Abstract
Introduction: The first-generation Watchman 2.5 (W 2.5)TM presented several limitations, such as challenges in implantation within complex left atrial appendage (LAA) anatomies, higher incidence of peri-device leak, device recapture, and device-related thrombus (DRT). The newer generation Watchman FLX (W-FLX)TM was introduced with a modified design aiming to overcome these limitations. The purpose of this meta-analysis is to conduct a comparative assessment of the safety and efficacy of the W-FLX and 2.5 devices in clinical practice. Method: The meta-analysis was conducted according to the preferred reporting items for systematic review and meta-analysis protocols (PRISMA). Studies were located through a search strategy utilizing PubMed, Cochrane, Google scholar and MEDLINE from inception to March 2023, with a primary objective to compare the safety and efficacy of the W-FLX and W 2.5 devices. After applying the selection criteria, five studies were included in this analysis. Results: The analysis included five studies comprising 54,727 patients. The W-FLX is associated with an increase in procedural success (OR 7.49 [95% CI 1.98-28.26, P = 0.02; I2 = 0%]), and a significant reduction in mortality (OR 0.52 [95% CI 0.51-0.54, P<0.01; I2 = 0%], major bleeding 0.57 [95% CI 0.51-0.64, P<0.01; I2 = 0%]), device embolism (OR 0.35 [95% CI 0.18-0.70, P = 0.02; I2 = 0%]), and pericardial effusion (OR 0.33 [95% CI 0.26-0.41, P<0.01; I2 = 0%]). The rates of DRT and stroke were similar between the two groups. Conclusion: Compared to the W 2.5, the W-FLX was associated with a higher procedural success rate and significantly reduced adverse outcomes including mortality, major bleeding, device embolization, and pericardial effusion.
Keywords: Left atrial appendage closure, left atrial appendage occlusion, Watchman, new generation Watchman
Introduction
Left atrial appendage closure (LAAC) has emerged as an alternative to oral anticoagulation in patients with non-valvular atrial fibrillation deemed at high risk for both thromboembolic and bleeding events. This procedure is particularly beneficial for patients who are intolerant or have contraindications for long-term anticoagulant therapy due to increased bleeding risks with a proven non-inferiority demonstrated in earlier studies [1,2]. The most frequently reported complications following LAAC are pericardial effusion, device implantation failure, device-related thrombus (DRT), and bleeding. The presence of peri-device leak is often encountered due to complex left atrial appendage (LAA) anatomy and morphological variations [3].
The Watchman device is a LAAC instrument with a small filter that is shaped like a parachute. It is delivered to the LAA via a catheter and self-expands to seal the area in order to prevent embolic migration of blood clots formed in the LAA while permitting blood to flow. It is used in non-valvular atrial fibrillation patients who are at high risk of stroke with a contraindication for long-term anticoagulant therapy [3]. The first-generation Watchman 2.5 (W 2.5) is the most widely used device worldwide; however, it is associated with few major limitations including difficult implantation in complex LAA anatomies, such as shallow LAA chicken-wing morphologies, peri-device leak, the need for repeated device recapture warranting the use of a new device, and the occurrence DRT [4]. The Watchman FLX (W-FLX), introduced in 2020, represents a promising significant advancement in the field and addressed these limitations. Observational studies have shown comparable efficacy and safety outcomes when compared to the W 2.5 [5,6]. The W-FLX has been designed and modified to improve procedural success rates, facilitate ease of positioning, and reduce procedural-related complications. We conducted this meta-analysis to compare safety and efficacy of the first and second generations of the Watchman devices.
Methods
This meta-analysis was conducted based on Cochrane collaboration guidelines and reported according to the preferred reporting items for systematic review and meta-analysis protocols (PRISMA) [7].
Study search and selection criteria
Two independent reviewers (MM, MRM) conducted a literature search of electronic databases including MEDLINE/PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials without language limitations from database inception through March, 2023. We also searched Google Scholar and two clinical trial registries (the World Health Organization’s International Clinical Trials Registry Platform and ClinicalTrials.gov). We used the following keywords: “Left atrial appendage closure, left atrial appendage exclusion, Watchman 2.5, Watchman FLX”. The references of the retrieved studies were screened for relevant studies that are appropriate for this meta-analysis.
