Abstract
Members of a panel of stress-responsive biosensors have been used to study the effect of megahertz frequency ultrasound on Escherichia coli. Insonification causes acoustic cavitation, the collapse of oscillating microbubbles in solution, which can damage bacterial cells. A focused 1-MHz ultrasound transducer, capable of generating a spatial peak pulse average intensity of 500 W/cm2, was used to treat liquid bacterial cultures. Stress-responsive promoters fused to luxCDABE allowed the continuous measurement of light produced as a result of protein damage, DNA damage, oxidative stress, and membrane perturbation. A promoter responsive to ammonia limitation was not transcriptionally activated under test conditions. In contrast to bacteria in exponentially growing cultures, those in stationary-phase cultures were more resistant to the effects of ultrasound treatment. Quantification of the degree of acoustic cavitation due to symmetric bubble collapse was measured by a 20-MHz passive transducer, the output of which appears to be only partially correlated with cellular damage and survival. The methods and results summarized here provide the basis for further investigation into applications, including the purification of water samples.
Bacteria have the capacity to respond to various types of stress encountered in their environments. Nutritional limitation as well as macromolecular damage result in intracellular signals that induce response programs. Many of the responses involve the transcriptional activation of specific sets of stress response genes, the products of which function to restore cellular “health,” establish a new steady-state balance, and decrease the level of the intracellular signals, resulting in a reduction of the stress response. While some genes are activated in response to only a limited number of stressors, the expression of others, most notably uspA (23), may be induced under many different conditions. Much of the research in the area of bacterial stress response has focused on growing cells and has been recently reviewed in the cases of DNA damage (26, 42), heat shock (14), and limitation for carbon (27), nitrogen (18), and phosphorus (43). However, a growing body of work in the area of stationary-phase responses indicates that stationary-phase cells have different means of stress response regulation and are generally more resistant (16, 28).
The use of stress-responsive reporters allows investigators to characterize the regulatory circuits involved in modulating the response. More recently, bioluminescence has provided a rapid viable alternative to the traditional colorimetric assays of promoter-lacZ fusions. Comprehensive characterization of enzymes of bacterial bioluminescence and its applications have been reviewed elsewhere (20, 30). The development of a panel of promoter-luxCDABE biosensors has allowed real-time, noninvasive detection of genotoxic agents (5, 40), protein-damaging solvents and toxins (35–37), and oxidative stressors (5–7). These biosensors report the stress response to pure components as well as to mixtures of known and unknown composition (6, 7, 40).
Ultrasound is known to damage biological cells and tissues through two primary mechanisms, heating and cavitation (33). Heating occurs as ultrasonic energy is absorbed by cells, tissues, or bone and produces biological effects indistinguishable from heating induced by other methods (22). Acoustic cavitation occurs when bubbles that are present naturally or added intentionally interact with the alternating compressions and rarefactions of the ultrasonic wave (2). Each bubble has a sharply defined resonance frequency that depends upon the bubble size, the composition of any contaminating surface layer, and the characteristics of the surrounding fluid (e.g., viscosity). If the bubble is driven near its resonance frequency, the resulting violent collapse may be symmetrical, producing temperatures in excess of 5,000 K (31) at the collapse point and pressures of thousands of atmospheres that can be measured as acoustic emissions. These conditions are sufficient to produce hydroxyl free radicals (24). If the bubble collapse is asymmetrical, however, as is the case when the collapsing bubble is within a few radii of a solid surface, a jet of liquid can form that pierces the bubble and strikes the surface at velocities up to 200 m/s (17). These microjets, which have been shown to be responsible for damage to both kidney and gallstones in lithotripsy (34), have the ability to puncture or shear cell walls (13).
Sonication is often used by investigators as a method of lysing bacterial cells. It depends upon bubble activity, heating, and the shear forces produced by the sonicator tip itself (“jackhammer effect”). Sonicators such as the Branson W-350 model operate at ultrasonic frequencies in the range of 20 to 25 kHz in a continuous wave or slowly pulsed mode (pulse duration of several seconds). Spatial and temporal average intensities for sonicators are typically a few watts per square centimeter. In contrast, diagnostic (imaging) ultrasound is typically composed of very short pulses (a few microseconds) in the frequency range of 1 to 10 MHz, with spatial peak pulse average intensities (ISPPA) of tens or hundreds of watts per square centimeter (11). The cavitation effects produced by the two ultrasound modalities, consequently, are qualitatively different, with sonication processes depending upon the heat and shear action of the sonicator tip while diagnostic ultrasound bioeffects depend largely upon the presence and characteristics of preexisting gas nuclei. At the lower frequencies, ultrasound has been used to introduce DNA into bacterial cells (3).
