Abstract
Stick insects (Phasmatodea) are quite diverse in the Neotropical region. Among them, Xerosoma Serville belongs to Pseudophasmatidae and comprises winged, roughly brownish phasmids that resemble bark or dry branches and inhabit the Atlantic Forest in Brazil. In this study, we present a redescription and revision of the genus that include three valid species, Xerosoma canaliculatum, Xerosoma michaelis, and Xerosoma nannospinus sp. nov. Xerosoma senticosum syn. nov. was found to be a junior synonym of X. canaliculatum. We also provide an identification key and geographic records for these three species. Additionally, we present a detailed study on the morphology and natural history of X. canaliculatum with the description of its nymphal stages, egg, male genitalia, ontogeny, oviposition method, life habits, defense mechanisms, mating behavior, and other aspects regarding its biology. The study also highlights the shortcomings related to the classification of Xerosomatinae, since its tribes find themselves without proper characterization and contain heterogeneous genera. We expect to provide a basis for a proper diagnosis of Xerosomatinae and encourage future studies on this group, as there is still much to be discovered about this lineage of Neotropical stick insects.
Keywords: Animal behavior, Atlantic Forest, Brazilian fauna, Mating behavior, Taxonomy
BACKGROUND
Phasmatodea is an order that comprises insects whose body shape is usually similar to plant parts –the stick and leaf insects. Stick and leaf insects are herbivores and mostly nocturnal, resting camouflaged during the day (Bedford 1978; Bradler et al. 2018). The order includes almost 3,500 species distributed predominantly in tropical and subtropical regions (Brock et al. 2022). The New World is inhabited by Occidophasmata, Cladomorphinae (Oriophasmata), and Timema (Timematodea) (Robertson et al. 2018; Simon et al. 2019; Cliquennois 2021), and contains 955 described species, representing 27% of the total diversity (Brock et al. 2022). Occidophasmata includes Agathemeridae, Pseudophasmatidae, Heteronemiinae, and Diapheromerinae (Bank and Bradler 2022). In Brazil, phasmids have received increasing attention and are currently represented by 228 species (Brock et al. 2022), but with estimations of approximately 600 species (Zompro 2012).
A large number of Brazilian species was described by foreign researchers in the 18th, 19th, and 20th centuries, resulting in several types being deposited in collections outside the country. Salvador de Toledo Piza (1898–1988) was the first Brazilian to study phasmids regularly. He published many studies between the 1930–1980s, describing numerous species and depositing types in Brazilian collections (Crispino et al. 2020 and references therein). Recently, both foreign and Brazilian researchers have studied Brazilian stick insects more frequently (Crispino et al. 2020; Madeira-Ott et al. 2020; Ghirotto 2021; Crispino et al. 2022 and references therein). Nonetheless, there is still a considerable deficit in the knowledge of Phasmatodea (see Cotterill and Foissner 2009; Hortal et al. 2015; Madeira-Ott et al. 2020).
Pseudophasmatidae is one of the most diverse lineages in the Neotropical region, with more than 300 described species, and consists of three subfamilies: Pseudophasmatinae, Stratocleinae, and Xerosomatinae. Although Xerosomatinae is not the most diverse of these subfamilies, it still includes three tribes, with 16 genera and 108 species, comprising 27% of the diversity of the family (Brock et al. 2022). Furthermore, Xerosomatinae monophyly was recently recovered in molecular studies, which included a good sampling of taxa (Simon et al. 2019; Bank and Bradler 2022). The type genus of the lineage, Xerosoma Serville 1831, is comprised of three species: Xerosoma canaliculatum Serville 1831, Xerosoma senticosum Stål 1875, and Xerosoma michaelis Redtenbacher 1906. Xerosoma is only recorded from Brazil and inhabits the Atlantic Forest, which despite being considered one of the most important areas of global biodiversity and endemism, is severely degraded and threatened (Tabarelli et al. 2005; Pinto et al. 2006).
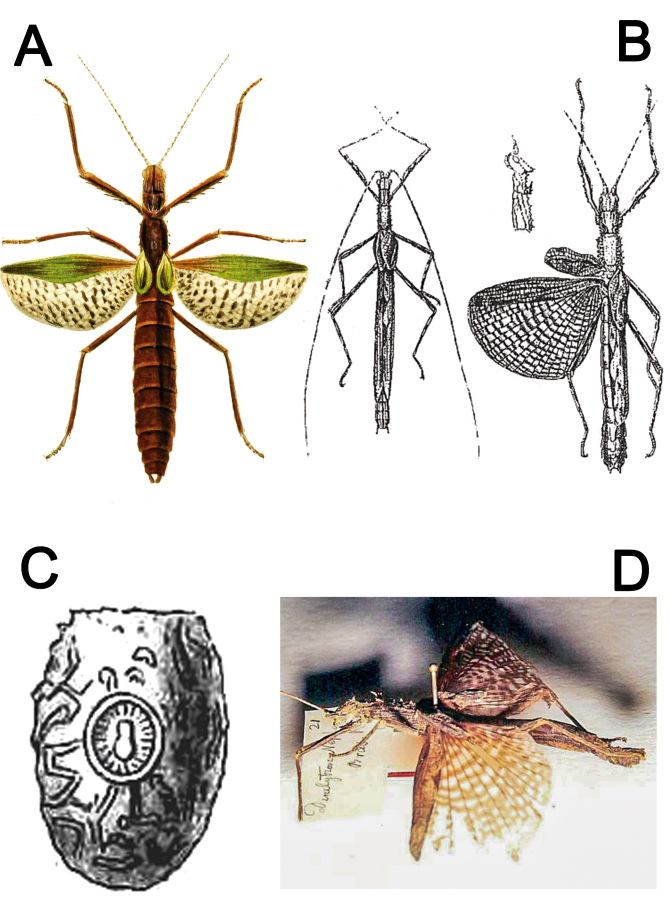
The type species of Xerosoma, X. canaliculatum, was originally briefly described and illustrated by Serville (1831) (Fig. 1A) based on a single female from Brazil deposited in the Muséum National d’Histoire Naturelle, Paris, France (MNHN). A more detailed description with illustrations of the male was published around 70 years later by Redtenbacher (1906) (Fig. 1B) from specimens also deposited in MNHN. The taxonomic history of X. canaliculatum is connected with the description of Dinelytron neptunus Kaup 1871 (Fig. 1D), which was first transferred to Prexaspes Stål 1875 by Kirby (1904) and then to a new genus, Harpuna Redtenbacher 1906, described to allocate Kaup’s species. Almost a century later, H. neptuna was synonymized under Xerosoma canaliculatum by Zompro (2004a). Zompro (2004a) did not find the egg illustrated by Kaup (1871) in the collection deposited in the Hessisches Landesmuseum, Darmstadt, Germany (HLDL), mentioning the egg based on Kaup’s illustration (Fig. 1C).
Fig. 1.
Historical illustrations and photographs from published literature. A: ♀ Xerosoma canaliculatum Serville, 1831 holotype illustrated by Serville (1838). B: Xerosoma canaliculatum ♂ and X. senticosum Stål, 1875 (= X. canaliculatum) ♀ syntype illustrated by Redtenbacher (1906). C: egg of Dinelytron neptunus Kaup, 1871 (= X. canaliculatum) illustrated by Kaup (1871), which does not correspond to eggs of Xerosoma. D: ♀ Dinelytron neptunus (= X. canaliculatum) holotype photographed by Oliver Zompro, available in Phasmida Species File Online (Brock et al. 2022, CCBY 4.0). Image not to scale.
The genus name comes from the Greek words “xero” and “soma”, which mean dry and body, respectively, likely an allusion to its similarity to a dry branch. The biological aspects of Xerosoma representatives are totally unreported in the literature. Despite being poorly studied, X. canaliculatum is one of several phasmids commonly found throughout the Atlantic Forest in Brazil. The lack of studies on Xerosoma exemplifies the poor knowledge regarding Brazilian phasmid species. Many taxa were never reviewed after the original descriptions (Madeira-Ott et al. 2020; Chiquetto-Machado and Cancello 2021) and the biology of most species are still unknown. Therefore, in this study we present a taxonomic revision of the genus Xerosoma by redescribing it and its species, in addition to describing a new species. We also provide an identification key for the species and characterize the morphological and biological aspects of Xerosoma canaliculatum. Along with the taxonomic revision, we aim to present basic life history traits that can enhance the understanding of biological aspects of Xerosoma in particular and Phasmatodea in general. With this work, we expect to provide a basis for a proper diagnosis of Xerosomatinae and encourage future studies on this group, as there is still much to be discovered about this lineage of Neotropical stick insects. Furthermore, we expect to inspire studies on Brazilian phasmids as well as general research on Phasmatodea with an integrative approach.
MATERIALS AND METHODS
Studied specimens
Types from the following institutions were examined: Muséum National d’Histoire Naturelle, Paris, France (MNHN); Natural History Museum, Vienna, Austria (MNW); and Zoologisches Institut und Zoologisches Museum, Universität von Hamburg, Germany (ZMUH). Additional specimens of X. canaliculatum were collected alive at the Reserva Natural SESC Bertioga, São Paulo, Brazil. This natural reserve consists of a continuous area with large patches of forest close to the Parque Estadual Restingas de Bertioga and the Parque Estadual da Serra do Mar, both important Atlantic Forest conservation units. Additional material available in the collections of the Museu de Zoologia da Universidade de São Paulo (MZUSP) and the Instituto Nacional de Pesquisas da Amazônia (INPA), as well as photographic records mostly from the online platform iNaturalist under the project tag “Bichos-Pau do Brasil” (inaturalist.org/projects/bichos-pau-do-brasil) created by Projeto Phasma (an independent phasmid research group to which most of the authors belong) and curated by several researchers, was also studied.
