Abstract
Developing chloroplasts of Cucumis sativus L., cv Beit Alpha which were incubated with either Mg-protoporphyrin IX or Mg-protoporphyrin IX monomethyl ester in darkness produced a partially phototransformable protochlorophyllide species that was tentatively identified as Mg-2,4-divinyl pheoporphyrin a5. S-Adenosylmethionine stimulated Mg-2,4-divinyl pheoporphyrin a5 formation irrespective of the starting material used. In the case of Mg-protoporphyrin IX monomethyl ester, this stimulation was attributed to the need to remethylate substrate that had been hydrolyzed by an endogenous methylesterase which converts part of the added Mg-protoporphyrin IX monomethyl ester to Mg-protoporphyrin IX.
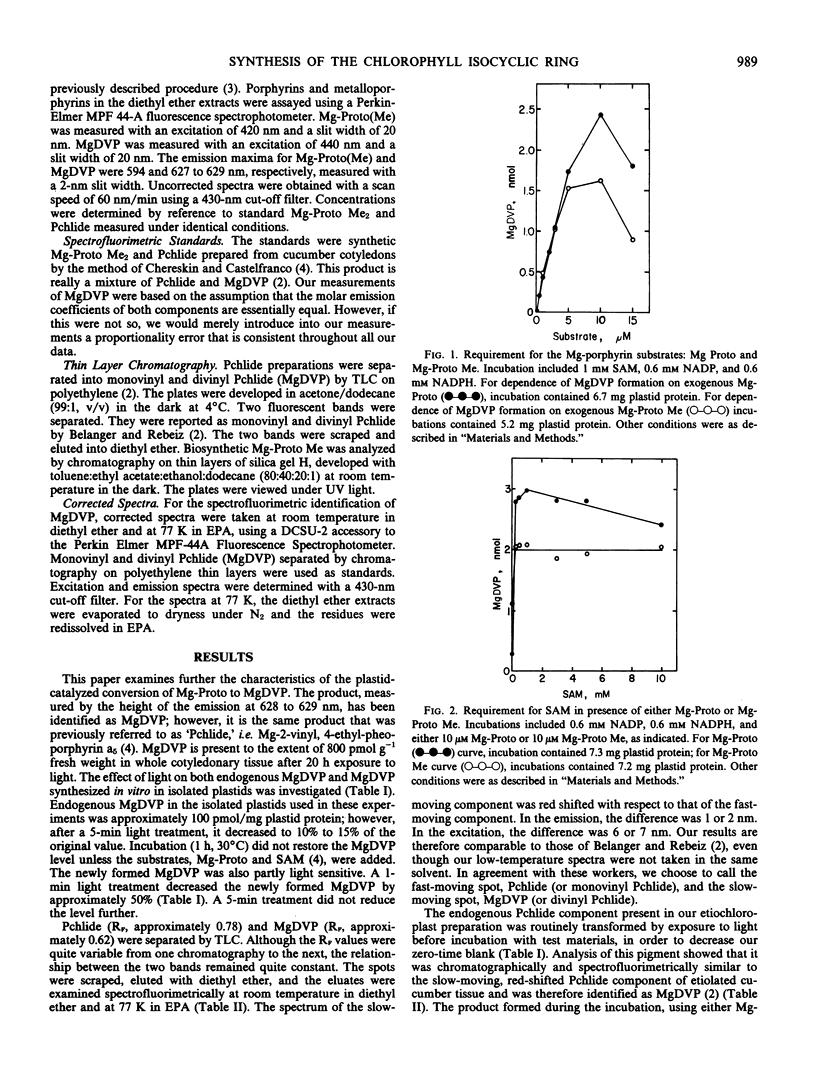
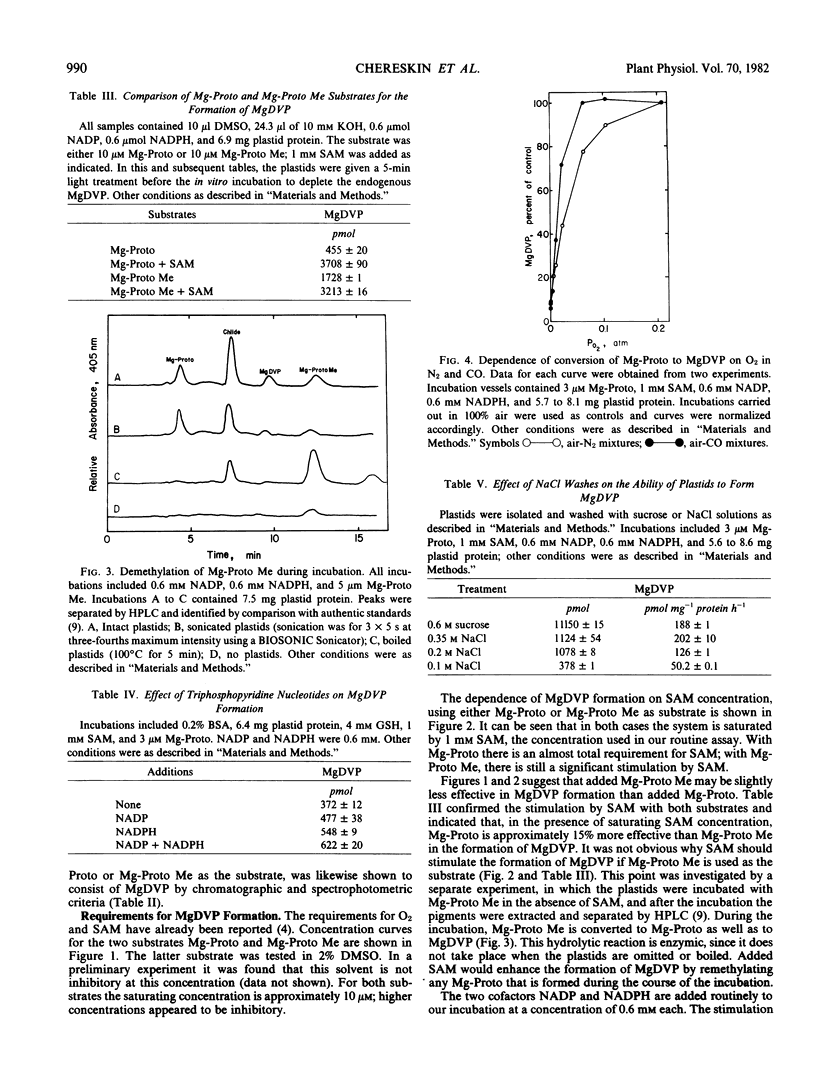
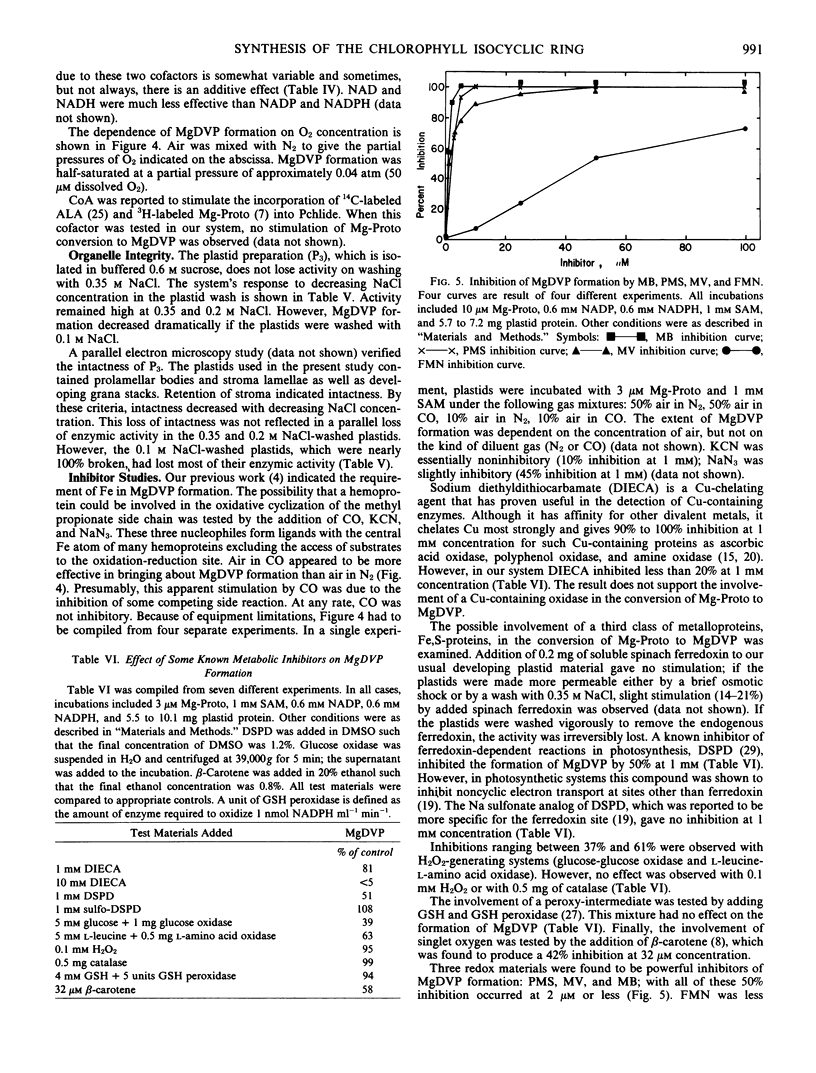
NADP and NADPH stimulated the conversion of Mg-protoporphyrin IX to Mg-2,4-divinyl pheoporphyrin a5. The conversion required oxygen and was half saturated at 50 micromolar dissolved O2. The conversion was insensitive to inhibitors of iron-sulfur and heme-containing proteins, to Cu chelators, H2O2, and peroxide scavengers. However, the conversion was extremely sensitive to phenazine methosulfate, methylene blue, and methyl viologen.