Recent studies have shown that damage to human erythrocytes (12) and platelets (13) can occur when gas nuclei are present in an intense ultrasonic field. Studies on the response of Chinese hamster ovary cells to ultrasound in the 1-MHz range argued that cell death was due to membrane damage and genotoxicity (3). The use of microorganisms to detect the effects of ultrasound is not unique to this study. Thacker (32) used yeast to determine the killing efficiency of ultrasound, while others have used luminous bacteria (19) and dinoflagellates (1) as generalized reporters of mechanical damage. In contrast, this paper elaborates on initial findings (41) of our investigations to determine whether treatment of bacterial cultures with high-frequency pulses of ultrasound induces specific stress responses. The end product of such an investigation would be to determine how much megahertz frequency ultrasound is necessary to kill bacteria. By analyzing the differential induction of members of a panel of stress responses, we may be able to delineate the mechanism(s) by which the viability of the population is affected.
MATERIALS AND METHODS
Bacterial culture and strains.
Escherichia coli transformants were grown on Luria-Bertani (LB) agar containing 50 μg of kanamycin sulfate ml−1. Overnight liquid cultures were prepared from these transformant plates in LB medium containing 50 μg of kanamycin sulfate ml−1. Fresh cultures were then inoculated from the overnight cultures into liquid LB medium without kanamycin for a higher level of expression (10). These cultures were incubated at 37°C with shaking until they reached an optical density at 600 nm between 0.20 and 0.30. One-milliliter aliquots of the liquid cultures were then transferred to 2-ml nylon centrifuge tubes (Beckman UltraClear) for induction (38).
As a positive control, a 1-ml aliquot of liquid culture was treated with a concentration of a known agent that induced 75 to 80% of the maximum response (see Table 2) in order to ensure that the E. coli cells were responsive. A 1-ml aliquot of liquid culture was left untreated by any inducer as a negative control in order to determine the background levels of induction that are present in the E. coli transformants. The untreated and positive control samples were incubated at room temperature with aeration to mimic conditions during ultrasound treatment.
TABLE 2.
Responses to ultrasound treatment
| Promoter | Response regulon | Maximum response ratioa at sublethal dose of:
|
|||||
|---|---|---|---|---|---|---|---|
| Ultrasound treatment | Cerulenin (20 mg/ml) | Medium without NH3 | Ethanol (1%) | H2O2 (0.001%) | UV (1,000 J/cm2) | ||
| fabA | Membrane damage | 7.2 | 15.1 | 1.0 | 1.0 | 1.0 | 1.0 |
| glnA | Ammonia starvation | 1.0 | 1.0 | 6.0 | 1.0 | 1.0 | 1.0 |
| grpE | Heat shock | 24.0 | 1.0 | 1.0 | 25.6 | 1.0 | 1.0 |
| katG | Oxidative damage | 4.5 | 1.0 | 1.0 | 1.0 | 12.5 | 1.0 |
| recA | SOS response | 4.1 | 1.0 | 1.0 | 1.0 | 1.0 | 16.1 |
Does not include viable cell count.
Several strains of bacteria that contain the pUCD615 plasmid (25), in which the luxCDABE cassette has been fused to a specific promoter, were used. In all cases, the host was E. coli RFM443. This strain and the relevant promoter-lux fusions are described in Table 1.
TABLE 1.
