Abstract
The importance of glucokinase (GK) in the regulation of insulin secretion has been highlighted by the phenotypes of individuals with activating and inactivating mutations in the glucokinase gene (GCK). Here we report 10 individuals with congenital hyperinsulinism (HI) caused by eight unique activating mutations of GCK. Six are novel and located near previously identified activating mutations sites. The first recognized episode of hypoglycemia in these patients occurred between birth and 24 years, and the severity of the phenotype was also variable. Mutant enzymes were expressed and purified for enzyme kinetics in vitro. Mutant enzymes had low glucose half-saturation concentration values and an increased enzyme activity index compared with wild-type GK. We performed functional evaluation of islets from the pancreata of three children with GCK-HI who required pancreatectomy. Basal insulin secretion in perifused GCK-HI islets was normal, and the response to glyburide was preserved. However, the threshold for glucose-stimulated insulin secretion in perifused glucokinase hyperinsulinism (GCK-HI) islets was decreased, and glucagon secretion was greatly suppressed. Our evaluation of novel GCK disease-associated mutations revealed that the detrimental effects of these mutations on glucose homeostasis can be attributed not only to a lowering of the glucose threshold of insulin secretion but also to a decreased counterregulatory glucagon secretory response.
Article Highlights
Our evaluation of six novel and two previously published activating GCK mutations revealed that the detrimental effects of these mutations on glucose homeostasis can be attributed not only to a lowering of the glucose threshold of insulin secretion but also to a decreased counterregulatory glucagon secretory response.
These studies provide insights into the pathophysiology of GCK-hyperinsulinism and the dual role of glucokinase in β-cells and α-cells to regulate glucose homeostasis.
Introduction
Glucokinase (GK), the rate-limiting enzyme for glucose entry into glycolysis and oxidation in the β-cell, plays a key role in determining the threshold of glucose stimulated insulin secretion (GSIS). The critical role of GK in the regulation of glucose homeostasis in humans has been demonstrated by the phenotype of individuals with inactivating mutations in the glucokinase (GCK) gene, which is characterized by stable, nonprogressive fasting hyperglycemia, classified as maturity diabetes of the young (MODY) type 2 (MODY2) (1–4). In contrast, activating GCK mutations result in persistent hypoglycemia due to hyperinsulinism (HI) (5–15).
Glucokinase hyperinsulinism (GCK-HI) is a rare form of congenital HI, accounting for ∼1–2% of individuals in a large series (16). GCK-HI–causing mutations result in activation of GK enzymatic activity and a lower glucose threshold for GSIS in β-cells. GK regulates the conversion of glucose to glycogen in the liver, in addition to having a role in other cell types that are important for glucose homeostasis, including neuroendocrine and enteroendocrine cells and α-cells (17). Studies in mouse models have shown that the activity of GK in α-cells plays an intrinsic role to suppress glucagon secretion. Increased activity of GK enhances glucagon suppression by glucose, while decreased activity leads to less suppression of glucagon secretion by glucose (18,19). Thus, the pathophysiology of GCK-HI is likely not limited to the β-cell. With very few exceptions, all of the reported HI-associated mutations are located in the allosteric site of the enzyme, which has been a target for type 2 diabetes drug development (17,20–23). The HI-causing mutations are characterized by a higher affinity for glucose and, for some mutations, a higher maximal enzyme velocity (1,24,25).
The phenotype of GCK-HI was initially described as mild and diazoxide responsive. However, as more GCK mutations have been identified, it is apparent that the phenotype is variable. A large proportion of patients are unresponsive to diazoxide (14,26–28), with some patients requiring pancreatectomy for intractable hypoglycemia. The lack of symptomatic hypoglycemia in some GCK-HI patients is likely the result of attenuation of symptoms due to hypoglycemia-associated autonomic failure resulting from repeated episodes of hypoglycemia (6,14,29).
Here, we report a series of 10 GCK-HI individuals from eight families with GCK mutations. Notably, we directly evaluated human GCK-HI islets isolated from surgical specimens after pancreatectomy by using perifusion studies to characterize the insulin and glucagon secretory responses to stimuli. These studies provide novel insights into the pathophysiology of GCK-HI and the dual role of GK in β-cells and α-cells to regulate glucose homeostasis.
Research Design and Methods
GCK-HI Case Subjects
Consent
Written informed consent was provided by all subjects or their parents. The Children’s Hospital of Philadelphia Institutional Review Board approved the study.
Probands
Probands were identified during evaluations in the Congenital Hyperinsulinism Center at The Children’s Hospital of Philadelphia. The diagnosis of HI was based on previously described criteria (30,31). Patients were defined as responsive to diazoxide when HI was completely controlled by treatment with diazoxide at doses ≤15 mg/kg/day, as demonstrated by maintaining plasma glucose concentrations ≥70 mg/dL for 12–18 h of fasting and/or by developing appropriate fasting hyperketonemia (β-hydroxybutyrate >2 mmol/L with concurrent plasma glucose <50 mg/dL).
Mutation Screening
Mutation screening was performed in commercial laboratories for all probands and parents in this study. Additional mutation analysis for other family members was performed on a research basis. Genomic DNA was isolated from peripheral blood (5 PRIME, Gaithersburg, MD) or from saliva (Oragene DNA self-collection kit; DNA Genotek, Kanata, Ontario, Canada). RNA was isolated from pancreatic tissue (AllPrep DNA/RNA Kit; Qiagen, Hilden, Germany) from the proband in family no. 8 (GCK:c.209-11t>a) and converted to cDNA (SuperScript II Reverse Transcriptase; Invitrogen, Waltham, MA) to confirm the amino acid consequence of this intronic variant. Coding sequences and intron/exon splice junctions were amplified and directly sequenced on an ABI 3730 capillary DNA analyzer (Applied Biosystems, Carlsbad, CA). The nucleotides of GCK and corresponding GK amino acids were numbered according to GenBank reference sequence NM_000162.3. The functional consequences of novel, missense mutations were predicted with bioinformatics software SIFT (32) and PolyPhen2 (33). Genetic variants were searched against the Genome Aggregation Database (gnomAD) Browser (v2.1) (34).
GK Mutant Expression and Enzyme Kinetics
GK Constructs
Human wild-type (WT) and mutant GK were created using site-directed mutagenesis, and the glutathione S-transferase (GST)-GK–tagged enzymes were expressed and purified as previously described (35). GK mutants evaluated were p.S64P, p.E67V, p.S69_E70insVPL, p.S69P, p.V91L, p.W99C, p.Y215C, and p.R447L. The negative control was inactivating mutation p.V367M.
GK Enzyme Kinetics Assay
Enzymatic activity of WT and mutant GK proteins was determined with an NADP+/NADPH-coupled assay using glucose-6-phosphate dehydrogenase as the secondary reaction, as previous described (35). The enzyme kinetics assays were conducted with the GK activator (GKA) piragliatin (5 μmol/L). For GK regulatory protein (GKRP) experiments, purified GK was added in a 1:1 or 1:2 molar ratio with GKRP with various concentrations of glucose (3, 6, and 12 mmol/L).