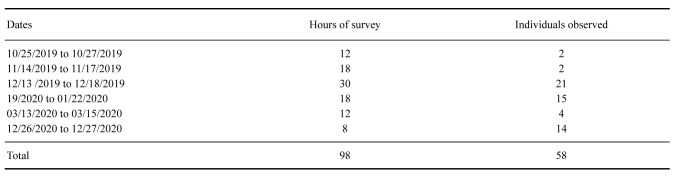
Field trips to Bertioga occurred in the rainy months (October–March) between 2019 and 2020 as part of a stick insect survey carried out in a partnership among SESC Bertioga, MZUSP, and Projeto Phasma. Xerosoma canaliculatum individuals were observed and collected by active nocturnal searching with flashlights. A suspended footbridge at SESC Bertioga allowed observation of up to approximately six meters from the ground (Fig. S1).
The expeditions comprised six campaigns, totaling 98 hours of active search and observations of specimens in situ. In total, 58 individuals were sighted (Table 1), of which the last 14 were collected and kept in captivity.
Table 1.
List of dates, hours and number of individuals of Xerosoma canaliculatum observed in field trips to SESC Bertioga, São Paulo, Brazil
Maintenance and preservation of X. canaliculatum specimens
Specimens of X. canaliculatum were bred in captivity for about 1 year in different sized screened containers, with 20 × 15 × 20 cm for nymphs and 40 × 60 × 40 cm for adults. They were kept under natural light and at room temperature ranging from 17°C to 32°C, and their food plant was kept in a flask with water, replaced weekly (Fig. S2A, C). To facilitate visualization, some individuals were kept outside the containers in tree trunks placed in an open glass pot (Fig. S2B, D). To maintain a moist environment, two simple house humidifiers and one ventilator were used, in addition to eventual manual water spraying. The air humidity was measured by a hygrometer and ranged between 43% and 85%. The eggs were incubated in small plastic containers with tissue covered holes containing sphagnum moss as a substrate, checked and moistened every three to four days to maintain a constant humidity of around 75%. The specimens were either raised until natural death or killed by freezing, and then placed in ethyl alcohol 70%, or pinned and dried; some of the eggs were dried for preservation. The specimens were deposited into MZUSP.
Taxonomy and morphology of the specimens
Morphological examination and image acquisition were made using a stereomicroscope (Leica M205A, equipped with a Leica DFC450 digital camera), an optical microscope (Leica DM2500, equipped with a Leica DFC295 digital camera) and a macro photography camera (Canon SL1 Camera, Canon EF 100 mm macro lens, Yongnuo YN560 III Flash) at the Laboratório de Biologia Aquática (UNESP, Assis, SP, Brazil) and the Laboratório de Aracnologia de Rio Claro (UNESP, Rio Claro, SP, Brazil). Schematic drawings followed Cala-Riquelme (2021). Measurements were taken using a Leica M205C stereomicroscope with LAS Core software or an analog caliper. The dissection of the genitalia followed Heleodoro and Rafael (2019), Chiquetto-Machado and Cancello (2021), and Ghirotto (2021). Some phallic organs (male genitalia) were further cleared in warm aqueous KOH solution. Morphological characterization and description followed Ragge (1955); Sellick (1997) for eggs; Helm et al. (2011), Chiquetto-Machado (2018), and Ghirotto (2021) for genitalia; and Cumming et al. (2021a) for wing venation. Scanning electron microscopy (SEM) of eggs of X. canaliculatum was carried out at MZUSP using a LEO 440 Carl-Zeiss microscope. The eggs were cleaned manually with a brush immersed in water and detergent solution, dehydrated in ethanol 100% for a few days and then critical point dried with CO2 using a CPD 030 critical point dryer. After mounting on stubs, the eggs were coated with a layer of gold using an SCD 050 sputter coater. Micrographs were recorded at an acceleration voltage of 15 kV.
For the SEM analysis of the tarsal morphology of juveniles and adults of X. canaliculatum, tarsi were stored in ethanol 70% and dehydrated in an ascending alcohol series. The tarsi were cut at the tibia, critical point dried with a Leica EM CPD300 critical point dryer (Leica, Germany), and sputter-coated with 10 nm gold-palladium (Leica Bal-TEC SCD500). Micrographs were recorded using a Hitachi TM3000 (Hitachi High-technologies Corp., Japan) at an acceleration voltage of 15kV.
The redescription and other observations on X. canaliculatum were based on type material as well as new material from several localities, mainly Bertioga in São Paulo state and Aracruz in Espírito Santo state. Measurements are given as the interval between the smallest and largest value obtained from the analyzed material.
Geographic distribution
Both examined material and photographic records from iNaturalist were used to produce the distribution map of Xerosoma, which was made with QGIS version 2.18.
RESULTS
TAXONOMY
Phasmatodea Jacobson and Bianchi 1902
Pseudophasmatidae Rehn 1904
Xerosomatinae Bradley and Galil 1977
Xerosomatini Bradley and Galil 1977
Xerosoma Serville 1831
Xerosoma Serville 1831 (61); Gray 1835 (26) [synopsis]; Serville 1838 [1839] (274), pl. 6, Fig. 3 [synopsis]; Blanchard 1840 (14, 18) [synopsis]; Westwood 1859 (103) [catalogue]; Stål 1875a (59, 99) [description of X. senticosa, X. spinosa transferred to Crexoylus]; Stål 1875c (20) [systematic]; Kirby 1890 (572) [systematic]; Rehn 1904 (101) [description of X. glypteomerion]; Kirby 1904 (416) [synopsis]; Redtenbacher 1906 (143) [key to genera, description of X. vignieri and X. michaelis, description of the male of X. canaliculatum]; Shelford 1909 (375) [synopsis in part]; Bradley and Galil 1977 (202) [synopsis in part]; Bragg 2001 (645) [type species data]; Zompro 2004a (111, 323) [Dinelytron neptunus Kaup 1871 synonymized under X. canaliculatum]; Otte and Brock 2005 (342 [catalogue]); Araujo and Garraffoni 2012 (236) [synopsis]; Conle et al. 2020 [X. glyptomerion Rehn 1904 and X. vignieri Redtenbacher, 1906 transferred to Isagoras].
Species included: Xerosoma canaliculatum, Xerosoma michaelis, and Xerosoma nannospinus sp. nov. The type species is X. canaliculatum, by original monotypy.
Diagnosis: Xerosoma is differentiated from other Xerosomatinae by a pair of spines in the posterior part of the pronotum, the presence of wings in both sexes, conspicuous keels in terga VIII–X in both sexes, and the prickly anterior femur of females.
Remarks: Monophyletic Xerosomatinae was recovered in molecular phylogenetic studies (Simon et al. 2019; Bank and Bradler 2022). However, there is a lack of a detailed evaluation of the morphological characteristics shared among its members for a decisive approach. Overall characteristics shared by Xerosomatinae and contrasting with other Pseudophasmatidae includes a more rugose or granulose body surface, a longer tegmina, a shorter subgenital plate showing the enlarged gonapophyses in females, and a poculum usually smaller and flattened in males. Xerosoma has all of these characteristics.
Key to ♀ of Xerosoma
(Fig. 2)
1a. Hindwings strongly reduced, not reaching the abdomen .................. X. michaelis
1b. Hindwings at least reaching abdominal segment VI .................. 2
2a. Spines in the posterior part of the pronotum are strongly pronounced forwardly, reaching a third of the pronotum in length .................. X. canaliculatum
2b. Spines in the posterior part of the pronotum are reduced, not reaching a third of the pronotum in length .................. X. nannospinus sp. nov.
Fig. 2.
Females of Xerosoma species, anterior region of the body in dorsal and lateral views. A: X. canaliculatum, black arrow indicating the strongly pronotal spines. B: holotype of X. nannospinus sp. nov. (MZUSP 1440), black arrow indicating the reduced pronotal spines. C: lectotype of X. michaelis, black arrow indicating the strongly prickly ventral margin of femur and the brachypterous wings. Photographs provided by Oskar Conle, Frank Hennemann and Pablo Riquelme, published with permission. Scale bar = 2 cm.
Key to ♂ of Xerosoma (males unknown for X. michaelis)
(Fig. 3)
1a. Spines in the posterior part of the pronotum are strongly pronounced forwardly, reaching a third of the pronotum in length .................. X. canaliculatum
1b. Spines in the posterior part of the pronotum are reduced, not reaching a third of the pronotum in length .................. X. nannospinus sp. nov.
Redescription: Brachypterous to fully winged. Sexual dimorphism marked with females significantly more robust than males. Male and eggs are not known for X. michaelis. Body length of 45.4–67 mm for ♂ and 55–65 mm for ♀.
Head slightly longer than wide, slightly dorsoventrally compressed; vertex almost flat, gently round or prominent posteriorly; surface covered with fairly prominent granules and frequently with a longitudinal line centrally. Ocelli reduced in ♂, absent in ♀. Antennae slender, reaching abdominal segments II or III in ♀, or segments VI to slightly longer than the body in ♂.
