Abstract
Hypertension and atherosclerosis are often found in one patient causing serious cardiovascular events. An animal model simultaneously expressing hypertension and atherosclerosis would be useful to study such a complex risk status. We therefore attempted to introduce a null mutation of the apolipoprotein E (ApoE) gene into the spontaneously hypertensive rat (SHR) using CRISPR/Cas9 to establish a genetic model for atherosclerosis with hypertension. We successfully established SHRApoE(−/−) having a 13-bps deletion in the 5′-end of ApoE gene. Deletion of ApoE protein was confirmed by Western blotting. Blood pressure of SHRApoE(−/−) was comparable to that of SHR. Feeding the rats with high fat high cholesterol diet (HFD) caused a significant increase in LDL cholesterol as well as in triglyceride in SHRApoE(−/−). After 8 weeks of HFD loading, superficial fat deposition was observed both in the aorta and the mesenteric arteries of SHRApoE(−/−) instead of mature atheromatous lesions found in humans. In addition, a null mutation of peroxiredoxin 2 (Prdx2) was introduced into SHRApoE(−/−) to examine the effect of increased oxidative stress on the development of atherosclerosis. SHR with the double depletion of ApoE and Prdx2 did not show mature atheroma either. Further, salt loading did not promote development of atheroma although it accelerated the development of fat deposition. These results indicated that when compared with ApoE-knockout mice, SHRApoE(−/−) was more resistant to atherosclerosis even though they have severe hypertension.
Keywords: apolipoprotein E, atherosclerosis, genome editing, peroxiredoxin 2, spontaneously hypertensive rat
Introduction
Atherosclerosis is one of the most prevalent arterial diseases that causes severe cardiovascular events such as myocardial and cerebral infarction [1,2,3]. It is important to clarify pathophysiological mechanisms of atherosclerosis to prevent severe cardiovascular events. In this context, it was of note that apolipoprotein E (ApoE)-depleted mice was developed in 1992, which has made tremendous contribution in basic studies of atherosclerosis [4, 5]. However, as ApoE-depleted mice had several limitations, it is worth to establish another model for atherosclerosis [6]. Rats are preferred in some biological studies because of their larger body size suitable for physiological experiments as well as repeated collection of biological samples such as serum. In addition, it is a great advantage that rats have genetic models for various diseases such as hypertension and diabetes [7, 8].
Hypertension is another important cardiovascular disease widely seen in the world. The two vascular diseases, i.e., hypertension and atherosclerosis, were often observed in one patient and their combination was thought to deteriorate arterial damages [9].
We therefore planned to establish a model for combined hypertension and atherosclerosis in rats to study effects of interaction between hypertension and atherosclerosis on the vasculature in detail. In this study, we employed the spontaneously hypertensive rat (SHR), the most popular genetic model for hypertension, to establish a model for combined hypertension and atherosclerosis through knocking out the ApoE gene by the genome editing strategy using CRISPR/Cas9. In addition, we attempted two manipulations to accelerate atheromatous changes in the ApoE-knockout SHR; (1) we made a double-knockout SHR by crossing the ApoE-knockout SHR with the peroxiredoxin 2 (Prdx2)-knockout SHR to examine whether oxidative stress would deteriorate atherosclerosis and hypertension. Prdx2 is one of key enzymes erasing reactive oxygen species in cells [10], and it was previously reported that Prdx2-depletion accelerated atheromatous lesions in ApoE-knockout mice [11]. (2) We examined effects of salt-loading on fat deposition in SHR and SHRApoE(−/−). A previous report showed that salt-loading aggravated fat deposition in the mesenteric artery in SHRSP under high fat high cholesterol diet (HFD) [12]. In this study, we constructed ApoE-knockout and ApoE-/Prdx2-double knockout SHR (SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−), respectively), and examine effects of those gene-knockouts on atherosclerosis in SHR.
