Abstract
Background: Transfusion of granulocytes obtained by apheresis is beneficial in febrile neutropenia (FN) but expensive and time-consuming. Buffy-coat-derived granulocytes could be an alternative. We studied the efficacy and safety of the administration of irradiated buffy-coat-derived granulocytes along with the standard of care in pediatric high-risk (HR) FN. Methods: Sixty children ≤18 years with malignancy and chemotherapy-induced HR FN were randomized to either the granulocyte transfusion (GT) arm which received irradiated buffy-coat derived granulocyte transfusion along with the standard treatment or the standard treatment (ST) arm. Results: Baseline characteristics, day-to-defervescence, antibiotic duration, hospital stay, and mortality were comparable between the groups. A significant difference was seen in days to achieve absolute neutrophil count (ANC) >500/mm3 in the 2 groups: 4.5 days (3-6.5) in the GT arm v/s 8 days (4-11) in the ST arm (P=0.01). Conclusion: Buffy-coat-derived granulocyte transfusion was safe and led to early hematological recovery but was not associated with survival benefits. Future studies with earlier initiation in the intended dose could be undertaken to generate more evidence.
Keywords: Febrile neutropenia, pediatrics, buffy-coat granulocytes
Introduction
Cancer is a major cause of morbidity and mortality in children [1]. The prognosis of pediatric cancers has improved due to the availability of modern chemotherapeutic agents and good supportive care. Febrile neutropenia (FN) constitutes a significant proportion of hospital admissions and FN-related mortality in India varies from 3%-10.3% [2]. In high-risk (HR) FN, the mortality can be as high as 23.7% [3]. FN is defined as a single oral temperature of more than 101°F or 100.4°F sustained over 1 hour with an absolute neutrophil count of less than 500/mm3 or expected to fall below 500/mm3 in the next 48 hours. Common infections include pneumonia, bloodstream infections, neutropenic enterocolitis, or fever without focus. Early initiation of antimicrobials and supportive care is the standard of care in FN.
Granulocyte transfusions (GT) have been used as a supportive measure in FN. Apheresis-derived granulocytes from a single donor are the preferred choice; however, the need for a group-specific donor, cost, and time taken for the procedure makes buffy coat-derived granulocytes an alternative to consider in resource-poor countries. Buffy coat is usually a by-product of blood component separation. Studies have shown that the chemotaxis, adhesion, reactive oxygen species production, and in vitro, killing of microorganisms by buffy coat-derived granulocytes are similar to apheresis-derived granulocytes [4]. Various trials have demonstrated the safety and feasibility of GT in children [5-8]. Granulocyte transfusion may help in bridging the gap between neutropenia and marrow recovery by replenishing the deficient granulocytes to combat infection. The paucity of prospective large studies in pediatric oncology has limited the use of GT in children.
Methods
Study design and patients
This open-label randomized controlled trial was conducted from August 2020 to March 2022 in a tertiary care teaching institute in India. The study was approved by the institute ethics committee (IECPG-426/26.08.20, RT-19/23.09.2020) and the study was prospectively registered in the clinical trial registry of India (CTRI/2020/10/028204).
Inclusion criteria: (1) Children with age less than or equal to 18 years with malignancy and chemotherapy-induced FN and (2) Any of the following High-risk FN features including: (a) who received strong myelosuppressive chemotherapy; (b) whose malignancy was not in remission; (c) those with any clinical focus of infection that would require prolonged systemic antibiotics; (d) presence of mucositis grade 3 or grade 4 and (e) those with profound neutropenia (absolute neutrophil count (ANC) <100/mm3).
Exclusion criteria: (1) Children in palliative care. (2) Hematopoietic stem cell transplantation (HSCT) recipient. (3) Those in the recovery phase of febrile neutropenia. (4) Non-availability of the patient’s blood group-specific buffy coat.
Children were assessed for enrolment after meeting inclusion criteria and written consent was obtained from parent/legally authorized representative.
Outcome assessment
The primary objective of the study was to evaluate the efficacy of the addition of irradiated buffy coat-derived GT to the standard of care (antimicrobials and best supportive care) in reducing the 28-day mortality. Secondary outcomes included the comparison of duration of hospital stay, antibiotic duration, days to defervescence, and occurrence of adverse events between the GT arm and standard of care treatment (ST) arm.
Sample size
Sample size calculation was based on the mortality rate in high-risk febrile neutropenia (primary outcome). Based on a retrospective analysis (unpublished data) of admitted children over the past 1 year, the mortality in children admitted with FN was documented to be 15%. To detect a decrease in the 28-day mortality of 15% in the control group to 5% in the intervention (GT) group at a power of 80% and alpha error of 5%, 138 children were required to be enrolled in each arm. This study was done as a pilot study and we included 30 participants in each arm.