Thorax and abdomen roughly cylindrical. Pronotum slightly longer than wide, slightly shorter to about the same length as the head; wrinkled and covered with granules to spines, at posterior half with a pair of acute spines, small or prominent, pointing upwards or curved anteriorly. Mesonotum stouter and 1.8–2.5× longer than the pronotum, with a pair of dorsolateral carinae. Mesonotum rugose and bearing small to large granules, with the largest presented in pairs centrally, one at each carina. Mesepimeron with a longitudinal rugose carina with larger granules near the posterior region. Metanotum and middle segment equally longer, in the same thickness, or slightly more robust than the posterior portion of mesonotum. Metanotum dorsally glabrous, darker, granulated ventrally.
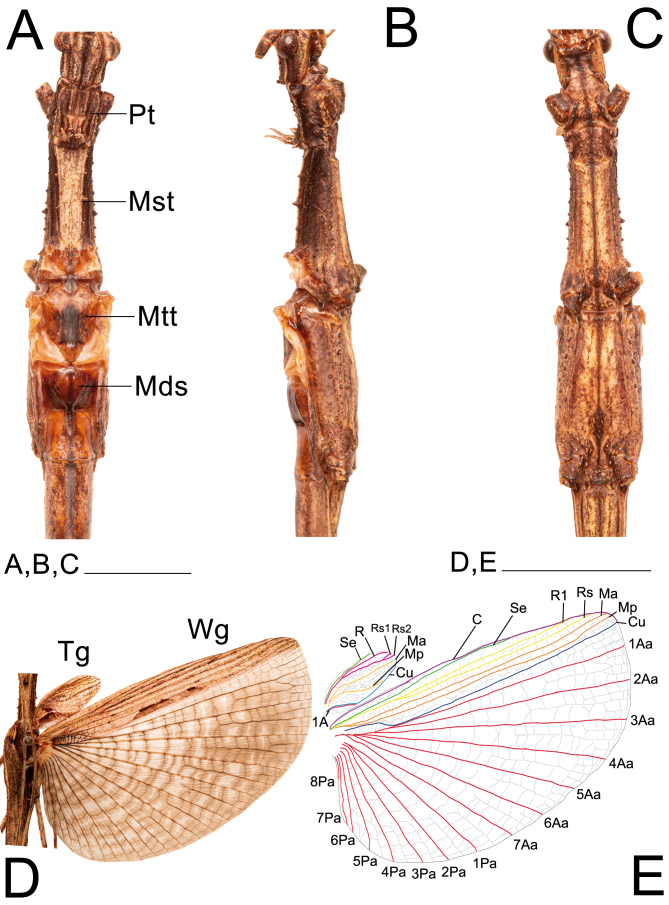
Brachypterous or fully winged. Tegmina suboval, posterior margin rounded or truncated; with marked venation and distinct shoulder pads; barely reaching the median segment to reaching tergum II. Hindwings reaching the center of the median segment or tergum VI in ♀ and tergum VIII in ♂; when fully winged, anal area tessellated.
Legs smooth and slender in ♂. In ♀, femora I and II with undulate to conspicuously sharp serrations laterally; femora III unarmed. Tibiae slightly granulate in ♀. Profemora basally curved, dorsoventrally compressed, slightly longer than the mesothorax; hind legs slightly longer than the abdomen. Basitarsi 2–2.5× longer than the respective following tarsomeres.
Abdominal segments longer than wide. When fully winged, terga I–V dorsally glabrous and centrally black. Terga laterally keeled, conspicuously larger forming lateral lobes in VII–IX. Tergum X with a pair of rough carinae, more pronounced in females; dorsally with elevations apically. Tergum X tectiform, slightly longer than IX, with two or three apical carinate projections in females. Apical inner surface of tergum X with about 10 in-curving teeth in ♂. Vomer significantly reduced, not sclerotized, showing as a basal bump 4× wider than long. Epiproct small, discreet. Paraprocts deeply incised medially in ♂ and posteromedially incised in ♀. Sternum IX of males (poculum) short, slightly pronounced, barely reaching tergum X, posterior margin rounded. Sternum VII of females with a small preopercular organ, forming a small lump-like median swelling at the posterior margin. Sternum VIII of females (subgenital plate) flat, reaching half the length of tergum IX, with the posterior margin rounded or slightly pointed. Gonapophyses VIII dorsoventrally flattened, linear to oblong, ventrally bearing setae, reaching the posterior region of tergum X. Gonoplac prominent, dorsoventrally flat, triangular, thicker and longer than both gonapophyses, ventrally bearing setae. Gonapophyses IX smooth, shiny, acute, shorter than VIII and concealed from ventral view by the enlarged gonoplac. Cerci short and small in ♀, tapered posteriorly with rounded apex, round in cross section, with third to half the length of tergum X. Cerci in ♂ slender, almost the same size as tergum X.
Eggs (not known for X. michaelis): Rounded, sub cylindrical, 1.5× longer than wide, nearly round in cross section, centrally widened in dorsal view; ventral surface flat (allowing gluing to flat surfaces), polar area slightly flat. Opercular aperture angled at approximately 45° towards dorsal region. Operculum round, slightly convex, with a distinct straight stalk with an apical irregular conical projection widening towards apex. Capsule and operculum densely covered with hairy setae of different sizes. Micropylar plate usually surrounded by a lighter, whitish color. Micropylar plate creamish, varying from round to oval to sub-rectangular, glabrous, with small sparse setae, and ridged margins, occupying almost 1/3 of the egg’s length. Micropylar cup dark, wide, elliptical, continuous with the short internal median line. Internal micropylar plate opened.
Fig. 3.
Males of Xerosoma species, anterior region of the body in dorsal and lateral views. A: X. canaliculatum in dorsal view. B: paratype (1442) of X. nannospinus sp. nov. in dorsal view. C: X. canaliculatum in lateral view, black arrow indicating the strongly pronotal spines. D: paratype (1442) of X. nannospinus sp. nov. in lateral view, black arrow indicating the reduced pronotal spines. Scale bar = 1 cm.
Geographic distribution
(Fig. 4)
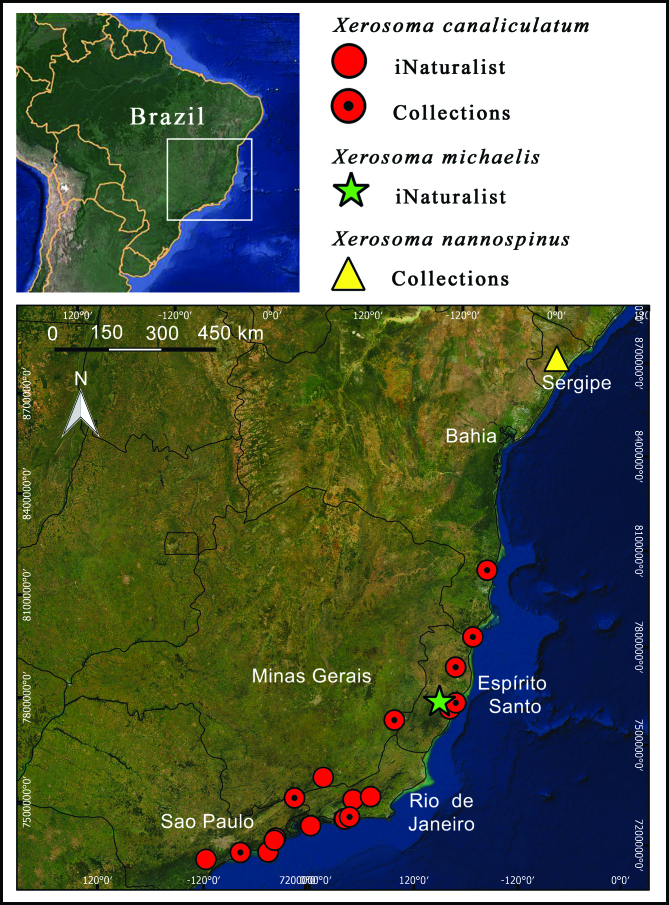
Xerosoma species are endemic to the Atlantic Forest in the southeast and northeast regions of Brazil, being recorded from six states: Sergipe, Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro, and São Paulo. It is expected that further sampling in other areas of the broad Atlantic Forest will increase the occurrence area of the genus. All of the gathered records comprise new or more precise occurrence records for the genus.
Fig. 4.
Distribution of Xerosoma species.
Xerosoma michaelis Redtenbacher 1906
(Figs. 5, 6)
Xerosoma michaelis Redtenbacher 1906 (145); Weidner 1966 (232) [catalogue]; Brock 1998 (42) [catalogue]; Zompro 2004a (112) [revision]; Otte and Brock 2005 (343) [catalogue].
