Abstract
Introduction: Low-level laser therapy (LLLT), also called Photobiomodulation, has gained widespread acceptance as a mainstream modality, particularly in the form of photobiostimulation (PBM). Here in our review, we aim to present the application of LLLT to help with depression, explore potential action mechanisms and pathways, discuss existing limitations, and address the challenges associated with its clinical implementation.
Methods: In biological systems, the visible light with a wavelength range of 400–700 nm activates photoreceptors involved in vision and circadian rhythm regulation. The near-infrared (NIR) light with a wavelength range of 800-1100 nm exhibits superior tissue penetration capabilities compared to the visible light, which enables the non-invasive application of LLLT to various tissues.
Results: By enhancing adenosine triphosphate (ATP) production using the respiratory chain, LLLT is able to enhance blood flow, reduce inflammation, support repair and healing, and enhance stem cell growth and proliferation. Preclinical studies using animal models have shown promising neuroprotective effects of the LLLT method on central nervous system (CNS) diseases, suggesting potential improvements in brain function for patients suffering from Alzheimer’s disease. In addition, it helps Parkinson’s patients with their movement problems and ameliorates mental disorders in individuals with depression.
Conclusion: patients’ quality of life can be significantly enhanced. A comprehensive understanding of the protective effects and underlying mechanisms of LLLT will facilitate its therapeutic application in the future.
Keywords: LLLT, Depression, Photoreceptor cell, Near-Infrared
Introduction
The utilization of sunlight for medicinal purposes has a long and interesting past, back to very old cultures and civilizations such as China, Egypt, and Greece, where it was known as heliotherapy.1,2 Shortly after the groundbreaking discoveries of the first laser systems, namely the ruby laser (1960) and the helium-neon laser (He-Ne) (1961), these novel devices found their way into the realm of medicine. The pioneering research of physicist Dr. Maiman resulted in the invention of the first practical laser in 1960. Subsequently, the field of photobiostimulation (PBM) emerged as an area of study to explore the medical and therapeutic applications of lasers. Unlike the lasers in high-power class, which are able to lead to tissue photothermal damage, the focus shifted towards low-power lasers with the potential for healing, tissue preservation, pain mitigation, inflammation reduction, and regenerative medicine across diverse medical disciplines. In the past few years, the utilization of low-level laser therapy (LLLT) as a non-pharmacological therapy and intervention approach has gained significant attention due to its potential beneficial effects on the brain.3-5 However, the emergence of LEDs, which are light-emitting diodes and alternative light sources for LLLT, caused some confusion in the field. While LEDs emit light similar to the available laser wavelengths, they lack the coherence characteristic of laser light and exhibit broader output peaks, making them less monochromatic. Consequently, the LLLT community is currently discussing the relative advantages of laser diodes versus LEDs. LEDs as low-power light sources on the milliwatt scale hold the advantage of being considerably more cost-effective than lasers.6 The primary mechanism underlying LLLT involves the absorption of red wavelength light (600–750 nm) and near-infrared (NIR) one (800–1100 nm) by cells through chromophores present in tissues, including Flavin. This process leads to the increased activity of cytochrome C oxidase and subsequent enhancement of ATP synthesis.2,7-11 Additionally, the absorption of low-energy light into ion channels leads to the release of calcium ions and thereby the activation of the transcription factors and the expression of the genes.12,13
The brain is an organ in the body that consumes the most energy.14 Normal brain function depends on optimal metabolism and energy supply.15 However, in many neurological diseases, energy metabolism and mitochondrial function are disintegrated, causing a vicious cycle of dysfunction. Depression, Alzheimer’s disease, and Parkinson’s disease are among the brain diseases characterized by a decrease in energy metabolism.16-20 A fact is that mitochondria provide the necessary energy for cells to perform their expressive functions. Studies have shown that ATP can mediate antidepressant-like effects through cortical P2X2 receptors. With the progress of nervous system diseases, there is an essential need for therapeutic strategies to strengthen and restore brain energy.16,21,22
Depression is a serious emotional disorder with a considerable prevalence and recurrence rate that affects the quality of life and, in some cases, causes suicide. Apathy, unpleasant negative emotions, and sleep or eating disorders are some of the main symptoms of depression,23 which can be caused by various factors such as biological, psychological, and social ones. The biology and mechanisms of depression are still unclear, and designing specific treatment plans has its own variety of challenges. Patients with more serious symptoms require longer treatment times and have a higher recurring rate. Consequently, there is a critical need for novel adaptable therapeutic approaches with minimal side effects.24-27
Low-power light therapy is a non-invasive photosynthetic approach that can be applied in the fields of neuroscience, psychiatry, and ophthalmology, and in recent years, the effects of LLLT on illnesses of the central nervous system (CNS) have been demonstrated in animal studies. LLLT can not only moderate oxidative stress but also raise ATP synthesis for improving mitochondrial function. It also acts on specific neural circuits to provide treatment as a depressant. However, its neuroprotective mechanism remains to be further elucidated.28-30 In this review, we aim to cover the probable mechanisms by which LLLT produces neuroprotectors and their impact on depression. In addition, we will discuss new approaches regarding the CNS and the benefits of LLLT for treatment, daily care, and disease prevention.
