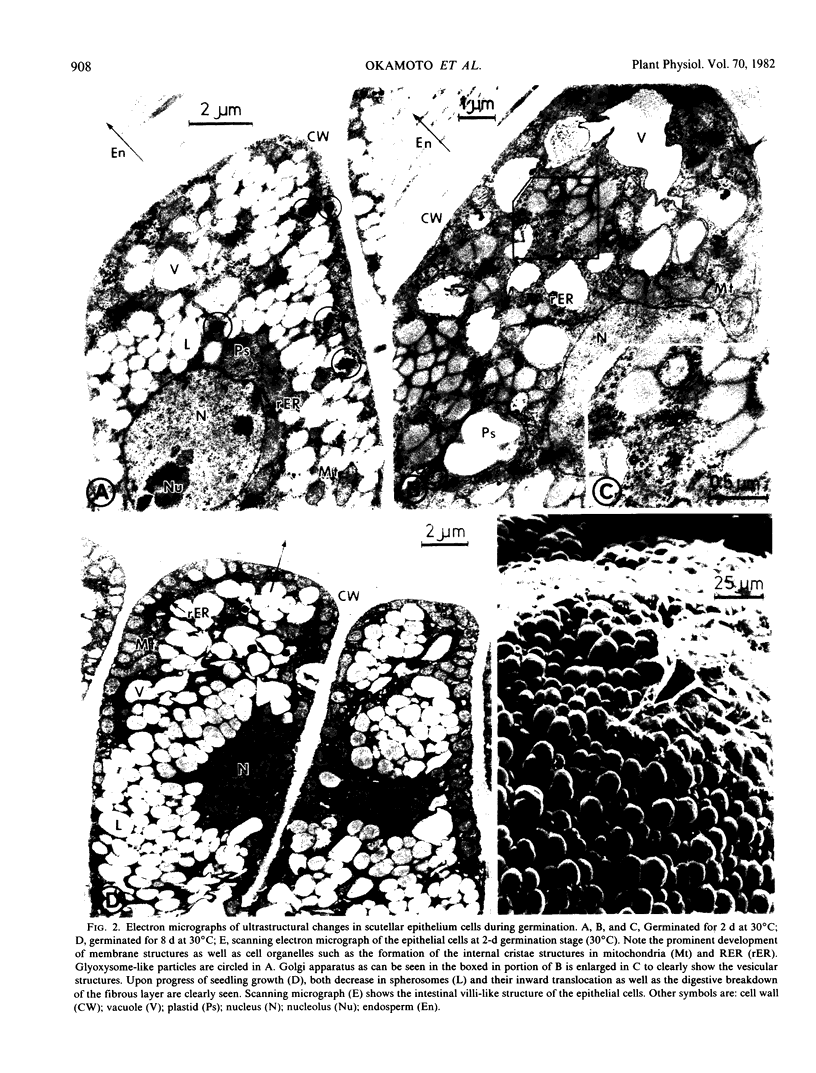
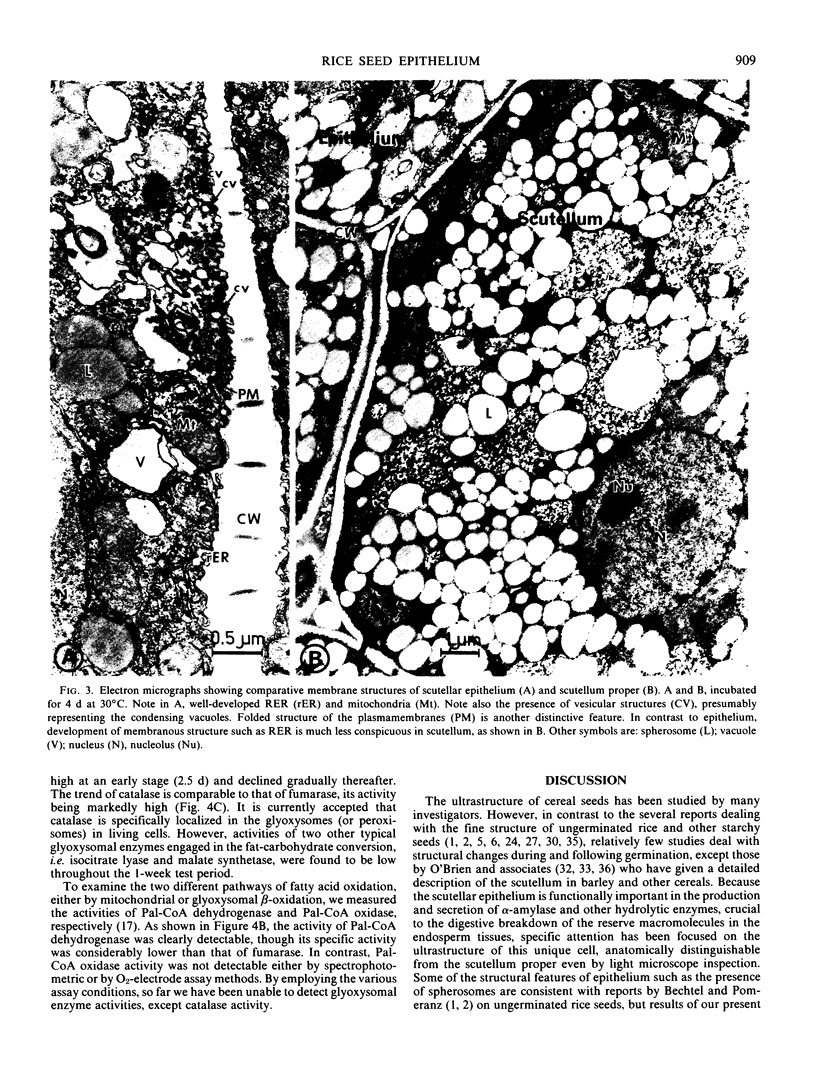
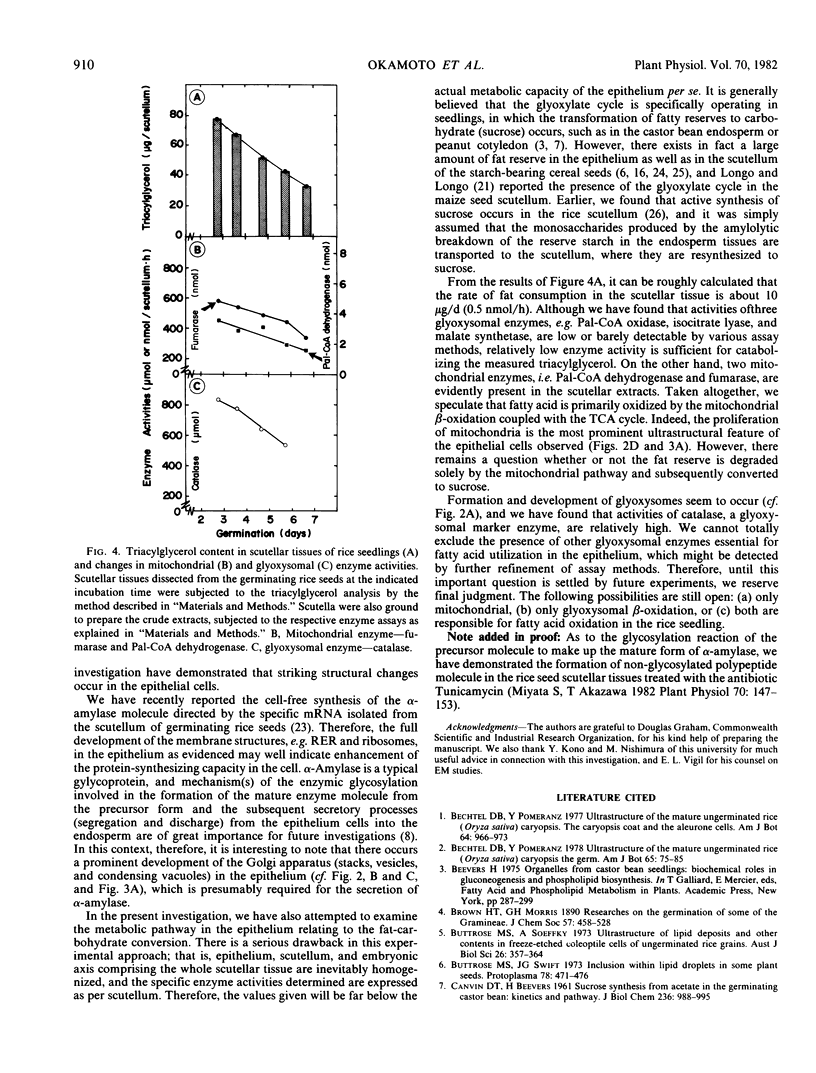
The ultrastructural changes occurring in the scutellar epithelium cells of rice seeds have been studied during germination and early seedling growth. During this time, several prominent structural changes occur, including (a) formation, development, and proliferation of organelles such as mitochondria, rough endoplasmic reticulum, free ribosomes, and Golgi apparatus; (b) folded structural modification of plasmamembranes in later stages; and (c) conspicuous decrease in lipid-storing spherosomes. Glyoxysome-like electron dense particles are detectable but their formation is much less prominent. It is conceivable that all these structural changes are related to the enhancement of the metabolic activities of the epithelial cells including the synthesis of hydrolytic enzymes such as α-amylase and their secretion into the endosperm tissues. Some enzyme activities characteristic of mitochondria and glyoxysomes have been determined using the crude scutellar