Abstract
The central nervous system is characterized by a peculiar vascularization termed blood–brain barrier (BBB), which regulates the exchange of cells and molecules between the cerebral tissue and the whole body. BBB dysfunction is a life-threatening condition since its presence corresponds to a marker of severity in most diseases encountered in the intensive care unit (ICU). During critical illness, inflammatory response, cytokine release, and other phenomena activating the brain endothelium contribute to alterations in the BBB and increase its permeability to solutes, cells, nutrients, and xenobiotics. Moreover, patients in the ICU are often old, with underlying acute or chronic diseases, and overly medicated due to their critical condition; these factors could also contribute to the development of BBB dysfunction. An accurate diagnostic approach is critical for the identification of the mechanisms underlying BBB alterations, which should be rapidly managed by intensivists. Several methods were developed to investigate the BBB and assess its permeability. Nevertheless, in humans, exploration of the BBB requires the use of indirect methods. Imaging and biochemical methods can be used to study the abnormal passage of molecules through the BBB. In this review, we describe the structural and functional characteristics of the BBB, present tools and methods for probing this interface, and provide examples of the main diseases managed in the ICU that are related to BBB dysfunction.
Keywords: Blood–brain barrier, ICU hepatic encephalopathy, Delirium, Extracorporeal membrane oxygenation (ECMO), Acute bacterial meningitis
Introduction
Brain homeostasis depends largely on specific interfaces between the blood and the brain. These interfaces ensure a constant supply of oxygen and nutrients to the cerebral tissue and remove waste products generated by cellular metabolism.[1,2] The blood–brain barrier (BBB), located at the level of cerebral microvessels, is the most important among those interfaces. It relies on a specific organization through the close interaction between brain endothelial cells (ECs) that express intercellular tight junctions (TJs).[3] Other characteristics distinguish brain ECs from other ECs, namely the absence of fenestration,[4] minimal pinocytic activity,[5] greater amount of mitochondria due to their intense metabolic activity,[6] higher intracellular vesicle transcytosis activity, and polarized expression of specific transporters. Brain ECs lay on a basal lamina in which pericytes are embedded and are surrounded by astrocytes’ end-feet that cover cerebral microvessels. A very similar cellular organization can be found on the blood–leptomeningeal barrier within the subarachnoid space, on the blood–cerebrospinal fluid (CSF) barrier within the choroid plexuses, and on the interface between blood and circumventricular organs located around the third and fourth ventricles.[7] Of note, post-capillary venules and veins harbor a pronounced perivascular space between pericytes and astrocytic end-feet layers, termed the Virchow–Robin space. This is filled with a fluid similar to CSF, which is referred to as interstitial fluid.
BBB dysfunction is frequently observed during critical illness. It is directly linked to a brain-related disease (severe multiple sclerosis, flare-up of neuromyelitis optica spectrum disorders [NMOSD], or acute disseminated encephalomyelitis [ADEM]),[8] brain infections (meningitis or abscess),[9] encephalitis (infectious or immune-mediated),[10] cerebral vasculitis,[11] acute ischemic stroke,[12] hypoxic ischemic encephalopathy,[13] brain trauma,[14] tumors,[15] and neurodegenerative diseases.[16] In addition, it can occur secondary to an extraneurological systemic condition, such as sepsis and cytokine release syndrome (CRS),[17,18] or toxic-metabolic disturbances (including medications).[19] Table 1 presents neurological and extraneurological pathological conditions associated with BBB dysfunction, which are encountered by intensivists.
Table 1.
Neurological and extraneurological pathological conditions associated with architectural and functional dysfunction of the blood–brain barrier, encountered by intensivists.
| Type of condition | Mechanism | Reference |
|---|---|---|
| Neurological | ||
| ADEM | Increased permeability to cells and solutes | Sonneville et al. 2008[8] |
| NMOSD | Increased permeability to cells and solutes | Zhao et al. 2021[103] |
| TBI | Increased permeability to cells and solutes | Guan et al. 2020[85] |
| Hypoxic ischemic encephalopathy | Increased permeability to solutes | Engelhardt et al. 2015[13] |
| Pharmacoresistant epilepsy and status epilepticus | Decreased permeability to drugs due to a high expression of BBB efflux pumps, leading to low concentrations of antiepileptic drugs in the CNS and increased permeability to solutes | Volk et al. 2005[100] Löscher and Potschka 2005[102] Nicita et al. 2014[101] |
| Acute bacterial meningitis | Hijacking of the machinery of normal BBB, leading to increased permeability to cells and solutes | Le Guennec et al. 2020[9] |
| Parasite-associated encephalitis | Trojan horse mechanism of normal BBB, leading to increased permeability to cells and solutes | Ross et al. 2021[126] |
| Viral encephalitis | Retrograde transport by peripheral nerve and Trojan horse mechanisms, leading to increased permeability to cells and solutes | Zegenhagen et al. 2016[77] |
| Extraneurological | ||
| Delirium | Increased permeability to nutrients and xenobiotics | Skrobik et al. 2013[92] Hughes et al. 2016[94] |
| Sepsis-associated encephalopathy | Increased levels of inflammatory cytokines and permeability to solutes | Gofton and Young 2012[36] Chaudhry et al. 2013[37] Lee and Hüttemann 2014[38] |
| CAR-T cell-related encephalopathy syndrome | Increased levels of inflammatory cytokines and permeability to cells and solutes | Davila et al. 2014[42] Gust et al. 2017[18] |
| COVID-19-associated encephalopathy | Increased levels of inflammatory cytokines and permeability to solutes | Altmayer et al. 2022[41] Younger 2020[81] |
| PRES | Increased permeability to solute | Lee et al. 2008[30] |
| Patients under ECMO | Loss of pulsatility and impairment of brain vessel carbon dioxide reactivity/neurovascular coupling | Veraar et al. 2019[55] |
| Hepatic encephalopathy | Increased permeability to nutrients and xenobiotics | Bouzbib et al. 2021[96] Schaefer et al. 2022[99] Weiss et al. 2016[98] Weiss et al. 2017[40] |
| Drug-induced encephalopathy | Increased permeability to nutrients and xenobiotics | Bauer et al. 2012[97] |
ADEM: Acute disseminated encephalomyelitis; BBB: Blood–brain barrier; CAR-T: Chimeric antigen receptor T-cells; CNS: Central nervous system; COVID-19: Coronavirus disease 2019; ECMO: Extracorporeal membrane oxygenatin; NMOSD: Neuromyelitis optica spectrum disorders; PRES: Posterior reversible encephalopathy syndrome; TBI: Traumatic brain injury.
In general, the common feature of BBB dysfunction is its increased permeability to plasma proteins and water. This phenomenon results in local or diffuse accumulation of extravascular fluid, the build-up of vasogenic edema, and an increase in brain volume, which can lead to elevation in intracranial pressure.[10] BBB alteration also promotes the activation of ECs, which is responsible for cytokine release from the endothelium and the recruitment of leukocytes on activated vascular cells. Moreover, BBB dysfunction facilitates the paracellular influx of cytokines and inflammatory cells into brain parenchyma, thereby triggering the activation of glial cells and neuroinflammation.[20,21] Figure 1 shows the cellular and molecular mechanisms involved in BBB alteration.[22]
Figure 1.
Structure of the human BBB: Normal BBB (A) andpathological BBB (B). The BBB is composed of brain ECs, a basal lamina, pericytes, and astrocytic end-feet. Brain ECs exhibit specific intercellular TJs at their luminal surface, which play a crucial role in restricting permeability. They are composed of different types of transmembrane proteins, such as JAMs, CLDNs, and OCLNs. These proteins are associated with the actin cytoskeleton by intermediate cytosolic proteins, including ZO proteins. Adherent junctions are located below TJs, and are mainly composed of cadherins, nectins, and catenins. Functional (e.g., endothelial dysfunction) and/or architectural (e.g., basal lamina and/or TJ degradation; and reduction of pericytes/astrocytes coverage) alterations of the BBB lead to increased permeability to cells and nutrients and to vascular leakage, which negatively affect brain homeostasis and functions. GCX is another factor which plays an important role in BBB integrity at the luminal cell surface in contact with the bloodstream. This factor protects ECs and reduces non-specific cell and pathogen interaction with ECs.