Materials and Methods
Construction of gene knockout SHR
SHR/Izm was provided by the Disease Model Cooperative Research Association (Kyoto, Japan). Deletion of the ApoE gene was done in SHR by the genome editing with CRISPR/Cas9 as described in the previous study [13]. Briefly, the target sequence was selected from the rat genome sequence using CRISPR Design Tool made by Dr. Feng Zhang et al. [https://zlab.bio/guide-design-resources].
The target sequence selected was 5′-GCTGTTGGTCCCATTGCTGA-3′ from +21 to +40 of the ApoE gene. Guide RNA and Cas9 mRNA were obtained through in vitro transcription on the cloned plasmid as a template using commercial kits [13]. Microinjection of Cas9 mRNA (100 µg/ml) and the guide RNA (50 µg/ml) was done at the pronuclear-stage of embryos. Thirty-six pups were obtained as a total. By direct sequencing of PCR products of the target region (forward primer; 5′-AGGACTGGGAGCTGGAATTT-3′, reverse primer; 5′-AGCTGCTCAGGGCTATTGAA-3′), two pups either with a 12- or a 13-bps-deletion were identified. All the procedures of genome editing were performed in Kyoto University. A heterozygous pup with a 13-bp deletion was selected and backcrossed with SHR to establish SHRApoE(−/−) (Supplementary Fig. 1)
ApoE protein expression was examined in SHRApoE(−/−) by the western blotting performed as previously described [14]. Briefly, serum and liver obtained from SHR/Izm and SHRApoE(−/−) at 14–16 weeks of age were used. RIPA buffer (Nakalai Tesque, Kyoto, Japan) and Protein BCA Assay Kit (FUJIFILM Wako, Osaka, Japan) were used for preparation of liver lysates and determination of protein concentration, respectively. Denatured serum (1 µl) or liver proteins (20 µg) were separated by SDS-PAGE on a 10% acrylamide gel and were transferred to a PVDF membrane (Immobilon-P, Merk Millipore, Burlington, MA, USA). An intermittent microwave irradiation method using a microwave processor (MI-77, Azumaya, Tokyo, Japan) [15] was employed for the antigen-antibody reactions with rabbit anti-ApoE polyclonal antibody (1:100, sc-98574, Santa Cruz, Dallas, TX, USA) and peroxidase conjugated goat anti-rabbit IgG polyclonal antibody (1:2,000, Dako, Glostrup, Denmark). Chemiluminescence reaction and image acquisition were performed using Clarity Peroxidase Solution (Bio-Rad, Hercules, CA, USA) and ImageQuant LAS-4000 (GE Healthcare, Chicago, IL, USA), respectively.
SHRApoE(−/−)Prdx2(−/−) was established through crossing Prdx2-knockout SHR with SHRApoE(−/−) [13]. SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) were deposited to the National BioResource Project-Rat under #0810 (SHR-ApoEem1Izm) and #0851 (SHR-ApoEem1IzmPrdx2em1Izm), respectively. The procedure of genome editing using CRISPR/Cas9 was approved by the local committee for Animal Research in Kyoto University (#160167).
Animal experiments
Male SHR and SHRApoE(−/−) at 12-weeks old were used in the experiments. Body weight (BW) and systolic blood pressure (SBP) by the tail-cuff method (BP-98A, Softron, Tokyo, Japan) were measured at the start of experiments. Rats were divided into 4 groups that were fed either (i) the normal diet (MF, Oriental Yeast Co., Ltd., Tokyo, Japan) and water, (ii) MF and 1% salt water, (iii) high fat high cholesterol diet containing 1% cholesterol, 0.25% cholic acid, 15% cocoa butter (HFD, Oriental Yeast Co., Ltd.) and water, or (iv) HFD and 1% salt water for 8 weeks. In the group (iii) and (iv), HFD was given to rats every second day (MF and HFD were given alternately) because continuous feeding of HFD induced sudden death of rats for an unknown reason.
At the end of experiments, urine was collected for 24 h with metabolic cages and then rats were fasted for 16 h and euthanized under anesthesia using 3% of isoflurane to collect the aorta, the mesenteric artery and blood samples. Blood samples were centrifugated at 3,000 rpm for 10 min at 4°C to obtain serum samples, which were stored at −80°C until use. Urine samples were briefly centrifugated and supernatants were stored at −80°C.