extracts, and the results dealing with the low activities of the glyoxylate cycle enzymes and palmitoyl-coenzyme A oxidase appear to indicate that fatty acid breakdown is possibly via mitochondrial β-oxidation, although we reserve a definitive conclusion on the glyoxysomes being nonfunctional in fatty acid oxidation in rice seedlings.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Dure L. S. Site of Origin and Extent of Activity of Amylases in Maize Germination. Plant Physiol. 1960 Nov;35(6):925–934. doi: 10.1104/pp.35.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hatch M. D. A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem. 1978 Mar;85(1):271–275. doi: 10.1016/0003-2697(78)90299-3. [DOI] [PubMed] [Google Scholar]
- Holmer G., Ory R. L., Hoy C. E. Changes in lipid composition of germinating barley embryo. Lipids. 1973 May;8(5):277–283. doi: 10.1007/BF02531905. [DOI] [PubMed] [Google Scholar]
- Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- Longo C. P., Longo G. P. The development of glyoxysomes in peanut cotyledons and maize scutella. Plant Physiol. 1970 Mar;45(3):249–254. doi: 10.1104/pp.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Akazawa T. Enzymic mechanism of starch breakdown in germinating rice seeds : 12. Biosynthesis of alpha-amylase in relation to protein glycosylation. Plant Physiol. 1982 Jul;70(1):147–153. doi: 10.1104/pp.70.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Okamoto K., Watanabe A., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. IN VIVO AND IN VITRO SYNTHESIS OF alpha-AMYLASE IN RICE SEED SCUTELLUM. Plant Physiol. 1981 Dec;68(6):1314–1318. doi: 10.1104/pp.68.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Kono Y., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds II. Scutellum as the Site of Sucrose Synthesis. Plant Physiol. 1969 May;44(5):765–769. doi: 10.1104/pp.44.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Akazawa T. Enzymic mechanisms of starch breakdown in germinating rice seeds: 7. Amylase formation in the epithelium. Plant Physiol. 1979 Feb;63(2):336–340. doi: 10.1104/pp.63.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]