Islet Isolation and Perifusion
The procedure for islet isolation from pancreatic surgical specimens was described previously (36,37). After 2 to 3 days of culture, islets were perifused with a physiological amino acid mixture (AAM) ramp (0 to 12 mmol/L increasing 0.3 mmol/L/min) or a glucose ramp (0 to 25 mmol/L increasing 0.625 mmol/L/min), and then 30 mmol/L KCl. As denoted, 0.3 μmol/L glyburide was added to perifusion experiments. In some perifusions, the dynamics of insulin and glucagon secretion were determined simultaneously, with the islets exposed to 4.0 mmol/L AAM, and then glucose was added from low to high (3 mmol/L then 16.7 mmol/L in control islets; 1, 3, 5, and 10 mmol/L in HI islets). For each perifusion, 1,000 islets were loaded per perifusion, and the results were presented as per 100 islets. Insulin and glucagon concentrations were measured using HTRF assay kits (catalog no. 62IN1PEH, Cisbio, Research Resource Identifiers [RRID]:AB2890910; catalog no. 62CGLPEG, Cisbio, RRID:AB_2936335). Control islets from humans without diabetes were obtained from the Integrated Islet Distribution Program (iidp.coh.org).
Protein Docking and Molecular Dynamics Analysis
GK structure models of 3IDH and 3VEV were used for indication of mutation sites and compound docking analysis. Compound docking was analyzed using Schrödinger 2022-4 (Schrödinger, New York, NY.). Molecular dynamics of GK was examined using Desmond 2021-2 software (Schrödinger). Root mean square deviation was calculated on carbon α-atoms for the selected loop residues for 500 ns: loop 1, residues from 41 to 71; loop 2, residues from 94 to 97; and loop 3, residues from 212 to 219.
Statistical Methods
Statistical analyses were performed on GraphPad Prism 9 software. Results are presented as mean ± SEM. Single-time end point data were analyzed using a single-factor ANOVA test.
Data and Resource Availability
All data generated or analyzed during this study are included in the published article and its online supplemental files.
Results
Clinical Characteristics
Clinical and genetic information on the 10 GCK-HI case subjects from eight families described in these studies is summarized in Table 1. The HI diagnosis was established based on established criteria demonstrating inappropriate insulin concentration/actions in the context of hypoglycemia (38). Some case subjects had a large birth weight for gestational age, but some had an appropriate weight for gestational age. The age of presentation and clinical severity were also variable among the patients, even within the same family. All 10 GCK-HI patients were treated with diazoxide at doses ranging from 6 to 20 mg/kg/day, with variable response; only 2 of the 10 could be controlled on diazoxide alone, while the rest required additional therapy for persistent hypoglycemia. Four patients underwent pancreatectomy, with two requiring additional therapy because of persistent and severe hypoglycemia following surgery.
Table 1.
Subject characteristics
| Subject no. | Family no. | Genotype | Gestational age (weeks)/birth weight (g) | Age at presentation | Diagnostic Data | Hyperinsulinism treatment | |||
|---|---|---|---|---|---|---|---|---|---|
| Plasma glucose (mg/dL) | Plasma insulin (μIU/mL) | Plasma C-peptide (ng/mL) | Plasma BOHB (mmol/L) | ||||||
| 1 | 1 | p.E67V | 39/2,892 (AGA) | 3 months | 49 | <2 | 0.290 | 0.49 | Diazoxide + lanreotide + investigational medication |
| 2 | 1 | p.E67V | FT/3,920 (AGA) | 24 years | 40 | Not obtained | Not obtained | Not obtained | Diazoxide |
| 3 | 2 | p.V91L | 39/3,771 (AGA) | Birth | 28 | 25.9 | NA | NA | Diazoxide |
| 4 | 3 | p.Y215C | 37/2,721 (AGA) | Birth | 47 | 2 | 0.750 | <0.3 | Diazoxide + continuous dextrose through G tube |
| 5 | 3 | p.Y215C | 38/4,400 (LGA) | Birth | 48 | NA | NA | 1.8 | Diazoxide + continuous dextrose through G tube |
| 6 | 4 | p.S64P (mosaic) | 36/3,685 (LGA) | 2 years | 42 | 8.1 | 0.9 | 0.26 | 60% pancreatectomy → cured (12 h, PG >70; 18 h, PG 41; BOHB, 2.1) |
| 7 | 5 | p.S69P (mosaic) | 38/3,970 (LGA) | Birth | 40 | 13.3 | 2.08 | 0.09 | 98% pancreatectomy → cured (10 h, PG 88; BOHB, 2) |
| 8 | 6 | p.W99C | 36/3,402 (LGA) | Birth | 45 | 9.7 | NA | NA | 98% pancreatectomy → persistent hypoglycemia – continuous dextrose through G tube + diazoxide + investigational medication |
| 9 | 7 | p. S69_E70insVPL | 38.5/4,052 (LGA) | Birth | 40 | 9.9 | 1.61 | <0.3 | 98% pancreatectomy → persistent hypoglycemia – continuous dextrose through G tube |
| 10 | 8 | p.R447L (mosaic) | 40/4,479 (LGA) | Birth | 40 | 10.2 | NA | NA | 98% pancreatectomy |
AGA, appropriate for gestational age; BOHB, β-hydroxybutyrate; FT, full-term; G tube, gastrostomy tube; LGA, large for gestational age; NA, not available; PG, plasma glucose.
Mutation Characteristics
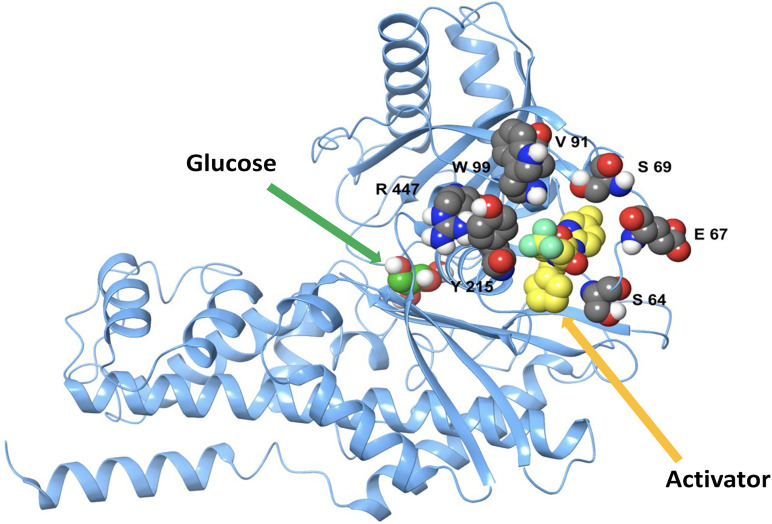
As shown in Fig. 1, the eight unique mutations identified in 10 GCK-HI case subjects are all located within the allosteric site of GK. Six of these mutations were novel: p.E67V, p.S64P, p.S69P, p.S69_E70insVPL, p.Y215C, and p.R447L. All of these, except the p.S64P mutation, involved amino acid residues not previously associated with activating mutations (8). The p.S69 position is particularly interesting, as we observed two different pathogenic variants at this site: p.S69P and p.S69_E70insVPL. The p.S69_E70insVPL involves insertion of three amino acids (valine [V], proline [P], and leucine [L]) between amino acid position 69 and 70 due to an intronic mutation that alters normal splicing (c.209-11t>a), confirmed by cDNA sequencing. Clinically, both p.S69 variants were associated with severe diazoxide-unresponsive HI presenting at birth, and both affected children required a 98% pancreatectomy for intractable hypoglycemia. After near-total pancreatectomy, the child with the p.S69P mutation achieved euglycemia. However, the child with the p.S69_E70insVPL mutation had persistent hypoglycemia. The remaining two mutations, p.V91L and p.W99C, have been previously reported (27,39).