Definitions
Phases of chemotherapy (supplement)
Intensive phase of chemotherapy
Chemotherapy phases which are strongly myelosuppressive and myeloablative chemotherapy.
Non-intensive chemotherapy
Chemotherapy that is minimally or briefly myelosuppressive.
Grade 3 mucositis
Patients with oral ulcers and able to take liquids alone orally.
Grade 4 mucositis
Patients with oral ulcers and not able to take both solids and liquids orally.
Randomization
We used computer-generated block randomization with variable block size. The randomization codes, thus generated, were kept with an individual not involved in the enrolment of the subjects, administration of the intervention, or the analysis of the data. Allocation concealment was achieved by the use of serially numbered opaque sealed envelopes.
Intervention arm (GT arm)
This group was given irradiated buffy coat derived granulocyte transfusion at 10 ml/kg every alternate day till the child was afebrile for >24 hrs without antipyretics and there was evidence of spontaneous neutrophil recovery (ANC≥500/mm3) for 2 consecutive days or occurrence of a life-threatening adverse event or till a maximum of 5 GTs. Granulocytes were procured from the blood bank after cross-matching for ABO, Rh groups, and X-ray irradiation (25 Gy). The granulocyte count of the final product was checked by the blood bank before issue. The children were premedicated with an injection of paracetamol dosed at 15 mg/kg, injection of hydrocortisone at 4 mg/kg, and chlorpheniramine maleate at 0.1 mg/kg, 30 minutes before transfusion. The granulocyte preparation was transfused within 6 hours of collection and given for 2-3 hours. The vital signs were monitored every 15 minutes for 1 hour and then for 30 minutes for the next 1 hour and then hourly till the end of transfusion to ensure safety. In the event of a life-threatening adverse reaction the transfusion had to be stopped immediately; however, none of the children had any serious events. If the child was also receiving amphotericin-B, the GT was given either before or after 6 hours to avoid pulmonary toxicity. The efficacy of transfusion was assessed by the rise in absolute neutrophil count. The intervention arm also received antimicrobials, chemotherapy, and G-CSF as per the standard treatment protocol.
Control arm
Standard therapy included antimicrobials, blood component support, and G-CSF as per the protocol. Children in both groups without any identified focus were started on intravenous antibiotics as per the institutional protocol which included a combination therapy with antipseudomonal penicillin (Piperacillin + Tazobactam - if platelet count was <20,000/mm3) or a third-generation cephalosporin (Cefoperazone + sulbactam - if platelet count was ≥20,000/mm3) and an aminoglycoside (Amikacin). The patients received these antibiotics at age and weight-appropriate dosages. The patients were admitted as an inpatient in case of a HR FN, however, if a hospital bed could not be made available. the antibiotics were administered in the day care on an ambulatory basis and randomization was done only after inpatient admission. The baseline investigations like complete blood counts (CBC), serum electrolytes, renal function tests (RFTs), liver function tests (LFTs), procalcitonin (PCT), chest X-ray (CXR), blood culture and sensitivity, and other microbiological samples if required were sent routinely as per unit policy before initiation of empirical antibiotics.
Urine microscopy and urine culture samples obtained by mid-stream clean catch in older children and children were not catheterized to collect the sample because of neutropenia.
The children were closely followed up clinically and CBC was done daily till afebrile and ANC recovery. PCT was repeated after 48 to 72 h of starting empirical antibiotics. If there was clinical worsening with no obvious focus with persistent fever and sterile microbial cultures after 48-72 h, antibiotics were upgraded to Meropenem and Vancomycin. In case of persistent fever for 5-7 days of starting antibiotics, antifungal therapy was added; preferably Liposomal Amphotericin-B. Relevant radiological investigations (CT chest) and serum galactomannan assay were done. CT paranasal sinuses and ultrasound abdomen were done as per requirement on a case-to-case basis. G-CSF, PRBC, and platelet transfusion were given as per protocol. Blood culture was taken before changing antibiotics. Antibiotics were modified according to the culture sensitivity report. The patient was discharged after an afebrile period of >48 h with stable vitals. Antibiotics were continued till an afebrile period of >24 h with neutrophil recovery in case of sterile blood culture. Occurrences of outcomes like microbiologically documented infection, radiologically defined pneumonia, hemodynamic instability, septic shock, transfusion reactions, and mortality were documented.
Adverse effect monitoring and safety
A data safety monitoring board (DSMB) was constituted which included a DSMB chair (senior professor of Pediatrics), a subject expert (Oncologist), and a bio-statistician. The first DSMB meeting was conducted before study enrolment. The second meeting was conducted (online through videoconferencing) after 50% enrolment and mortalities in both groups were discussed for causality assessment. The board was communicated periodically through e-mail and they reviewed and evaluated the accumulated study data for participant safety, study conduct and progress, and when appropriate, efficacy. The final meeting was done after the completion of the study (full enrolment).