E. coli strain and plasmids used in this study
| Bacterial strain or plasmid | Genotype or description | Reference |
|---|---|---|
| RFM443 | F−galK2 lac-74 rpsL200 | 10 |
| pUCD615 | 17.55-kbp broad-host-range Apr Knr plasmid carrying promoterless luxCDABE from V. fischeri | 25 |
| pGrpELux | 206-bp fragment from promoter region of E. coli grpE cloned into the BamHI-EcoRI site of pUCD615 | 36 |
| pRecALux | 279-bp fragment from promoter region of E. coli recA cloned into the BamHI-EcoRI site of pUCD615 | 40 |
| pGlnALux | 227-bp fragment from glnAp2 promoter region of E. coli glnA cloned into the BamHI-EcoRI site of pUCD615 | 39 |
| pKatGLux | 654-bp fragment from promoter region of E. coli katG cloned into the BamHI-EcoRI site of pUCD615 | 6 |
| pFabALux | 248-bp fragment from promoter region of E. coli fabA cloned into the BamHI-EcoRI site of pUCD615 | 4 |
Light measurement and cell viability.
Duplicate 50-μl aliquots of the samples were pipetted into colorless 1.5-ml microcentrifuge tubes (without caps), which were placed in standard capped scintillation vials. Samples (at 26°C) were read approximately every 20 min for at least 3 h by using a 1219 RackBeta liquid scintillation counter (LKB/Wallac), according to the manufacturer’s instructions for measuring chemiluminescence. Bioluminescence was measured in relative light units (RLU) (38). Viabilities of both induced and untreated cells were measured by plating appropriately diluted samples (with sterile saline) onto LB agar containing 50 μg of kanamycin sulfate ml−1.
Ultrasound apparatus.
A 1-MHz transducer and a narrow-band 20-MHz probe transducer, both with 5.08-cm focal lengths, were arranged confocally in the walls of a 30.5- by 20.3- by 20.3-cm Plexiglas tank filled with degassed water at 26°C. A rotatory sample holder was positioned so that an attached sample could be positioned at the cofocus of the two transducers. The 1-MHz transducer induced cavitation in the sample while the 20-MHz transducer passively recorded the 20-MHz component of acoustic emissions produced by collapsing bubbles. The voltage output from the probe transducer represented the sum of the pressure wave emissions of thousands of uncorrelated bubble implosions occurring in the sample in response to each 1-MHz pulse (12). The voltage signal was amplified by a broad-band amplifier (Matec model 625) and then filtered by a 20-MHz filter (WaveTek Ultramin AB4BB20/1) to remove electrical noise. The resulting signal was recorded by a digital oscilloscope (model 9354AM; LeCroy Corp.), which calculated the root mean square (RMS) values of cavitation signals and downloaded them to a computer approximately three times per second. The time-averaged RMS values during the 5-min exposure were used as indicators of cavitation activity.
Ultrasound treatment.
Two types of microbubble cavitation nuclei were used: Albunex, a commercially available agent (Mallinckrodt Medical Inc., St. Louis, Mo.) consisting of protein-encapsulated air bubbles, and ST68, a research agent supplied by Margaret Wheatley (Drexel University) consisting of surfactant-coated air bubbles.
One-milliliter samples consisting of E. coli in LB medium and 50 μl of microbubble agent were placed into 2-ml Beckman UltraClear centrifuge tubes, which were the exposure vessels. The sample was insonified for 5 min with an acoustic field produced by the 1-MHz transducer, operated at an intensity of 500 W/cm2 (ISPPA). The acoustic field consisted of 1-ms-duration pulses at a pulse repetition frequency of 20 Hz to minimize sample heating. The sample tube was rotated at 200 rpm to ensure recirculation of the bubbles by acoustic radiation force (21, 44). Cavitation occurring during insonification was recorded by a passive 20-MHz transducer.
A sham-exposed sample was treated identically in series with the experimental sample, except that the acoustic field was turned off to provide a baseline for cell viability and bioluminescence. The passive cavitation detection system was time gated to ensure that the signal recorded was due only to collapsing bubbles and not to reflections of ultrasound within the exposure tank (12).
Data collection and analysis.
For each sample, following exposure to ultrasound, duplicate light emission measurements (in RLU) were averaged together at each time point and divided by the number of viable cells to determine RLU/viable cell. Similar to specific activity, specific response ratios were calculated by dividing the bioluminescent response of the experimental sample by that of the untreated (sham) sample at a particular time point, that with the maximum response. By doing so, one can compare responses of promoters under different stress conditions or one can compare the responses of different stress-responsive promoters. This type of calculation contrasts with response ratios as previously described (36), where the number of viable cells was not taken into account at sublethal doses of stressor.