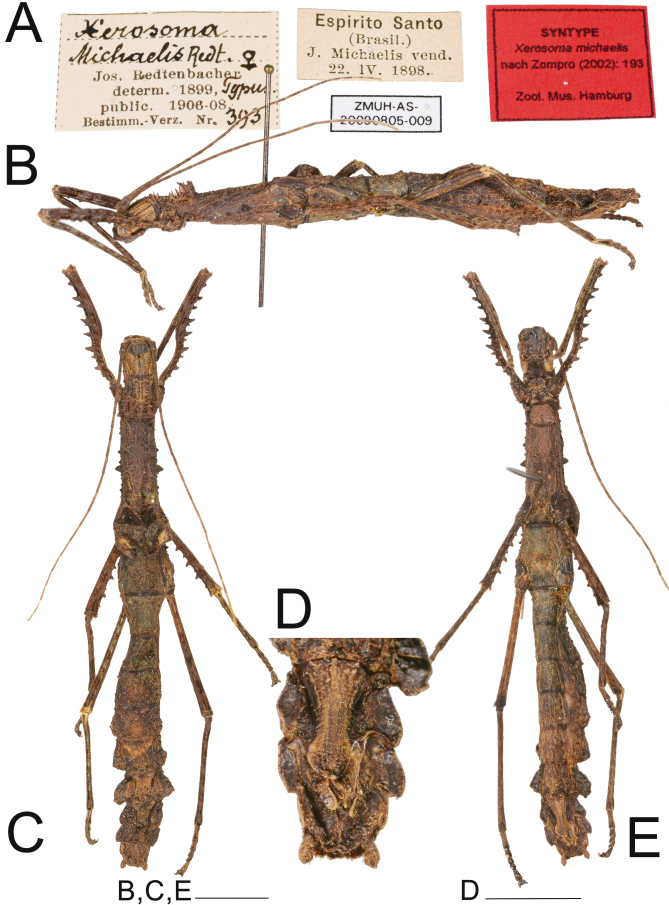
Examined material: Lectotype (here designated) ♀ (ZMUH AS-20090805-009) “Jos. Retenbacher determ. 1899, Typus, public. 1906-08, Bestimm. -Verz. Nr. 393. Espirito Santo (Brasil) J. Michaelis vend. 22 IV 1898” (examined by photo).
Paralectotype (here designated) ♀ (NMW 241) “Espírito Santo, Brasil, ex. coll. Fruhstorfer, J. Michaelis vend., 24.486.” (examined).
Type condition: (Fig. 5). Redtenbacher (1906) did not specify how many specimens he examined. There are two females known to be the syntypes of the species (Brock et al. 2022). The designated lectotype is well preserved and complete.
Fig. 5.
Lectotype ♀ of Xerosoma michaelis. A: labels. B: lateral view. C: dorsal view D: end of abdomen in ventral view. E: ventral view. Scale bars: B, C, E: 1 cm; D: 5 mm. Photographs provided by Oskar Conle, Frank Hennemann and Pablo Riquelme, published with permission.
Remarks: We selected a lectotype to stabilize nomenclature choosing the specimen in the best conditions and with better diagnostic characters. A living female was photographed by a citizen and uploaded to iNaturalist, perfectly matching the characteristics of the species (Fig. 6 and Supplementary link); we used this record to compose the redescription of color and the updated geographical occurrence for the species.
Fig. 6.
Live Xerosoma michaelis ♀ found in Santa Teresa, Espírito Santo, Brazil. Photographs by Felipe Afonso. A: full body in dorsolateral view. B: same as previous, zoomed. C: dorsal view, placed on a palm tree for photography. Image not to scale.
Diagnosis: Known only from females, Xerosoma michaelis females differ from those of X. canaliculatum and X. nannospinus sp. nov. by the strongly reduced wings (brachypterous) and stronger spines or projections on the lateral margins of the mesothorax and pro-and mesofemora.
Comments on the etymology: The etymology as given by Redtenbacher (1906) is dedicated to the collector of the type material, J. Michaelis (Fig. 5A).
Redescription (female only): Body length of 55 mm.
Head slightly longer than wide, dorsoventrally compressed, slightly rounded in dorsal view; vertex flat, surface covered with small granules. Head bearing a poorly marked longitudinal sulcus. Antennae reaching tergum II.
Thorax and abdomen roughly cylindrical. Pronotum slightly longer than wide and slightly smaller than the head; rugose and covered with spines, with a posterior larger pair pointing forward. Mesonotum wider and 2.5× longer than the pronotum, with a pair of dorsolateral carinae. Mesonotum rugose and bearing two pairs of well-marked rounded spines centrally at each carina. Mesepimeron with a longitudinal rugose carina with larger granules near the posterior region. Metanotum and median segment as wide as or slightly wider and 1.2× longer than the posterior region of the mesonotum.
Tegmina reduced, posterior margin truncated, venation strong and with distinctly raised sub-basal elevations, slightly reaching the middle of the median segment. Hindwings reduced, only reaching the middle of the median segment.
Legs with conspicuous spines on femora I–II, femora III unarmed. Tibiae bearing small granules. Profemora slightly longer than the mesothorax and hind legs slightly longer than the abdomen; profemora basally curved and compressed. Basitarsi 2× longer than the following respective tarsomeres.
Abdominal segments longer than wide, slightly less granulated than the rest of the body. Terga with lateral waved projections that become larger and more pronounced in VII. Tergum X with an apical pair of rugose carinae. Tergum X tectiform, slightly longer than IX, with three weak apical carinate projections. Epiproct small. Paraprocta with short incision. Sternum VII with a small preopercular organ, forming a small lump-like median swelling in the posterior margin. Subgenital plate flat, roundly prominent apically, slightly longer than half the length of segment IX. Gonaplacs and gonapophyses VIII reaching half the length of tergum X. Cerci short and small, tapered towards a rounded apex, round in cross section, with a third of the length of tergum X.
Color: (Fig. 6). Body mostly stained with irregular shades of brown, complemented by black, white, and green spots. Head with a small centralized dorsal longitudinal black line; lateral region of the head bearing a dark brown band with a longitudinal creamish hairline just behind the eyes. Antennae with dorsal green spots in the scapus and pedicellus; the rest of the antennomeres alternating with light and dark shades of brown, except the whitish last five ones. Prothorax brown, with all spines light green. Mesothorax similar to the prothorax, whitish at the base dorsally; dorsolateral spines green, and ventro-lateral spines dark brown. Abdomen dorsally lighter in the first four terga and greenish in terga V–VII dorsally, with the latter stained with brown like the rest of the body. Costal area of tegmina irregularly brownish with lighter and darker spots and green spots apically. Legs in different shades of brown, similar to the body, but with black spots mainly on the anterior femora and greenish spots on the joints of the femur and tibia.
Geographic distribution: Xerosoma michaelis is recorded for the first time from a precise location, in the municipality of Santa Teresa, Espírito Santo state, from a photographic record available on iNaturalist (2022), taken on 16/01/2022 (Fig. 6) (Supplementary link).
Xerosoma nannospinus sp. nov. Engelking and Ghirotto 2022
(Figs. 7–15)
urn:lsid:zoobank.org:act:###
Examined material: Holotype ♀ (MZUSP 1440): Brasil, Sergipe, Areia Branca, Parque Nacional de Itabaiana, 10°46'02.6"S, 37°20'14.6"W, 01.V.2022, P. H. Martins, M.D.F. Magalhães & Gonzalez-Filho H.M.O, Paratypes 3♂ (MZUSP 1441, 1442, 1443): same collection data as for holotype; ♀ (MZUSP 1830): Brasil, Alagoas, Passo do Camaragibe, Mata a beira de pista, 9°16'57.0"S, 35°31'28.1"W, 18.iii.2023, V.M. Ghirotto, P.W. Engelking, E.W. Engelking col.; ♀ (MZUSP 1831): Brasil, Alagoas, Rio Largo, Cedro do Itu, Mata Atlântica, 9°31'07.4"S, 35°53'34.6"W, 19.iii.2023, V.M. Ghirotto, P.W. Engelking, E.W. Engelking col.; ♀ (MZUSP 1832): Brasil, Sergipe, Santa Luzia do Itanhy, Mata Atântica à beira da pista, 11°22'32.1"S, 37°25'18.2"W, 21.iii.2023, V.M. Ghirotto, P.W. Engelking, E.W. Engelking col. Additional material: ♀ nymph (MZUSP 1444): fourth instar, same collection data as for holotype; 4 eggs.
Remarks: Known only from a few individuals from a few populations. As this species shares similarities with X. canaliculatum, the description is concise and is compared to that of X. canaliculatum. Mouthparts were not examined.
Diagnosis: Xerosoma nannospinus sp. nov. differs from X. michaelis by the fully developed hindwings and the reduced number of serrations on femora I–II in females, the only sex known for the latter. Both sexes of X. nannospinus differ from X. canaliculatum by the more pointed vertex, the smaller eyes, the smaller spines on pronotum, the smaller and rounder tegmina, the shorter wings, and the less pronounced lateral tergal projections. Females can be further differentiated from those of X. canaliculatum by the presence of only two apical projections in the posterior margin of tergum X (instead of three) and the enlarged gonapophysis, while males can be further differentiated by the narrower edge of tergum X, the shorter cerci, the more triangular, wider and thinner vomer and the wider base and shorter length of the dorsal sclerite. X. nannospinus can also be distinguished from X. canaliculatum by the shorter and rounder eggs.
Comments on the etymology: “nannospinus” is a junction of the Greek word “nánnos” = small, reduced, diminutive, and the Latin word “spinus” = spines, in allusion to the smaller spines at the base of the pronotum in comparison with the other two Xerosoma species.
Fig. 7.