Statistical analysis
Data analysis was done by STATA/SE 11.2 (Stata Corp, College Station, TX). Analysis was done on an intention-to-treat basis. For the primary outcome variable (incidence of mortality), Fischer’s exact test was used to compare the proportion of categorical variables. For the categorical variables in the secondary outcome, Fisher’s exact test/Chi-square test was used to compare the proportions. For continuous variables in the secondary objective mean ± standard deviation was done and compared using the Student t-test for normative distribution, and median with interquartile range with Mann Whitney U test for non-normative distribution. Two-sided P<0.05 was considered statistically significant.
Results
Eighty-three children with high-risk febrile neutropenia were screened and sixty-two children were found to be eligible (Figure 1). Two eligible children could not be enrolled because of the non-availability of group-specific granulocytes.
Figure 1.
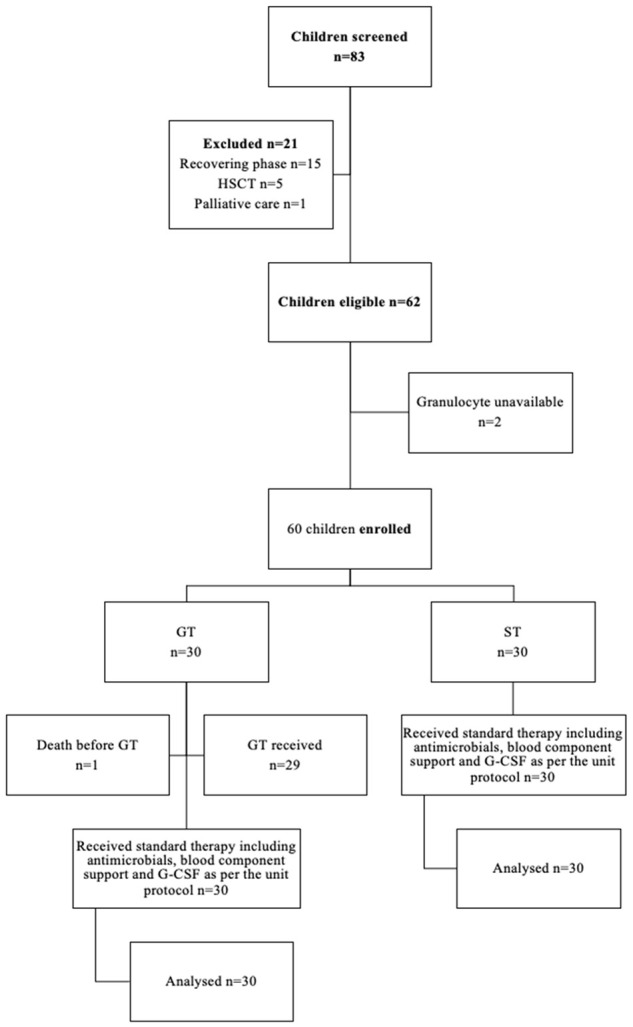
Study flow diagram.
Baseline characteristics and investigations were comparable between both the groups and have been depicted in Tables 1 and 2. More than 50% of the subjects in both groups received intravenous antibiotics on an ambulatory basis before admission.
Table 1.
Baseline characteristics
| Characteristics | GT n=30 | ST n=30 | p value |
|---|---|---|---|
| Age | 5.5 (4-10.2) | 5 (4-9.2) | 0.70 |
| Gender | 0.59 | ||
| Male | 18 (60%) | 21 (70%) | |
| Female | 12 (40%) | 9 (30%) | |
| Primary diagnosis | |||
| Hematological malignancies | |||
| ALL | 10 (33.3%) | 14 (46.7%) | 0.54 |
| AML | 6 (20%) | 7 (23.3%) | |
| ALL relapse | 3 (10%) | 1 (3.3%) | |
| AML relapse | 1 (3.3%) | 1 (3.3%) | |
| Lymphomas | 3 (10%) | 5 (16.7%) | |
| Solid tumors | |||
| Retinoblastoma | 1 (3.3%) | 1 (3.3%) | |
| Ewing’s sarcoma | 4 (13.3%) | 1 (3.3%) | |
| Others | 2 (6.7%) | 0 | |
| Focus of infection | |||
| Respiratory | 10 (33%) | 10 (33%) | 0.21 |
| GI | 17 (57%) | 11 (37%) | |
| Both | 0 | 1 (3%) | |
| No focus | 3 (10%) | 8 (27%) | |
| CXR abnormality at admission | 2 (7.1%) | 4 (14.3%) | 0.67 |
| Total febrile period before admission (days) | 3 (1-5.2) | 3 (1-7) | 0.32 |
| Antibiotic received before admission | |||
| No antibiotics | 13 (43.3%) | 14 (46.7%) | 0.90 |
| First line antibiotics | 4 (13.3%) | 4 (13.3%) | |
| Second line antibiotics | 12 (40%) | 10 (30.3%) | |
| Empirical Antifungals | 1 (3.3%) | 2 (6.7%) |
Categorical variables were compared using Chi-square test and continuous variables using Mann-Whitney U test.