Figure 1.
Schematic of GK. Location of activating mutations in relation to the catalytic site (glucose) and allosteric site (GKA) in the open conformation based on GK structure model 3VEV.
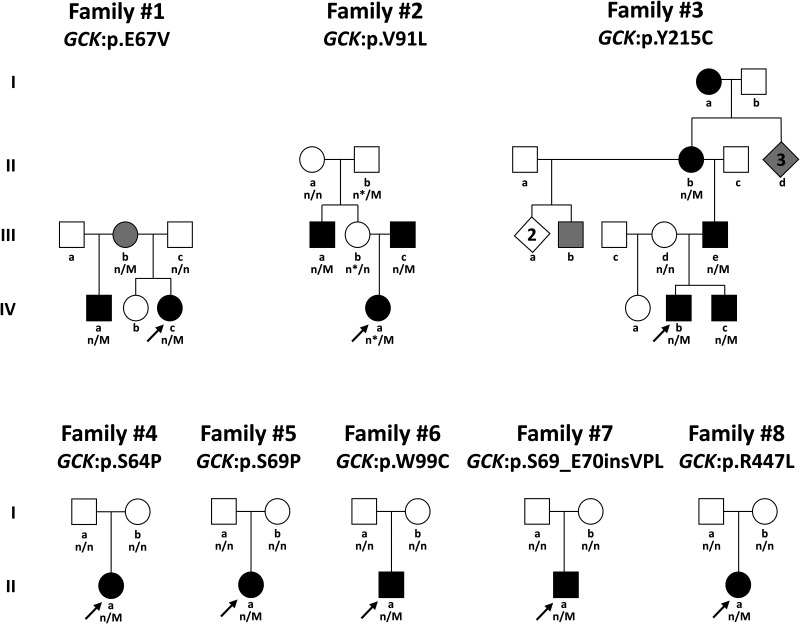
As shown in Fig. 2, the GCK mutations arose de novo in the probands from five of the eight families. In three of these, the GCK mutation was found to be mosaic, in that it was detected at low levels of mosaicism in DNA from resected pancreas while testing in blood was negative (family nos. 4, 5, and 8) (40). Paternity was not excluded in the two individuals with a de novo GCK mutation detectable in peripheral blood DNA. In the remaining three families, the GCK mutation was transmitted from a carrier parent. In two of these (family nos. 2 and 3), the carrier parent was known to be affected and had previously required treatment for HI. In addition to the GCK mutation (p.V91L) inherited from her affected father, the proband in family no. 2 inherited a novel intronic GCK variant (c.45 + 4 t>a) from her mother (2-III-b) and grandfather (2-II-b), who were both unaffected. This GCK c.45 + 4 t>a variant appears to be a benign polymorphism, since it is not predicted to alter splicing (41), has a population frequency of 1:7,837 individuals in control populations (42), and carriers in this family did not have hypoglycemia.
Figure 2.
Pedigrees of eight families with HI associated with mutations in GCK. Arrows indicate probands. Circles, females; squares, males; diamonds, multiple individuals of unspecified sex. Black shapes, hypoglycemia diagnosed; gray shapes, hypoglycemia suspected. n/M, mutation positive; n/n mutation negative. In family no. 2, (*) indicates carriers of a novel intronic GCK variant (c.45 + 4t>a).
In family no. 3, individuals from four generations are known to be affected with HI. The proband’s younger brother (3-IV-c) is affected; the proband’s father (3-III-e) and paternal great-grandmother (3-I-a) had both undergone pancreatectomy for hypoglycemia. DNA from the great-grandmother was not available for mutation analysis. Although not originally thought to be affected, the paternal grandmother (3-II-b) was found to be hypoglycemic during a fasting test, and four other individuals are suspected of also having hypoglycemia in multiple generations of this family. DNA was not available on these individuals for mutation analysis.
In family no. 1, the carrier mother (1-III-b) had not been clinically diagnosed with HI but had a history of hypoglycemia consistent with being a carrier of the GCK mutation. The proband’s half-brother (1-IV-a) also carried the GCK mutation and was recognized to be affected when routine laboratory studies at age 20 years revealed a fasting glucose of 40 mg/dL. He reported a history of chronic headaches and episodes of lightheadedness and disorientation. He was started on diazoxide with good glycemic control, although he continues to have frequent episodes of mild hypoglycemia.
Enzyme Kinetics of GCK Mutations Demonstrate Gain-of-Function
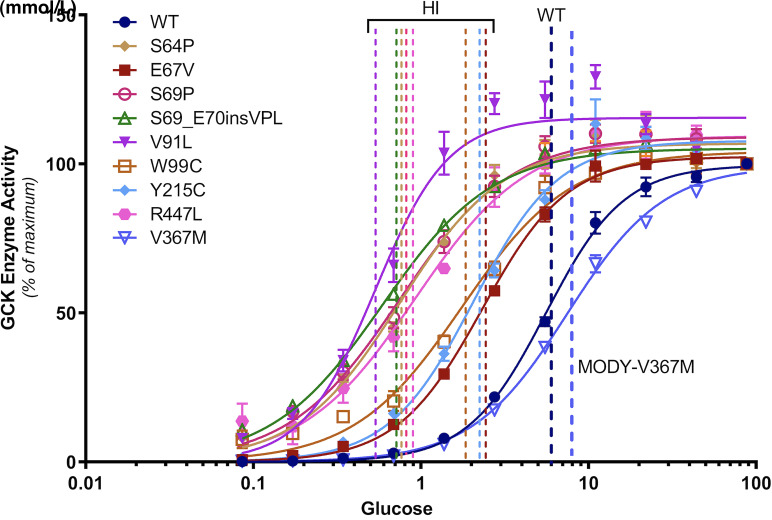
We produced GST-tagged mutant GK proteins for each of the variants to examine the impact on enzyme kinetics and to confirm their pathogenicity. The glucose half-saturation concentration (S0.5) (43) was decreased for all eight GCK variants, confirming that these are activating, gain-of-function mutations (Fig. 3). This is in contrast to the inactivating MODY GCK variant p.V367M, which results in an increase of the glucose S0.5. The GK mutants p.S69P and p.S69_E70insVPL resulted in the greatest downward shift in glucose S0.5, with values of 0.6 mmol/L compared with 7.0 mmol/L for WT GK (Fig. 3 and Table 2). The clinical phenotype of the infants carrying these mutations (p.S69P and p.S69_E70insVPL) was very severe; both were unresponsive to diazoxide treatment and required a 98% pancreatectomy (Table 1). Patients with the p.R447L and p.W99C mutations required a 98% pancreatectomy for severe hypoglycemia, although the enzyme kinetics studies indicated that the glucose S0.5 was not as severely decreased, with values of 1.1 and 2.4 mmol/L, respectively (Tables 1 and 2).