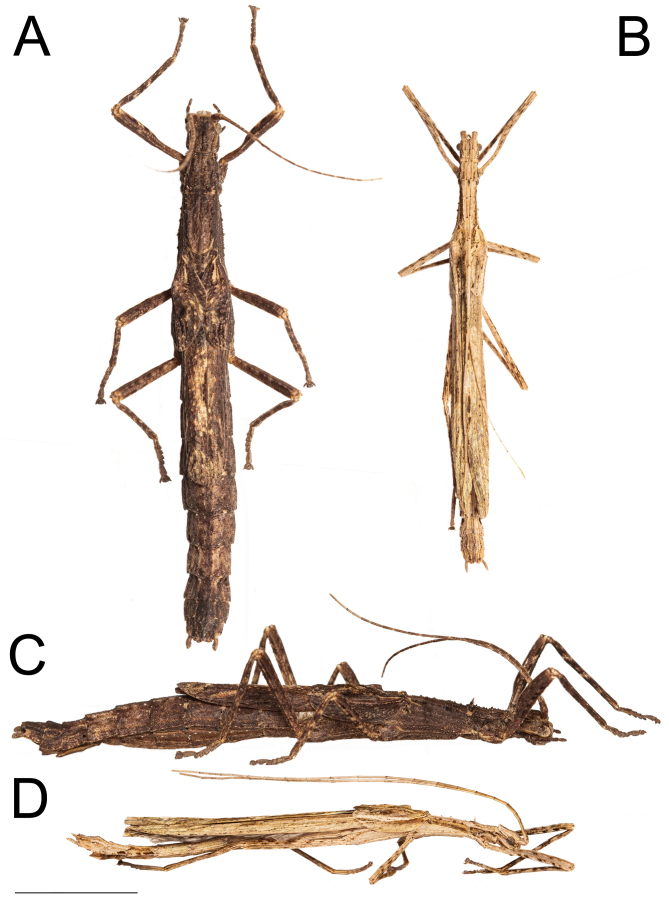
Xerosoma nannospinus sp. nov. couple: holotype ♀ MZUSP 1440, and paratype ♂ MZUSP 1442. A: dorsal view of ♀. B: dorsal view of ♂. C: lateral view of ♀. D: lateral view of ♂. Scale bar = 1 cm.
Description: Female (Figs. 8–10). Total body size of 55.4 mm, winged. Measurements are given in table 3. Description in comparison with X. canaliculatum.
Table 2.
Measurements (mm) of four Xerosoma nannospinus sp. nov.
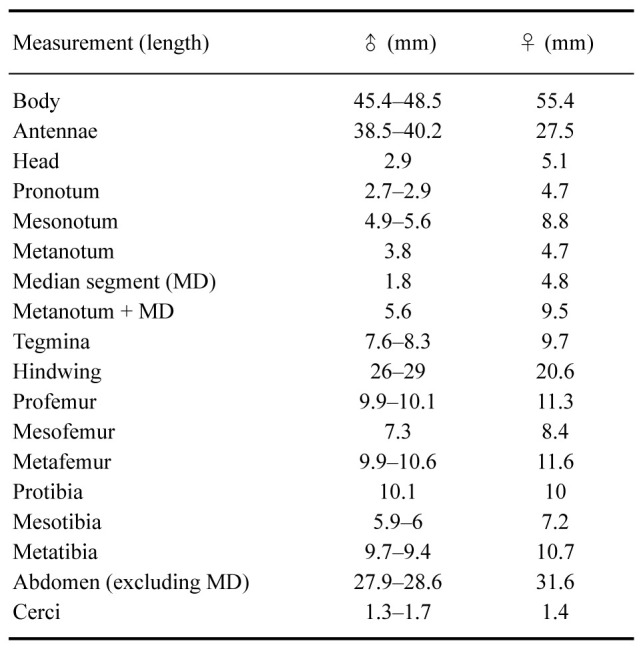
Fig. 8.
Head and prothorax of ♀ Xerosoma nannospinus sp. nov. holotype MZUSP 1440. A: head and prothorax in lateral view. B: dorsal view; C: ventral view. Scale bar = 5 mm.
Fig. 9.
Wings of ♀ Xerosoma nannospinus sp. nov. holotype MZUSP 1440. A: dorsal view with wings opened. B: dorsal view of tegmina closed. Scale bars = 5 mm.
Fig. 10.
Posterior region of the abdomen of ♀ Xerosoma nannospinus sp. nov. holotype MZUSP 1440. A: dorsal view. B: lateral view. C: ventral view. Scale bar = 5 mm.
Head with prominent vertex forming a pointed apex, less pronounced tubercles, presenting a marked longitudinal blackened line but without forming sulci. Head slightly broader, width about 1.4× the length. Eyes slightly elongated, about 0.37 of the length of the head. Antennae slightly shorter, reaching tergum II.
Pro-and mesothorax slightly broader. Prothorax about 1.7× longer than wide, bearing a pair of pointed dorsal spines near the posterior margin, accompanied by smaller spines dorsally; all spines less pronounced. Small granules scattered on the dorsal surface and along the dorsolateral carinae of the pronotum. Mesothorax, metathorax and median segment as in X. canaliculatum.
Tegmina shorter and rounder, about 2× longer than wide, reaching the median segment, with less pronounced shoulder pads. Hindwings slightly shorter, just reaching tergum V. The venation of tegmina and hindwings are fairly similar to that of X. canaliculatum, also presenting Rs subdivided into Rs1 and Rs2, reaching the apical costal area of the tegmina.
Femora and tibiae with about the same proportions as those of X. canaliculatum. Femora III slightly more serrated in the ventral-posterior carinae.
Abdomen 1.3× longer than the combined length of the head, thorax and median segment. Terga in the same proportion and smoother than in X. canaliculatum. Terga with less pronounced carinae. Lateral terga keels in the posterior region less pronounced. Apical posterior terga projections less pronounced. Sternum VIII (subgenital plate) not reaching the median length of IX, broader. Epiproct, paraprocta and cerci as in X. canaliculatum. Praeopercular organ wide, short, less pronounced. Gonapophyses VIII slightly longer and gonapophyses IX slightly broader.
Color: Body mostly with irregular shades of brown, complemented by black and creamish stains. Most of the head and thorax spines dark brown basally and creamish apically. Posterior region of the head irregularly light brown with a creamish line just behind the eyes. Head with a well-marked dorsal longitudinal black line. Pro-and mesothorax mainly dark brown, lighter dorsally. Abdomen similar to thorax, darker dorsally in the first 5 segments covered by wings and brownish laterally; terga VII–VIII with lateral black spots. Antennae similar in color as the rest of the body, becoming lighter from the 7th antennomere. Costal area of tegmina irregularly brownish with lighter and darker spots, anal region with black spots, proximal ventral area reddish. Hindwings with costal area similar to the tegmina and including a red stain basally. Anal area with light, translucent cells centrally and near the costal area; area near veins brownish, veins dark brown to blackish. Legs in different shades of brown similar to the body, but with black spots, mainly on the anterior femora.
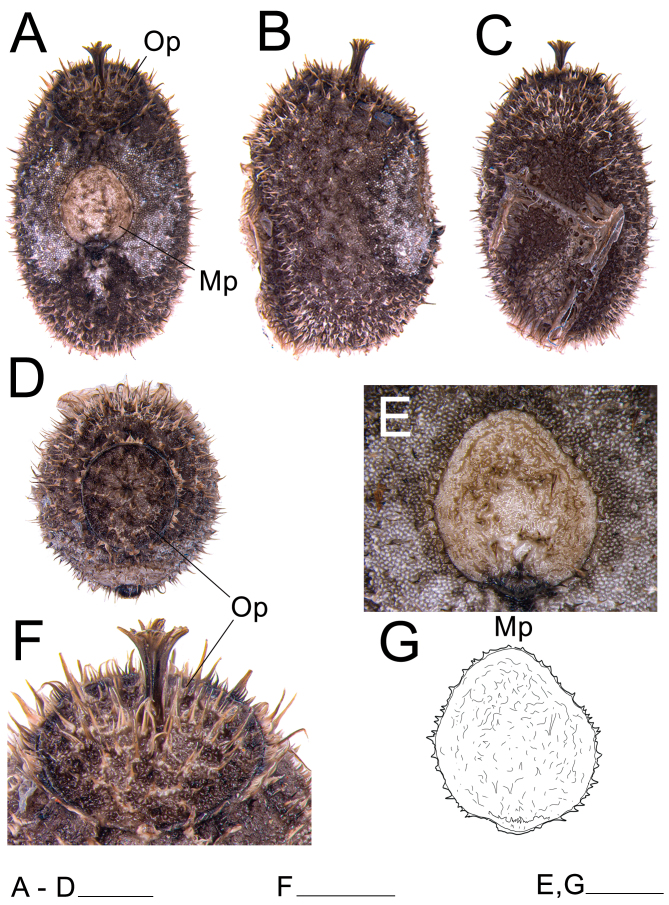
Eggs: (Fig. 11). As for the genus. Rounder than those of X. canaliculatum, 1.5× longer than tall. Micropylar plate longer, micropylar cup less pronounced than in X. canaliculatum. Capsule with longer hairy setae.
Fig. 11.
Egg of Xerosoma nannospinus sp. nov. A: dorsal view. B: lateral view. Scale bar = 1 mm.
Male: (Figs. 12–14). Body size of 45.4–48.5 mm. Measurements are given in table 3.