All animals were kept in an animal room at a constant temperature (23 ± 2°C) and humidity (55 ± 10%), with a mean ventilation frequency of 10–13/h and a 12 h light/dark cycle (light: 7:00–19:00). The rats were housed in TPX cages (W270 × L440 × H187 mm, Natsume Seisakusho Co., Ltd., Tokyo, Japan) containing woodchips (Clean Chip, Shimizu Laboratory Supplies, Co., Ltd., Kyoto, Japan). All experiments were performed according to the Guide for the Care and Use of Laboratory Animals in Shimane University and approved by the Institutional Review Board of the University (# IZ2-22).
Biochemical analysis
Total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C) and triglycerides (TG) were measured using commercial kits (Determiner L TC II, MetaboLead HDL-C and Determiner L TG II for TC, HDL-C and TG, respectively, Kyowa Medex Co., Ltd., Tokyo, Japan) on an automated analyzer (JCA-BM6070, JEOL Ltd., Tokyo, Japan). Serum lipoprotein cholesterol composition were analyzed using high-performance liquid chromatography (Skylight Biotech Inc., Akita, Japan) [16]. Urinary isoprostane (IsoP) was measured by ELISA using a commercial kit following the manufacturer’s protocol (Nikken Seil Co., Ltd., Shizuoka, Japan).
Fat deposition in the aorta and the mesenteric artery
Aorta and mesenteric artery were collected and fatty tissue outside of vessels were removed in ice-cold PBS. For quantitative evaluation of fat deposition, the aorta and the mesenteric artery were stained with Oil red O and images were recorded using a digital camera. For the aorta, a ratio of fat-deposited area / the total area was calculated using NIH Image-J [6]. For the mesenteric artery, a number of ring-like depositions / the length of artery (/cm) was counted.
Severity of atheromatous lesion was examined by microscopic observation of fat-deposited lesions in the aorta. Aortic tissue was stained either with H&E or with oil-red O for microscopic observation (show only SHRApoE(−/−) aortic tissue in Supplementary Fig. 2).
Statistical analysis
All values were expressed as mean ± SD. Inter-group differences were analyzed by Student’s t-test or ANOVA. Analyses were conducted with Prism (v.8, GraphPad Prism Software Inc., San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
Serum lipid profiles
Table 1 summarizes lipid profiles of the rats. Even under MF, SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) showed a significantly greater level of TC and TG than those in SHR. In contrast, HDL-C was lower than that in SHR. Under HFD, greater increase of TC was observed in both SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−). On the other hand, TG did not increase from the level of the rats under MF. In addition, HDL-C increased modestly in both SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) while no increase was observed in SHR. These profiles were consistent with those in ApoE-knockout mice, indicating the role of ApoE in HDL [6]. It was of note that Prdx2-depletion did not influence the profile significantly.
Table 1. Lipid profile of SHR, SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) under normal chow or HFD.
| SHR | SHRApoE(−/−) | SHRApoE(−/−)Prdx2(−/−) | P | ||
|---|---|---|---|---|---|
| (A) MF | N | 3 | 7 | 7 | |
| TC (mg/dl) | 63.3 ± 2.7 | 171.7 ± 28.7 | 172.4 ± 13.4 | <0.01*† | |
| HDL-C (mg/dl) | 45.6 ± 1.3 | 23 ± 1.3 | 23.1 ± 1.3 | <0.01*† | |
| TG (mg/dl) | 43.1 ± 6.1 | 131.9 ± 41.8 | 127.4 ± 23.8 | <0.01*† | |
| (B) HFD | N | 3 | 7 | 7 | |
| TC (mg/dl) | 73 ± 2.2 | 710.4 ± 77.9 | 680.6 ± 104.0 | <0.01*† | |
| HDL-C (mg/dl) | 44 ± 1.8 | 41.9 ± 15.4 | 61.1 ± 16.0 | n.s. | |
| TG (mg/dl) | 40.9 ± 3.6 | 98.9 ± 36.2 | 82.8 ± 19.0 | <0.01† | |
Male rats at 12 weeks of age were fed normal chow or HFD. After 8 weeks of feeding, serum samples were taken and total cholesterol (TC), high density lipoprotein cholesterol (HDL-C)) and triglyceride (TG) were measured as described in Methods. Each value represents mean ± SD. *: SHR vs. SHRApoE(−/−), †: SHR vs. SHRApoE(−/−)Prdx2(−/−). No significant differences were observed between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−).