Figure 3.
Effect of GCK-HI associated GCK mutations on enzyme activity. WT GK, eight GCK-HI mutants, and a MODY2 mutant were expressed and GST-GCK was purified. Glucose dose-dependent GCK enzyme activity was evaluated based on NADP+/NADPH-coupled enzyme assay. The vertical lines indicate glucose S0.5 values.
Table 2.
Enzyme kinetics of WT and mutant GK
| Case subject no. | Mutation site | Glucose S0.5 (mmol/L) | ATP Km (mmol/L) | Hill coefficients | Activity index | |
|---|---|---|---|---|---|---|
| No GKA | 5 μmol/L GKA | |||||
| Normal | WT | 7.0 | 2.7 | 0.32 | 1.86 | 1 |
| 1, 2 | p.E67V | 2.8 | 0.8 | 0.30 | 1.72 | 3 |
| 3 | p.V91L | 1.2 | 0.5 | 0.35 | 1.99 | 39 |
| 4, 5 | p.Y215C | 2.8 | 0.8 | 0.34 | 1.71 | 7 |
| 6 | p.S64P | 0.9 | 1.0 | 0.38 | 1.42 | 10 |
| 7 | p.S69P | 0.6 | 0.3 | 0.42 | 1.31 | 7 |
| 8 | p.W99C* | 2.4 | 0.5 | 0.40 | 1.35 | 7 |
| 9 | p.S69_E70insVPL* | 0.6 | 0.6 | 0.36 | 1.31 | 15 |
| 10 | p.R447L* | 1.1 | 1.0 | 0.37 | 1.29 | 5 |
| MODY | p.V367M | 8.9 | 2.0 | 0.35 | 1.52 | 0.3 |
Pancreatic islets were isolated and studied.
To further assess the impact of the mutations on enzyme activation, we used a GKA, piragliatin (5 μmol/L), which binds to the allosteric site of the GK protein (Fig. 1), to determine the effect on enzyme kinetics. Piragliatin lowered the glucose S0.5 in WT GK, the MODY p.V367M mutant, and in most of the activating mutations, including, p.E67V, p.V91L, p.Y215C, p.S69P, and p.W99C (Table 2). Piragliatin had little or no effect on the glucose S0.5 of the p.S64P, p.S69_E70insVPL, and p.R447L GK mutants, suggesting that these variants may interfere with activator binding (Table 2). As shown in Fig. 1 and Supplementary Fig. 2, the amino acid residues p.S64, p.S69, and p.R447 are critical for piragliatin binding; interrupting these binding sites eliminates the activating effect on GK. ATP Km and Hill coefficients were not significantly altered in any of the mutants (Table 2). The calculated enzyme activity index (1) of the p.V91L mutation in our study is similar to that which was previously reported (39).
GCK-Activating Mutations Have Impaired GKRP Inhibition
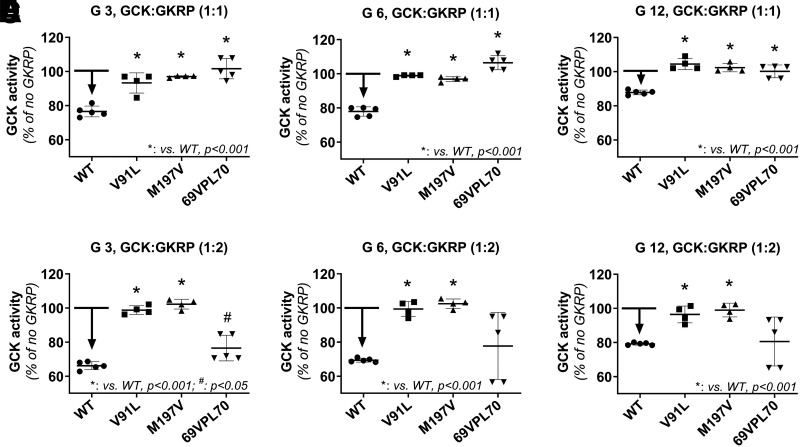
Next, we investigated the inhibitory effects of GKRP on mutant GK enzyme kinetics. At 3 and 6 mmol/L glucose with GKRP at a 1:1 molar ratio with GK, there was a 20% inhibition of WT GK activity (Fig. 4A and B). When the glucose concentration was increased to 12 mmol/L, the inhibitory effects of GKRP (1:1 ratio) on WT GK were reduced by half to ∼10% (Fig. 4C). GKRP (1:1 ratio) had no inhibitory effect on enzyme activity in two of the activating GK mutants (p.V91L and p.S69_E70insVPL) at any of the glucose concentrations (Fig. 4A–C). When GKRP was increased to a molar ratio of 2:1 relative to GK, there was an ∼30% inhibition of WT GK enzyme activity at 3 and 6 mmol/L glucose, and an ∼20% inhibition at 12 mmol/L glucose (Fig. 4D–F). Interestingly, the increased GKRP ratio showed no inhibition of the p.V91L mutant and only a partial inhibition of the p.S69_E70insVPL variant (Fig. 4D–F). The decreased sensitivity to GKRP inhibition in some of these GK mutants suggests that GCK-HI may result not only from islet dysfunction but that the liver, where GKRP regulates GK activity, may also play a role in the pathophysiology of HI.
Figure 4.
GKRP inhibition of GK activity in the p.V91L and p.S69_E70insVPL GCK-HI mutants. GK enzyme activity of GK mutants (p.V91L and p.S69_E70insVPL) was assessed in the presence of different molar ratio of GK to GKRP (1:1 and 1:2) at glucose (G) concentrations of 3 mmol/L (A and D), 6 mmol/L (B and E), and 12 mmol/L (C and F). The error bars represent ± SEM. Statistics were calculated using single-factor ANOVA (n = 4–5). *P < 0.001; #P < 0.05 compared with WT.