Table 3.
Measurements (mm) of four eggs of Xerosoma nannospinus sp. nov.
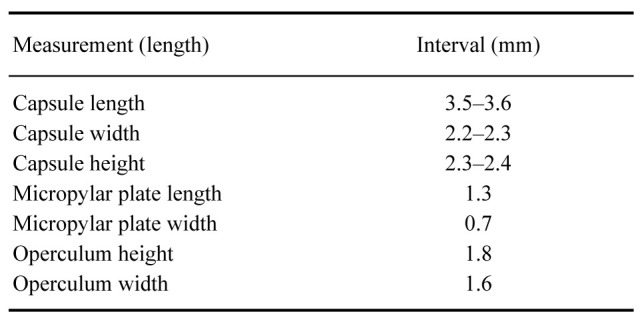
Fig. 12.
Head and prothorax of ♂ Xerosoma nannospinus sp. nov. paratype MZUSP 1441. A: head and prothorax in lateral view. B: dorsal view; C: ventral view. Scale bar = 2.5 mm.
Fig. 13.
Wings of ♂ Xerosoma nannospinus sp. nov. paratype MZUSP 1441. A: dorsal view with wings opened. B: dorsal view of tegmina closed. Scale bars:A= 1 cm; B = 3 mm.
Fig. 14.
Posterior region of the abdomen of ♂ Xerosoma nannospinus sp. nov. paratype MZUSP 1441. A: dorsal view. B: lateral view. C: ventral view. C: detail of vomer and paraprocts in ventral view. Scale bars: A, B, C = 5 mm; D = 3 mm.
Head broader, 1.2× longer than wide; eyes smaller, vertex more pointed, and height shorter basally; granulations less pronounced; longitudinal blackened line weaker. Ocelli present, reduced as in X. canaliculatum. Antennae shorter, reaching tergum VI.
Thorax fairly similar to that of X. canaliculatum, only slightly thinner, bearing a pair of pointed dorsal spines near the posterior margin, accompanied by smaller granules dorsally, all of these less pronounced. Mesothorax, metathorax and median segment as in X. canaliculatum.
Tegmina shorter and rounder, about 2× longer than wide, reaching tergum I with shoulder pads less pronounced. Hindwings as in X. canaliculatum, reaching tergum VIII. Venation of tegmina and hindwings fairly similar to that of X. canaliculatum.
Legs similar to those of the X. canaliculatum, except for slightly shorter anterior pair.
Abdomen 1.6× longer than the combined length of the head, thorax and median segment. Terga in the same proportions as in X. canaliculatum, lateral terga keels weakly projected to the posterior region, less pronounced. Apical posterior terga projections also weakly projected, less pronounced. Cerci shorter and broader. Tergum X with narrower projections and posterior ventral margin as in X canaliculatum. Epiproct and paraprocta as in X. canaliculatum. Poculum (posterior portion of sternum IX) dorsoventrally flattened, posterior margin slightly more truncated.
Color: Similar to that of X. canaliculatum, but less colorful, presenting only shades of brown, gray, cream, black, and white.
Nymph, fourth instar: Fairly similar in color to the female holotype, general structures partly developed.
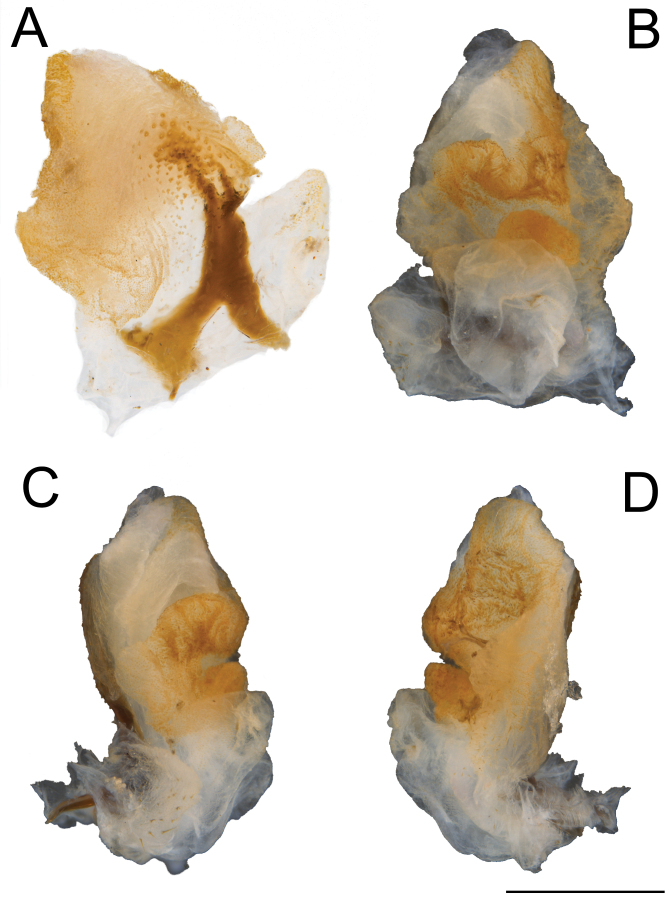
Genitalia (phallic organ): (Fig. 15). General composition and morphology of lobes similar to those of X. canaliculatum. Dorsal sclerite shorter and broader, bifurcated basally, irregularly fanning out to the membrane apically and slightly tilting to the right side. Apical dorsal surface with several circular sclerotized granules, larger than those in X. canaliculatum.
Fig. 15.
Male genitalia of Xerosoma nannospinus sp. nov. paratype MZUSP 1441 treated with KOH. A: latero-dorsal view. B: ventral view. C: lateral view. D: lateral view. Scale bar = 1 mm.
Xerosoma canaliculatum Serville 1831
(Figs. 16–48)
Fig. 16.
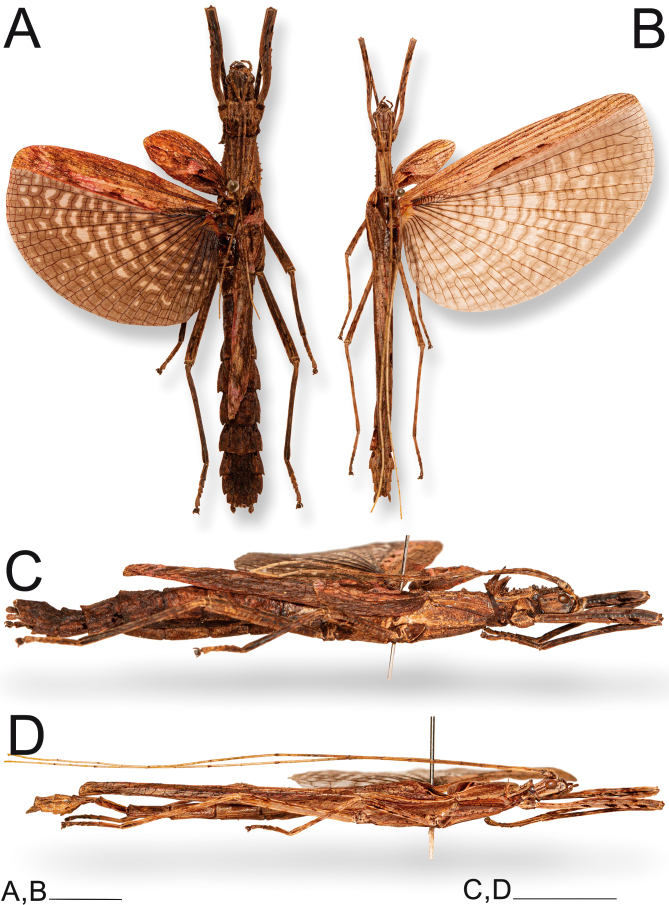
Mounted ♀ and ♂ Xerosoma canaliculatum specimens from Bertioga, São Paulo, Brazil. A: dorsal view of ♀. B: dorsal view of ♂. C: lateral view of ♀. D: lateral view of ♂. Scale bars = 1 cm.
Xerosoma canaliculatum Serville 1831 (61); Gray 1835 (27); Serville 1838[1839] (3) pl. 6, Fig. 3; Burmeister 1840 (40); Blanchard 1840 (18); Westwood 1859 (104); Stål 1875a (100); Kirby 1904 (416); Redtenbacher 1906 (9); Klante 1969 (3); Zompro 2004b (93); Otte and Brock 2005 (342); Araujo and Garraffoni 2012 (236); Delfosse et al. 2019 (193).
Dinelytron neptuna Kaup 1871 (41); Kaup 1871 (23); Kirby 1904 (414) [Prexaspes neptunus]; Redtenbacher 1906 (131) [Harupuna neptunus]; Zompro 2001 (138), Fig. 13 [Harupuna neptuna]; Zompro 2004b (93) [X. canaliculatum].
Xerosoma senticosa Stål 1875a (99); Kirby 1904 (416); Redtenbacher 1906 (144), pl. 5:10 [X. senticosum]; Brock 1998 (57); Zompro 2004a (112) Otte and Brock 2005 (343); Araujo and Garraffoni 2012 (236). syn. nov.