A ratio value of >1 indicates transcriptional activation of the lux operon; a ratio equal to 1 represents no response; and a ratio of <1 indicates toxicity, because the amount of light produced by the uninduced cells (background, low-level transcription) is greater than what the induced sample can produce. The killing percentage for each ultrasound-treated sample was calculated by dividing its colony forming capacity by the colony forming capacity of the sham-exposed control, subtracting this value from 1, and multiplying this fraction by 100.
RESULTS
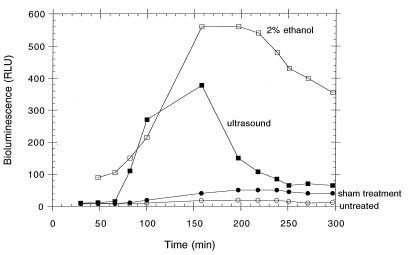
The kinetics of transcriptional activation of the grpE promoter are shown in Fig. 1. Bioluminescence is due to the activity of the lux proteins, which results from induction of the heat shock response and for which the signal can be denatured proteins. The advantage of using the luxCDABE reporter is evident, as other reporters, such as lacZ, would require permeabilizing cells with solvent or detergent (which might induce stress responses) in order to supply a colorimetric substrate to the reporter enzyme. In contrast, endogenously supplied substrate by the luxCDE gene products provides the fatty aldehyde required by luciferase, the luxAB gene product; thus, a real-time reporter allows the measurement of response in intact cells. In addition, activity of luciferase requires reduced electron carriers (flavin mononucleotide) and ATP. Change (oxidation) with the redox state of the cell or the availability of ATP pools would result in a loss of luciferase, or “lights off,” response, indicating toxicity (40). Ultrasound appears to elicit a response comparable to that of a known heat shock inducer (ethanol) in terms of the increase in bioluminescence. The response to ultrasound decreases while the response to ethanol is more prolonged, due to the fact that the ethanol remains in the system while the acoustic cavitation ceases once the insonifier is turned off. There are two negative controls displayed in Fig. 1: one is a sample that was placed in the ultrasound apparatus and subjected to all manipulations except the insonification itself; the other is a sample that remained in the incubator during the same period of time. The two controls do not differ significantly in their responses.
FIG. 1.
Kinetic graph showing induction of bioluminescence as a function of time for grpE′::lux promoter fusion bacteria after exposure to 5 min of 1-MHz pulsed ultrasound (ISPPA, 500 W cm−2). RLU are directly proportional to stress response. The sham-treated control was subjected to transport and all manipulations, as was the ultrasound-treated sample, without being subjected to the 1-MHz transducer. The untreated control was placed at 26°C and shaken but was not transported or placed in the apparatus.
Table 2 shows specific response ratios calculated from kinetic data (not shown) of different stress-response promoters. Each promoter was challenged with a “cognate” stressor as well as with ultrasound. With the exception of the nitrogen starvation sensor, all of the other promoters tested did respond to ultrasound. In most cases, the responses were comparable but lower than the response to the specific stressor. Response ratios were determined at 200 min for fabA (which responds comparatively slowly) and at 120 min for glnA, grpE, katG, and recA. Each promoter responded to its own cognate stressor but did not respond to stressors that stimulated a different promoter.
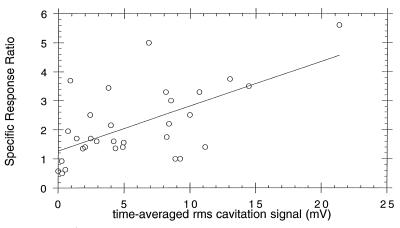
Figure 2 shows the relationship between specific response ratios from individual experiments in which the grpE′::luxCDABE fusion was studied and the time-averaged RMS voltage from the inertial cavitation detector, a value that reflects symmetrical bubble collapse in solution. There is a general increase in the specific response ratio with an increase in the amount of cavitation as measured by the RMS voltage. The R value for best-fit straight line (shown) was 0.6112. Percent killing also increases generally with elevated cavitation activity (R, 0.5477 [data not shown]) and specific response ratio (R, 0.6374 [data not shown]). The two different types of microbubble cavitation nuclei were indistinguishable (data not shown).