Fat deposition in the aorta and the mesenteric artery
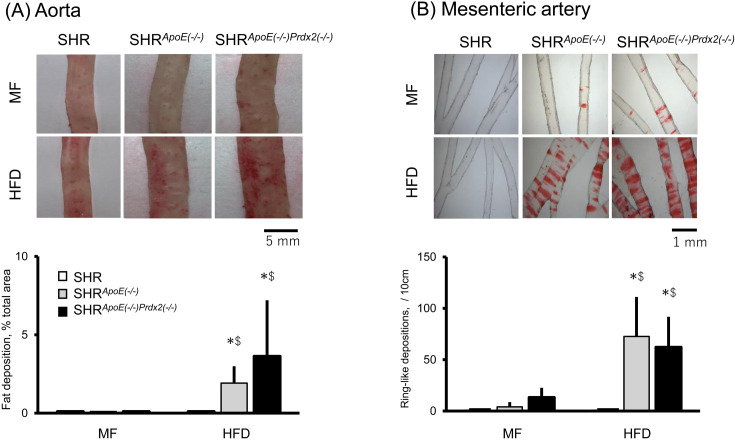
As shown in Fig. 1, little deposition was found either in the aorta or in the mesenteric artery of the three rat strains examined. In contrast, under HFD, both SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) had significantly greater fat deposition in the two arteries when compared with SHR. No significant differences were observed in severity of fat deposition between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−).
Fig. 1.
Arterial fat deposition in spontaneously hypertensive rat (SHR), SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−). Fat deposition in the aorta (A) and in the mesenteric artery (B) are shown in the figure. Rats were fed either normal diet (MF) or high-fat high-cholesterol diet (HFD) for 8 weeks. Fat deposition was quantified as described in Methods. Each column and error bar shows mean and SD, respectively. In contrast to SHR that showed no fat deposition under either MF or HFD, the two knockout SHRs showed significantly greater fat deposition both in the aorta (A) and in the mesenteric artery (B). No significant differences were observed between the two knockout SHRs. *: P<0.05 vs. the deposition under MF. $: P<0.05 vs. SHR. No significant difference was observed between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) either under MF or HFD.
Neither SHRApoE(−/−) nor SHRApoE(−/−)Prdx2(−/−) showed severe atheromatous changes as observed in ApoE- and Prdx2-double knockout mice [11]. Instead, we found fat deposition and foamy histiocytes positive in oil-red O stain on the intimal surface of the aorta without intimal thickening in both SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) (show only SHRApoE(−/−) in Supplementary Fig. 2). After eight weeks under HFD, SHR showed no depositions either in the aorta or in the mesenteric artery.