The pre-determined inclusion criteria were all articles published in peer-reviewed journals comparing the W 2.5 to the W-FLX. There were no restrictions on sample size, language, or follow-up durations. We excluded all retrieved reviews, abstracts, letters, editorials, observational studies, and non-human studies.
Data extraction
The data abstraction was performed by two independent reviewers (SM and MN) and all discrepancies were resolved by a third reviewer (MME). We extracted the following information: First author name, population size, mean age in years, gender, percentages of hypertension, diabetes, CAD, heart failure, stroke/TIA, and the mean CHA2DS2-VASc and HAS-BLED scores.
Outcomes of interest
The primary outcome was a composite of device thrombosis, device embolism, and major bleeding. Secondary outcomes were mortality, stroke, pericardial effusion, and procedural success rate.
Statistical analysis
The outcomes of interest were pooled using a random effects Mantel-Haenszel model. We used the DerSimonian and Laird method for estimation of τ2. We reported the effect sizes as odds ratio (OR) with 95% confidence interval (CI), given the included studies were observational. We considered the 95% CIs that did not cross zero statistically significant. We used I2 statistics to assess statistical heterogeneity; I2 >50% considered significant heterogeneity. Analyses were performed using RStudio (Posit Software, PBC, Boston, MA).
Study quality and risk of bias
The quality of the included studies was assessed using the Newcastle-Ottawa Scale for cohort studies, as shown in Supplementary Table 1. In the Newcastle-Ottawa Scale, the maximum points awarded are two for comparability, and one for all other parameters. A score of <5 is considered low quality, 5-6 medium quality, and 7-9 high quality.
Results
Summary of studies
A total of 84 articles were screened from the electronic database search. After a thorough review, five studies met the inclusion criteria. The search process is detailed in Figure 1. A total of 54,727 patients were included in the analysis from five retrospective observational studies [8-12]. The baseline characteristics are outlined in Table 1. The details of the included studies are illustrated in Table 2.
Figure 1.
Flow diagram.
Table 1.
Baseline characteristics of the study population
| Study ID | Device | Number of patients | Age, y | Female, n (%) | CHF, n (%) | LVEF | HTN | DM, n (%) | Stroke, n (%) | CHADS-VASC | HAS-BLED |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paitazoglou et al. | W 2.5 | 1025 | 73.4 (8.8) | 408 (40.1) | 349 (34.1) | NA | 885 (86.6) | 304 (29.7) | 199 (19) | 4.5 (1.6) | 2.3 (1.2) |
| W-FLX | 164 | 73.7 (8.3) | 66 (40) | 54 (32.3) | NA | 147 (89.6) | 58 (35.3) | 56 (34.1) | 4.6 (1.4) | 3.2 (0.8) | |
| Vizzari et al. | W 2.5 | 100 | 77.8 (5.93) | NA | NA | NA | NA | NA | NA | NA | NA |
| W-FLX | 200 | 77 (7.18) | 65 (32.5) | 109 (54.5) | 47 (11.3) | 186 (93) | 83 (41.5) | 58 (29) | 5 (1.4) | 4 (1.01) | |
| Price et al. | W 2.5 | 48999 | 76.2 (7.7) | 20191 (41.2) | 18691 (38.1) | 54 (10.1) | 44981 (91.6) | 17439 (35.5) | 10887 (22.2) | 4.8 (1.5) | 2.9 (1.1) |
| W-FLX | 27455 | 76.2 (7.7) | 11228 (40.9) | 10620 (38.7) | 54 (10.1) | 25125 (91.6) | 9799 (35.7) | 6005 (21.9) | 4.8 (1.5) | 2.8 (1.1) | |
| Fukuda et al. | W 2.5 | 49 | 76.4 (7.4) | 13 (26) | 28 (57) | 56.6 (12.2) | 40 (82) | 17 (35) | 20 (41) | 4.9 (1.6) | 3.2 (1) |
| W-FLX | 44 | 80.8 (6.6) | 17 (39) | 28 (64) | 61.4 (14.6) | 35 (80) | 11 (25) | 15 (34) | 4.9 (1.4) | 2.8 (0.8) | |
| Galea et al. | W 2.5 | 71 | 77.1 (7.3) | 20 (28.2) | 12 (16.9) | NA | 61 (85.9) | 17 (23.9) | 20 (28.2) | 4 | 3 |
| W-FLX | 73 | 76.5 (8.4) | 23 (31.5) | 14 (19.2) | NA | 60 (82.2) | 28 (38.4) | 22 (30.1) | 4 | 3 |
CHF: Congestive Heart Failure; LVEF: Left Ventricular Ejection Fraction; HTN: Hypertension; DM: Diabetes Mellitus; W-FLX: Watchman FLX; W 2.5: Watchman 2.5.
