Abstract
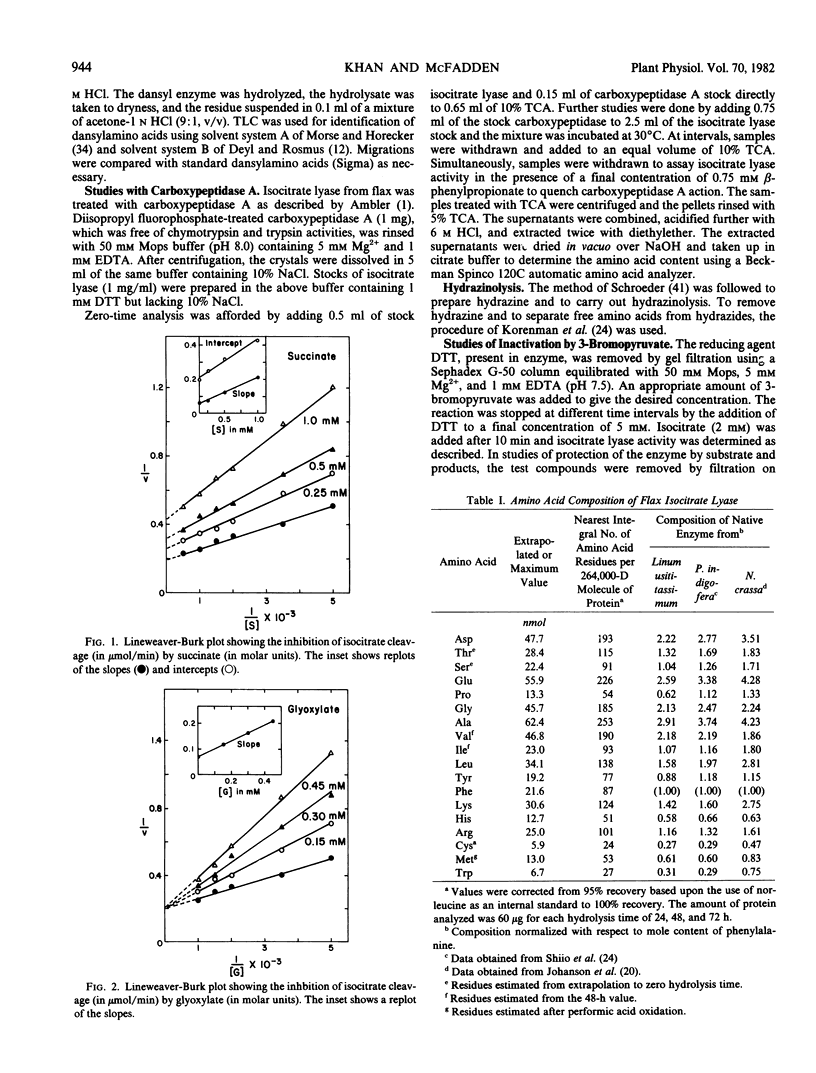
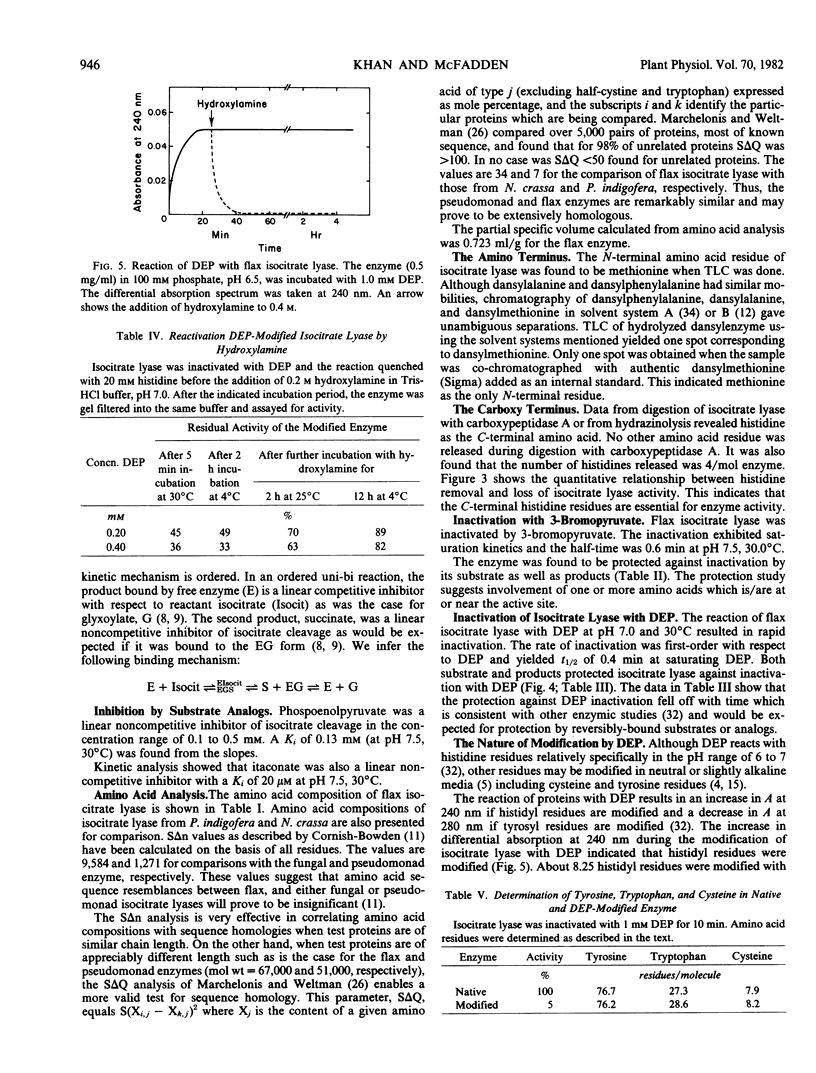
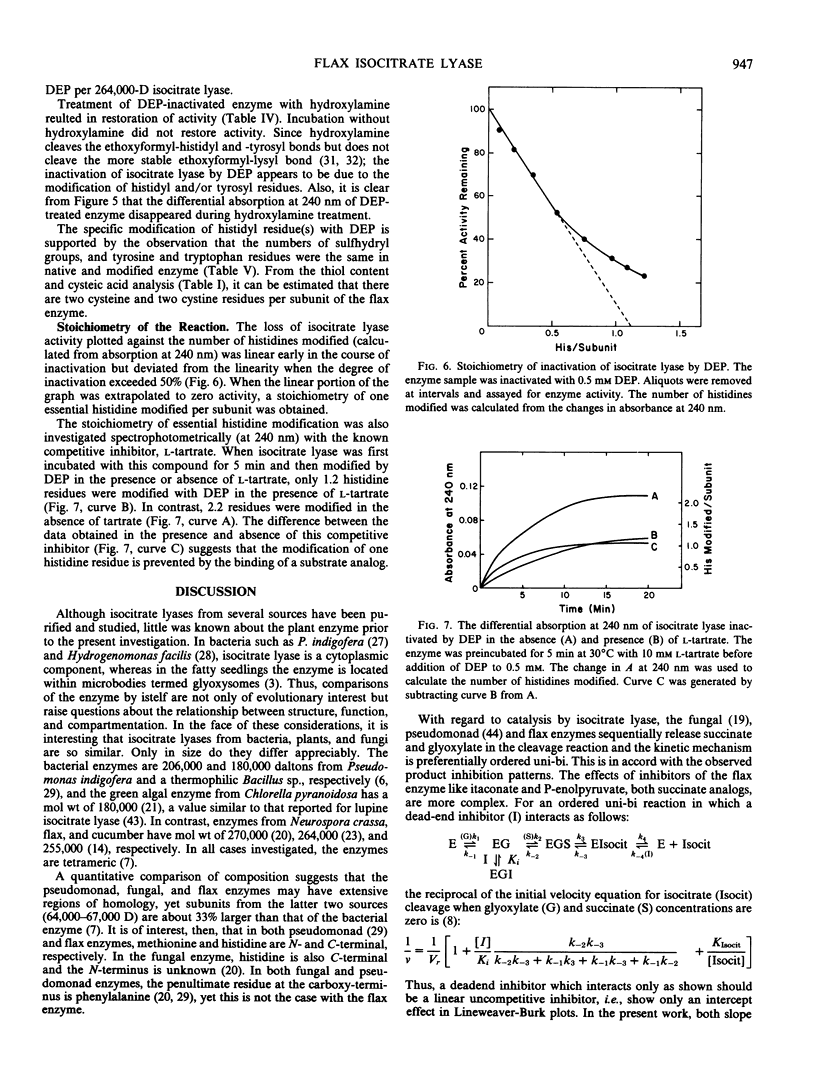
The cleavage of Ds-isocitrate catalyzed by isocitrate lyase from Linum usitatissimum results in the ordered release of succinate and glyoxylate. The glyoxylate analog 3-bromopyruvate irreversibly inactivates the flax enzyme in a process exhibiting saturation kinetics and protection by glyoxylate or isocitrate or the competitive inhibitor l-tartrate. Succinate provides considerably less protection. Results with 3-bromopyruvate suggest that this reagent modifies plant and prokaryotic isocitrate lyases differently. Treatment of the tetrameric 264,000-dalton flax enzyme with carboxypeptidase A results in a release of one histidine/subunit which is concordant with loss of activity. The only N-terminal residue is methionine. Treatment of flax enzyme with diethylpyrocarbonate at pH 6.5 selectively modifies two histidines per 67,000-dalton subunit. The reaction of one histidine residue is abolished by the binding of l-tartrate and the modification of one is coincident with inactivation. The carboxy-terminal and active-site modifications establish that one histidine residue/monomer is essential in the flax enzyme and considerably extend information heretofore available only for fungal and bacterial isocitrate lyase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Berger S. L. Diethyl pyrocarbonate: an examination of its properties in buffered solutions with a new assay technique. Anal Biochem. 1975 Aug;67(2):428–437. doi: 10.1016/0003-2697(75)90315-2. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Walsh K. A., Neurath H. Evidence of an essential histidine residue in thermolysin. Biochemistry. 1974 Jan 1;13(1):205–210. doi: 10.1021/bi00698a030. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Chell R. M., Sundaram T. K., Wilkinson A. E. Isolation and characterization of isocitrate lyase from a thermophilic Bacillus sp. Biochem J. 1978 Jul 1;173(1):165–177. doi: 10.1042/bj1730165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna W. J., McFadden B. A. Isocitrate lyase from parasitic and free-living nematodes. Arch Biochem Biophys. 1975 Oct;170(2):608–619. doi: 10.1016/0003-9861(75)90156-3. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. How reliably do amino acid composition comparisons predict sequence similarities between proteins? J Theor Biol. 1979 Feb 21;76(4):369–386. doi: 10.1016/0022-5193(79)90007-9. [DOI] [PubMed] [Google Scholar]
- Deyl Z., Rosmus J. Thin layer chromatography of Dansyl amino acid derivatives. J Chromatogr. 1965 Dec;20(3):514–520. doi: 10.1016/s0021-9673(01)97453-9. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Frevert J., Kindl H. Plant microbody proteins. Purification and glycoprotein nature of glyoxysomal isocitrate lyase from cucumber cotyledons. Eur J Biochem. 1978 Dec 1;92(1):35–43. doi: 10.1111/j.1432-1033.1978.tb12720.x. [DOI] [PubMed] [Google Scholar]
- Garrison C. K., Himes R. H. The reaction between diethylpyrocarbonate and sulfhydryl groups in carbocylate buffers. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1251–1255. doi: 10.1016/0006-291x(75)90807-4. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros C., Labouesse B. Study of the dansylation reaction of amino acids, peptides and proteins. Eur J Biochem. 1969 Feb;7(4):463–470. doi: 10.1111/j.1432-1033.1969.tb19632.x. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. I. Purification, kinetic mechanism, and interaction with inhibitors. Biochim Biophys Acta. 1974 Oct 17;364(2):327–340. doi: 10.1016/0005-2744(74)90018-7. [DOI] [PubMed] [Google Scholar]