Table 2.
Baseline investigations
| Investigation | GT n=30 | ST n=30 | p value |
|---|---|---|---|
| Hemoglobin (g/dL) | 6.9 (6.1-7.7) | 7 (6-8.9) | Not significant |
| Total leucocyte count (×109/L) | 520 (275-870) | 405 (200-850) | |
| Absolute neutrophil count (ANC) (×109/L) | 70 (37.5-127.5) | 60 (40-122) | |
| Platelet count (×109/L) | 13.5 (5.75-35) | 27 (9.25-116.25) | |
| Procalcitonin (ng/ml) | 3.45 (0.49-17.25) | 1.9 (0.55-7) |
Categorical variables were compared using Chi-square test and continuous variables using Mann-Whitney U test.
Granulocyte transfusion details
Median number of granulocyte transfusions was 1 (1-4). Granulocyte transfusion was done on a median of 2 days from the day of randomization. One child was randomized in the GT arm but the child died before receiving granulocyte transfusion. The average dose of granulocytes given to a child on the GT arm was 0.27×109/kg.
Patients in both groups received the standard treatment as per the unit’s policy, consisting of intravenous antibiotics, antifungals, blood component support, granulocyte colony-stimulating factor support, and other supportive measures as necessary.
Primary outcome
There were 8 deaths in the GT arm and 2 deaths in the ST arm, however it was not statistically significant (P=0.08). In the GT arm, the primary diagnosis in non-survivors was AML (n=3), and ALL (n=3) with two of them in the induction phase and 1 relapse each. Four of the 8 mortalities in the GT arm had microbiologically documented infection. It included 2 bloodstream infections: Acinetobacter baumanni and Klebsiella pneumoniae, one disseminated fungal infection (Mucormycosis), and one influenza virus lower respiratory tract illness leading to multiorgan dysfunction. In the ST arm, there were 2 deaths; both patients were in the ALL consolidation phase and succumbed to clinically diagnosed sepsis with no blood culture positivity. None of the deaths in the GT arm were attributed to the intervention after the causality assessment and DSMB review (Table 3).
Table 3.
Primary and secondary outcome parameters
| Outcomes | GT n=30 | ST n=30 | p value |
|---|---|---|---|
| 28 day mortality | 8 (26.7%) | 2 (6.7%) | 0.08 |
| Day to defervescence from randomisation | 8 (5-12) | 8 (5-11) | 0.86 |
| Days to achieve ANC>500/mm3 | 4.5 (3-6.5) | 8 (4-11) | 0.01 |
| Antibiotic days | 23 (15.8-30.5) | 19 (12.8-28) | 0.18 |
| Duration of hospital stay | 11 (8-5) | 9 (6-14.25) | 0.51 |
| Hemodynamic instability | 2 (6.7%) | 2 (6.7%) | 1.00 |
| Need for oxygen | 16 (53.3%) | 14 (46.7%) | 0.79 |
| Need for ventilation | 8 (26.7%) | 2 (6.7%) | 0.08 |
| Need for pediatric intensive care unit admission | 6 (20%) | 1 (3.3%) | 0.10 |
| Need for high dependency unit admission | 10 (33.3%) | 4 (13.3%) | 0.12 |
| Need for inotropes | 9 (30%) | 7 (23.3%) | 0.77 |
| Microbiologically documented infection | 9 (30%) | 7 (23.3%) | 0.77 |
| Radiologically defined pneumonia | 10 (33.3%) | 10 (33.3%) | 1.00 |
| Volume overload | 4 (13.3%) | 0 | 0.11 |
| Transfusion adverse events | 1 (3.3%) | 0 | 1.00 |
| PRBC requirement | 27 (90%) | 25 (83.3%) | 0.70 |
| RDP requirement | 24 (80%) | 21 (70%) | 0.55 |
| GCSF administered | 22 (73.3%) | 18 (60%) | 0.41 |
| Antivirals received | 4 (13.3%) | 3 (10%) | 1.00 |
| Antifungals received | 18 (60%) | 16 (53.3%) | 0.79 |
Categorical variables were compared using Chi-square test/Fischer-exact test and continuous variables using Mann-Whitney U test.