A decrease of the plastids' ability to convert Mg-protoporphyrin IX to Mg-2,4-divinyl pheoporphyrin a5 after lysis in 0.1 molar NaCl suggested a requirement for plastid integrity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis, XXVII. Detection of novel chlorophyll and chlorophyll precursors in higher plants. Biochem Biophys Res Commun. 1979 May 28;88(2):365–371. doi: 10.1016/0006-291x(79)92057-6. [DOI] [PubMed] [Google Scholar]
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis. Detection of divinyl protochlorophyllide in higher plants. J Biol Chem. 1980 Feb 25;255(4):1266–1272. [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations of the biogenesis of chlorophyll a. IV. Isolation and partial characterization of some biosynthetic intermediates between Mg-protoporphine IX monomethyl ester and Mg-vinylpheoporphine a5, obtained from Chlorella mutants. Arch Biochem Biophys. 1969 Mar;130(1):374–383. doi: 10.1016/0003-9861(69)90047-2. [DOI] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations on the biogenesis of chlorophyll a. 3. Biosynthesis of Mg-vinylpheoporphine a5 methylester from Mg-protoporphine IX monomethylester as observed in Chlorella mutants. Arch Biochem Biophys. 1968 Apr;125(1):269–277. doi: 10.1016/0003-9861(68)90661-9. [DOI] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- GRANICK S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948 1949;Series 44:220–245. [PubMed] [Google Scholar]
- Griffiths W. T., Jones O. T. Magnesium 2,4-divinylphaeoporphyrin alpha-5 as a substrate for chlorophyll biosynthesis in vitro. FEBS Lett. 1975 Feb 15;50(3):355–358. doi: 10.1016/0014-5793(75)80526-6. [DOI] [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. MAGNESIUM 2,4-DIVINYLPHAEOPORPHYRIN A5 MONOMETHYL ESTER, A PROTOCHLOROPHYLL-LIKE PIGMENT PRODUCED BY RHODOPSEUDOMONAS SPHEROIDES. Biochem J. 1963 Nov;89:182–189. doi: 10.1042/bj0890182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. THE INHIBITION OF BACTERIOCHLOROPHYLL BIOSYNTHESIS IN RHODOPSEUDOMONAS SPHEROIDES BY 8-HYDROXYQUINOLINE. Biochem J. 1963 Aug;88:335–343. doi: 10.1042/bj0880335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. A protein-protochlorophyll complex obtained from inner seed coats of Cucurbita pepo. The resolution of its two pigment groups into true protochlorophyll and a pigment related to bacterial protochlorophyll. Biochem J. 1966 Oct;101(1):153–160. doi: 10.1042/bj1010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laasch N., Kaiser W., Urbach W. Effects of disalicylidenepropanediamines on photosynthetic electron transport of isolated spinach chloroplasts. Plant Physiol. 1979 Apr;63(4):605–608. doi: 10.1104/pp.63.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN P. J. Purification and properties of the amine oxidase of pea seedlings. Biochem J. 1955 Apr;59(4):609–620. doi: 10.1042/bj0590609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheis J. R., Rebeiz C. A. Chloroplast biogenesis. Net synthesis of protochlorophyllide from magnesium-protoporphyrin monoester by developing chloroplasts. J Biol Chem. 1977 Jun 25;252(12):4022–4024. [PubMed] [Google Scholar]
- Mattheis J. R., Rebeiz C. A. Chloroplast biogenesis. Net synthesis of protochlorophyllide from protoporphyrin IX by developing chloroplasts. J Biol Chem. 1977 Dec 10;252(23):8347–8349. [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel J., Clement-Metral J. D. A 4-vinylprotochlorophyllide complex accumulated by "phofil" mutant of Rhodopseudomonas spheroides. An authentic intermediate in the development of the photosynthetic apparatus. Biochim Biophys Acta. 1976 May 14;430(2):253–264. doi: 10.1016/0005-2728(76)90083-9. [DOI] [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Protochlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):24–32. doi: 10.1104/pp.47.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Recent studies on plant alpha-oxidation systems. Arch Biochem Biophys. 1974 May;162(1):147–157. doi: 10.1016/0003-9861(74)90113-1. [DOI] [PubMed] [Google Scholar]