Low-Level Light-Tissue Interaction
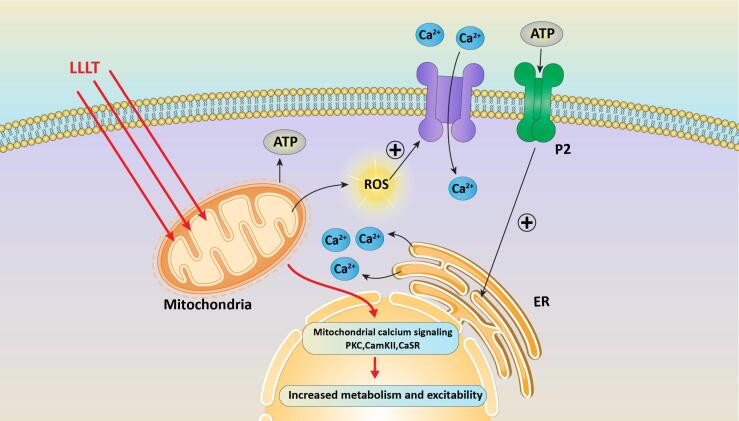
Concurring with the primary law of photobiology, photons of the light must be absorbed by the electronic bands of the cells’ chromophores for low-power light to have an impact on a living biological system. Investigating these pigments using optical spectroscopy is one way to determine their identity. Similar to the absorption spectra of photoreceptor molecules, the absorption spectra of the chromophores represent the distinct nature of tissue or living cells at various wavelengths. The fact that the spectrum describes the quantum structure of target molecules supports the idea of the existence of cellular receptors and signaling in light-stimulated pathways. Some studies confirm that the red to NIR wavelengths can be absorbed by cytochrome C oxidase (CCO).31 Primary cellular effects are generally related to the interaction of photons with intracellular components like cytochromes (Figure 1). Visible-NIR light radiation can be absorbed by cytochromes, which are located in mitochondria.32 It is also assumed that light can act as a catalyst and affect molecules, organelles, and cells without absorption.33
Figure 1.
The Cellular Pathway in LLL-Tissue/Cell Interaction
As it is presented in Figure 1, low-level light is absorbed by the mitochondrial chroromophore, most probably CCO. The interaction of light and CCO increases mitochondrial membrane potential, leading to a rise in ATP synthesis and causing some amounts of reactive oxygen species (ROS), Ca2+, and nitric oxide (NO).Additionally, there is communication between the mitochondria and the nucleus, which is caused by changes in the mitochondria. These alterations modify ATP synthesis, intracellular redox potential, pH, and concentration of the cyclic adenosine monophosphate (cAMP). Mitochondrial calcium signaling changes ion flux at the cell membrane and membrane permeability, leading to an increase in metabolism and excitability.34
The interaction of LLLT with tissues is influenced by such parameters as tissue absorption coefficient, laser wavelength, energy density (including pulse length and frequency), polarization, interaction time, and wave properties due to the intrinsic wave of light.35 There are some facts indicating that pulsed light (laser or LED) is also affective for treatment differently from continuous wave (CW) mode. Previous research has defined that pulsed light appears to be more effective than others in triggering desired biological pathways and processes.36,37 Hence, it is vital to select optimized irradiation parameters in a treatment plan to obtain a more substantial therapeutic outcome.