Secondary outcomes
The median day to defervescence from randomization was 8 (5-12) d in GT and 8 (5-11) d in ST (P=0.86). A significant difference was observed in the days to achieve ANC>500/mm3 between the GT arm 4.5 (3-6.5) d and ST arm 8 (4-11) d; (P=0.01). The median number of antibiotic days was 23 (15.8-30.5) d in the GT group and 19 (12-28) in the ST group (P=0.18). The duration of hospital stay was comparable between the groups (11 d in granulocyte and 9 d in standard arm, P=0.51). There were no differences in oxygen requirement, intensive care admission, or ventilatory requirement between GT and ST arm.
One child developed volume overload secondary to granulocyte transfusions which were managed and resolved completely. No other adverse events were documented secondary to buffy coat-derived granulocyte transfusion (Table 3).
Predictors of mortality (Tables 4 and 5)
Table 4.
Comparison of baseline characteristics between survivors and non-survivors
| Baseline Characteristics | Survivors n=50 | Not survivors n=10 | p value |
|---|---|---|---|
| Gender | |||
| Males | 31 (62%) | 8 (80%) | 0.47 |
| Females | 19 (38%) | 2 (20%) | |
| Primary Diagnosis | |||
| ALL | 19 (38%) | 5 (50%) | 0.56 |
| AML | 10 (20%) | 3 (30%) | |
| ALL relapse | 3 (6%) | 1 (10%) | |
| AML relapse | 1 (2%) | 1 (10%) | |
| Lymphomas | 8 (16%) | 0 | |
| Retinoblastoma | 2 (4%) | 0 | |
| Ewing’s sarcoma | 5 (10%) | 0 | |
| Others | 2 (4%) | 0 | |
| Antibiotic received before admission | |||
| No antibiotics | 26 (52%) | 1 (10%) | 0.02 |
| First line antibiotics | 7 (14%) | 1 (10%) | |
| Second line antibiotics | 15 (30%) | 7 (70%) | |
| Empirical Antifungals | 2 (4%) | 1 (10%) | |
| Focus of infection | |||
| Respiratory | 16 (32%) | 4 (40%) | 0.84 |
| GI | 23 (46%) | 5 | |
| Both | 1 (2%) | 0 | |
| No focus | 10 (20%) | 1 | |
| Chest X ray abnormality at admission | 6 (12%) | 0 | 0.57 |
Table 5.
Comparison of outcomes between survivors and non-survivors
| Outcomes | Survived n=50 | Not survived n=10 | p value |
|---|---|---|---|
| Blood culture positivity | 3 (6%) | 4 (40%) | 0.99 |
| Need for oxygen | 20 (40%) | 10 (100%) | 0.01 |
| Need for ventilation | 0 | 10 (100%) | <0.01 |
| Need for PICU | 3 (6%) | 4 (40%) | 0.01 |
| Need for HDU | 6 (12%) | 8 (80%) | <0.01 |
| Need for inotropes | 6 (12%) | 10 (100%) | <0.01 |
| Microbiologically documented infection | 11 (22%) | 5 (50%) | 0.08 |
| Radiologically defined pneumonia | 12 (24%) | 8 (80%) | 0.01 |
| Volume overload | 1 (2%) | 3 (30%) | 0.01 |
| Transfusion adverse events | 1 (2%) | 0 | 0.99 |
| PRBC | 42 (84%) | 10 (100%) | 0.33 |
| RDP | 35 (70%) | 10 (100%) | 0.05 |
Categorical variables were compared using Chi-square test/Fischer-exact test and continuous variables using Mann-Whitney U test.
Previous antibiotic administration on an ambulatory basis before admission was associated with mortality. Other baseline characteristics like age, primary diagnosis, and chest x-ray abnormality at admission didn’t differ between the ones who survived and the ones who died. Oxygen requirement, inotrope requirement, intensive care admission, and need for ventilation were significantly more in the participants who died.
Discussion
In this study, we evaluated the safety and efficacy of buffy coat-derived granulocyte transfusion in HR FN. Good supportive care is needed for favorable outcomes in FN. Mortality due to FN ranges from 10.3% to 23.7% in developed countries [3,9]. The overall mortality in our study was 16.7%.
GT has been used in FN; however, mixed results in efficacy have resulted in it being considered an underutilized intervention [10]. Until recently, it remained a largely unexplored area barring some retrospective and very few prospective studies which identified its beneficial effect [6,10,11]. Granulocytes are derived predominantly from apheresis of the ABO blood group and Rh-matched donor after dexamethasone and G-CSF-based mobilisation [12]. Buffy coat is a reasonable alternative in a resource-limited setting. We studied the use of buffy coat because of the easy availability of the product and its cost-effectiveness.