BBB: Blood–brain barrier; CLDNs: Claudins; ECs: Endothelial cells; GCX: Glycocalyx; JAMs: Junctional adhesion molecules; OCLNs: Occludins; TJs: Tight junctions; ZO: Zonula occludens.
Recent progress made in fundamental and clinical science enabled researchers to unravel the importance of BBB dysfunction during critical illness. In this review, we briefly present the most common clinical and pathological features related to the architectural or functional dysfunction of the BBB observed in the intensive care unit (ICU). We classified those features according to their pathophysiological mechanisms: (1) increased permeability to solutes; (2) hijacking of BBB-crossing mechanisms by cells and pathogens; and (3) increased permeability to nutrient and xenobiotics.
Increased BBB Permeability to Solutes and Vasogenic Edema
Brain ECs express, as in every microvessel in the body, adherent junctions produced by the homophilic interaction of cadherins. These junctions develop early during cerebral angiogenesis and are essential for the establishment of TJs that are specific to brain ECs.[1,[22], [23], [24]] TJs are expressed at the apical pole of brain ECs and are produced by the homophilic interaction of occludins (OCLNs), claudins (CLDNs; mainly CLDN-3 and CLDN-5), and junctional adhesion molecules (JAMs). These proteins are associated with the endothelial actin cytoskeleton by intermediate cytosolic proteins, including zonula occludens (ZO) proteins and cingulins (CGNs).[25] In CLDN-5 knockout mice, which die within hours after birth, the BBB is permeable to small molecules without alteration of angiogenesis or the occurrence of edema or cerebral bleeding.[26] JAMs belonging to the superfamily of immunoglobulins are involved in the establishment of TJs, as well as in the diapedesis of leukocytes through the brain microvessels.[23] Thus, these TJs are responsible for the integrity of the BBB, and play a fundamental role in reducing capillary permeability by limiting paracellular diffusion.[25] However, the permeability of cerebral microvessels is regulated. In response to a transient increase in the levels of vascular endothelial growth factor (VEGF), CLDN-5 molecules are temporally internalized, thereby resulting in a transient increase in vascular permeability to solutes.[27] If VEGF levels remain high, CLDN-5 is internalized and destroyed, leading to an increase in permeability for several days. Inflammation and the immune response also play a role in the control of vascular permeability. Indeed, inflammatory molecules, such as thrombin, histamine, and some cytokines (interferon-γ [IFN-γ], tumor necrosis factor-α [TNF-α]), induce the transient formation of paracellular pores, thus increasing endothelial permeability within minutes. The basement membrane acts as another mechanical filter, allowing only the passage of small molecules. Its physical properties can be altered by the action of matrix metalloproteinase-2 (MMP-2) and MMP-9.[25] Aquaporine-4 (AQP-4) water channels are expressed on astrocytes’ end-feet and contribute to movement of water through the BBB. Lipophilic molecules with a molecular weight <400 Da and fewer than eight hydrogen bonds can freely cross the BBB.[28] Other small molecules (e.g., glucose and amino acids) need specific transporters, while larger molecules (e.g., insulin and iron transferrin) require receptor-mediated endocytosis to cross the BBB.[29]
Different factors involved in this increased permeability are also implicated in pathophysiology. In clinics, increased permeability to solutes is responsible for the development of brain vasogenic edema. Thus, during posterior reversible encephalopathy syndrome, vasogenic cerebral edema explains the occurrence of neurological symptoms and imaging findings.[30] Altered or overpassed cerebral autoregulation is considered responsible for the disease.[31] Nevertheless, it has been proposed that dysfunction of the VEGF receptor FMS-like tyrosine kinase 1 (Flt1), which binds to placental growth factor (PlGF), could increase BBB permeability, resulting in brain edema. This is frequently observed in some etiologies, such as hemolysis, elevated liver enzymes, and low platelets count (HELLP) syndrome, which occurs during pregnancy.[32] Moreover, during malignant glioma, brain angiogenesis is overly stimulated by tumoral cells, which secrete several pro-angiogenic factors (e.g., VEGF).[33] This leads to constant remodeling of brain microvessels, which remain into an immature state with increased permeability, thus explaining the local edema observed within malignant gliomas.[34]
In septic shock and particularly in patients with sepsis-associated encephalopathy, increased levels of inflammatory cytokines have been linked to altered TJ protein expression or function.[35,36] Cytokine-mediated oxidative phosphorylation in the context of sepsis[37,38] might promote the phosphorylation of OCLN, which has been shown to increase permeability by compromising TJ structural integrity.[39]
Thus, all situations associated with systemic inflammation and cytokine release in the ICU might impair BBB permeability. This has been shown by our research group and others in acutely ill cirrhotic patients, particularly in those displaying acute-on-chronic liver failure.[40] More recently, the neurological presentation of patients with acute respiratory distress syndrome (ARDS) secondary to coronavirus disease 2019 (COVID-19) has attracted considerable attention. It has been demonstrated that the BBB function was impaired probably secondary to CRS.[41] The use of lymphodepletion chemotherapy, followed by infusion of genetically modified autologous T cells that express a CD19-specific chimeric antigen receptor (CD19 CAR-T cells), is increasing.[42] A frequent complication observed after infusion of CAR-T cells is a cytokine storm termed CRS; this condition is characterized by a sepsis-like presentation with fever and hypotension.[43] A frequently observed neurologic adverse event of CAR-T cell infusion is called CAR-T cell-related encephalopathy syndrome or immune effector cell-associated neurotoxicity syndrome (ICANS). The severity of ICANS can range from focal isolated and reversible neurological symptoms to severe diffuse brain edema and multifocal brain hemorrhage.[42,44] Moreover, it is strongly related to the importance of CRS and BBB dysfunction.[18] It has been shown that grading of ICANS was correlated to factors known to activate brain ECs, such as interleukin-6 (IL-6), IFN-γ, and TNF-α.[18] Moreover, it has been reported that the angiopoietin-2 (Ang-2):Ang-1 ratio is higher in patients with severe ICANS. An increase in this ratio reflects the activation of microvascular permeability.[18] In addition, increased levels of cytokines (e.g., IFN-γ, TNF-α, and IL-6) have been found in the CSF of patients with ICANS, further suggesting the existence of BBB dysfunction in these patients.[18]
Alteration in BBB permeability is also observed in patients undergoing extracorporeal membrane oxygenation (ECMO), which is increasingly used to overcome cardiac and/or pulmonary dysfunction that is refractory to conventional therapy.[45,46] Schematically, there are two types of ECMO that always involve a centrifugal pump ensuring a continuous laminar flow and an extracorporeal oxygenation membrane: a veno-arterial (VA) and a veno-venous configuration. Patients receiving ECMO are exposed to numerous risk factors for BBB dysfunction (e.g., hypoxia, hypercapnia, acidosis, tissue hypoperfusion, or hemostasis disorders),[[47], [48], [49]] which promote ischemic and/or hemorrhagic brain injuries.[50,51] These complications are more frequently observed in this population, burdened by a poor prognosis in terms of morbidity and mortality.[52,53] Typically, heart beat-induced flow pulsatility in distal arteries is low due to kinetic energy dissipation within compliant proximal arteries. Nevertheless, the total absence of heart pulsatility in more severe patients under VA-ECMO might exert a negative effect on BBB integrity. ECs are physiologically subject to stretching tension generated by pulsatile changes in blood pressure. These cells can sense this pulse wave and adapt to such constraints by undergoing remodeling and maturation of their intercellular junction to improve barrier integrity. This is achieved through a series of intracellular signaling events called mechanotransduction.[54] It has been shown that the exclusive laminar blood flow caused by the ECMO pump plays a role in the occurrence of cerebral ischemia in patients under VA-ECMO through an alteration of cerebral autoregulation induced by the absence of pulsatility.[55] This condition was associated with a steep decrease in brain vessel carbon dioxide reactivity and impairment of neurovascular coupling, which aims to increase the cerebral blood flow regionally in response to the metabolic demands of brain structures.[55] Moreover, multiple studies reported that an excessively rapid correction of hypercapnia in patients under veno-venous- or VA-ECMO was associated with intracranial bleeding.[50,51] Furthermore, in patients under VA-ECMO, the sudden restoration of cerebral flow might be associated with ischemic-reperfusion syndrome. The latter could contribute to BBB leakage and intracranial edema, leading to a hemorrhagic transformation, particularly in patients who previously experienced ischemic stroke.[56]