FIG. 2.
Specific response ratio plotted versus time-averaged RMS cavitational signal (in millivolts) for all exposures in this study. Data shown are for individual experiments, not group means. Specific response ratio is defined as the ratio of the maximum RLU per viable cell for ultrasound-exposed bacteria to the maximum RLU per viable cell for sham-exposed controls. RMS signal from the 20-MHz passive cavitation detector is a measure of average acoustic emissions produced by collapsing bubbles occurring throughout the 5-min insonification.
Table 3 shows the results from three trials. Cells exhibited differential heat shock response in different phases of growth. Response ratios close to 1.0 indicate very little bioluminescence above the background (untreated) levels, and percent killing was diminished from a range of 4 to 50% to a range of 10 to 15% after more than 22 h of growth.
TABLE 3.
Differential heat shock responses of bacteria from exponential and stationary phases of growth
| Time after inoculation (h) | % Killing | Specific response ratio |
|---|---|---|
| 1 | 43.3 ± 8.5 | 2.9 ± 0.6 |
| 2 | 49.7 ± 6.2 | 3.2 ± 0.7 |
| 2a | 44.5 ± 7.6 | 3.4 ± 0.4 |
| 8 | 23.5 ± 6.1 | 1.8 ± 0.5 |
| 24 | 13.7 ± 2.1 | 1.2 ± 0.3 |
| 48 | 10.4 ± 1.0 | 1.3 ± 0.4 |
Two-hour cells resuspended to an optical density at 600 nm (2.0) comparable to that of 24-h cells.
DISCUSSION
The results of this study show that short pulses of 1-MHz ultrasound have the ability to induce some stress responses in E. coli and, under some conditions, to cause bacterial death. The agent responsible for the stress appears to be acoustic cavitation, the activation of preexisting microbubbles by the ultrasonic wave, since without the addition of exogenous microbubbles, stress responses are statistically indistinguishable from sham (no ultrasound) exposures (data not shown). Activation of four of the five promoter-lux fusions (Table 2) is most likely due to the action of liquid jets generated by asymmetric bubble collapses and presence of hydroxyl free radicals produced by very hot, symmetrical bubble collapses (31). The lack of transcriptional activation of the lux operon in the glnA promoter fusion shows, not unexpectedly, that ammonia starvation is not a stress factor for ultrasound-bacterium interaction and illustrates the specificity of the luminescent stress response biosensor panel. The response profiles of the grpE promoter to ultrasound and to ethanol (Fig. 1) are similar between 80 and 120 min. After that point, the ethanol response continues to increase, due to the fact that added ethanol remains during the course of the bioluminescence measurement while the stress due to ultrasound is transient, ceasing once the apparatus is shut down. It is not feasible to use the increase in temperature for the positive control of the grpE heat shock response because of the thermal instability of the Vibrio fischeri lux gene products. The response of fabA was particularly slow, probably due to the fact that the activation of its transcription requires a number of intervening physiological steps. Recently, we cloned another promoter that is sensitive to membrane damage, the phage shock protein (pspA) promoter (9), and are determining the kinetics and magnitude of its response to ultrasound (39). Compared to fabA, this new fusion may react more rapidly to membrane perturbances.
Figure 2 shows that the time-averaged RMS voltage from the inertial cavitation detector is only a moderately useful descriptor of stress response. Only 61% of the variation in specific response ratio is attributable to this descriptor, which provides a measure of the number of symmetrically collapsing bubbles during a 5-min exposure. However, such collapses are likely only when bubbles are at least a few radii from a solid surface. With bacterial concentrations as high as 3.4 × 1011 cells/ml, a simple calculation shows that every Albunex bubble (8) has, on average, 35 bacterial cells on or very near its surface. Perfectly symmetrical bubble collapses are therefore less likely than asymmetrical ones, which would produce fewer acoustic emissions. Therefore our detector records a measure of inertial cavitational activity (emissions from symmetrical collapses) that differs from the activity (liquid jets from asymmetrical collapse) that produces the observed protein damage and membrane damage stress responses. Techniques have not yet been developed for measuring the number and maximum velocities of bubble-produced jets in a cavitating bubble field as complex as those produced in this study.