The Neuroscience of Depression
Major depressive disorder is a common impairing mental illness that has a significant effect on the quality of life and negative effects on mood, behavior, and mental perception.38 Globally, approximately 5% of the world’s population suffers from depression.39 In recent decades, various mechanisms in the pathophysiology of depression have been investigated, including changes in noradrenergic, dopaminergic, and glutamatergic systems, escalated inflammation, abnormalities of the hypothalamic-pituitary-adrenal axis, vascular changes, decreased neurogenesis, and neuroplasticity.40
The ketamine-induced glutamate burst stimulates signaling pathways that promote synaptic growth. This includes the activation of the complex that is the mammalian target of complex 1, which regulates the translation of synaptic proteins required for the formation of new synapses. It is considered to have caused synaptic plasticity in long-term memory.41
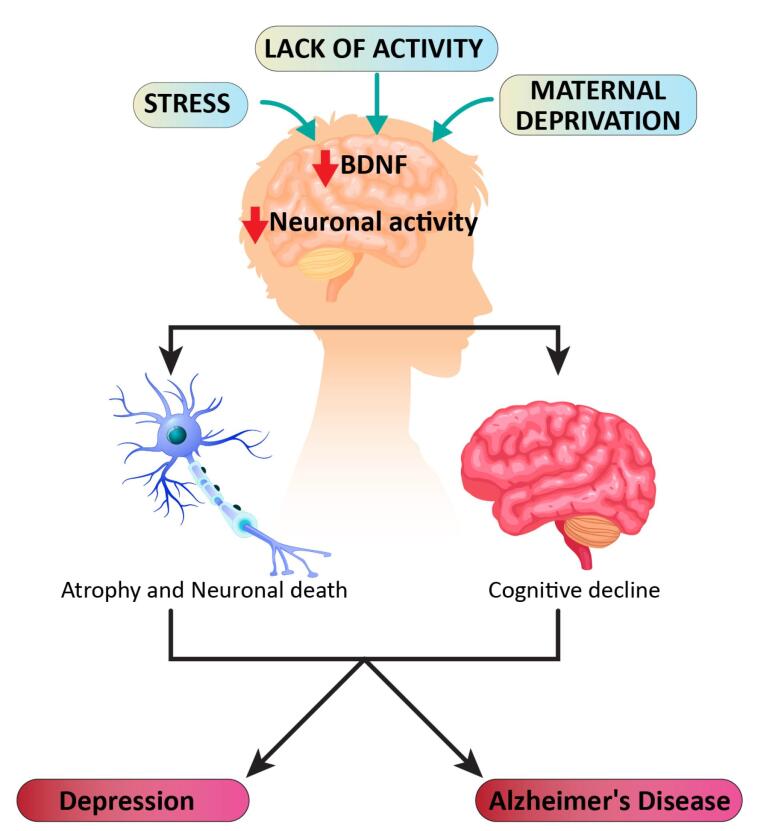
The behavioral indications of sadness and depression are wide and broad. They cover affective, motivational, cognitive, and physiological domains and include apathy, abnormal reward-related perception, and memory changes.42-45 Currently, major depressive disorder is considered to be a multifactorial disease with various causes and triggers, including genetic susceptibility, stress, and other pathological processes such as inflammation. For example, in some cases, genetic factors can cause depression. It should be emphasized that depression is a heterogeneous disorder, including many subtypes (melancholic, atypical, psychotic, etc.) with different characteristics in terms of symptoms, neurobiology, reproductive function physiology, and endocrine.45 A multitude of symptoms associated with depression are most likely the result of anomalies in numerous elements of normal brain processes, which can range from the molecular to the neural circuit level 46. Lack of activity, stress, and maternal deprivation can reduce both brain-derived neurotrophic factors (BDNFs) and neural activity in the brain (Figure 2). This leads to cognitive decline or atrophy and neuronal cell death, in which the final result might be depression or Alzheimer’s.47
Figure 2.