Table 2.
Summary of the included studies
| Study ID | Country | Study Design | Number of participants | Follow up duration | Conclusion |
|---|---|---|---|---|---|
| Paitazoglou et al. | 4 European centers | Retrospective registry | W-FLX: 164 | 3 months | Improved sealing rate with similar safety profile. |
| W 2.5: 1025 | |||||
| Vizzari et al. | Italy | Prospective nonrandomized double-center registry | W-FLX: 200 | 272 ± 172.76 days | High success rates with W-FLX with good sealing and low rates of complications including DRT, distal embolization. |
| W 2.5: 100 | |||||
| Price et al. | USA | NCDR LAAO | W-FLX: 27,013 | In-hospital and peri-procedural | W-FLX had Lower rates of MAE, mortality, embolization, bleeding and cardiac arrest. |
| W 2.5: 27,013 | |||||
| Fukuda et al. | Japan | Retrospective single-center study | W-FLX: 44 | 45 days | W-FLX was as safe and effective as conventional W 2.5 during the short-term period. |
| W 2.5: 49 | |||||
| Galea et al. | Switzerland | Prospective cohort | W-FLX: 73 | 6 months | W-FLX as compared to W 2.5, was associated with similar procedure-related complications and 6-month NACE, but with improved LAA neck coverage, and lower IDL and DRT. |
| W 2.5: 71 |
W-FLX: Watchman FLX; W 2.5: Watchman 2.5; DRT: Device-Related Thrombus; NCDR LAAO Registry: The National Cardiovascular Data Registry Left Atrial Appendage Occlusion; MAE: Major Adverse Events; NACE: Net Adverse Cardiovascular Events; LAA: Left Atrial Appendage; IDL: Intra-Device Leak.
Outcomes
DRT
An analysis of three studies with 237 patients in the W-FLX group and 953 patients in the W 2.5 group showed no statistical difference between the two arms (OR 0.55 [95% CI 0.07, 4.48, P = 0.35]). No heterogeneity was observed with I2 = 0% (Figure 2).
Figure 2.

Forest plot of device thrombosis.
Device embolism
An analysis of four studies with 27,736 patients in the W-FLX group and 50,139 patients in the W 2.5 group showed a statistically significant reduction in device embolism favoring W-FLX (OR 0.35 [95% CI 0.18, 0.70, P = 0.02]). No heterogeneity was observed with I2 = 0% (Figure 3).
Figure 3.

Forest plot of device embolism.
Major bleeding
An analysis of five studies with 27,936 patients in the W-FLX group and 50,239 patients in the W 2.5 group showed a statistically significant reduction in major bleeding favoring the W-FLX (OR 0.57 [95% CI 0.51, 0.64, P<0.01]). No heterogeneity was observed with I2 = 0% (Figure 4).
Figure 4.
Forest plot of major bleeding.
Mortality
An analysis of five studies with 27,936 patients in the W-FLX group and 50,239 patients in W 2.5 group showed a statistically significant reduction in mortality favoring W-FLX (OR 0.52 [95% CI 0.51, 0.54, P<0.01]). No heterogeneity was observed with I2 = 0% (Figure 5).