Evaluation of oxidative stress
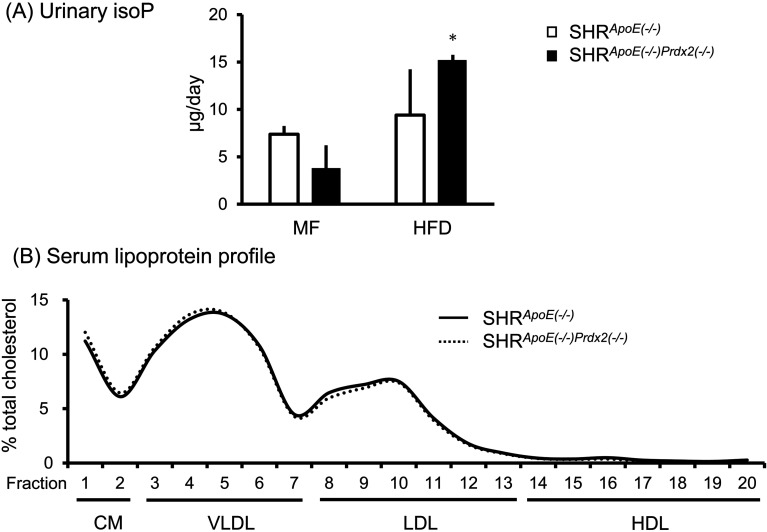
Oxidative stress measured by urinary IsoP secretion did not differ significantly between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) though IsoP tended to be greater under HFD. HFD increased urinary IsoP significantly in SHRApoE(−/−)Prdx2(−/−) when compared with that under MF (Fig. 2A). Although we expected that oxidative stress induced by Prdx2-depletion would modify the lipoprotein profile through oxidization of lipoprotein, a detailed analysis of the lipid profile indicated no apparent changes in the relative pattern of cholesterol level among the lipoprotein fractions between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) (Fig. 2B).
Fig. 2.
Effects of high fat high cholesterol diet (HFD) on urinary isoprostane (IsoP) (A) and on serum lipoprotein profile (B). Blood and urine samples were collected after 8-weeks of HFD feeding. (A) IsoP in urine collected for 24 h was measured by ELISA as described in Methods. Each column and error bar shows mean and SD, respectively. *: P<0.05 vs. normal diet (MF). No significant difference was observed between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) either under MF or HFD. (B) Serum lipoprotein was fractionated by HPLC, and cholesterol was measured in each fraction as described in Methods. Percent cholesterol against total cholesterol is indicated in the panel. No significant difference in the lipoprotein profile was observed between SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−).
Effects of salt-loading
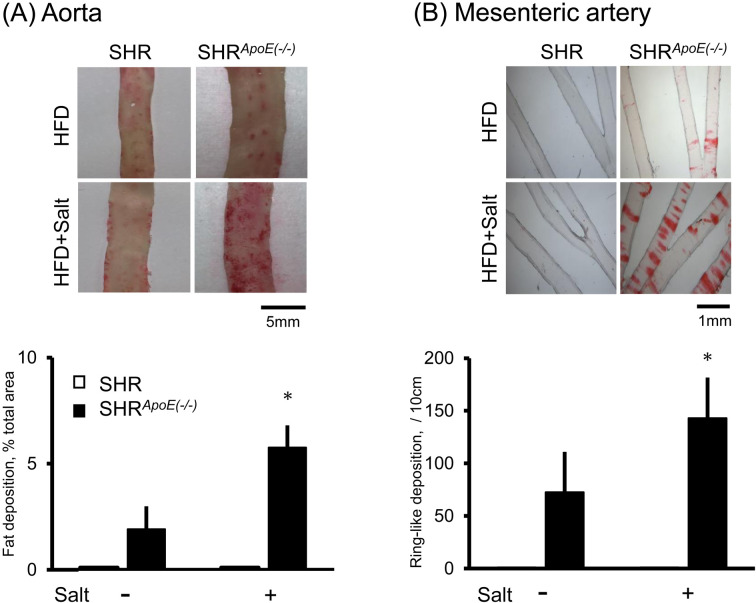
As shown in Fig. 3, salt-loading accelerated the fat deposition both in the aorta and in the mesenteric artery only in SHRApoE(−/−). Urinary IsoP excretion increased in SHRApoE(−/−) under salt-loading (Supplementary Fig. 3), suggesting that salt-loading accelerated the fat deposition probably through the increase in oxidative stress.
Fig. 3.
Effect of salt loading on arterial fat deposition. Rats were fed high-fat high-cholesterol diet (HFD) with or without 1% salt water for 8 weeks. Fat deposition was evaluated in the aorta (A) and in the mesenteric artery (B) as described in Methods. No fat depositions were observed in spontaneously hypertensive rat (SHR) under all the condition examined here. Each column and error bar indicates the mean and SD, respectively. Salt-loading accelerated fat deposition significantly in SHRApoE(−/−) both in the aorta (A) and in the mesenteric artery (B). *: P<0.05 vs. rats without salt.