Despite the low correlation coefficient of the data in Fig. 2, an analysis of variance showed that the probability of obtaining the trends by a random process is 0.0003. If 0.05 is chosen as the alpha level, it is clear that acoustic emissions and bacterial stress response are strongly related. A cavitation field consisting of millions of bubbles collapsing, shattering, regrowing, and collapsing again is likely to contribute both symmetrical and asymmetrical collapses that produce emissions and membrane damage, respectively. Free radical production is more likely to accompany symmetrical collapses due to elevated collapse temperatures in the bubble interior, and so better correlation between DNA damage or oxidative damage stress responses and acoustic emissions might be expected.
The degree of killing and the level of response were comparable. In cases where a significant proportion of the population was killed, the remaining members of the population were brighter; that is, they emitted more light. In a few instances, the number of survivors did not change after ultrasound treatment. Either the members of this population were successful in mounting a stress response that allowed full recovery or there was a significant amount of cell division during the time interval. The latter explanation is not likely, since the temperature of the samples was maintained at 26°C from the time samples were placed in aliquots in the ultrasound tubes. Positive and negative controls were also transferred to 26°C at that time. Since it is difficult to duplicate exactly the stage of growth at which the sample is taken, one explanation for the lower correlation between killing and response may be the variability in the culture. While every effort was made to inoculate and remove cells in the same manner each time, it is unlikely that the cells were identical. One way to circumvent such variability would be to used lyophilized aliquots of one master culture. Additionally, the growth phase affects the responsiveness as well as the survival of the population. All of the data shown in this study were derived from the study of exponentially growing cells. Additional studies confirm what others (15, 29) have found: stationary-phase cells (24 h or more) are more resistant to the same amount of ultrasound, in that they do not emit as much light and have a much higher survival rate (Table 3). Since stationary-phase cells are more resistant to ultrasound, a continuation of current studies should allow us to determine the dosage and treatment regimen most effective at killing these cells, which may be more representative of what is found in nature. By combining a knowledge of cell death by acoustic cavitation with an understanding of bacterial stress responses, we may be able to specify parameters by which water, other liquids, and items such as surgical implants may be sterilized rapidly, effectively, and relatively inexpensively with ultrasound alone or in combination with other treatments. Finally, initial studies using the recA′::luxCDABE reporter have provided the foundation for continued investigation of possible genotoxic effects due to acoustic cavitation.
ACKNOWLEDGMENTS
This work was supported by NSF bioengineering grant BES-9528168 (A.C.V., E.C.E.) and Swarthmore College Faculty Research Funds, as well as by an American Society for Microbiology Sustaining Member Undergraduate Research Fellowship (M.H.) and NSF Presidential Faculty Fellowship 92537777 (E.C.E., S.K.).
We thank members of the Vollmer and Everbach research groups, Tom Baldwin and Robert A. LaRossa, for their invaluable input and encouragement and Margaret Wheatley, Department of Chemical Engineering, Drexel University, for supplying surfactant-based echo-contrast microbubble agent.
REFERENCES
- 1.Anderson D M, Nosenchuck D M, Reynolds G T, Walton A J. Mechanical stimulation of bioluminescence in the dinoflagellate Gonyaulux ployhedra Stein. J Exp Mar Biol Ecol. 1988;122:277–288. [Google Scholar]
- 2.Apfel R E. Sonic effervescence: a tutorial on acoustic cavitation. J Acoust Soc Am. 1997;101:1227–1237. [Google Scholar]
- 3.Armour E P, Corry P M. Cytotoxic effects of ultrasound in vitro dependence on gas content, frequency, radical scavengers, and attachment. Radiat Res. 1982;89:369–380. [PubMed] [Google Scholar]
- 4.Belkin, S. Unpublished results.