Glucose Threshold for Insulin Secretion Is Decreased in Islets Isolated From Patients With GCK-HI
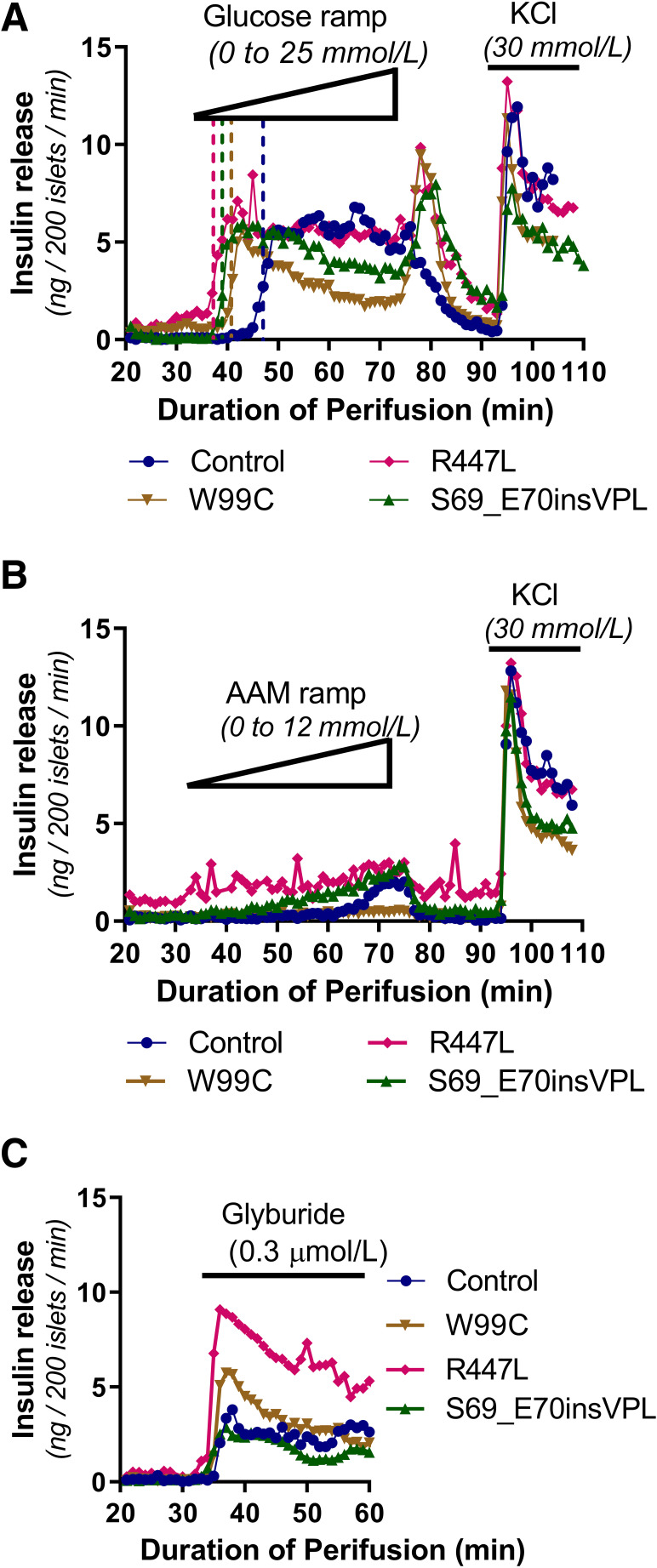
To evaluate the effect of the GCK mutations on insulin secretion dynamics, we isolated islets from the pancreata of three individuals with GCK-HI (p.S69_E70insVPL, p.W99C and p.R447L) who required pancreatectomy due to intractable hypoglycemia, and the dynamics of insulin secretion were studied by perifusion. Overall, the maximum insulin secretion, the insulin off-response after glucose withdrawal, and the response to 30 mmol/L KCl of the GCK-HI islets were similar to that seen in normal control human islets (Fig. 5A). The response to high glucose (25 mmol/L) was increased, and the threshold of GSIS was decreased in all three sets of GCK-HI islets (Fig. 5A). The glucose threshold for normal control islets was 6.9 mmol/L, while the GCK-HI islets all had reduced glucose thresholds with p.S69_E70insVPL at 1.9 mmol/L, p.W99C at 3.1 mmol/L, and p.R447L at 1.2 mmol/L. Unlike islets isolated from children with inactivating mutations of KATP channels (KATP-HI) (36,37), the basal insulin secretion in GCK-HI islets was not considerably elevated. Since gain of responsiveness to amino acids-stimulated insulin secretion (AASIS) is a characteristic feature of KATP-HI islets (36,37,44), we examined AASIS in GCK-HI islets. Control human islets showed only a small rise in insulin release when concentrations of AAM reached >10 mmol/L (Fig. 5B). Similar to control islets, islets with the p.S69_E70insVPL and p.R447L mutations showed a small insulin response to AASIS, while islets with the p.W99C mutation showed no response (Fig. 5B). In contrast to islets from KATP-HI, which showed no response to the KATP channel antagonist glyburide (37), GCK-HI islets showed a normal response to glyburide stimulation similar to control islets, demonstrating that the function of KATP channels was not altered (Fig. 5C).
Figure 5.
Functional evaluation of insulin secretion of islets isolated from GCK-HI patients. Perifusion of primary islets isolated from surgical pancreatic specimens from GCK-HI patients (W99C, S69_E70insVPL, or R447L) to assess insulin release compared with normal control subjects. Stimulated with glucose ramp (0–25 mmol/L) and KCl (30 mmol/L) (A), AAM ramp (0–12 mmol/L) and KCl (30 mmol/L) (B), or glyburide (0.3 μmol/L) (C). Glucose threshold is indicated by dashed vertical lines.