Figure 5.
Forest plot of mortality.
Pericardial effusion
An analysis of four studies with 27,863 patients in the W-FLX group and 50,168 patients in the W 2.5 group showed a statistically significant reduction in pericardial effusion favoring the W-FLX (OR 0.33 [95% CI 0.26, 0.41, P<0.01]). No heterogeneity was observed with I2 = 0% (Figure 6).
Figure 6.

Forest plot of pericardial effusion.
Procedural success
An analysis of three studies with 408 patients in the W-FLX group and 1169 patients in the W 2.5 group showed a statistically significant increase of more than 7 fold in procedural success favoring W-FLX with OR 7.49 [95% CI 1.98, 28.26, P = 0.02]. No heterogeneity was observed with I2 = 0% (Figure 7).
Figure 7.

Forest plot of procedure success.
Stroke
An analysis of five studies with 27,936 patients in W-FLX group and 50,239 patients in W 2.5 group showed no statistical difference between the two arms (OR 0.90 [95% CI 0.16, 5.16, P = 0.59]). No heterogeneity was observed with I2 = 0% (Figure 8).
Figure 8.
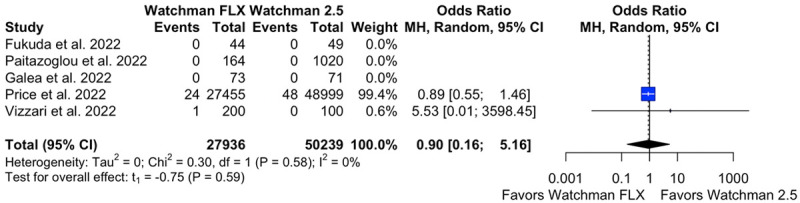
Forest plot of stroke.
Discussion
The W-FLX is a newer generation of trans-catheter left atrial appendage occlusion (LAAO) device. Few reports showed that it offers potential clinical advantages when compared to the W 2.5 device. In this meta-analysis that included 54,727 patients, we identified superior outcomes with W-FLX in terms of mortality rates (OR = 0.52 [95% CI: 0.51-0.54]; P<0.01), major bleeding (OR = 0.57 [95% CI: 0.51-0.64]; P<0.01), pericardial effusion (OR = 0.33 [95% CI: 0.26-0.41]; P<0.01), and device embolism (OR = 0.35 [95% CI: 0.18-0.70]; P<0.02) when compared to W 2.5. Furthermore, W-FLX showed a higher procedural success rate than W 2.5 (OR = 7.49 [95% CI: 1.98-28.26]; P = 0.02).
The implantation of the LAAO devices has potential risks that can result in adverse outcomes. Previous research conducted by Reddy et al. reported major procedure-related complications observed with W 2.5 that raised some safety concerns, especially during the early period of the study. These complications included cardiac effusion and tamponade, procedure-related stroke, device embolization, and DRT [3].
The modified design of W-FLX offers an overall better safety profile. The smaller metal surface area helps in reducing the risk of device-related thrombosis. Additionally, the soft, closed, atraumatic distal end of the device facilitate smoother navigation within the LAA that mitigates the risk of pericardial effusion. By increasing number of struts (18 instead of 10) and adopting an open architecture configuration, the device can now conform more efficiently to the LAA ostium and ensure adequate sealing due to the dual-row anchors [8]. Furthermore, the modified design of W-FLX can lower the risk of peri-procedure leak due to the lengthening of the fabric membrane. The deployment mechanism, consisting of a pair of J-shaped fixation anchors, not only provides improved control and stability but also offers the capability for both complete and partial retrieval, allowing for repositioning and potentially reducing the risk of device embolization. The device’s shorter length is another crucial improvement, as it enables the effective closure of even shallow LAAs, leading to enhanced procedural outcomes [5,10]. The PINNACLE FLX trial has demonstrated these benefits clinically in patients with non-valvular atrial fibrillation [5].