Model of Depression Progression
Major depressive symptoms appear to be associated with the disruption of a widespread neural network that encompasses cortical and limbic areas rather than a functional breakdown of a particular brain region.48
Low-level Light Therapy in Depression
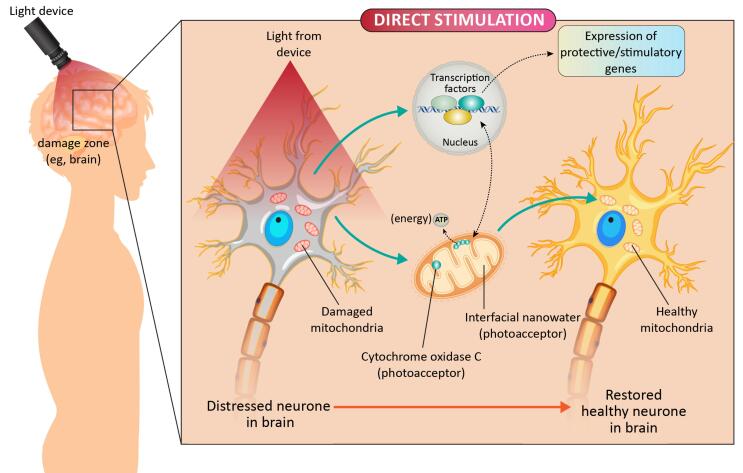
Currently, antidepressant medications are based on the monoaminergic neurotransmission theory, which results in a rise in the 5-hydroxytryptamine (5-HT) or norepinephrine brain levels as antidepressants with side effects.49 Patients suffering from depression exhibit mitochondrial dysfunction and energy metabolism abnormalities in many areas of the brain,50,51 as well as trouble focusing and weariness, which may be characterized by a lack of energy.52 As a result, CCO might be an implicit target for LLLT to mitigate depression through the skull, in which NIR photon energy is transferred via the scalp by LLLT to the cerebral cortex (Figure 3). Some preclinical investigations have shown that following LLLT, the expression and activity of ATP synthase and mitochondrial complex greatly increased in the prefrontal cortex (PFC) and may improve the depression-like tendency in mice.53 To achieve antidepressant effects, LLLT can increase neurotransmitter levels, specifically 5-HT in the PFC, as well as NO levels.54 BDNF has antidepressant and neurogenesis properties that can help with the illness.55,56 Some researchers have discovered that LLLT increases BDNF expression in hippocampus neurons via the oxidative stress mechanism.57 Furthermore, depression is thought to be linked to inflammation and oxidative stress.57 Because LLLT has been proven to have anti-inflammatory properties as well as the potential to reduce the excessive formation of ROS in the oxidative damage process, it may be useful for treating depression.58-60
Figure 3.
Schematic Diagram Showing the Direct Stimulation Mechanism of LLLT
For depression receptors, LLLT may improve glutamate receptor activity via glutamate transporter-1 (GLT-1)-mediated glutamate uptake in the cerebral cortex, and hippocampus by boosting and stimulating the expression of α- amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. Thus, it can reduce glutamate excitotoxicity and improve depression side effects and complications.61
We can take advantage of the unique low-energy light to execute the non-invasive combination approach or to boost favorable signaling pathways by engaging molecular signals. Deeper penetration is vital for the application of LLLT to reach deep brain tissues and activate preventive or regenerative processes, as well as preventing muscular atrophy in individuals who have lost their capacity to move normally. Furthermore, boosting the production capacity of mitochondria in healthy cells might cause sick cells’ living spaces to be compressed. Non-specific photoreceptor components have a wide range of applications in photodynamic and optogenetic treatment.62,63 Specific wavelengths are able to be tuned using up-convergent nanoparticle materials. NIR light that penetrates deeper into tissue is thought to either activate the ventral tegmental area to deliver dopamine or suppress and control brain cell activity and the habenular nucleus, on which there are numerous studies demonstrating the hyperactivity of lateral habenula neurons in patients with depression.64 CCO, light-sensitive chromophores in critical pathways, appears to be the target of LLLT in neuronal tissue. It should be noted that, for brain tissue, which is a complex biological system composed of various chromophores in a deeper targeted area, it is critical to apply light photons in a longer wavelength regimen to deliver the desired light, and penetration facilitates clinical applications. LLLT is now integrated into traditional medicine, along with ongoing research to verify its effectiveness. There are downstream signal pathways following the LLLT in which neuroprotective mechanisms are initiated for the improvement of neural disorders. Until now, there have been NIR physical therapy tools for the rehabilitation of musculoskeletal diseases, while the light energy density should be optimized based on the patient’s conditions for achieving the goals based on precision medicine. In other words, the key point is that the effects of LLLT appear to be influenced by specific light irradiation parameters. The main function and role of LLLT in the treatment of CNS abnormalities have not yet been generally understood. More clinical facts and evidence are crucial for a deeper understanding of the improvements. The neuroprotective mechanisms and psychological benefits due to LLLT are interesting research areas from bench to bed.