Discussion
In this study, we constructed two knockout SHR strains, SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) in an attempt to establish a model for combined hypertension and atherosclerosis. As SHRApoE(−/−) was shown to have a 13-bps deletion in the coding sequence just after the initiation codon, we expected that this rat harbored a flame-shift of the coding sequence resulting in ‘functional’ knockout of ApoE, which was supported by the much greater cholesterol level in SHRApoE(−/−) as well as no specific bands identified in western blotting analysis (see Table 1 and Supplementary Fig. 1). In spite of that, it may be necessary to examine whether SHRApoE(−/−) expresses (1) a partial ApoE protein without the epitopes recognized by the antibody we used or (2) no ApoE protein at all.
Although we expected that Prdx2-depletion in addition to hypertension would accelerate atherosclerosis in SHRApoE(−/−)Prdx2(−/−), this study clearly showed that those were not enough to make severe atheromatous lesions in SHR (see Supplementary Fig. 2), and that SHRApoE(−/−)Prdx2(−/−) would not be a good model for atherosclerosis observed in humans.
In rodents, it is well known that the cholesterol metabolism differs from that in humans; rodents lack the cholesteryl ester transfer protein (CETP) that transfers cholesterol from HDL to LDL. Accordingly, rodents are known to be resistant to atheromatous diseases [17]. However, after the establishment of ApoE-knockout mice, many studies showed development of atheromatous lesions in the proximal part of the aorta of ApoE-knockout mice [18, 19], indicating that this mouse model was, at least partly, useful as a model of atherosclerosis.
The present study implied that SHR was more resistant to atherosclerosis than was the mouse model. It was repeatedly shown that phenotypes of transgenic or knockout animals were largely dependent on the genetic background of the animals [20]. Even though SHR seemed to have an advantage due to its hypertension, its genetic background might make this strain particularly resistant to atherosclerosis.
A few attempts have been done to evaluate ApoE-depletion on atherosclerosis in rats [21,22,23]. As they did not provide quantitative information about fat-deposited area, it is not possible to compare severity of atherosclerosis in terms of the size of fat-deposited area between SHRApoE(−/−) and the ApoE-depleted rats reported previously. However, as far as microscopic photographs in the reports were examined, fat deposition was limited on the intimal surface of the aorta, which was similar to our observation in SHRApoE(−/−) (references 6 and 21, and see Supplementary Fig. 2). This observation suggested that ApoE-depleted rats were resistant to atherosclerosis in general even if hypertension was added to promote endothelial damage. On the other hand, it is interesting that some studies successfully induced intimal thickening by implanting stents in the aorta or by keeping the rats under germ-free condition [22, 23]. These observations suggested that additional environmental factors modifying inflammatory and/or metabolic conditions in rats would be essential to induce mature atherosclerosis in rats.
Recently, several studies pointed out that activation of the inflammasome might be a key process in atherogenesis [24]. It may be of interest whether activators of inflammasome such as LPS would accelerate atherosclerosis in SHRApoE(−/−)Prdx2(−/−).
Instead of mature atheromatous lesions, we observed superficial fat depositions in arteries both in SHR ApoE(−/−)Prdx2(−/−) and SHRApoE(−/−). However, Prdx2 depletion in SHRApoE(−/−) did not accelerate the fat depositions significantly. In a previous study, Prdx2 depletion deteriorated atherosclerosis in ApoE-knockout mice [11]. In addition, Prdx2 was reported to play an important role in the regulation of intracellular oxidative stress [25, 26], which was confirmed in our previous study [13]. Unexpectedly, however, the present study could not replicate those observations. Effects of constitutive depletion of Prdx2 might have only a marginal effect due to potential compensation by alternative systems against oxidative stress.