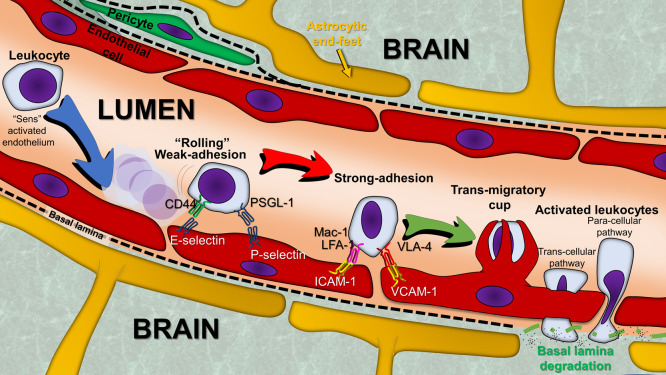
The hijacking of BBB-crossing mechanisms by cells and pathogens, despite the specific organization of the BBB, and immune surveillance, are key physiological processes. The trans endothelial migration (TEM) or diapedesis of leukocytes through the BBB increases in pathological circumstances, and the types of leukocytes (neutrophils, monocytes, or lymphocytes) depend on the chemokines released in the brain lesion.[57,58] TEM is highly regulated and classically divided in different steps (Figure 2). Those steps depend on the heterophilic interaction between cell adhesion molecules (CAMs) expressed by leukocytes and their counter-receptor expressed on brain ECs. The expression of these cell surface molecules and the force of their interaction are stimulated by inflammatory cytokines.[59] During the early step termed “rolling,” leukocytes weakly and transiently adhere to ECs. This step depends on the expression of CAMs of the selectin family, mainly P-selectins, which are expressed by ECs, stored in organelles called Weibel–Palade bodies, and address at the cell surface in response to factors such as histamine, thrombin, and complement proteins.[59] Other selectins, such as E-selectins, are expressed exclusively by activated ECs under induction by IL-1 and TNF-α.[59] P-selectin expression on the cell surface is very rapid and transient; it becomes undetectable in the blood within 30 min after cytokine stimulation.[60] Unlike P-selectin, E-selectin is not stored, and its expression requires de novo synthesis. This explains its delayed involvement in leukocyte “rolling” vs. P-selectin.[22,60] This step is followed by a strong cell adhesion stage, in which leukocytes adhere more firmly to ECs via interaction between integrins and members of the immunoglobulin superfamily. Endothelial surface immunoglobulins, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion protein 1 (VCAM-1) actively participate to this strong adhesion phase through interaction with leukocyte integrins lymphocyte function-associated antigen 1 (LFA-1) and macrophage-1 antigen (Mac-1) for endothelial ICAM-1, as well as leukocyte integrins very late antigen-4 (VLA-4) and lymphocyte Peyer's patch adhesion molecule 1 (LPAM-1) for endothelial VCAM-1. Although ICAM-1, VCAM-1, and leukocyte integrins are weakly expressed at the basal state, their expression is upregulated under inflammatory conditions by TNF-α and IL-1.[59] Once firmly adherent to the ECs, leukocytes induce modifications in the cytoskeleton of ECs that enable TEM as a third step. It is currently debatable whether TEM on cerebral ECs occurs mainly through a paracellular route (going through TJ by an interception with JAMs like a zipper) or a transcellular route (going through the EC cytoplasm though the less thick part of the ECs).[61,62] The last step in this process is the entry into the central nervous system (CNS).
Figure 2.
Overview of the different steps that comprise leukocytes TEM. Under the chemo-attractive effect of chemokines produced by activated ECs or other cell types within the vessel wall and/or the inflammatory zone in the adjacent tissue, a migration of circulating leukocytes takes place toward the vascular wall near the lesion. These cells migrate from the vascular lumen to the tissues, through a process of extravasation called diapedesis, which consists of different steps. The first step is a weak and transient adhesion termed “rolling.” The second step involves stronger adhesion to ECs. Following the remodeling of their cytoskeleton, leukocytes initiate diapedesis through the monolayer of ECs, toward the inflammatory site. The process is guided by the cytokine gradient and occurs through either a trans-cellular or paracellular pathway.
ECs: Endothelial cells; ICAM-1: Intercellular adhesion molecule 1; LFA-1: Lymphocyte function-associated antigen 1; Mac-1: Macrophage-1 antigen; PSGL-1: P-selectin glycoprotein ligand-1; TEM: Trans endothelial migration; VCAM-1: Vascular cell adhesion protein 1; VLA-4: Very late antigen-4.
The role of TEM within a normal BBB in mainly linked to immunosurveillance.[58] During pathological conditions (neuroinflammatory conditions [multiple sclerosis, NMOSD, or ADEM], brain infection, traumatic brain injury [TBI], etc.), brain ECs undergo architectural remodeling (e.g., reduction in the expression of TJ molecules and increase in paracellular permeability), accompanied by an increased expression of CAMs and proinflammatory cytokines/chemokines. These effects lead to the recruitment of circulating leukocytes and their crossing of the BBB.[58]
In specific pathological conditions, in which BBB is not dysfunctional per se, metastatic cells, bacteria, viruses, and fungi, can cross the BBB. This occurs through mechanisms that overcome the BBB defense by hijacking these TEM processes. Bacteria, fungi, and parasites primarily enter the CNS following a hematogenous dissemination by crossing the BBB transcellularly, paracellularly, and/or through infected phagocytes (Trojan horse mechanism). However, they can also spread contiguously from adjacent tissues (sinuses) or gain entry as a complication of trauma, surgery, or developmental malformations. Viruses can infect the brain through a retrograde axonal transport mechanism from the periphery via peripheral nerves, as well as the Trojan horse mechanism.
The pathophysiology of bacterial brain infection (Figure 3) is best described in acute bacterial meningitis with Streptococcus pneumoniae, Neisseria meningitidis, and Hemophilus influenzae in children and adults, and Escherichia coli and group B Streptococcus in neonates.[63] Several studies have revealed the specificity of interaction between these bacteria and ECs.[64,65] Several bacterial receptors on the surface of ECs have been discovered in the last decades. These include the platelet-activating factor receptor (PAFR) for S. pneumoniae[65] or CD147 (also termed basigin [BSG]) or extracellular matrix metalloproteinase inducer (EMPRINN) for N. meningitidis.[64,66] Bacterial adhesion to ECs further induces intracellular signaling that leads to the disruption of the intercellular TJs and facilitates bacterial paracellular crossing of the endothelial monolayer. Some bacteria, such as S. pneumoniae,[67] E. coli,[68] group B Streptococcus,[69] and H. influenzae, can cross the BBB through transcytosis.[70] Although in vitro studies have shown that N. meningitidis is internalized by human brain microvascular ECs,[71] the pathway used by meningococci to cross the BBB remains a matter of debate.[72]
Figure 3.
Strategies used by bacterial pathogens to cross the BBB. Streptococcus pneumoniae adheres to ECs by binding a cell receptor called PAF-R. This interaction allows transcytosis through the endothelium. In addition, the release of pneumolysin and H2O2 promotes cell wall degradation and TJ disassembly, allowing bacterial invasion through a paracellular pathway. The Escherichia coli K1 strain adheres to brain ECs by binding to CNTNAP1, which promotes bacterial transcytosis across the endothelium. In addition, the Hcp1 toxin, a component of the T6SS of E. coli K1, can be injected into brain ECs, thereby leading to cell injury, monolayer disassembly, and bacterial crossing through a paracellular pathway. Group B Streptococcus interacts with brain ECs through integrins (integrin-α2β1) and secretes a pore-forming protein termed β-hemolysin. This protein is cytolytic for brain ECs, leading to cell injury, BBB monolayer disassembly, and bacterial invasion through a paracellular pathway. Neisseria meningitidis interacts with ECs through interaction with the cellular receptor CD147. Following initial bacterial adhesion, intracellular signaling events lead to the formation of membrane protrusions, thereby allowing bacteria to resist the shear stress exerted by the bloodstream. In addition, these events promote the modification of TJ organization, thus opening the path for the crossing of the BBB by bacteria through a paracellular pathway.