Acknowledgments
Z.E would like to acknowledge the support with the scientific research grant of the European Union Horizon 2020 research and innovation program, Grant Agreement No. 653782 (EuPRAXIA).
Authors’ Contribution
Conceptualization: Afshan Shirkavand.
Data curation: Maryam Akhavan Tavakoli, Zeinab Ebrahimpour.
Formal analysis: Zeinab Ebrahimpour, Maryam Akhavan Tavakoli.
Investigation: Afshan Shirkavand, Maryam Akhavan Tavakoli.
Methodology: Afshan Shirkavand.
Project administration: Afshan Shirkavand- Zeinab Ebrahimpour.
Software: Afshan Shirkavand.
Supervision: Afshan Shirkavand.
Validation: Maryam Akhavan Tavakoli.
Visualization: Afshan Shirkavand, Maryam Akhavan Tavakoli.
Writing–original draft: Afshan Shirkavand, Maryam Akhavan Tavakoli- Zeinab Ebrahimpour.
Writing–review & editing: Afshan Shirkavand, Maryam Akhavan Tavakoli-Zeinab Ebrahimpour.
Competing Interests
The authors declare no conflict of interest
Ethical Approval
Not applicable.
Funding
This study had no external funding.
Please cite this article as follows: Shirkavand A, Akhavan Tavakoli M, Ebrahimpour Z. A brief review of low-level light therapy in depression disorder. J Lasers Med Sci. 2023;14:e55. doi:10.34172/jlms.2023.55.
References
- 1.Gheita TA, Eesa NN. Rheumatology in Egypt: back to the future. Rheumatol Int. 2019;39(1):1–12. doi: 10.1007/s00296-018-4192-0. [DOI] [PubMed] [Google Scholar]
- 2.Wirz-Justice A, Skene DJ, Münch M. The relevance of daylight for humans. Biochem Pharmacol. 2021;191:114304. doi: 10.1016/j.bcp.2020.114304. [DOI] [PubMed] [Google Scholar]
- 3.Saucedo CL, Courtois EC, Wade ZS, Kelley MN, Kheradbin N, Barrett DW, et al. Transcranial laser stimulation: mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimul. 2021;14(2):440–9. doi: 10.1016/j.brs.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos Cardoso F, Mansur FCB, Lopes-Martins RÁB, Gonzalez-Lima F, Gomes da Silva S. Transcranial laser photobiomodulation improves intracellular signaling linked to cell survival, memory and glucose metabolism in the aged brain: a preliminary study. Front Cell Neurosci. 2021;15:683127. doi: 10.3389/fncel.2021.683127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pchelin P, Shkarupa D, Smetanina N, Grigorieva T, Lapshin R, Schelchkova N, et al. Red light photobiomodulation rescues murine brain mitochondrial respiration after acute hypobaric hypoxia. J Photochem Photobiol B. 2023;239:112643. doi: 10.1016/j.jphotobiol.2022.112643. [DOI] [PubMed] [Google Scholar]
- 6.Heiskanen V, Hamblin MR. Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci. 2018;17(8):1003–17. doi: 10.1039/c8pp90049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrage H, Heiskanen V, Palin WM, Cooper PR, Milward MR, Hadis M, et al. Under the spotlight: mechanisms of photobiomodulation concentrating on blue and green light. Photochem Photobiol Sci. 2019;18(8):1877–909. doi: 10.1039/c9pp00089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–58. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Lunova M, Smolková B, Uzhytchak M, Janoušková K, Jirsa M, Egorova D, et al. Light-induced modulation of the mitochondrial respiratory chain activity: possibilities and limitations. Cell Mol Life Sci. 2020;77(14):2815–38. doi: 10.1007/s00018-019-03321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49(1):1–17. doi: 10.1016/s1011-1344(98)00219-x. [DOI] [PubMed] [Google Scholar]
- 11.Karu TI, Pyatibrat LV, Kalendo GS. Cell attachment modulation by radiation from a pulsed light diode (lambda = 820 nm) and various chemicals. Lasers Surg Med. 2001;28(3):227–36. doi: 10.1002/lsm.1043. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nin-Hill A, Mueller NPF, Molteni C, Rovira C, Alfonso-Prieto M. Photopharmacology of Ion channels through the light of the computational microscope. Int J Mol Sci. 2021;22(21):12072. doi: 10.3390/ijms222112072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faria-Pereira A, Morais VA. Synapses: the brain’s energy-demanding sites. Int J Mol Sci. 2022;23(7):3627. doi: 10.3390/ijms23073627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci. 2018;11:216. doi: 10.3389/fnmol.