Determining the cause of thrombosis or embolism during LAAO procedure can be challenging. A comprehensive evaluation of the patient’s medical history, risk factors, and peri-procedural events is crucial in identifying potential causes. Advanced imaging techniques, like transesophageal echocardiography (TEE) and cardiac CT, can aid in visualizing the device’s position and integrity post-implantation. In cases of DRT, adjusting anticoagulation strategies may help reduce the risk of embolic events [13]. However, further research is required to understand the precise mechanisms and risk factors associated with DRT and embolism following LAAO.
The reported incidence of DRT following LAAO in the literature is around 3.8%, with most events happening within the first year post-implantation [4]. Paitazoglou et al. reported an even lower incidence of 2.4% [6]. Although DRT may potentially increase the risk of stroke, we do not have robust data to support this hypothesis [4,14]. W-FLX has some unique characteristics that can theoretically reduce the risk of DRT, such as fewer exposed knobs to the atrial side and more proximal implantation than W 2.5, leading to improved flow [15,16]. However, our data did not show any significant difference in the rate of DRT (OR = 0.55 [95% CI: 0.07-4.48]; P = 0.35) or stroke (OR = 0.9 [95% CI: 0.16-5.16]; P = 0.59) between the W-FLX and W 2.5 groups. We believe that more research is needed to investigate these outcomes.
Most of our results were driven by Price et al.’s article, which is the main limitation of our study. The follow-up period of the constituent articles was relatively short, mostly limited to three months at most, which could potentially mask potential late complications. The learning curve associated with any new therapeutic modality requiring hand skills is another important consideration. Since W 2.5 was introduced early in the market, the higher rate of complications reported in the literature could have been overestimated due to lack of experience.
In conclusion, our findings indicate improved safety outcomes with the use of W-FLX compared to its predecessor, and favor its use in clinical practice. Nevertheless, further studies examining the long-term safety outcomes of this device are warranted.
Disclosure of conflict of interest
None.
Abbreviations
- LAAC
left atrial appendage closure
- W-FLX
Watchman FLX
- W 2.5
Watchman 2.5
- DRT
device-related thrombus
Supporting Information
References
- 1.Reddy VY, Sievert H, Halperin J, Doshi SK, Buchbinder M, Neuzil P, Huber K, Whisenant B, Kar S, Swarup V, Gordon N, Holmes D PROTECT AF Steering Committee and Investigators. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–98. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–24. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 4.Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol. 2018;4:1629–37. doi: 10.1016/j.jacep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, Stone J Jr, Shah M, Dukkipati SR, Adler S, Nair DG, Kim J, Wazni O, Price MJ, Asch FM, Holmes DR Jr, Shipley RD, Gordon NT, Allocco DJ, Reddy VY PINNACLE FLX Investigators. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation. 2021;143:1754–62. doi: 10.1161/CIRCULATIONAHA.120.050117. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-González I, Korsholm K, Trejo-Velasco B, Thambo JB, Mazzone P, Rioufol G, Grygier M, Möbius-Winkler S, Betts T, Meincke F, Sandri M, Schmidt B, Schmitz T, Nielsen-Kudsk JE. Procedural and short-term results with the new Watchman FLX left atrial appendage occlusion device. JACC Cardiovasc Interv. 2020;13:2732–41. doi: 10.1016/j.jcin.2020.