In a study by Mastronardi et al., buffy coat-derived granulocytes were analyzed for neutrophil content, viability, and function (measured by reactive oxygen species production), which was comparable to apheresis derived and they recommended it as a viable source [13]. Van de Geer et al. demonstrated that in buffy coat-derived granulocyte chemotaxis, adhesion, degranulation, reactive oxygen species production, in vitro killing was comparable with G-CSF/Dexamethasone stimulated granulocytes [4]. In the NHS clinical guidelines, the Granulocyte working group has also recommended the use of buffy coat-derived granulocyte transfusion as an alternative [12]. In a case report by Gupta et al., both apheresis-derived and buffy coat-derived granulocyte showed a comparable rise in ANC [14]. Buffy coat-derived GT has not been studied prospectively or compared with apheresis-derived granulocyte transfusion. We studied the use of buffy coat-derived granulocytes versus standard care in high-risk FN.
The commonest malignancy in our study was acute leukemia which was similar to other studies [5,8,15]. Gram-negative organisms are the commonest organisms isolated, among which E. coli was the commonest isolate similar to the previous study from our centre [2]. Blood culture positivity was comparable to other studies [2]. In a study by Bothra et al., in children who were on oral antibiotics, chest x-ray abnormalities at presentation were identified as predictors for adverse outcomes [2]. A significant proportion of children received intravenous antibiotics before admission on an ambulatory basis, with the majority of them already on second-line antibiotics at the time of enrolment. We identified that those who received antibiotics on an ambulatory basis before admission, which was a surrogate for delayed admission, were associated with mortality. We started GT soon after randomization however there was a time-lapse from the onset of FN to admission for those who received antibiotics on an ambulatory basis. This may indicate a delay in the initiation of GT in a particular febrile episode which could have led to inferior results. But in a setup like ours, the majority of patients with febrile neutropenia are treated on an outpatient basis due to a shortage of admission beds often without a need for admission. In a study by Meena et al., 86% of febrile neutropenia children recovered with outpatient treatment in a resource-limited setting [16].
The survival outcomes of the granulocyte transfusion cohort were comparable with other pediatric studies with febrile neutropenia who received granulocyte transfusion. It was 73% in the GT arm which was comparable to other studies including viz Siedel et al. (72%), Atay et al. (77%), Ozturkmen et al. (60%) and Sach et al. (81.5%) [5,6,17,18]. The mortality rate was higher in the GT arm compared to the standard arm. There were 8 deaths in the GT arm and 2 deaths in the ST arm. All deaths were in children with hematological malignancies. In the GT arm deaths, the primary diagnosis included 2 relapsed ALL and 2 AML cases; however, the ST arm had no relapse cases. General sickness levels could be higher in relapse cases receiving intensive chemotherapy. When we compared the children who died with those who survived we found that a greater proportion of those who succumbed had been on intravenous antibiotics on an ambulatory basis before admission compared to the ones who survived.
There was a significant difference in the time to attain an ANC>500/mm3. The GT arm had a faster neutrophil recovery compared to the standard arm among those who achieved hematological response however, there was no difference in the duration of hospital stay, antibiotic days, and occurrence of adverse events. Even though there was faster neutrophil recovery, it did not translate into a clinical and mortality benefit. It was similar to the observation in previous studies where a faster hematological recovery was not converted into a clinical benefit [19,20].
The dose of granulocyte transfusions, initiation time, and its correlation with disease outcomes in previous studies has shown conflicting results. Kim et al. in a retrospective review of adult hematological cases found that there was no correlation between the dose of granulocyte transfusion and clinical outcomes [19]. On the contrary in a retrospective study, Luciana et al. found that a standard dose of 1.5-3×108/kg is more beneficial, which has been proven in other studies including the RING study where post-hoc analysis demonstrated that a dose of more than 0.6×109/kg was beneficial [21]. Siedel et al. found that early initiation and a dose of at-least 3×108/kg were desirable for good outcomes [17]. The dose of granulocyte transfusion (0.27×109/kg) in our cohort was lower compared to what other studies have found, which may be related to granulocytes derived from buffy coat compared to those stimulated by dexamethasone and G-CSF in apheresis derived granulocyte transfusion.
Garg et al. found that the early initiation of GT within 7 days of neutropenic sepsis led to an improvement in 30-day survival, and also had a decreased febrile period [11]. A multicentric prospective trial in the pediatric population by Sach et al. showed that the early initiation of granulocyte transfusion was beneficial [5]. Uppuluri et al. demonstrated a 13% survival benefit in a retrospective analysis when GT was initiated within 48 hours of septicemia compared to late initiation [22]. In our study, even though the median day of initiation of granulocyte transfusion was day 2 of randomization, a greater proportion of patients were on ambulatory care antibiotics on an outpatient basis before enrolment which was not uniform within the cohort.