In contrast to Prdx2 depletion, salt-loading resulted in a significant increase of fat deposition in HFD-fed SHRApoE(−/−). As a significant increase in urinary IsoP excretion was observed in the salt-loaded rats, oxidative stress might be responsible for the acceleration of deposition. Alternatively, salt-induced increase in BP was the causative factor. Periodical ring-like fat depositions observed in the mesenteric artery implied that a kind of mechanical stress induced those pathological changes. Increased BP would aggravate sheer stress on the endothelial cells of arteries, which might induce fat deposition [27]. Although we planned to examine BP of SHRApoE(−/−) in the present study, it was not possible because SHRApoE(−/−) under HFD was somehow vulnerable to stress and a substantial number of rats suffered from sudden death after tail-cuff measurement of BP.
We need to refer to a limitation of the present study; potential off-target modifications have not been examined in SHRApoE(−/−) yet. We expected a low incidence of off-target modifications in SHRApoE(−/−) because we did not use cDNA but used mRNA in the microinjection of Cas9 and the guide RNA [28, 29]. However, it would be necessary to examine off-target effects in SHRApoE(−/−) before further phenotype analyses.
In conclusion, two knockout SHR strains, SHRApoE(−/−) and SHRApoE(−/−)Prdx2(−/−) were successfully established. These knockout SHRs, however, did not show severe atheromatous lesions even though they showed severe hypercholesterolemia and hypertension. Other environmental and/or genetic factors would be necessary to promote atherosclerosis in these rat models.
Conflict of Interests
The authors declare that have no conflict of interests.
Supplementary
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685–1695. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 2.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011; 17: 1410–1422. doi: 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 3.Björkegren JLM, Lusis AJ. Atherosclerosis: Recent developments. Cell. 2022; 185: 1630–1645. doi: 10.1016/j.cell.2022.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992; 71: 343–353. doi: 10.1016/0092-8674(92)90362-G [DOI] [PubMed] [Google Scholar]
- 5.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992; 258: 468–471. doi: 10.1126/science.1411543 [DOI] [PubMed] [Google Scholar]
- 6.Gao M, Xin G, Qiu X, Wang Y, Liu G. Establishment of a rat model with diet-induced coronary atherosclerosis. J Biomed Res. 2016; 31: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto K. Spontaneously hypertensive rat (SHR) an animal model for hypertension research. Jpn Heart J. 1973; 14: 182–183. doi: 10.1536/ihj.14.182 [DOI] [Google Scholar]
- 8.Yokoi N, Hoshino M, Hidaka S, Yoshida E, Beppu M, Hoshikawa R, et al. A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J Diabetes Res. 2013; 2013: 103731. doi: 10.1155/2013/103731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995; 25: 155–161. doi: 10.1161/01.HYP.25.2.155 [DOI] [PubMed] [Google Scholar]
- 10.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005; 435: 347–353. doi: 10.1038/nature03587 [DOI] [PubMed] [Google Scholar]
- 11.Park JG, Yoo JY, Jeong SJ, Choi JH, Lee MR, Lee MN, et al. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2011; 109: 739–749. doi: 10.1161/CIRCRESAHA.111.245530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamori Y, Horie R, Sato M, Fukase M. Hypertension as an important factor for cerebrovascular atherogenesis in rats. Stroke. 1976; 7: 120–125. doi: 10.1161/01.STR.7.2.120 [DOI] [PubMed] [Google Scholar]
- 13.Mahal Z, Fujikawa K, Matsuo H, Zahid HM, Koike M, Misumi M, et al. Effects of the Prdx2 depletion on blood pressure and life span in spontaneously hypertensive rats. Hypertens Res. 2019; 42: 610–617. doi: 10.1038/s41440-019-0207-9 [DOI] [PubMed] [Google Scholar]