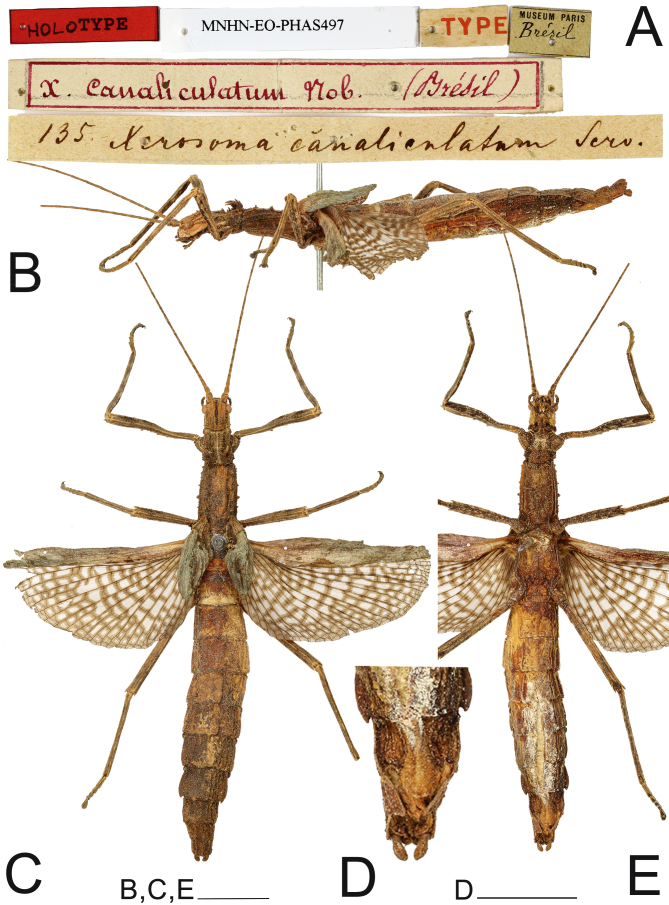
Examined material: 1♀ (MNHN-EO-PHAS497): “Brésil, 135” (holotype, examined by photo); 1♂ (MNHN) “Brasilien” (examined by illustration); 1♀ (MNW 239): Brazil, Bahia, “Xerosoma senticosa, Coll. Br. V. W., Coll. Sommer” (holotype of Xerosoma senticosum, examined). 1♂ (MNW 7654): Brazil, Bahia, “Coll. Br. V. W., Coll. Sommer” (same coll. and locality as the holotype of Xerosoma senticosa, examined); 1♀ (SMFD (examined by photo)): “Brasilien”; 1 egg (HLDL (examined by illustration)); 1♂ (MZUSP 799): Brasil, Bahia, Parque Nacional do Pau Brasil, 16°28'24"S, 39°16'20"W, 18–19. XI.2016, P. I. Chiquetto‐Machado, J. P. Constantini, N. C. C. P. Barbosa. 2♂1♀ (MZUSP 751, 764, 845): Brasil, Espírito Santo, Reserva Biológica Córrego Grande, 18°14'32"S, 39°40'00"W, 20–21.XI.2016, P. I. Chiquetto‐Machado, J. P. Constantini, N. C. C. P. Barbosa. 1♂1♀ (MZUSP 489, 490): Brasil, Minas Gerais, Parque Nacional do Caparaó, Núcleo Alto Caparaó, 20°25'10"S, 41°50'53"W, 30.xi-1.xii.2015, P. I. Chiquetto‐Machado, A. Z. Ramin, J. P. Constantini. 1♂ (MZUSP 851) Brasil, Rio de Janeiro, Parque Nacional de Itatiaia, 22°25'35"S, 44°37'05"W, 31.x-2.xi.2016, P. I. Chiquetto-Machado. 2♂ (MZUSP 1203, 1204): Brasil, Espírito Santo, Aracruz, Itaparica, 9–11.i.2019, E.B. Crispino, E. Travassos Júnior, P.W. Engelking, I.M. Cunha, S. Harumi. 5♂, 5♀, eggs, nymphs (MZUSP): raised in captivity by V. M. Ghirotto, origin: Brasil, Espírito Santo, Aracruz. 1♀ (last instar nymph) (MZUSP 389): Brasil, Espírito Santo, Reserva Biológica de Sooretama, 19°03'15"S, 40°08'48"W, 24–26.xi.2014, P.I.C. Machado, T.F. Carrijo, R.G. Santos; 2♂1♀ (INPA) Brasil, Espírito Santo, Reserva Vale, R. Heleodoro.
Type condition: (Fig. 17). The type ♀ is very well preserved. Ventrally, it is possible to see that the specimen had its abdomen filled with cotton through an incision, which made it impossible to see the preopercular organ. The wings are opened.
Fig. 17.
♀ Xerosoma canaliculatum holotype. A: labels. B: lateral view. C: dorsal view D: end of abdomen in ventral view. E: ventral view. Scale bars: B, C, E = 1 cm; D = 5 mm.
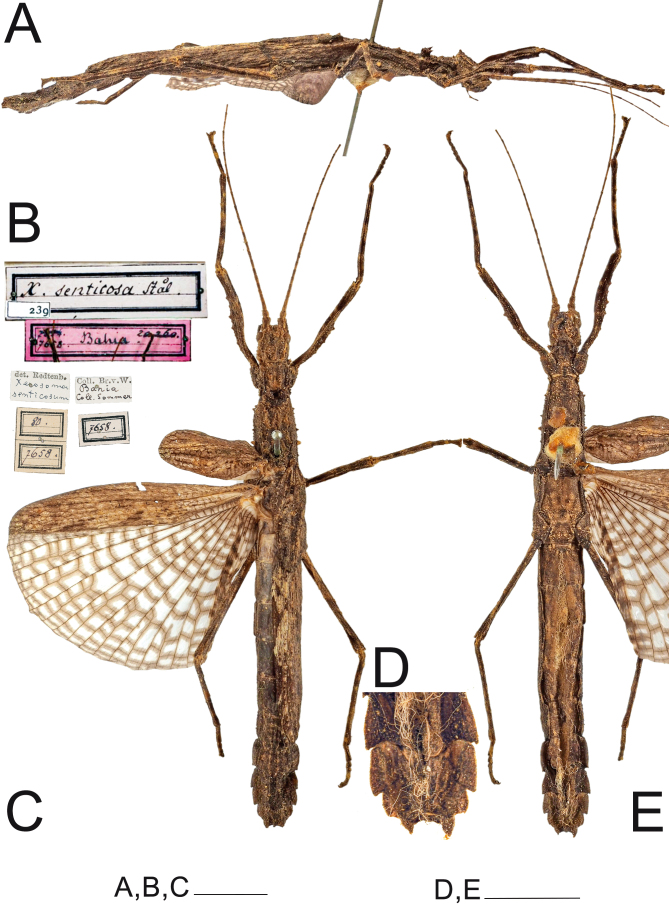
Remarks: Xerosoma senticosum (Stål, 1875) is solely represented by the female holotype, deposited in NMW (Fig. 18). However, a male also from NMW from the same locality and collector label, highly likely from the same population as the X. senticosum holotype was also analyzed (Fig. 19). The only observable difference of this material from most Xerosoma canaliculatum is the absence of the black longitudinal line on the head of the female. As this line appeared poorly marked in some X. canaliculatum female specimens, in addition to all other morphological characteristics matching those of X. canaliculatum, we synonymized Xerosoma senticosum (Stål, 1875) syn. nov. under Xerosoma canaliculatum. The etymology of X. senticosum was not given by Stål (1875a), however, the epithet likely comes from the Latin words “sentis” and “cosa”, which mean thorns and hip, respectively, probably referring to the “lateral projections on the last terga”, as mentioned by Stål (1875a). Based on current knowledge and considering that no voucher material was specified, we disregard Bradley and Galil’s (1977) record of X. canaliculatum from Costa Rica.
Fig. 18.
Holotype ♀ of the synonym Xerosoma senticosum (= X. canaliculatum) (NMW). A: lateral view. B: labels. C: dorsal view. D: end of abdomen in ventral view. E: ventral view. Scale bars: A, C, E = 1 cm; D = 5 mm. Photographs provided by Natural History Museum Vienna, NOaS Image Collection /H. Bruckner; published with permission.
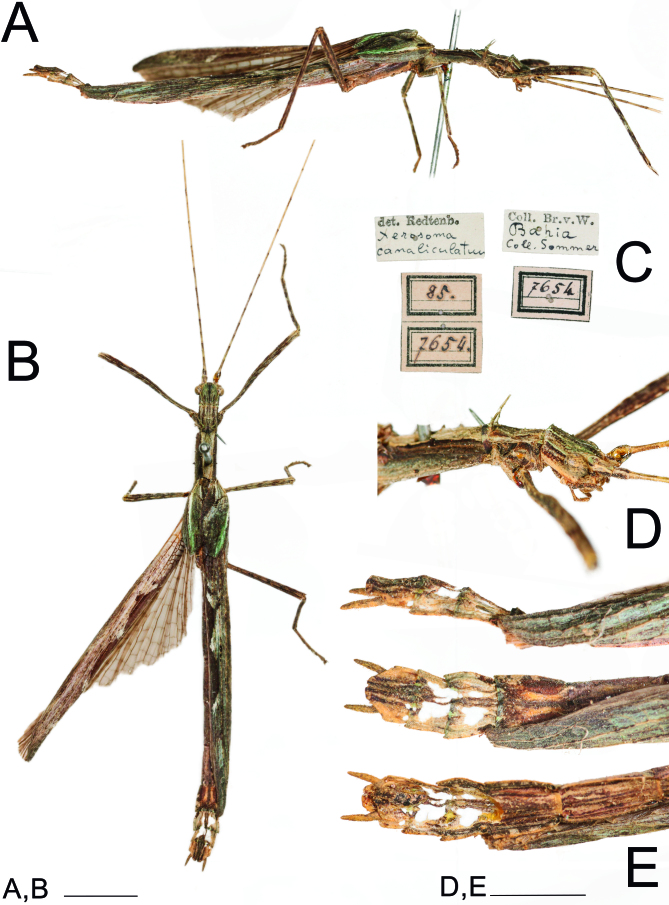
Fig. 19.