BBB: Blood–brain barrier; CNTNAP1: Contactin-associated protein 1; CSF: Cerebrospinal fluid; ECs: Endothelial cells; Hcp1: Hemolysin-co-regulated protein 1; PAF-R: Platelet-activating factor receptor; T6SS: Type VI secretion system; TJ: Tight junction.
Other complex mechanisms might facilitate the crossing of BBB by bacteria, as a direct cytotoxic effect on the endothelium provoked by the bloodstream infection and the sepsis-induced cytokine storm. This effect leads to the disruption of the endothelial barrier and BBB leakage. However, subarachnoid hemorrhage or microbleeds, which are anecdotally associated with meningitis, can be observed as a result of this pathological mechanism. In most cases, these pathogens do not affect the architecture of the BBB.[73] Mycobacterium tuberculosis is simultaneously an extracellular and intracellular microorganism. It can cross the BBB via the bloodstream either directly or through the Trojan horse mechanism in infected monocytes/neutrophils.[74] It can also cross the BBB through infection of the choroid plexus and ventricle walls.[74] During the initial bacteremic phase, it has been suggested that bacteria deposited within the meninges and the cortex create foci (termed Rich foci), which are small granulomas. These Rich foci may also develop within the choroid plexus and the ventricular wall. Rupture of these foci into the subarachnoid spaces causes diffuse inflammatory meningoencephalitis and triggers a strong inflammatory T cell response. High levels of TNF-α and IFN-γ have been found in the CSF of patients with tuberculous meningoencephalitis.[75] Ultimately, the obstruction of the CSF by inflammatory infiltrates and pus leads to the development of hydrocephalus with poor prognosis. Listeria monocytogenes is an intracellular bacterium and a leading cause of meningoencephalitis in adults.[76] The L. monocytogenes surface protein internalin B interacts with EC receptors (e.g., E‐cadherin and a tyrosine kinase receptor called Met) to promote bacterial entry into ECs and invade the CNS.[76]
Viruses mainly infect the CNS via retrograde transport by peripheral nerves, or by circulating peripheral immune cells. Nevertheless, BBB dysfunction can also occur following viral infection in the brain. For instance, rabies virus, poliovirus, coxsackievirus, equine encephalitis virus, enterovirus, flavivirus, West-Nile virus, tick-borne encephalitis virus, Zika virus, and Japanese encephalitis virus can infect neurons and astrocytes. This leads to apoptosis of these cells, thus injuring the astrocytic end-feet sealing the BBB.[77] Moreover, they can indirectly cause cellular death as a consequence of the inflammatory response responsible for neurotoxicity and BBB dysfunction through IFN type I response and its related downstream signaling activation cascade.[77] Massive recruitment of T cells after viral CNS invasion also plays a major role in BBB dysfunction. This has been observed during the occurrence of immune reconstitution inflammatory syndrome in patients with human immunodeficiency virus and ongoing progressive multifocal leukoencephalopathy due to infection of the CNS with John Cunningham virus.[78]
A recent example of BBB dysfunction during viral infection is COVID-19, which is caused by the severe acute respiratory disease coronavirus 2. Endothelial dysfunction is a major pathological mechanism during COVID-19, and responsible for thrombosis. It has been described that patients with COVID-19 ARDS had increased levels of plasma endothelial activation markers Ang-2 and E-selectin, which were predictive of respiratory worsening and admission to the ICU.[79] Cases of COVID-19-related encephalitis have been reported. Brain necropsies of these patients have revealed the presence of cerebral lesions related to oxygen deprivation and severe systemic inflammation, such as hypoxic ischemic injury, neuronal loss, perivascular parenchymal infiltrates of T-cell and macrophages, activated microglia, and hypertrophic astrocytes associated with a marked axonal injury.[[80], [81], [82], [83]] In addition, ADEM-like presentations, as well as presentations with necrotic-hemorrhagic white matter lesions within the perivascular space, have been reported.[[81], [82], [83]] These lesions most likely result from cerebrovascular insults secondary to systemic inflammation rather than direct viral CNS invasion associated with BBB dysfunction. Moreover, it has been found that S-100β protein expression, which is correlated with BBB leakage, was associated with brain injury in COVID-19 patients.[84] Furthermore, a recent study conducted by our group highlighted a strong correlation between blood brain endothelial activation markers (E-selectin, TNF-α, IL-6, PlGF, and thrombomodulin [THBD]) and TNF-α levels in critically ill patients with COVID-19 ARDS and encephalitis. These findings suggested that COVID-19-induced encephalitis is a cytokine-associated acute brain dysfunction.[41]
Altered BBB permeability is also present in TBI. Following the initial injury, a secondary injury begins through specific cellular and molecular processes, namely the release of excitatory molecules, inflammation cascade, and cell apoptosis. Large amounts of damage associated molecular pattern (DAMP) molecules are released from apoptotic cells, causing gliosis and activation of innate immune response and leading to immune cell infiltration. The unregulated immune response worsens the inflammation process and induces the progression of injury. Altered expression of AQP-4 water channels, which could lead to more severe cerebral edema, has also been shown in TBI models.[85]
Increased BBB Permeability to Nutrients and Xenobiotics
Liposoluble molecules cross the endothelial plasma membrane by passive diffusion. In contrast, water-soluble molecules can only cross the BBB through specific mechanisms involving active transport by protein complexes (Figure 4). However, passive diffusion is limited in cerebral capillaries via an active efflux phenomenon due to the presence of multiple pumps and transporters on the surface of cerebral ECs. The BBB has numerous proteins that regulate the passage of molecules in both directions (i.e., from the bloodstream to the CNS and vice versa).[86] The passage of drugs is also regulated by these proteins.[86] The solute carrier (SLC) family and ATP-binding cassette (ABC) transporter family are the main superfamilies of transporters.[[87], [88], [89]]
Figure 4.
Polarized expression of specific transporters of brain ECs. Exchanges between the intravascular and extravascular compartments are essential for brain homeostasis. The brain vascular endothelium is the interface allowing these continuous exchanges. Some transporters allow the passage of essential molecules from the bloodstream to the brain, such as carbohydrates GLUT1, lactate MCT1, amino acids LAT-1, and carnitine/drugs OCTN2. Other transporters, such as P-gp or BCRP, act as pumps and restrict the passage of xenobiotics. BCRP: Breast cancer resistance protein; ECs: Endothelial cells; GLUT1: Glucose transporter 1; LAT-1: Large neutral amino acid transporter-1; MCT1: Monocarboxylic acid transporter-1; OCTN2: Organic cation/carnitine transporter; P-gp: P-glycoprotein.
Some members of the SLC family are involved in the transport of energetic substrates, such as carbohydrates (glucose transporter 1 [GLUT1]), lactate (monocarboxylic acid transporter-1 [MCT1]), amino acids (large neutral amino acid transporter-1 [LAT-1]), creatine, etc., and are highly represented. Amino acids (e.g., glutamate, aspartate, glycine, and γ-aminobutyric acid), which are the main CNS neurotransmitters, are synthesized within the brain. In contrast, other essential amino acids originate from the systemic circulation.