GCK-HI Islets Have Impaired Glucagon Secretion
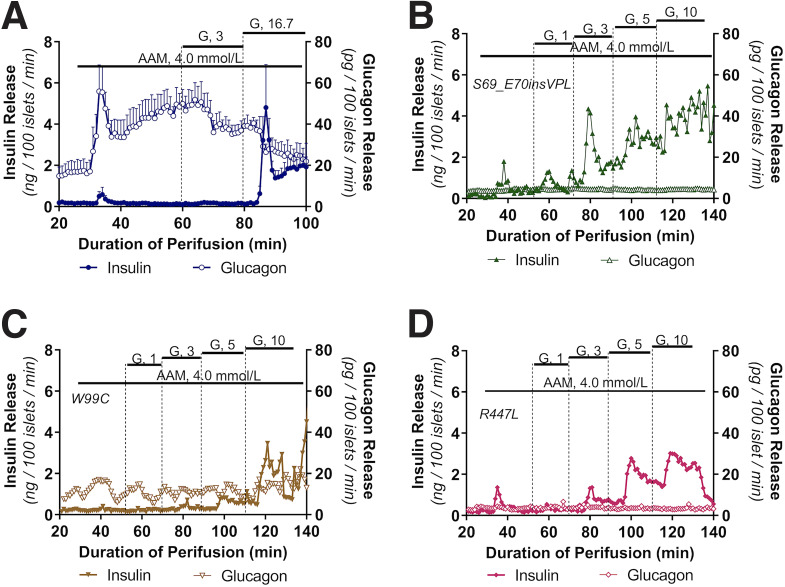
Impaired glucagon secretion during hypoglycemia has been reported in HI patients (45). All three GCK-HI patients (p.S69_E70insVPL, p.R447L, and p.W99C) were receiving an infusion of intravenous glucagon prior to surgery; thus, we were unable to measure plasma glucagon concentrations prior to pancreatectomy. However, we were able to evaluate glucagon and insulin secretion in cultured islets isolated from these patients in response to stimulation with AAM alone or AAM in combination with increasing concentrations of glucose (Fig. 6). In normal human control islets, 4 mmol/L AAM stimulated nearly a fourfold increase of glucagon secretion with minimal effect on insulin secretion (Fig. 6A). When 3 mmol/L glucose, a glucose concentration below the threshold for insulin secretion, was added to the 4 mmol/L AAM, we observed suppression of glucagon secretion, similar to previous results (37). Addition of 16.7 mmol/L glucose suppressed glucagon secretion even further, while simultaneously stimulating biphasic insulin secretion. Assessment of the GCK-HI islets demonstrated low basal glucagon secretion, and stimulation with 4 mmol/L AAM did not stimulate glucagon secretion from these islets (Fig. 6B–D). The threshold for GSIS in islets from all three individuals with GCK-HI using a stepwise increase of glucose concentration was decreased, similar to the data observed with glucose-ramp stimulated insulin secretion shown in Fig. 5A. The maximum insulin secretion response to high glucose in control and GCK-HI islets was similar, suggesting comparable insulin secretion capacity.
Figure 6.
Glucagon and insulin secretion in GCK-HI islets in presence of amino acids and glucose. Glucagon and insulin release were measured simultaneously in perifused normal human islets and islets isolated from GCK-HI patients (p.W99C, p.S69_E70insVPL, or p.R447L). Each perifusion used 1,000 islets, and data are presented at ng/100 islets/min for insulin and pg/100 islets/min for glucagon. Islets were perifused first with AAM (4 mmol/L) alone and then in combination with increasing glucose (G) concentration steps as denoted in WT control (A), p.S69_E70insVPL (B), p.W99C (C), and p.R447L (D).
Together, the findings of decreased basal glucagon and impaired AAM-stimulated glucagon secretion provide indirect evidence of inhibition of α-cell glucagon secretion in GCK-HI islets. To ascertain whether there was a difference in total α-cell mass in the GCK-HI case subjects, we stained pancreatic sections from two GCK-HI case subjects and from two age-matched control subjects (Supplementary Fig. 1A–D). The architecture of GCK-HI islets was very similar to normal control islets; thus, the reduced glucagon secretion is unlikely to be explained by a decreased α-cell mass in GCK-HI islets. We also costained pancreatic sections with the proliferative marker Ki67 and insulin or glucagon. The similar number of Ki67/insulin+ cells in GCK-HI (1.12%) and controls islets (1.37%) and Ki67/glucagon+ cells in GCK-HI (1.75%) and controls islets (1.38%) indicate that there was no significant increase in β- and α-cell proliferation. Taken together, our evaluation of GCK-HI islets indicates that these mutations result in a remarkable decrease in the glucose threshold for insulin secretion as well as a suppression of glucagon secretion.
Molecular Dynamic Analysis of p.S69_E70insVPL
The mutant GK p.S69_E70insVPL variant is of particular interest since the insertion of three amino acids between amino acid residues 69 and 70 will increase the loop size from residues 41 to 71. The predicted structure of this mutant enzyme makes the allosteric pocket inaccessible to the activator piragliatin (Supplementary Fig. 2). Using computer model simulation (Supplementary Fig. 3), we calculated the molecular dynamics of the p.S69_E70insVPL mutant and WT GK at three locations: loop 1, 41–71; loop 2, 93–97; and loop 3, 212–219. As shown in Supplementary Fig. 4, compared with WT GK, the movements of loop 1 and loop 3 were increased in the p.S69_E70insVPL mutant GK; in contrast, the movements of loop 2 were unaltered.
Discussion
We report and characterize a series of eight activating GCK mutations associated with HI. All are located near the GK allosteric site, similar to previously reported HI-associated GCK mutations (35) and in contrast to inactivating GCK mutations that cause MODY2, which can occur throughout the GK protein (1–4). The allosteric site is also the target of many GKAs. We performed direct functional analysis of pancreatic islets from patients with GCK-HI, including insulin and glucagon secretion. These results demonstrate a decrease in the glucose threshold for insulin secretion, providing direct evidence of the pathophysiology of GCK-HI. Interestingly, we also observed suppression of glucagon secretion in these GCK-HI islets. These observations provide novel insights to the understanding of GCK-HI: inappropriate insulin secretion from β-cells at a lower glucose threshold is the key pathogenic feature of HI, but decreased glucagon secretion during hypoglycemia may also play a role in the pathophysiology of GCK-HI. Although not examined in this study, it is likely that activating GK mutations affect glucose metabolism through effects not only on islets but also on liver and other tissues that express GK (17).
As shown by these studies, examination of enzyme kinetics of mutant GK can provide important information for clinical interpretation of genetic results and may also help to explain the severity of clinical hypoglycemia phenotypes. Our data show that all of the HI-associated GCK mutations studied here have increased sensitivity to glucose and an increased activity index, in line with previously published studies of GCK-activating mutations. There seems to be some correlation between the plasma glucose concentration during hypoglycemia and the GK enzyme activity index, with an r2 of 0.42 (Supplementary Fig. 5). As our results demonstrate, glucose S0.5 values may correlate with severity of clinical presentation similar to the activity index. Our data indicate that a glucose S0.5 of <2.5 mmol/L or an activity index of >5 seem to be associated with a clinical presentation severe enough to require pancreatectomy. Interestingly, the most variable phenotypes pertaining to severity of clinical presentation only occurred in mild GCK-activating mutations such as p.Y215C. We also noticed that four mutations (p.S64P, p. E67V, p.S69P, and p.S69_E70insVPL) are located in the loop structure of the GK protein, the connection loop from residues 41 to 71 facilitating the cooperativity between the large and small domains of GK, suggesting that alteration of this loop will have profound effects on GK enzyme function. A shorter loop reduces the large and small domain cooperativity and impairs enzyme function (46). In contrast, elongation of this loop at the site of 69 and 70 will greatly increase enzyme activity, as evidenced by mutation of p.S69_E70insVPL. Although we recognize the limitation of computer-based molecular dynamics calculation, we attempt to explain the different degree of enzyme activation for these mutations at the molecular level, which may improve the understanding of GK function.