- Johanson R. A., Hill J. M., McFadden B. A. Isocitrate lyase from Neurospora crassa. II. Composition, quaternary structure, C-terminus, and active-site modification. Biochim Biophys Acta. 1974 Oct 17;364(2):341–352. doi: 10.1016/0005-2744(74)90019-9. [DOI] [PubMed] [Google Scholar]
- John P. C., Syrett P. J. The purification and properties of isocitrate lyase from Chlorella. Biochem J. 1967 Oct;105(1):409–416. doi: 10.1042/bj1050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn F. R., McFadden B. A. Embryogenesis and the glyoxylate cycle. FEBS Lett. 1980 Jun 30;115(2):312–314. doi: 10.1016/0014-5793(80)81195-1. [DOI] [PubMed] [Google Scholar]
- Khan F. R., Saleemuddin M., Siddiqi M., McFadden B. A. Purification and properties of isocitrate lyase from flax seedlings. Arch Biochem Biophys. 1977 Sep;183(1):13–23. doi: 10.1016/0003-9861(77)90413-1. [DOI] [PubMed] [Google Scholar]
- Korenman S. G., Craven G. R., Anfinsen C. B. Determination of the carboxyl-terminal amino acid residue of the beta-galactosidase of Escherichia coli K 12. Biochim Biophys Acta. 1966 Jul 27;124(1):160–165. doi: 10.1016/0304-4165(66)90324-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McFadden B. A., Rao G. R., Cohen A. L., Roche T. E. Isocitrate lyase from Pseudomonas indigofera. V. Subunits and terminal residues and the relation to catalytic activity. Biochemistry. 1968 Oct;7(10):3574–3582. doi: 10.1021/bi00850a035. [DOI] [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Miles E. W. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]
- RODGERS K. Estimation of succinic acid in biological materials. Biochem J. 1961 Aug;80:240–244. doi: 10.1042/bj0800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche T. E., McFadden B. A. Active site modification of isocitrate lyase. Biochem Biophys Res Commun. 1969 Oct 8;37(2):239–246. doi: 10.1016/0006-291x(69)90725-6. [DOI] [PubMed] [Google Scholar]