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paitazoglou C, Meincke F, Bergmann MW, Eitel I, Fink T, Vireca E, Wohlmuth P, Veliqi E, Willems S, Markiewicz A, Grygier M. The ALSTER-FLX registry: 3-month outcomes after left atrial appendage occlusion using a next-generation device, a matched-pair analysis to EWOLUTION. Heart Rhythm. 2022;19:917–26. doi: 10.1016/j.hrthm.2022.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Vizzari G, Grasso C, Sardone A, Mazzone P, Laterra G, Frazzetto M, Sacchetta G, Micari A, Tamburino C, Contarini M. Real-world experience with the new Watchman FLX device: data from two high-volume Sicilian centers. The FLX-iEST registry. Catheter Cardiovasc Interv. 2022;100:154–60. doi: 10.1002/ccd.30237. [DOI] [PubMed] [Google Scholar]
- 10.Price MJ, Friedman DJ, Du C, Wang Y, Lin Z, Curtis JP, Freeman JV. Comparative safety of transcatheter LAAO with the first-generation watchman and next-generation Watchman FLX devices. JACC Cardiovasc Interv. 2022;15:2115–23. doi: 10.1016/j.jcin.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda N, Imamura T, Tanaka S, Kataoka N, Ushijima R, Ueno H, Kinugawa K. Comparison in short-term safety and efficacy between new-generation WATCHMAN FLX and conventional WATCHMAN 2.5 for percutaneous left atrial appendage closure. J Clin Med. 2022;11:1618. doi: 10.3390/jcm11061618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galea R, Mahmoudi K, Gräni C, Elhadad S, Huber AT, Heg D, Siontis GCM, Brugger N, Sebag F, Windecker S, Valgimigli M, Landolff Q, Roten L, Amabile N, Räber L. Watchman FLX vs. Watchman 2.5 in a dual-center left atrial appendage closure cohort: the WATCH-DUAL study. Europace. 2022;24:1441–50. doi: 10.1093/europace/euac021. [DOI] [PubMed] [Google Scholar]
- 13.Dukkipati SR, Kar S, Holmes DR, Doshi SK, Swarup V, Gibson DN, Maini B, Gordon NT, Main ML, Reddy VY. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. 2018;138:874–85. doi: 10.1161/CIRCULATIONAHA.118.035090. [DOI] [PubMed] [Google Scholar]
- 14.Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, Gori T, Meincke F, Protopopov AV, Betts T, Mazzone P, Foley D, Grygier M, Sievert H, De Potter T, Vireca E, Stein K, Bergmann MW following investigators and institutions participated in the EWOLUTION study. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: final 2-year outcome data of the EWOLUTION trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol. 2019;12:e006841. doi: 10.1161/CIRCEP.118.006841. [DOI] [PubMed] [Google Scholar]
- 15.Simard T, Jung RG, Lehenbauer K, Piayda K, Pracoń R, Jackson GG, Flores-Umanzor E, Faroux L, Korsholm K, Chun JKR, Chen S, Maarse M, Montrella K, Chaker Z, Spoon JN, Pastormerlo LE, Meincke F, Sawant AC, Moldovan CM, Qintar M, Aktas MK, Branca L, Radinovic A, Ram P, El-Zein RS, Flautt T, Ding WY, Sayegh B, Benito-González T, Lee OH, Badejoko SO, Paitazoglou C, Karim N, Zaghloul AM, Agrawal H, Kaplan RM, Alli O, Ahmed A, Suradi HS, Knight BP, Alla VM, Panaich SS, Wong T, Bergmann MW, Chothia R, Kim JS, Pérez de Prado A, Bazaz R, Gupta D, Valderrabano M, Sanchez CE, El Chami MF, Mazzone P, Adamo M, Ling F, Wang DD, O’Neill W, Wojakowski W, Pershad A, Berti S, Spoon D, Kawsara A, Jabbour G, Boersma LVA, Schmidt B, Nielsen-Kudsk JE, Rodés-Cabau J, Freixa X, Ellis CR, Fauchier L, Demkow M, Sievert H, Main ML, Hibbert B, Holmes DR Jr, Alkhouli M. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. 2021;78:297–313. doi: 10.1016/j.jacc.2021.04.098. [DOI] [PubMed] [Google Scholar]
- 16.Aminian A, Schmidt B, Mazzone P, Berti S, Fischer S, Montorfano M, Lam SCC, Lund J, Asch FM, Gage R, Cruz-Gonzalez I, Omran H, Tarantini G, Nielsen-Kudsk JE. Incidence, characterization, and clinical impact of device-related thrombus following left atrial appendage occlusion in the prospective global AMPLATZER amulet observational study. JACC Cardiovasc Interv. 2019;12:1003–14. doi: 10.1016/j.jcin.2019.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