Our data demonstrating loss of sensitivity to GKRP for some GK mutants suggest that lack of an inhibitory response to GKRP inhibition in the liver may play a role in the pathophysiology of GCK-HI (47); however, more studies are required to fully understand the role of activating GCK mutations in liver glucose metabolism and its contribution to the hypoglycemia phenotype. It is improbable that activating mutations of GCK in the liver alone can cause hypoglycemia, since a recent study showed that a liver-specific GKA, TTP399, lowers plasma glucose levels in patients with type 2 diabetes without any hypoglycemia adverse effects (48). This is dissimilar to nontissue-selective GKAs, in which hypoglycemia is a common adverse effect (49,50).
Recent studies showed that knockout of GCK in mouse α-cells results in hyperglucagonemia with loss of glucose inhibition of glucagon secretion (19), which provides evidence that GK plays an important role in α-cells function. Studies of GCK-MODY2 also observed that MODY2 patients have a high glucose threshold for glucagon suppression (51). In addition, genetically increased α-cell GK leads to enhanced glucose suppression of glucagon secretion (18). In line with these studies, our glucagon secretion data in GCK-HI islets show that the GCK-activating mutations lead to significant suppression of α-cell function. Importantly, our findings indicate that suppressed α-cell function and lack of counterregulatory hormone response (i.e., impaired glucagon secretion during hypoglycemia) may contribute to the severity and rate of recovery of hypoglycemia in GCK-HI patients. Measurements of glucagon during hypoglycemia may help determine whether there is impaired glucagon secretion as we observed in perifused isolated islets. Pancreatic histology from two individuals was examined. However, the data are not sufficient to draw meaningful conclusions about the role of GK in the regulation of β- and α-cell proliferation.
Overall, the study of activating GCK mutations and particularly, the study of islets isolated from patients carrying these mutations, offer a unique opportunity to establish a better understanding of the pathophysiology of HI and of GK function, which may facilitate the development of novel therapies for both diabetes and HI.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24147438.
Article Information
Acknowledgments. The authors dedicate this work to Dr. Franz Matschinsky, a pioneer in the field of GK research, an outstanding scientist, and a great mentor to us. The authors thank the families that participated in this study.
Funding. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK098517 and R01DK056268 to D.D.D.L. and by the University of Pennsylvania Diabetes Research Center National Institute of Health grant P30DK19525.
Duality of Interest. C.L. and Y.Y. are employees of Nanjing AscendRare Pharmaceutical Technology Co. D.D.D.L. has received consulting fees from Zealand Pharma A/S, Crinetics Pharmaceuticals, Hanmi Pharmaceutical, and Eiger Pharma and has received funding/research contracts from Zealand Pharma A/S, Rezolute, Crinetics Pharmaceuticals, Twist, Hanmi Pharmaceutical, and Ultragenyx for studies not included in this manuscript. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.L. designed and performed experiments and cowrote the manuscript. C.A.J. cowrote the manuscript. Y.Y. performed all the protein docking and modeling, and molecular dynamic analysis. M. Li expressed GK mutants and performed enzyme kinetics experiments. M. Lu expressed GK mutants and performed enzyme kinetics experiments. P.C. expressed GK mutants and performed enzyme kinetics experiments. K.E.B. collected clinical data and performed mutation analysis of family members. N.M.D. performed and analyzed perifusion studies. T.R.B. contributed clinical data. N.S.A. contributed clinical data. C.A.S. contributed to the design of the studies and analysis of results. D.D.D.L. contributed to the design and overall direction of the study. All coauthors reviewed and edited the manuscript and approved the submitted version. D.D.D.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK098517 and R01DK056268 to D.D.D.L. and by the University of Pennsylvania Diabetes Research Center National Institute of Health grant P30DK19525.
References
- 1. Davis EA, Cuesta-Muñoz A, Raoul M, et al. Mutants of glucokinase cause hypoglycaemia- and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 1999;42:1175–1186 [DOI] [PubMed] [Google Scholar]
- 2. Barbetti F, Cobo-Vuilleumier N, Dionisi-Vici C, et al. Opposite clinical phenotypes of glucokinase disease: Description of a novel activating mutation and contiguous inactivating mutations in human glucokinase (GCK) gene. Mol Endocrinol 2009;23:1983–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Njølstad PR, Søvik O, Cuesta-Muñoz A, et al. Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med 2001;344:1588–1592 [DOI] [PubMed] [Google Scholar]
- 4. Vionnet N, Stoffel M, Takeda J, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature 1992;356:721–722 [DOI] [PubMed] [Google Scholar]
- 5. Ajala ON, Huffman DM, Ghobrial II. Glucokinase mutation–a rare cause of recurrent hypoglycemia in adults: a case report and literature review. J Community Hosp Intern Med Perspect 2016;6:32983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christesen HB, Brusgaard K, Beck Nielsen H, Brock Jacobsen B. Non-insulinoma persistent hyperinsulinaemic hypoglycaemia caused by an activating glucokinase mutation: hypoglycaemia unawareness and attacks. Clin Endocrinol (Oxf) 2008;68:747–755 [DOI] [PubMed] [Google Scholar]
- 7. Christesen HB, Jacobsen BB, Odili S, et al. The second activating glucokinase mutation (A456V): implications for glucose homeostasis and diabetes therapy. Diabetes 2002;51:1240–1246 [DOI] [PubMed] [Google Scholar]
- 8. Christesen HB, Tribble ND, Molven A, et al. Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: prevalence in children and characterisation of a novel GCK mutation. Eur J Endocrinol 2008;159:27–34 [DOI] [PubMed] [Google Scholar]
- 9. Challis BG, Harris J, Sleigh A, et al. Familial adult onset hyperinsulinism due to an activating glucokinase mutation: implications for pharmacological glucokinase activation. Clin Endocrinol (Oxf) 2014;81:855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cuesta-Muñoz AL, Huopio H, Otonkoski T, et al. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes 2004;53:2164–2168 [DOI] [PubMed] [Google Scholar]
- 11. Dullaart RP, Hoogenberg K, Rouwé CW, Stulp BK. Family with autosomal dominant hyperinsulinism associated with A456V mutation in the glucokinase gene. J Intern Med 2004;255:143–145 [DOI] [PubMed] [Google Scholar]
- 12. Glaser B, Kesavan P, Heyman M, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 1998;338:226–230 [DOI] [PubMed] [Google Scholar]