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19(9):609–33. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148–60. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, et al. Glutamate metabolism in major depressive disorder. Am J Psychiatry. 2014;171(12):1320–7. doi: 10.1176/appi.ajp.2014.14010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ammal Kaidery N, Thomas B. Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem Int. 2018;117:91–113. doi: 10.1016/j.neuint.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge P, Dawson VL, Dawson TM. PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol Neurodegener. 2020;15(1):20. doi: 10.1186/s13024-020-00367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong W, Cao X, Zeng Y, Qin X, Zhu M, Ren J, et al. Astrocytic epoxyeicosatrienoic acid signaling in the medial prefrontal cortex modulates depressive-like behaviors. J Neurosci. 2019;39(23):4606–23. doi: 10.1523/jneurosci.3069-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao YF, Verkhratsky A, Tang Y, Illes P. Astrocytes and major depression: the purinergic avenue. Neuropharmacology. 2022;220:109252. doi: 10.1016/j.neuropharm.2022.109252. [DOI] [PubMed] [Google Scholar]
- 23.Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–312. doi: 10.1016/s0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 24.Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4(1):7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price RB, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. 2020;25(3):530–43. doi: 10.1038/s41380-019-0615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JQ, Mao L. The ERK pathway: molecular mechanisms and treatment of depression. Mol Neurobiol. 2019;56(9):6197–205. doi: 10.1007/s12035-019-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155–61. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong N. Photobiomodulation as a treatment for neurodegenerative disorders: current and future trends. Biomed Eng Lett. 2019;9(3):359–66. doi: 10.1007/s13534-019-00115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnstone DM, Moro C, Stone J, Benabid AL, Mitrofanis J. Turning on lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer’s and Parkinson’s disease. Front Neurosci. 2015;9:500. doi: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR. Brain photobiomodulation therapy: a narrative review. Mol Neurobiol. 2018;55(8):6601–36. doi: 10.1007/s12035-017-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirkavand A, Akhavan Tavakkoli M. Review of LLLT in repair process. Laser Medicine. 2020;17(1):33–9. [Google Scholar]
- 32.Lanzafame RJ. Low level laser therapy: clinical practice and scientific background. J Clin Laser Med Surg. 1999;17(4):179. doi: 10.1089/clm.1999.17.179. [DOI] [Google Scholar]
- 33.Norregaard K, Metzler R, Ritter CM, Berg-Sørensen K, Oddershede LB. Manipulation and motion of organelles and single molecules in living cells. Chem Rev. 2017;117(5):4342–75. doi: 10.1021/acs.chemrev.6b00638. [DOI] [PubMed] [Google Scholar]
- 34.de Freitas LF, Hamblin MR. proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. doi: 10.1109/jstqe.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalik M, Szymańczyk J, Stajnke M, Ochrymiuk T, Cenian A. Medical applications of diode lasers: pulsed versus continuous wave (cw) regime. Micromachines (Basel) 2021;12(6):710. doi: 10.3390/mi12060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, et al. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6(10):e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5’-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010;1306:100–5. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 38.Colucci-D’Amato L, Speranza L, Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci. 2020;21(20):7777. doi: 10.3390/ijms21207777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Depressive disorder (depression). WHO website. https://www.who.int/news-room/fact-sheets/detail/depression.