- Roche T. E., McFadden B. A., Williams J. O. Modification of the active site of isocitrate lyase from Pseudomonas indigofera. Arch Biochem Biophys. 1971 Nov;147(1):192–200. doi: 10.1016/0003-9861(71)90327-4. [DOI] [PubMed] [Google Scholar]
- Roche T. E., Williams J. O., McFadden B. A. Effect of pH and buffer upon Km and inhibition by phosphoenolpyruvate of isocitrate lyase from Pseudomonas indigofera. Biochim Biophys Acta. 1970 Apr 22;206(1):193–195. doi: 10.1016/0005-2744(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Rogers J. E., McFadden B. A. Isocitrate lyase from Neurospora crassa: pH dependence of catalysis and interaction with substrates and inhibitors. Arch Biochem Biophys. 1977 Apr 30;180(2):348–353. doi: 10.1016/0003-9861(77)90048-0. [DOI] [PubMed] [Google Scholar]
- Rogers J. E., McFadden B. A. Isocitrate lyase from Pseudomonas indigofera: pH dependence of catalysis and binding of substrates and inhibitors. Arch Biochem Biophys. 1976 Jun;174(2):695–704. doi: 10.1016/0003-9861(76)90400-8. [DOI] [PubMed] [Google Scholar]
- SHIIO I., SHIIO T., MCFADDEN B. A. ISOCITRATE LYASE FROM PSEUDOMONAS INDIGOFERA. I. PREPARATION, AMINO ACID COMPOSITION AND MOLECULAR WEIGHT. Biochim Biophys Acta. 1965 Jan;96:114–122. doi: 10.1016/0005-2787(65)90615-5. [DOI] [PubMed] [Google Scholar]
- Vanni P., Vincenzini M. T., Nerozzi F. M., Sinha S. P. Studies on isocitrate lyase isolated from Lupinus cotyledons. Can J Biochem. 1979 Sep;57(9):1131–1137. doi: 10.1139/o79-145. [DOI] [PubMed] [Google Scholar]
- Williams J. O., Roche T. E., McFadden B. A. Mechanism of action of isocitrate lyase from Pseudomonas indigofera. Biochemistry. 1971 Apr 13;10(8):1384–1390. doi: 10.1021/bi00784a017. [DOI] [PubMed] [Google Scholar]