P-glycoprotein (P-gp) is the most important among ABC transporters (also called multidrug resistance proteins). It was initially described as the transporter responsible for the development of resistance to chemotherapy.[90] Its various known substrates include chemotherapeutic agents (doxorubicin, daunorubicin, vinblastine, vincristine, etoposide, etc.), immunosuppressive drugs (ciclosporin, tacrolimus, corticosteroids, etc.), antibiotics (cephalosporins, fluoroquinolones, etc.), antiepileptic drugs (phenytoin, carbamazepine, lamotrigine, phenobarbital, etc.), and antiretroviral therapies directed against the human immunodeficiency virus. Breast cancer resistance protein (BCRP) and multidrug resistance proteins are other efflux pumps of the ABC family expressed on ECs that can remove toxic and pharmacological substances from ECs.[88,91] Their expression can be modulated by different pathophysiological situations. It should be noted that P-gp expression, and probably that of BCRP, decreases with aging.
Delirium is frequently encountered in the ICU; its physiopathology is particularly linked to an altered clearance of nutrients and xenobiotics by the BBB.[92] The pathophysiology of this condition implicates several causes of BBB dysfunction, and studies have shown an association between markers of EC activation and the duration of delirium.[93] Increased blood levels of circulating S-100β protein and E-selectin are observed in these patients, attesting the altered BBB permeability.[94] Furthermore, polymorphisms in transporters and the coadministration of P-gp inhibitors (amiodarone, azithromycin, cyclosporine, isavuconazole, lapatinib, quinine, ritonavir, tamoxifen, ticagrelor, verapamil, etc.[95]) are associated with the duration of delirium.[92] Similarly, efflux pumps are implicated in toxic and metabolic encephalopathies, especially in drug-induced cases following the simultaneous administration of efflux pump agonists.[96] Using photon emission tomography or cerebral scintigraphy, in vivo animal and human studies have demonstrated that the coadministration of such agonists induced a 10-fold increase in drug accumulation in the CNS.[97] In our previous metabolomics analysis of CSF samples obtained from cirrhotic patients with hepatic encephalopathy, the results revealed that two-thirds of the patients displayed abnormal levels of efflux pump agonists that could have contributed to the development of neurological symptoms.[98,99] In pharmacoresistant epilepsy and status epilepticus, efflux pump expression was increased due to intractable seizures.[100] Numerous antiepileptic drugs are substrates of efflux pumps; this rapidly leads to a decreased concentration of antiepileptic drugs in the CNS and, consequently, treatment failure. Preliminary data regarding pediatric refractory status epilepticus and pharmacoresistant epilepsy suggested that inhibition of efflux pumps through the use of specific or less specific P-gp or BCRP blockers enabled control of seizure activity for several weeks. This finding suggested that efflux pump expression returned to normal levels.[101,102]
Perspectives
Recent progress achieved in cellular biology and immunology has unraveled the pathophysiological importance of BBB dysfunction during critical illness. Nevertheless, there is a need to develop new tools for deciphering the pathophysiological mechanisms of BBB alteration in different settings in the ICU, as well as novel clinical strategies for its management.
Currently, there is a growing interest in developing compounds that are able to prevent BBB leakage or restore its integrity. This can be achieved by targeting molecules at the luminal surface of ECs, such as component of the glycocalyx (GCX), which plays many critical roles in the regulation of BBB integrity. GCX is a complex molecular dynamic structure synthesized by ECs, and mainly composed of proteoglycans, glycoproteins, and glycosaminoglycans.[103] It provides a negative charge to the luminal surface of ECs, thereby repelling the negatively charged plasma proteins and activated platelets, and plays a role in leukocyte adhesion.[104] GCX in the BBB is a target of preclinical research[105,106] as this structure is often impaired during TBI,[105] status epilepticus,[106] and sepsis.[107] Thus, current research on this field might lead to the development of new molecules that can help to restore BBB integrity.
There is a continuous development of methods for the exploration of the BBB. Among them, microdialysis sampling is a promising, yet invasive technique. It allows the monitoring of neurotransmitters in the brain and has been used to analyze the metabolism of molecules at the BBB level.[108] This technique permits the simultaneous estimation of the concentrations of several chemicals in the interstitial fluid. The device utilized in this method consists of a probe that has been surgically implanted in the brain. The probe is composed of a hollow-fiber dialysis membrane linked to inlet and outlet tubing. A liquid similar to the brain interstitial fluid circulates within this membrane. Small molecules in the extracellular space diffuse in this fluid depending on their concentration, and are collected and analyzed by the device system. This device can be simultaneously implanted within a blood vessel, allowing a comparative analysis of a substance in both sides of the BBB. This approach is extensively used in animal models to determine the pharmacokinetic profiles of multiple drugs and neuropeptides crossing through the BBB.[109]
A few studies utilizing this technique in humans have been published thus far. These mainly involved patients undergoing brain surgery (i.e., the probe was placed during the procedure) due to glioblastoma,[110] traumatic spinal cord injury,[111] or severe subarachnoid hemorrhage.[112] This device allows the investigation of the glial-lymphatic system – a recently discovered and poorly understood vascular network within the brain.[113] Previously, it was thought that there is no lymphatic network within the CNS (and its corollary of an immune sanctuary with immune privilege). However, over the past decade, this concept has been gradually rejected. The glial-lymphatic system is an organized network[114] set in parallel with brain vessels within their perivascular spaces; this system communicates with the systemic lymphatic system of meninges and the skull.[115] Ultimately, as with the CSF, lymph enters into the venous blood.[116] This vascular network is implicated in the immune surveillance of the brain, and plays an important role during bacterial meningitis to promote rapid regional cranial leucopoiesis[117]; it is also involved in sensing pathological antigens in brain tumors.[118] This structure also participates in the clearance of metabolic brain waste. It has been suggested that impairment of this network plays an important role in the occurrence of neurodegenerative disease, such as Alzheimer's disease.[119]
It has been shown that waste clearance through the glial-lymphatic system was mainly active during the quiescent sleep phase, whereas it was inactive during wakefulness.[120,121] This might be of importance for patients in the ICU, in whom the sleep pattern is markedly disturbed due to sleep deprivation (noise due to staff activity and alarms, light exposure, nurse care activities, anxiety, fear, and pain), as well as the use of artificial sedatives and analgesics.[122]
Among the techniques used to study BBB, proton magnetic resonance spectroscopy is increasingly used to investigate the transfer of chemicals across the BBB.[123,124] This method allows the non-invasive detection and quantification of metabolites in specific parts/voxels within the CNS. A recent advance in magnetic field technology has allowed researchers to use magnetic resonance spectroscopy with 10.5 T in humans and up to 11.7 T in animals.[125] This approach improves spatial resolution imaging and increases accuracy to assist in the diagnosis and monitoring of several diseases.
Conclusions
BBB dysfunction is a life-threatening condition associated with multiple diseases encountered by intensivists. This condition should be rapidly diagnosed and managed; hence, an accurate diagnostic approach is critical for the proper management of such patients. During critical illness, inflammatory response, cytokine release, and other phenomena activating the brain endothelium contribute to BBB dysfunction and increase its permeability to solutes, cells, nutrients, and xenobiotics.
Author Contributions
Loic Le Guennec and Nicolas Weiss: Conceptualization, Loic Le Guennec and Nicolas Weiss: Writing- Original draft preparation. Nicolas Weiss: Supervision.: Loic Le Guennec and Nicolas Weiss: Writing- Reviewing and Editing.
Acknowledgments
Acknowledgements
The authors wish to thank Alexander Balcerac for English editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics Statement
Not applicable.
Conflict of Interest
Nicolas Weiss has received consultant fees from MedDay Pharmaceuticals and Owkin. Loïc Le Guennec has nothing to disclose.
Data Availability
Not applicable.