The discrepancy between hematological and clinical response could be related to irreversible damage caused by sepsis that could not have been reversed even after the restoration of neutrophils. In some studies, it was found that clinical response was obtained even in the absence of a hematological response because neutrophil migration to diseased tissue sites occurs before ANC recovery [23].
Severe adverse effects are rare with GT (5% of the recipients) which include hypotension, pulmonary infiltrates, and respiratory distress [24], Price et al. documented up to 20% grade 3-4 reactions in a randomised adult study where apheresis-derived granulocyte transfusions were used [21]. We found that buffy coat-derived GT was safe to administer. There was one child who developed volume overload secondary to granulocyte transfusion. Hypotension, fluctuation in arterial saturation, and TRALI were not documented in our study which was reported in apheresis-derived GT [11,18].
The effect of the addition of granulocyte transfusion derived from buffy coat to standard of care in pediatric malignancy with FN has not been explored till now. We chose buffy coat-derived granulocyte which is economical compared to apheresis-derived GT and its use has not been studied in a controlled setting. We were able to demonstrate a faster ANC recovery with the use of granulocytes and were able to demonstrate their safety.
The shortcomings of our study were the small sample size and the buffy coat given according to the weight which may not contain the required dose. There was some delay in the initiation of buffy coat in some cases because of late admission due to the non-availability of in-patient hospital beds. Future studies with earlier initiation of buffy coat granulocytes in the intended dose could be undertaken to generate more evidence to either support or refute this modality of supportive care which is easily available and cheaper compared to the apheresis granulocyte transfusions.
Conclusions
Buffy coat-derived granulocyte transfusion was safe to administer in children. Granulocyte transfusion leads to early hematological recovery but it did not lead to survival benefit.
Acknowledgements
The authors acknowledge the children who participated in the study and the staff of the blood bank involved in the preparation of the buffy coat granulocytes. The authors also acknowledge the members of the data safety monitoring board namely Prof. Sushil Kabra, Dr. Akash Jha, and Dr. Maroof A Khan.
Disclosure of conflict of interest
None.
Abbreviations
- FN
Febrile neutropenia
- GT
Granulocyte transfusion
- ST
Standard treatment
- HSCT
Hematopoietic stem cell transplant
- ANC
Absolute neutrophil count
- Gy
grays
- PCT
procalcitonin
References
- 1.Bashar MA, Thakur JS. Incidence and pattern of childhood cancers in India: findings from population-based cancer registries. Indian J Med Paediatr Oncol. 2017;38:240–241. doi: 10.4103/ijmpo.ijmpo_163_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bothra M, Seth R, Kapil A, Dwivedi SN, Bhatnagar S, Xess I. Evaluation of predictors of adverse outcome in febrile neutropenic episodes in pediatric oncology patients. Indian J Pediatr. 2013;80:297–302. doi: 10.1007/s12098-012-0925-3. [DOI] [PubMed] [Google Scholar]
- 3.Lai HP, Hsueh PR, Chen YC, Lee PI, Lu CY, Lu MY, Lin WC, Hsieh YC, Lee CY, Lin KH, Huang LM. Bacteremia in hematological and oncological children with febrile neutropenia: experience in a tertiary medical center in Taiwan. J Microbiol Immunol Infect. 2003;36:197–202. [PubMed] [Google Scholar]
- 4.van de Geer A, Gazendam RP, Tool AT, van Hamme JL, de Korte D, van den Berg TK, Zeerleder SS, Kuijpers TW. Characterization of buffy coat-derived granulocytes for clinical use: a comparison with granulocyte colony-stimulating factor/dexamethasone-pretreated donor-derived products. Vox Sang. 2017;112:173–182. doi: 10.1111/vox.12481. [DOI] [PubMed] [Google Scholar]
- 5.Sachs UJ, Reiter A, Walter T, Bein G, Woessmann W. Safety and efficacy of therapeutic early onset granulocyte transfusions in pediatric patients with neutropenia and severe infections. Transfusion. 2006;46:1909–1914. doi: 10.1111/j.1537-2995.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 6.Atay D, Ozturk G, Akcay A, Yanasik M, Anak S, Devecioglu O. Effect and safety of granulocyte transfusions in pediatric patients with febrile neutropenia or defective granulocyte functions. J Pediatr Hematol Oncol. 2011;33:e220–225. doi: 10.1097/MPH.0b013e31821ffdf1. [DOI] [PubMed] [Google Scholar]