- 14.Ferdaus MZ, Xiao B, Ohara H, Nemoto K, Harada Y, Saar K, et al. Identification of Stim1 as a candidate gene for exaggerated sympathetic response to stress in the stroke-prone spontaneously hypertensive rat. PLoS One. 2014; 9: e95091. doi: 10.1371/journal.pone.0095091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyokuni S, Kawaguchi W, Akatsuka S, Hiroyasu M, Hiai H. Intermittent microwave irradiation facilitates antigen-antibody reaction in Western blot analysis. Pathol Int. 2003; 53: 259–261. doi: 10.1046/j.1320-5463.2003.01465.x [DOI] [PubMed] [Google Scholar]
- 16.Usui S, Mizuno T, Okazaki M, Nakamura M, Sakurabayashi I. Evaluation of a gel-permeation high-performance liquid chromatography for determining triglyceride levels in serum major lipoproteins, compared with the ultracentrifugation/precipitation method. Clin Biochem. 2009; 42: 114–117. doi: 10.1016/j.clinbiochem.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 17.de Grooth GJ, Klerkx AH, Stroes ES, Stalenhoef AF, Kastelein JJ, Kuivenhoven JA. A review of CETP and its relation to atherosclerosis. J Lipid Res. 2004; 45: 1967–1974. doi: 10.1194/jlr.R400007-JLR200 [DOI] [PubMed] [Google Scholar]
- 18.Imaizumi K. Diet and atherosclerosis in apolipoprotein E-deficient mice. Biosci Biotechnol Biochem. 2011; 75: 1023–1035. doi: 10.1271/bbb.110059 [DOI] [PubMed] [Google Scholar]
- 19.Ivanovski O, Szumilak D, Nguyen-Khoa T, Dechaux M, Massy ZA, Phan O, et al. Dietary salt restriction accelerates atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2005; 180: 271–276. doi: 10.1016/j.atherosclerosis.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 20.Wait JM, Tomita H, Burk LM, Lu J, Zhou OZ, Maeda N, et al. Detection of aortic arch calcification in apolipoprotein E-null mice using carbon nanotube-based micro-CT system. J Am Heart Assoc. 2013; 2: e003358. doi: 10.1161/JAHA.112.003358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ardestani BS, Eftedal I, Pedersen M, Jeppesen PB, Nørregaard R, Matchkov VV. Endothelial dysfunction in small arteries and early signs of atherosclerosis in ApoE knockout rats. Sci Rep. 2020; 10: 15296. doi: 10.1038/s41598-020-72338-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cornelissen A, Simsekyilmaz S, Liehn E, Rusu M, Schaaps N, Afify M, et al. Apolipoprotein E deficient rats generated via zinc-finger nucleases exhibit pronounced in-stent restenosis. Sci Rep. 2019; 9: 18153. doi: 10.1038/s41598-019-54541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuang HL, Chiu CC, Lo C, Hsu CC, Liu JY, Hung SW, et al. Circulating gut microbiota-related metabolites influence endothelium plaque lesion formation in ApoE knockout rats. PLoS One. 2022; 17: e0264934. doi: 10.1371/journal.pone.0264934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang T, Liu J, Chen X, Zhang L, Pi J, Sun H, et al. Endothelial Foxp1 suppresses atherosclerosis via modulation of Nlrp3 inflammasome activation. Circ Res. 2019; 125: 590–605. doi: 10.1161/CIRCRESAHA.118.314402 [DOI] [PubMed] [Google Scholar]
- 25.Stresing V, Baltziskueta E, Rubio N, Blanco J, Arriba MC, Valls J, et al. Peroxiredoxin 2 specifically regulates the oxidative and metabolic stress response of human metastatic breast cancer cells in lungs. Oncogene. 2013; 32: 724–735. doi: 10.1038/onc.2012.93 [DOI] [PubMed] [Google Scholar]
- 26.Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci USA. 2014; 111: 12157–12162. doi: 10.1073/pnas.1401712111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik J, Novakova L, Valerianova A, Chytilova E, Lejsek V, Buryskova Salajova K, et al. Wall shear stress alteration: a local risk factor of atherosclerosis. Curr Atheroscler Rep. 2022; 24: 143–151. doi: 10.1007/s11883-022-00993-0 [DOI] [PubMed] [Google Scholar]
- 28.Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods. 2014; 11: 399–402. doi: 10.1038/nmeth.2857 [DOI] [PubMed] [Google Scholar]
- 29.Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M, et al. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol. 2013; 31: 681–683. doi: 10.1038/nbt.2661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.