Managing Editor: Jingling Bao
References
- 1.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Weiss N., Miller F., Cazaubon S., Couraud P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788(4):842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Kniesel U., Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20(1):57–76. doi: 10.1023/a:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenstermacher J., Gross P., Sposito N., Acuff V., Pettersen S., Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 5.Sedlakova R., Shivers R.R., Del Maestro R.F. Ultrastructure of the blood-brain barrier in the rabbit. J Submicrosc Cytol Pathol. 1999;31(1):149–161. [PubMed] [Google Scholar]
- 6.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol. 1977;1(5):409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 7.Saunders N.R., Ek C.J., Habgood M.D., Dziegielewska K.M. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31(6):279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Sonneville R., Demeret S., Klein I., Bouadma L., Mourvillier B., Audibert J., et al. Acute disseminated encephalomyelitisin the intensive care unit:clinical features and outcome of 20 adults. Intensive Care Med. 2008;34(3):528–532. doi: 10.1007/s00134-007-0926-2. [DOI] [PubMed] [Google Scholar]
- 9.Le Guennec L., Coureuil M., Nassif X., Bourdoulous S. Strategies used by bacterial pathogens to cross the blood-brain barrier. Cell Microbiol. 2020;22(1):e13132. doi: 10.1111/cmi.13132. [DOI] [PubMed] [Google Scholar]
- 10.Nass R.D., Akgün K., Dague K.O., Elger C.E., Reichmann H., Ziemssen T., et al. CSF and serum biomarkers of cerebral damage in autoimmune epilepsy. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.647428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demiselle J., Auchabie J., Beloncle F., Gatault P., Grangé S., Du Cheyron D., et al. Patients with ANCA-associated vasculitis admitted to the intensive care unit with acute vasculitis manifestations: a retrospective and comparative multicentric study. Ann Intensive Care. 2017;7(1):39. doi: 10.1186/s13613-017-0262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazmierski R., Michalak S., Wencel-Warot A., Nowinski W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. 2012;79(16):1677–1685. doi: 10.1212/WNL.0b013e31826e9a83. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt S., Huang S.F., Patkar S., Gassmann M., Ogunshola O.O. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: a comparative study. Fluids Barriers CNS. 2015;12:4. doi: 10.1186/2045-8118-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellander B.M., Olafsson I.H., Ghatan P.H., Bro Skejo H.P., Hansson L.O., Wanecek M., et al. Secondary insults following traumatic brain injury enhance complement activation in the human brain and release of the tissue damage marker S100B. Acta Neurochir. 2011;153(1):90–100. doi: 10.1007/s00701-010-0737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavard J., Gutkind J.S. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 16.Noe C.R., Noe-Letschnig M., Handschuh P., Noe C.A., Lanzenberger R. Dysfunction of the blood-brain barrier-a key step in neurodegeneration and dementia. Front Aging Neurosci. 2020;12:185. doi: 10.3389/fnagi.2020.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ait-Oufella H., Maury E., Lehoux S., Guidet B., Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36(8):1286–1298. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 18.Gust J., Hay K.A., Hanafi L.A., Li D., Myerson D., Gonzalez-Cuyar L.F., et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Guennec L., Marois C., Demeret S., Wijdicks E., Weiss N. Toxic-metabolic encephalopathy in adults: critical discussion and pragmatical diagnostic approach. Rev Neurol. 2022;178(1–2):93–104. doi: 10.1016/j.neurol.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Minagar A., Alexander J.S. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9(6):540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 21.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 22.Toborek M., Kaiser S. Endothelial cell functions. Relationship to atherogenesis. Basic Res Cardiol. 1999;94(5):295–314. doi: 10.1007/s003950050156. [DOI] [PubMed] [Google Scholar]
- 23.Ballabh P., Braun A., Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Dejana E., Corada M., Lampugnani M.G. Endothelial cell-to-cell junctions. FASEB J. 1995;9(10):910–918. doi: 10.1096/fasebj.9.10.7615160. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso F.L., Brites D., Brito M.A. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64(2):328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gavard J., Gutkind J.S. VE-cadherin and claudin-5: it takes two to tango. Nat Cell Biol. 2008;10(8):883–885. doi: 10.1038/ncb0808-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardridge W.M. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19(8):1059–1072. doi: 10.1517/14728222.2015.1042364. [DOI] [PubMed] [Google Scholar]
- 29.Pardridge W.M., Eisenberg J., Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44(6):1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee V.H., Wijdicks E.F., Manno E.M., Rabinstein A.A. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–210. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- 31.Lin J.T., Wang S.J., Fuh J.L., Hsiao L.T., Lirng J.F., Chen P.M. Prolonged reversible vasospasm in cyclosporin a-induced encephalopathy. AJNR Am J Neuroradiol. 2003;24(1):102–104. [PMC free article] [PubMed] [Google Scholar]
- 32.Schreurs M.P., Houston E.M., May V., Cipolla M.J. The adaptation of the blood-brain barrier to vascular endothelial growth factor and placental growth factor during pregnancy. FASEB J. 2012;26(1):355–362. doi: 10.1096/fj.11-191916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmeliet P., Collen D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann N Y Acad Sci. 2000;902:249–262. doi: 10.1111/j.1749-6632.2000.tb06320.x. discussion 262-4. [DOI] [PubMed] [Google Scholar]
- 34.Lafuente J.V., Adán B., Alkiza K., Garibi J.M., Rossi M., Cruz-Sánchez F.F. Expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor-beta (PDGFR-beta) in human gliomas. J Mol Neurosci. 1999;13(1–2):177–185. doi: 10.1385/JMN:13:1-2:177. [DOI] [PubMed] [Google Scholar]
- 35.Benchenane K., López-Atalaya J.P., Fernández-Monreal M., Touzani O., Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27(3):155–160. doi: 10.1016/j.tins.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 36.Gofton T.E., Young G.B. Sepsis-associated encephalopathy. Nat Rev Neurol. 2012;8(10):557–566. doi: 10.1038/nrneurol.2012.183. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhry H., Zhou J., Zhong Y., Ali M.M., McGuire F., Nagarkatti P.S., et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27(6):669–684. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee I., Hüttemann M. Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta. 2014;1842(9):1579–1586. doi: 10.1016/j.bbadis.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raleigh D.R., Boe D.M., Yu D., Weber C.R., Marchiando A.M., Bradford E.M., et al. Occludin S408 phosphorylation regulates tight junction protein interactions and barrier function. J Cell Biol. 2011;193(3):565–582. doi: 10.1083/jcb.201010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss N., Rosselli M., Mouri S., Galanaud D., Puybasset L., Agarwal B., et al. Modification in CSF specific gravity in acutely decompensated cirrhosis and acute on chronic liver failure independent of encephalopathy, evidences for an early blood-CSF barrier dysfunction in cirrhosis. Metab Brain Dis. 2017;32(2):369–376. doi: 10.1007/s11011-016-9916-9. [DOI] [PubMed] [Google Scholar]
- 41.Altmayer V., Ziveri J., Frère C., Salem J.E., Weiss N., Cao A., et al. Endothelial cell biomarkers in critically ill COVID-19 patients with encephalitis. J Neurochem. 2022;161(6):492–505. doi: 10.1111/jnc.15545. [DOI] [PubMed] [Google Scholar]
- 42.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224) doi: 10.1126/scitranslmed.3008226. 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355) doi: 10.1126/scitranslmed.aaf8621. 355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luyt C.E., Combes A., Becquemin M.H., Beigelman-Aubry C., Hatem S., Brun A.L., et al. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012;142(3):583–592. doi: 10.1378/chest.11-2196. [DOI] [PubMed] [Google Scholar]
- 46.Combes A., Brodie D., Bartlett R., Brochard L., Brower R., Conrad S., et al. Position paper for the organization of extracorporeal membrane oxygenation programs for acute respiratory failure in adult patients. Am J Respir Crit Care Med. 2014;190(5):488–496. doi: 10.1164/rccm.201404-0630CP. [DOI] [PubMed] [Google Scholar]