- 13. Henquin JC, Sempoux C, Marchandise J, et al. Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of β-cells. Diabetes 2013;62:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morishita K, Kyo C, Yonemoto T, Kosugi R, Ogawa T, Inoue T. Asymptomatic congenital hyperinsulinism due to a glucokinase-activating mutation, treated as adrenal insufficiency for twelve years. Case Rep Endocrinol 2017;2017:4709262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pal P, Miller BG. Activating mutations in the human glucokinase gene revealed by genetic selection. Biochemistry 2009;48:814–816 [DOI] [PubMed] [Google Scholar]
- 16. Snider KE, Becker S, Boyajian L, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab 2013;98:E355–E363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matschinsky FM, Magnuson MA, Zelent D, et al. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006;55:1–12 [PubMed] [Google Scholar]
- 18. Bahl V, Lee May C, Perez A, Glaser B, Kaestner KH. Genetic activation of α-cell glucokinase in mice causes enhanced glucose-suppression of glucagon secretion during normal and diabetic states. Mol Metab 2021;49:101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basco D, Zhang Q, Salehi A, et al. α-cell glucokinase suppresses glucose-regulated glucagon secretion. Nat Commun 2018;9:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J, Lin S, Myers RW, et al. Novel, highly potent systemic glucokinase activators for the treatment of type 2 diabetes mellitus. Bioorg Med Chem Lett 2017;27:2069–2073 [DOI] [PubMed] [Google Scholar]
- 21. Lloyd DJ, St Jean DJ, Jr, Kurzeja RJ, et al. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature 2013;504:437–440 [DOI] [PubMed] [Google Scholar]
- 22. Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003;301:370–373 [DOI] [PubMed] [Google Scholar]
- 23. Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 2009;8:399–416 [DOI] [PubMed] [Google Scholar]
- 24. Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 1990;39:647–652 [DOI] [PubMed] [Google Scholar]
- 25. Nessa A, Rahman SA, Hussain K. Hyperinsulinemic hypoglycemia–the molecular mechanisms. Front Endocrinol (Lausanne) 2016;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wabitsch M, Lahr G, Van de Bunt M, et al. Heterogeneity in disease severity in a family with a novel G68V GCK activating mutation causing persistent hyperinsulinaemic hypoglycaemia of infancy. Diabet Med 2007;24:1393–1399 [DOI] [PubMed] [Google Scholar]
- 27. Martínez R, Gutierrez-Nogués Á, Fernández-Ramos C, et al.; Spanish Congenital Hyperinsulinism Group . Heterogeneity in phenotype of hyperinsulinism caused by activating glucokinase mutations: a novel mutation and its functional characterization. Clin Endocrinol (Oxf) 2017;86:778–783 [DOI] [PubMed] [Google Scholar]
- 28. Meissner T, Marquard J, Cobo-Vuilleumier N, et al. Diagnostic difficulties in glucokinase hyperinsulinism. Horm Metab Res 2009;41:320–326 [DOI] [PubMed] [Google Scholar]
- 29. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005;54:3592–3601 [DOI] [PubMed] [Google Scholar]
- 30. Stanley CA, Baker L. Hyperinsulinism in infancy: diagnosis by demonstration of abnormal response to fasting hypoglycemia. Pediatrics 1976;57:702–711 [PubMed] [Google Scholar]
- 31. Finegold DN, Stanley CA, Baker L. Glycemic response to glucagon during fasting hypoglycemia: an aid in the diagnosis of hyperinsulinism. J Pediatr 1980;96:257–259 [DOI] [PubMed] [Google Scholar]
- 32. Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res 2012;40:W452–W457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010;7:248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lek M, Karczewski K, Minikel E, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayed S, Langdon DR, Odili S, et al. Extremes of clinical and enzymatic phenotypes in children with hyperinsulinism caused by glucokinase activating mutations. Diabetes 2009;58:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calabria AC, Li C, Gallagher PR, Stanley CA, De León DD. GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes 2012;61:2585–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li C, Ackermann AM, Boodhansingh KE, et al. Functional and metabolomic consequences of KATP channel inactivation in human islets. Diabetes 2017;66:1901–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrara C, Patel P, Becker S, Stanley CA, Kelly A. Biomarkers of insulin for the diagnosis of hyperinsulinemic hypoglycemia in infants and children. J Pediatr 2016;168:212–219 [DOI] [PubMed] [Google Scholar]
- 39. Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med 2010;362:1348–1350 [DOI] [PubMed] [Google Scholar]
- 40. Boodhansingh KE, Yang Z, Li C, et al. Localized islet nuclear enlargement hyperinsulinism (LINE-HI) due to ABCC8 and GCK mosaic mutations. Eur J Endocrinol 2022;187:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol 1997;268:78–94 [DOI] [PubMed] [Google Scholar]
- 42. Lek M, Karczewski KJ, Minikel EV, et al.; Exome Aggregation Consortium . Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matschinsky FM, Glaser B, Magnuson MA. Pancreatic beta-cell glucokinase: closing the gap between theoretical concepts and experimental realities. Diabetes 1998;47:307–315 [DOI] [PubMed] [Google Scholar]
- 44. Fourtner SH, Stanley CA, Kelly A. Protein-sensitive hypoglycemia without leucine sensitivity in hyperinsulinism caused by KATP channel mutations. J Pediatr 2006;149:47–52 [DOI] [PubMed] [Google Scholar]
- 45. Debreceni L, Mészáros I. Persistent hypoglycemia due to hyperinsulinemia, hypoglucagonemia and mild adrenal insufficiency. Exp Clin Endocrinol 1987;90:221–226 [DOI] [PubMed] [Google Scholar]
- 46. Martinez JA, Larion M, Conejo MS, Porter CM, Miller BG. Role of connecting loop I in catalysis and allosteric regulation of human glucokinase. Protein Sci 2014;23:915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Veiga-da-Cunha M, Xu LZ, Lee YH, Marotta D, Pilkis SJ, Van Schaftingen E. Effect of mutations on the sensitivity of human beta-cell glucokinase to liver regulatory protein. Diabetologia 1996;39:1173–1179 [DOI] [PubMed] [Google Scholar]
- 48. Vella A, Freeman JLR, Dunn I, Keller K, Buse JB, Valcarce C. Targeting hepatic glucokinase to treat diabetes with TTP399, a hepatoselective glucokinase activator. Sci Transl Med 2019;11:eaau3441. [DOI] [PubMed] [Google Scholar]
- 49. Bonadonna RC, Heise T, Arbet-Engels C, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J Clin Endocrinol Metab 2010;95:5028–5036 [DOI] [PubMed] [Google Scholar]
- 50. Zhi J, Zhai S. Effects of piragliatin, a glucokinase activator, on fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. J Clin Pharmacol 2016;56:231–238 [DOI] [PubMed] [Google Scholar]
- 51. Guenat E, Seematter G, Philippe J, Temler E, Jequier E, Tappy L. Counterregulatory responses to hypoglycemia in patients with glucokinase gene mutations. Diabetes Metab 2000;26:377–384 [PubMed] [Google Scholar]