- 5.Belkin S, Vollmer A C, Van Dyk T K, Smulski D R, Reed T R, LaRossa R A. Oxidative and DNA damaging agents induce luminescence in E. coli harboring lux fusions to stress promoters. In: Campbell A K, Kricka L J, Stanley P E, editors. Bioluminescence and chemiluminescence: fundamentals and applied aspects. Chichester, England: John Wiley and Sons; 1995. pp. 509–512. [Google Scholar]
- 6.Belkin S, Smulski D R, Vollmer A C, Van Dyk T K, LaRossa R A. Oxidative stress detection with Escherichia coli harboring a katG′::lux fusion. Appl Environ Microbiol. 1996;62:2252–2256. doi: 10.1128/aem.62.7.2252-2256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkin S, Smulski D R, Dadon S, Vollmer A C, Van Dyk T K, LaRossa R A. A panel of stress-responsive luminous bacteria for toxicity detection. Water Res. 1997;31:3009–3016. [Google Scholar]
- 8.Bleeker H K, Shung K K, Barnhart J L. Ultrasonic characterization of Albunex®, a new contrast agent. J Acoust Soc Am. 1990;87:1792–1797. [Google Scholar]
- 9.Brissette J L, Weiner L, Ripmaster T L, Model P. Characterization and sequence of the Escherichia coli stress-induced psp operon. J Mol Biol. 1991;220:35–48. doi: 10.1016/0022-2836(91)90379-k. [DOI] [PubMed] [Google Scholar]
- 10.Drolet M, Phoenix P, Menael R, Massé E, Liu L F, Crouch R J. Overexpression of RNase H partially complements the growth defect of an Escherichia coli ΔtopA mutant: R-loop formation is a major problem in the absence of DNA topoisomerase I. Proc Natl Acad Sci USA. 1995;92:3527–3530. doi: 10.1073/pnas.92.8.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duck F A, Starritt H C, Aindow J D, Perkins M A, Hawkins A J. The output of pulse-echo ultrasound equipment: a survey of powers, pressures, and intensities. Br J Radiol. 1985;58:989–1001. doi: 10.1259/0007-1285-58-694-989. [DOI] [PubMed] [Google Scholar]
- 12.Everbach E C, Makin I R S, Azadniv M, Meltzer R S. Correlation of ultrasound-induced hemolysis with cavitation detector output in vitro. Ultrasound Med Biol. 1997;23:619–624. doi: 10.1016/s0301-5629(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 13.Everbach E C, Makin I R S, Francis C F, Meltzer R S. Effect of acoustic cavitation on platelets in the presence of an echo-contrast agent. Ultrasound Med Biol. 1998;24:129–136. doi: 10.1016/s0301-5629(97)00233-0. [DOI] [PubMed] [Google Scholar]
- 14.Gross C A. Function and regulation of heat shock proteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1382–1399. [Google Scholar]
- 15.Hengge-Aronis R. The role of rpoS in early stationary phase gene regulation in Escherichia coli K12. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 171–200. [Google Scholar]
- 16.Hengge-Aronis R. Regulation of gene expression during entry into stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1497–1512. [Google Scholar]
- 17.Lauterborn W, Bolle H. Experimental investigations of cavitation-bubble collapse in the neighbourhood of a solid boundary. J Fluid Mech. 1975;72:391–399. [Google Scholar]
- 18.Magasanik B. Regulation of nitrogen utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1344–1356. [Google Scholar]
- 19.McInnes C, Engel D, Martin R W. Bacterial luminescence: a new tool for investigating the effects of acoustic energy and cavitation. J Acoust Soc Am. 1990;88:2527–2532. doi: 10.1121/1.399974. [DOI] [PubMed] [Google Scholar]
- 20.Meighen E M, Dunlap P V. Physiological, biochemical and genetic control of bacterial bioluminescence. Adv Microbiol Physiol. 1993;34:1–67. doi: 10.1016/s0065-2911(08)60027-2. [DOI] [PubMed] [Google Scholar]
- 21.Miller D L, Williams A R. Nucleation and evolution of ultrasonic cavitation in a rotating exposure chamber. J Ultrasound Med. 1992;11:407–412. doi: 10.7863/jum.1992.11.8.407. [DOI] [PubMed] [Google Scholar]
- 22.National Council on Radiation Protection and Measurements. Biological effects of ultrasound: mechanisms and clinical implications, report no. 74. Bethesda, Md: National Council on Radiation Protection and Measurements; 1983. [Google Scholar]
- 23.Nyström T, Neidhardt F C. Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli. Mol Microbiol. 1992;6:3187–3198. doi: 10.1111/j.1365-2958.1992.tb01774.x. [DOI] [PubMed] [Google Scholar]