- 7.Aktekin E, Bay A, Yılmaz M. Granulocyte transfusion therapy in childhood. Indian J Hematol Blood Transfus. 2017;33:417–420. doi: 10.1007/s12288-016-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidel MG, Minkov M, Witt V, Matthes-Martin S, Pötschger U, Worel N, Leitner G, Stary J, Gadner H, Peters C. Granulocyte transfusions in children and young adults: does the dose matter? J Pediatr Hematol Oncol. 2009;31:166–172. doi: 10.1097/MPH.0b013e318196a6f9. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Trehan A, Bansal D. Risk factors for microbiologically-documented infections, mortality and prolonged hospital stay in children with febrile neutropenia. Indian Pediatr. 2018;55:859–864. [PubMed] [Google Scholar]
- 10.Estcourt LJ, Stanworth S, Doree C, Blanco P, Hopewell S, Trivella M, Massey E. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2015;2015:CD005341. doi: 10.1002/14651858.CD005341.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg A, Gupta A, Mishra A, Singh M, Yadav S, Nityanand S. Role of granulocyte transfusions in combating life-threatening infections in patients with severe neutropenia: experience from a tertiary care centre in North India. PLoS One. 2018;13:e0209832. doi: 10.1371/journal.pone.0209832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elebute M, Massey E, Benjamin S, Stanworth S, Lucas G. Clinical guidelines for the use of granulocyte transfusions. 2016 [Google Scholar]
- 13.Mastronardi R, Cleophax S, Begué S, Hurtado-Nedelec M, Gross S, Bocquet T, Djoudi R. Preparation of pooled granulocytes concentrates from whole blood buffy coats (PGC) as an alternative to apheresis. Transfus Clin Biol. 2019;26:164–170. doi: 10.1016/j.tracli.2019.06.188. [DOI] [PubMed] [Google Scholar]
- 14. https://www.gjtmonline.com/temp/GlobJTransfusMed61106-5150996_141829.pdf.
- 15.Prasad M, Chinnaswamy G, Arora B, Vora T, Hawaldar R, Banavali S. Risk predictors for adverse outcome in pediatric febrile neutropenia: single center experience from a low and middle-income country. Indian J Cancer. 2014;51:432–7. doi: 10.4103/0019-509X.175321. [DOI] [PubMed] [Google Scholar]
- 16.Meena JP, Gupta AK, Seth R. Outcomes of febrile neutropenia in children with cancer managed on an outpatient basis: a report from tertiary care hospital from a resource-limited setting. J Pediatr Hematol Oncol. 2020;42:467–473. doi: 10.1097/MPH.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 17.Seidel MG, Peters C, Wacker A, Northoff H, Moog R, Boehme A, Silling G, Grimminger W, Einsele H. Randomized phase III study of granulocyte transfusions in neutropenic patients. Bone Marrow Transplant. 2008;42:679–684. doi: 10.1038/bmt.2008.237. [DOI] [PubMed] [Google Scholar]
- 18.Öztürkmen S, Altuntaş F, Olcay L. Granulocyte transfusion therapy in paediatric patients with severe neutropenic infection. Transfus Apher Sci. 2013;48:381–385. doi: 10.1016/j.transci.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Lim HJ, Kim JS, Kim BS, Bang SM, Kim I, Han KS, Kim BK, Lee SM, Yoon SS. Therapeutic granulocyte transfusions for the treatment of febrile neutropenia in patients with hematologic diseases: a 10-year experience at a single institute. Cytotherapy. 2011;13:490–498. doi: 10.3109/14653249.2010.529889. [DOI] [PubMed] [Google Scholar]
- 20.Díaz R, Soundar E, Hartman SK, Dreyer Z, Teruya J, Hui SK. Granulocyte transfusions for children with infection and neutropenia or granulocyte dysfunction. Pediatr Hematol Oncol. 2014;31:425–434. doi: 10.3109/08880018.2013.868562. [DOI] [PubMed] [Google Scholar]
- 21.Price TH. The RING study: a randomized controlled trial of GCSF-stimulated granulocytes in granulocytopenic patients. Blood. 2014;124:SCI-16. [Google Scholar]
- 22.Uppuluri R, Ramachandrakurup S, Vaidhyanathan L, Kandath S, Subburaj D, Raj R. Changing trends in the use of granulocyte transfusions in neutropenic children with sepsis in India. Indian J Hematol Blood Transfus. 2017;33:207–210. doi: 10.1007/s12288-016-0727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price TH, Bowden RA, Boeckh M, Bux J, Nelson K, Liles WC, Dale DC. Phase I/II trial of neutrophil transfusions from donors stimulated with G-CSF and dexamethasone for treatment of patients with infections in hematopoietic stem cell transplantation. Blood. 2000;95:3302–3309. [PubMed] [Google Scholar]
- 24.Pizzo PA, Poplack DG. Principles and Practice of Padiatric Oncology. In: Pizzo PA, Poplack DG, editors. Philadelphia: Wolters Kluwer; 2021. [Google Scholar]