- 40.Castrén E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017;97(Pt B):119–26. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Duman RS. Neurobiology of stress, depression, and rapid acting antidepressants: remodeling synaptic connections. Depress Anxiety. 2014;31(4):291–6. doi: 10.1002/da.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 43.Spellman T, Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177(5):381–90. doi: 10.1176/appi.ajp.2020.20030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Juruena MF, Werne Baes CV, Menezes IC, Graeff FG. Early life stress in depressive patients: role of glucocorticoid and mineralocorticoid receptors and of hypothalamic-pituitary-adrenal axis activity. Curr Pharm Des. 2015;21(11):1369–78. doi: 10.2174/1381612821666150105125500. [DOI] [PubMed] [Google Scholar]
- 46.Chaudhury D, Liu H, Han MH. Neuronal correlates of depression. Cell Mol Life Sci. 2015;72(24):4825–48. doi: 10.1007/s00018-015-2044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao Z, Wang Y, Sun Z. The relationships between stress, mental disorders, and epigenetic regulation of BDNF. Int J Mol Sci. 2020;21(4):1375. doi: 10.3390/ijms21041375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraus C, Castrén E, Kasper S, Lanzenberger R. Serotonin and neuroplasticity - links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. 2017;77:317–26. doi: 10.1016/j.neubiorev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE, et al. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl Psychiatry. 2014;4(6):e397. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164(5):778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 52.Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154(1):116–8. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 53.Xu Z, Guo X, Yang Y, Tucker D, Lu Y, Xin N, et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol Neurobiol. 2017;54(6):4551–9. doi: 10.1007/s12035-016-9983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eshaghi E, Sadigh-Eteghad S, Mohaddes G, Rasta SH. Transcranial photobiomodulation prevents anxiety and depression via changing serotonin and nitric oxide levels in brain of depression model mice: a study of three different doses of 810 nm laser. Lasers Surg Med. 2019;51(7):634–42. doi: 10.1002/lsm.23082. [DOI] [PubMed] [Google Scholar]
- 55.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7(2):137–51. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 56.Dinoff A, Herrmann N, Swardfager W, Gallagher D, Lanctôt KL. The effect of exercise on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF) in major depressive disorder: a meta-analysis. J Psychiatr Res. 2018;105:123–31. doi: 10.1016/j.jpsychires.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Heo JC, Park JA, Kim DK, Lee JH. Photobiomodulation (660 nm) therapy reduces oxidative stress and induces BDNF expression in the hippocampus. Sci Rep. 2019;9(1):10114. doi: 10.1038/s41598-019-46490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Madungwe NB, Zilberstein NF, Feng Y, Bopassa JC. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. Am J Cardiovasc Dis. 2016;6(3):93–108. [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaura M, Yao M, Yaroslavsky I, Cohen R, Smotrich M, Kochevar IE. Low level light effects on inflammatory cytokine production by rheumatoid arthritis synoviocytes. Lasers Surg Med. 2009;41(4):282–90. doi: 10.1002/lsm.20766. [DOI] [PubMed] [Google Scholar]
- 60.Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal. 2012;5(244):pe45. doi: 10.1126/scisignal.2003579. [DOI] [PubMed] [Google Scholar]
- 61.Xu S, Wan B. Recent advances in low-level laser therapy on depression. Stress Brain. 2022;2(4):123–38. doi: 10.26599/sab.2022.9060026. [DOI] [Google Scholar]
- 62.Moro C, Valverde A, Dole M, Hoh Kam J, Hamilton C, Liebert A, et al. The effect of photobiomodulation on the brain during wakefulness and sleep. Front Neurosci. 2022;16:942536. doi: 10.3389/fnins.2022.942536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MMS, Xu W, Zheng L, Yu B, Leung ACS, Kwok RTK, et al. Ultrafast discrimination of gram-positive bacteria and highly efficient photodynamic antibacterial therapy using near-infrared photosensitizer with aggregation-induced emission characteristics. Biomaterials. 2020;230:119582. doi: 10.1016/j.biomaterials.2019.119582. [DOI] [PubMed] [Google Scholar]
- 64.Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science. 2018;359(6376):679–84. doi: 10.1126/science.aaq1144. [DOI] [PubMed] [Google Scholar]