- 24.Riesz P, Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic Biol Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 25.Rogowsky P M, Close T J, Chimera J A, Shaw J J, Kado C I. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J Bacteriol. 1987;169:5101–5112. doi: 10.1128/jb.169.11.5101-5112.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rupp W D. DNA repair mechanisms. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 2277–2294. [Google Scholar]
- 27.Saier M H, Jr, Ramseier T M, Reizer J. Regulation of carbon utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1325–1343. [Google Scholar]
- 28.Siegele D A, Zambrano M M, Kolter R. Morphological and physiological changes during stationary phase. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1672–1782. [Google Scholar]
- 29.Small P, Blankenhorn D, Welty D, Zinser E, Slonczewski J L. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J Bacteriol. 1994;176:1729–1737. doi: 10.1128/jb.176.6.1729-1737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart G S A B, Williams P. lux genes and the applications of bacterial luminescence. J Gen Microbiol. 1992;138:1289–1300. doi: 10.1099/00221287-138-7-1289. [DOI] [PubMed] [Google Scholar]
- 31.Suslick K S. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 32.Thacker J. An approach to the mechanism of killing of cells in suspension by ultrasound. Biochim Biophys Acta. 1973;304:240–248. doi: 10.1016/0304-4165(73)90241-9. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. Diagnostic ultrasound guidance for 1993, revised ed. 510(k). Rockville, Md: Center for Devices and Radiologic Health; 1993. [Google Scholar]
- 34.Vakil N, Everbach E C. Transient acoustic cavitation in gallstone fragmentation: a study of gallstones fragmented in vivo. Ultrasound Med Biol. 1993;19:331–342. doi: 10.1016/0301-5629(93)90105-w. [DOI] [PubMed] [Google Scholar]
- 35.Van Dyk T K, Belkin S, Vollmer A C, Smulski D R, Reed T R, LaRossa R A. Fusions of Vibrio fischeri lux genes to Escherichia coli stress promoters: detection of environmental stress. In: Campbell A K, Kricka L J, Stanley P E, editors. Bioluminescence and chemiluminescence: fundamentals and applied aspects. Chichester, England: John Wiley and Sons; 1995. pp. 147–150. [Google Scholar]
- 36.Van Dyk T K, Majarian W R, Konstantinov K B, Young R M, Dhurjati P S, LaRossa R A. Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl Environ Microbiol. 1994;60:1414–1420. doi: 10.1128/aem.60.5.1414-1420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Dyk T K, Reed T R, Vollmer A C, LaRossa R A. Synergistic induction of the heat shock response in Escherichia coli by simultaneous treatment with chemical inducers. J Bacteriol. 1995;177:6001–6004. doi: 10.1128/jb.177.20.6001-6004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vollmer A C. Genotoxic sensors. In: LaRossa R A, editor. Methods in molecular biology: bioluminescence methods and protocols. Totowa, N.J: Humana Press; 1998. pp. 145–151. [DOI] [PubMed] [Google Scholar]
- 39.Vollmer, A. C. Unpublished results.
- 40.Vollmer A C, Belkin S, Smulski D R, Van Dyk T K, LaRossa R A. Detection of DNA damage by use of Escherichia coli carrying recA′::lux, uvrA′::lux, or alkA′::lux reporter plasmids. Appl Environ Microbiol. 1997;63:2566–2571. doi: 10.1128/aem.63.7.2566-2571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmer A C, Makin I R S, Everbach E C. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Induction of the heat shock response and the SOS response in Escherichia coli by the effects of acoustic cavitation from ultrasound, abstr. I-85; p. 317. [Google Scholar]
- 42.Walker G C. The SOS response of Escherichia coli. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1400–1416. [Google Scholar]
- 43.Wanner B L. Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 1357–1381. [Google Scholar]
- 44.Williams A R, Miller D L. The role of non-acoustic factors in the induction and proliferation of cavitational activity in vitro. Phys Med Biol. 1989;34:1561–1569. doi: 10.1088/0031-9155/34/11/005. [DOI] [PubMed] [Google Scholar]