- 47.Lago P., Rebsamen S., Clancy R.R., Pinto-Martin J., Kessler A., Zimmerman R., et al. MRI, MRA, and neurodevelopmental outcome following neonatal ECMO. Pediatr Neurol. 1995;12(4):294–304. doi: 10.1016/0887-8994(95)00047-j. [DOI] [PubMed] [Google Scholar]
- 48.Black M.D., Coles J.G., Williams W.G., Rebeyka I.M., Trusler G.A., Bohn D., et al. Determinants of success in pediatric cardiac patients undergoing extracorporeal membrane oxygenation. Ann Thorac Surg. 1995;60(1):133–138. doi: 10.1016/S0003-4975(95)00238-3. [DOI] [PubMed] [Google Scholar]
- 49.Barrett C.S., Bratton S.L., Salvin J.W., Laussen P.C., Rycus P.T., Thiagarajan R.R. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10(4):445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 50.Le Guennec L., Cholet C., Huang F., Schmidt M., Bréchot N., Hékimian G., et al. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8(1):129. doi: 10.1186/s13613-018-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luyt C.E., Bréchot N., Demondion P., Jovanovic T., Hékimian G., Lebreton G., et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(5):897–907. doi: 10.1007/s00134-016-4318-3. [DOI] [PubMed] [Google Scholar]
- 52.Lewandowski K., Rossaint R., Pappert D., Gerlach H., Slama K.J., Weidemann H., et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med. 1997;23(8):819–835. doi: 10.1007/s001340050418. [DOI] [PubMed] [Google Scholar]
- 53.Mateen F.J., Muralidharan R., Shinohara R.T., Parisi J.E., Schears G.J., Wijdicks E.F. Neurological injury in adults treated with extracorporeal membrane oxygenation. Arch Neurol. 2011;68(12):1543–1549. doi: 10.1001/archneurol.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gulino-Debrac D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers. 2013;1(2):e24180. doi: 10.4161/tisb.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veraar C.M., Rinösl H., Kühn K., Skhirtladze-Dworschak K., Felli A., Mouhieddine M., et al. Non-pulsatile blood flow is associated with enhanced cerebrovascular carbon dioxide reactivity and an attenuated relationship between cerebral blood flow and regional brain oxygenation. Crit Care. 2019;23(1):426. doi: 10.1186/s13054-019-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hingorani A., Ascher E., Tsemekhim B., Markevich N., Kallakuri S., Schutzer R., et al. Causes of early post carotid endartectomy stroke in a recent series: the increasing importance of hyperperfusion syndrome. Acta Chir Belg. 2002;102(6):435–438. doi: 10.1080/00015458.2002.11679347. [DOI] [PubMed] [Google Scholar]
- 57.Sage P.T., Carman C.V. Settings and mechanisms for trans-cellular diapedesis. Front Biosci. 2009;14(13):5066–5083. doi: 10.2741/3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marchetti L., Engelhardt B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol. 2020;2(1):H1–18. doi: 10.1530/VB-19-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen J., T-To S.S., Schrieber L., King N.J. Early E-selectin, VCAM-1, ICAM-1, and late major histocompatibility complex antigen induction on human endothelial cells by flavivirus and comodulation of adhesion molecule expression by immune cytokines. J Virol. 1997;71(12):9323–9332. doi: 10.1128/JVI.71.12.9323-9332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramaniam M., Koedam J.A., Wagner D.D. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol Biol Cell. 1993;4(8):791–801. doi: 10.1091/mbc.4.8.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cazaubon S., Weiss N., Couraud P.O. [Transendothelial migration through the neurovascular unit: “a cup to cross the barrier”] Med Sci. 2012;28(2):125–127. doi: 10.1051/medsci/2012282002. [DOI] [PubMed] [Google Scholar]
- 62.Mickael M.E., Kubick N., Klimovich P., Flournoy P.H., Bieńkowska I., Sacharczuk M. Paracellular and transcellular leukocytes diapedesis are divergent but interconnected evolutionary events. Genes. 2021;12(2):254. doi: 10.3390/genes12020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Beek D., de Gans J., Tunkel A.R., Wijdicks E.F. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354(1):44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 64.Bernard S.C., Simpson N., Join-Lambert O., Federici C., Laran-Chich M.P., Maïssa N., et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nat Med. 2014;20(7):725–731. doi: 10.1038/nm.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cundell D.R., Gerard C., Idanpaan-Heikkila I., Tuomanen E.I., Gerard N.P. PAf receptor anchors Streptococcus pneumoniae to activated human endothelial cells. Adv Exp Med Biol. 1996;416:89–94. doi: 10.1007/978-1-4899-0179-8_16. [DOI] [PubMed] [Google Scholar]
- 66.Le Guennec L., Virion Z., Bouzinba-Ségard H., Robbe-Masselot C., Léonard R., Nassif X., et al. Receptor recognition by meningococcal type IV pili relies on a specific complex N-glycan. Proc Natl Acad Sci USA. 2020;117(5):2606–2612. doi: 10.1073/pnas.1919567117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ring A., Weiser J.N., Tuomanen E.I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Invest. 1998;102(2):347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stins M.F., Badger J., Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30(1):19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 69.Nizet V., Kim K.S., Stins M., Jonas M., Chi E.Y., Nguyen D., et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65(12):5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Virji M., Käyhty H., Ferguson D.J., Alexandrescu C., Moxon E.R. Interactions of Haemophilus influenzae with human endothelial cells in vitro. J Infect Dis. 1992;165(Suppl 1):S115–S116. doi: 10.1093/infdis/165-supplement_1-s115. [DOI] [PubMed] [Google Scholar]
- 71.Nikulin J., Panzner U., Frosch M., Schubert-Unkmeir A. Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells. Int J Med Microbiol. 2006;296(8):553–558. doi: 10.1016/j.ijmm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Eugène E., Hoffmann I., Pujol C., Couraud P.O., Bourdoulous S., Nassif X. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J Cell Sci. 2002;115(Pt 6):1231–1241. doi: 10.1242/jcs.115.6.1231. [DOI] [PubMed] [Google Scholar]
- 73.Koedel U., Scheld W.M., Pfister H.W. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis. 2002;2(12):721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 74.Donald P.R., Schaaf H.S., Schoeman J.F. Tuberculous meningitis and miliary tuberculosis: the rich focus revisited. J Infect. 2005;50(3):193–195. doi: 10.1016/j.jinf.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Mastroianni C.M., Paoletti F., Lichtner M., D'Agostino C., Vullo V., Delia S. Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin Immunol Immunopathol. 1997;84(2):171–176. doi: 10.1006/clin.1997.4367. [DOI] [PubMed] [Google Scholar]
- 76.Bijlsma M.W., Brouwer M.C., Kasanmoentalib E.S., Kloek A.T., Lucas M.J., Tanck M.W., et al. Community-acquired bacterial meningitis in adults in the Netherlands, 2006-14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339–347. doi: 10.1016/S1473-3099(15)00430-2. [DOI] [PubMed] [Google Scholar]
- 77.Zegenhagen L., Kurhade C., Koniszewski N., Överby A.K., Kröger A. Brain heterogeneity leads to differential innate immune responses and modulates pathogenesis of viral infections. Cytokine Growth Factor Rev. 2016;30:95–101. doi: 10.1016/j.cytogfr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 78.Harypursat V., Zhou Y., Tang S., Chen Y. JC Polyomavirus, progressive multifocal leukoencephalopathy and immune reconstitution inflammatory syndrome: a review. AIDS Res Ther. 2020;17(1):37. doi: 10.1186/s12981-020-00293-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smadja D.M., Guerin C.L., Chocron R., Yatim N., Boussier J., Gendron N., et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Younger D.S. Postmortem neuropathology in COVID-19. Brain Pathol. 2021;31(2):385–386. doi: 10.1111/bpa.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee M.H., Perl D.P., Nair G., Li W., Maric D., Murray H., et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2021;384(5):481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeKosky S.T., Kochanek P.M., Valadka A.B., Clark R.S.B., Chou S.H., Au A.K., et al. Blood biomarkers for detection of brain injury in COVID-19 patients. J Neurotrauma. 2021;38(1):1–43. doi: 10.1089/neu.2020.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guan Y., Li L., Chen J., Lu H. Effect of AQP4-RNAi in treating traumatic brain edema: multi-modal MRI and histopathological changes of early stage edema in a rat model. Exp Ther Med. 2020;19(3):2029–2036. doi: 10.3892/etm.2020.8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohtsuki S., Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24(9):1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 87.de Lange E.C. Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56(12):1793–1809. doi: 10.1016/j.addr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Löscher W., Potschka H. Blood-brain barrier active efflux transporters: aTP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuji A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2005;2(1):54–62. doi: 10.1602/neurorx.2.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordon-Cardo C., O'Brien J.P., Casals D., Rittman-Grauer L., Biedler J.L., Melamed M.R., et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86(2):695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deeley R.G., Cole S.P. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580(4):1103–1111. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 92.Skrobik Y., Leger C., Cossette M., Michaud V., Turgeon J. Factors predisposing to coma and delirium: fentanyl and midazolam exposure; CYP3A5, ABCB1, and ABCG2 genetic polymorphisms; and inflammatory factors. Crit Care Med. 2013;41(4):999–1008. doi: 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- 93.Shehabi Y., Riker R.R., Bokesch P.M., Wisemandle W., Shintani A., Ely E.W., et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 94.Hughes C.G., Pandharipande P.P., Thompson J.L., Chandrasekhar R., Ware L.B., Ely E.W., et al. Endothelial activation and blood-brain barrier injury as risk factors for delirium in critically ill patients. Crit Care Med. 2016;44(9):e809–e817. doi: 10.1097/CCM.0000000000001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sudsakorn S., Bahadduri P., Fretland J., Lu C. 2020 FDA drug-drug interaction guidance: a comparison analysis and action plan by pharmaceutical industrial scientists. Curr Drug Metab. 2020;21(6):403–426. doi: 10.2174/1389200221666200620210522. [DOI] [PubMed] [Google Scholar]
- 96.Bouzbib C., El Mourabit H., Wendum D., Lasnier E., Mouri S., Housset C., et al. ATP-binding cassette transporters expression in rats with cirrhosis and hepatic encephalopathy. Clin Res Hepatol Gastroenterol. 2022;46(9) doi: 10.1016/j.clinre.2021.101784. [DOI] [PubMed] [Google Scholar]
- 97.Bauer M., Zeitlinger M., Karch R., Matzneller P., Stanek J., Jäger W., et al. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: a comparison with rat data. Clin Pharmacol Ther. 2012;91(2):227–233. doi: 10.1038/clpt.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weiss N., Barbier Saint Hilaire P., Colsch B., Isnard F., Attala S., Schaefer A., et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J Hepatol. 2016;65(6):1120–1130. doi: 10.1016/j.jhep.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 99.Schaefer A., Journaux M., Mourabit H.E., Mouri S., Wendum D., Lasnier E., et al. A systemic mechanism of increased transendothelial migration of leukocytes through the blood-brain barrier in hepatic encephalopathy. Clin Res Hepatol Gastroenterol. 2022;46(3) doi: 10.1016/j.clinre.2021.101801. [DOI] [PubMed] [Google Scholar]
- 100.Volk H., Potschka H., Löscher W. Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem. 2005;53(4):517–531. doi: 10.1369/jhc.4A6451.2005. [DOI] [PubMed] [Google Scholar]
- 101.Nicita F., Spalice A., Papetti L., Nikanorova M., Iannetti P., Parisi P. Efficacy of verapamil as an adjunctive treatment in children with drug-resistant epilepsy: a pilot study. Seizure. 2014;23(1):36–40. doi: 10.1016/j.seizure.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Löscher W., Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 103.Zhao F., Zhong L., Luo Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci Ther. 2021;27(1):26–35. doi: 10.1111/cns.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shireman P.K., Pearce W.H. Endothelial cell function: biologic and physiologic functions in health and disease. AJR Am J Roentgenol. 1996;166(1):7–13. doi: 10.2214/ajr.166.1.8571908. [DOI] [PubMed] [Google Scholar]
- 105.Genét G.F., Bentzer P., Hansen M.B., Ostrowski S.R., Johansson P.I. Effects of propranolol and clonidine on brain edema, blood-brain barrier permeability, and endothelial glycocalyx disruption after fluid percussion brain injury in the rat. J Trauma Acute Care Surg. 2018;84(1):89–96. doi: 10.1097/TA.0000000000001708. [DOI] [PubMed] [Google Scholar]
- 106.Li X., Zhu J., Liu K., Hu Y., Huang K., Pan S. Heparin ameliorates cerebral edema and improves outcomes following status epilepticus by protecting endothelial glycocalyx in mice. Exp Neurol. 2020;330 doi: 10.1016/j.expneurol.2020.113320. [DOI] [PubMed] [Google Scholar]
- 107.Lee W.L., Slutsky A.S. Sepsis and endothelial permeability. N Engl J Med. 2010;363(7):689–691. doi: 10.1056/NEJMcibr1007320. [DOI] [PubMed] [Google Scholar]
- 108.Chaurasia C.S., Müller M., Bashaw E.D., Benfeldt E., Bolinder J., Bullock R., et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res. 2007;24(5):1014–1025. doi: 10.1007/s11095-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 109.Stangler L.A., Kouzani A., Bennet K.E., Dumee L., Berk M., Worrell G.A., et al. Microdialysis and microperfusion electrodes in neurologic disease monitoring. Fluids Barriers CNS. 2021;18(1):52. doi: 10.1186/s12987-021-00292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lynes J., Jackson S., Sanchez V., Dominah G., Wang X., Kuek A., et al. Cytokine microdialysis for real-time immune monitoring in glioblastoma patients undergoing checkpoint blockade. Neurosurgery. 2019;84(4):945–953. doi: 10.1093/neuros/nyy392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gallagher M.J., Hogg F.R.A., Zoumprouli A., Papadopoulos M.C., Saadoun S. Spinal cord blood flow in patients with acute spinal cord injuries. J Neurotrauma. 2019;36(6):919–929. doi: 10.1089/neu.2018.5961. [DOI] [PubMed] [Google Scholar]
- 112.Torné R., Culebras D., Sanchez-Etayo G., García-García S., Muñoz G., Llull L., et al. Double hemispheric microdialysis study in poor-grade SAH patients. Sci Rep. 2020;10(1):7466. doi: 10.1038/s41598-020-64543-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abbott N.J. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45(4):545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 114.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ringstad G., Eide P.K. Molecular trans-dural efflux to skull bone marrow in humans with CSF disorders. Brain. 2022;145(4):1464–1472. doi: 10.1093/brain/awab388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Plog B.A., Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13:379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pulous F.E., Cruz-Hernández J.C., Yang C., Kaya Ζ., Paccalet A., Wojtkiewicz G., et al. Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis. Nat Neurosci. 2022;25(5):567–576. doi: 10.1038/s41593-022-01060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Graham M.S., Mellinghoff I.K. Meningeal lymphatics prime tumor immunity in glioblastoma. Cancer Cell. 2021;39(3):304–306. doi: 10.1016/j.ccell.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ding D., Wang X., Li Q., Li L., Wu J. Research on the glial-lymphatic system and its relationship with Alzheimer's disease. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jessen N.A., Munk A.S., Lundgaard I., Nedergaard M. The glymphatic system: a beginner's guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reddy O.C., van der Werf Y.D. The sleeping brain: harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020;10(11):868. doi: 10.3390/brainsci10110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Patel M., Chipman J., Carlin B.W., Shade D. Sleep in the intensive care unit setting. Crit Care Nurs Q. 2008;31(4):319–320. doi: 10.1097/01.CNQ.0000336816.89300.41. 309–18; quiz. [DOI] [PubMed] [Google Scholar]
- 123.Rao M.R., Norquay G., Stewart N.J., Wild J.M. Measuring 129 Xe transfer across the blood-brain barrier using MR spectroscopy. Magn Reson Med. 2021;85(6):2939–2949. doi: 10.1002/mrm.28646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Towner R.A., Gulej R., Zalles M., Saunders D., Smith N., Lerner M., et al. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience. 2021;43(2):563–578. doi: 10.1007/s11357-021-00363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garin C.M., Nadkarni N.A., Pépin J., Flament J., Dhenain M. Whole brain mapping of glutamate distribution in adult and old primates at 11.7T. Neuroimage. 2022;251 doi: 10.1016/j.neuroimage.2022.118984. [DOI] [PubMed] [Google Scholar]
- 126.Ross E.C., Ten Hoeve A.L., Barragan A. Integrin-dependent migratory switches regulate the translocation of Toxoplasma-infected dendritic cells across brain endothelial monolayers. Cell Mol Life Sci. 2021;78(12):5197–5212. doi: 10.1007/s00018-021-03858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.