Male X. canaliculatum from the same collection and locality as the holotype ♀ of the synonym Xerosoma senticosum (= X. canaliculatum). Photographs taken at the Natural History Museum Vienna. A: lateral view. B: dorsal view C: labels. D: Head, pro-and mesothorax in lateral view. E: end of abdomen in lateral, dorsal and ventral views. Scale bars: A, C, E = 5 mm; D = 2 mm.
Diagnosis: Xerosoma canaliculatum differs from X. michaelis by the fully developed hindwings and the reduced spination on femora I–II. It differs from X. nannospinus sp. nov. by the flattened vertex, larger eyes, stronger spines, longer tegmina, longer wings, lateral terga projections more pronounced, presence of three apical projections on tergum X in ♀ and broad projections on tergum X in ♂, compressed gonapophysis in ♀, longer cerci in ♂, flattened vomer in ♂, longer eggs, and male genitalia with smaller dorsal sclerite basal projection, as the more schlerotized apical of dorsal sclerite.
Comments on the etymology: The etymology as given by Serville (1831) comes from the Latin word “canaliculatum”, which means small canal (canaliculus), referring to the marked longitudinal black line on the head.
Redescription (based on type and additional material).
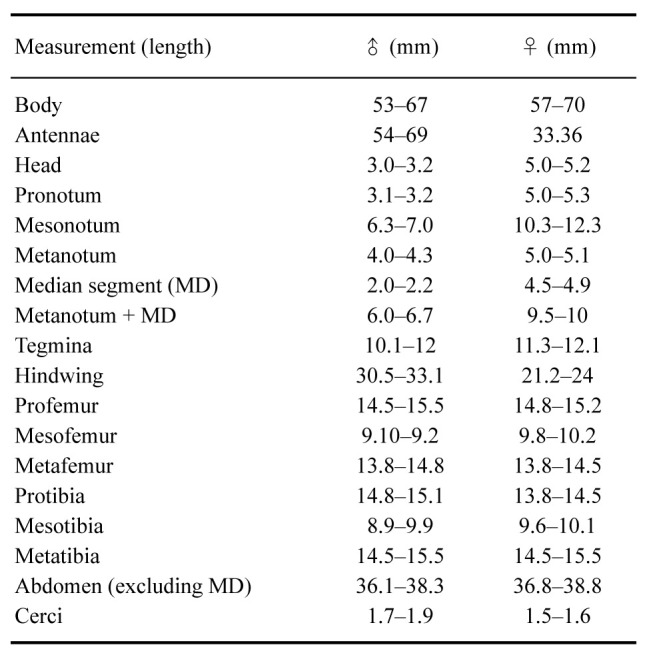
Female: Total body size of 57–70 mm, winged (Figs. 20–24). Measurements are given in table 4.
Table 4.
Measurements (mm) of 10 males and 10 females of Xerosoma canaliculatum
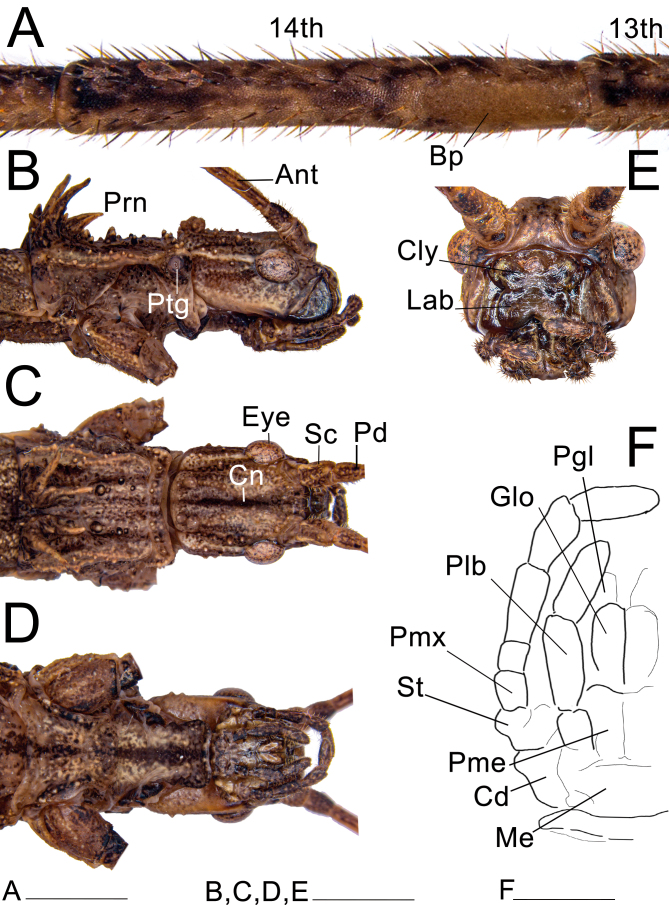
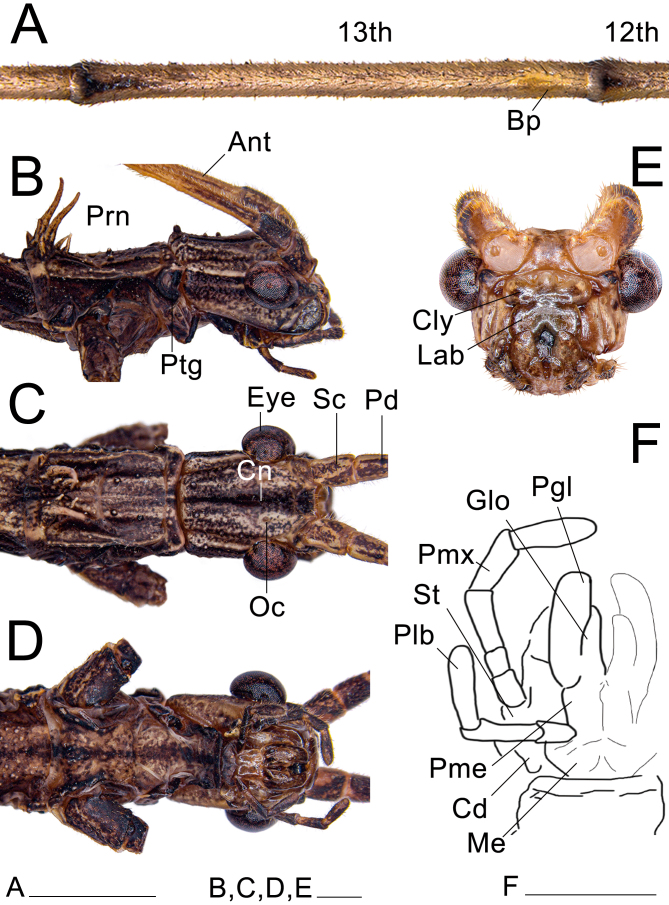
Head: (Fig. 20). Elongate, sub-rectangular in dorsal view, and slightly longer than wide; length and width approximately the same as the prothorax. Vertex slightly convex, with a pronounced longitudinal blackened line (canaliculus), less marked in some few specimens, appearing only as a stain in northern populations or depressed into a sulcus on the posterior half in southern populations. Head with a pronounced pair of carinae basally and 8–10 small granules dorsolaterally. Eyes large, pronounced, slightly elongated, about a third the length of the head. Labial and maxillary palps elongated; when extended, the labial palp slightly exceeds the head, while the maxillary palp entirely exceeds the head. Clypeus convex, with two paramedian deep triangular depressions. Lacinia with three distal teeth; mesal edge bearing a restricted bundle of strong setae. Galea broad, spatulate, bearing rows of setae along the outer lateral and the anterior edge; inner laterally and slightly apical with two dense patches of elongate, stout microtrichia, ventralmost patch long, distributed along the edge of the galea, dorsalmost patch smaller, elliptical; between this patch and the lateral rows of setae on both surfaces, several distinct circular granules around the same diameter of the base of nearby setae. Galealobulus well developed, conical, but only gradually tapering, very elongated, almost as long as the galea. Left mandible with two cutting edges, dorsal undulate and blunt, ventral straight and slightly sharper; right mandible with dorsal cutting edge undulate and blunt, ventral blunter, almost entirely straight, except for a convexity basally; both mandibles with a dense row of large setae at the base of the dorsal margin; mesal surfaces smooth without protuberances. Antennae filiform, reaching tergum III; composed of about 30 antennomeres; covered with fine bristles; scapus compressed dorsoventrally, 1.3× longer than wide; pedicel cylindrical, slightly shorter than scapus; first flagellomere (third antennomere) about 2.2× longer than the pedicel. Antennal bump distinct, present on the 14th antennomere.
Fig. 20.
Head and prothorax of ♀ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: 13th and 14th antennomeres. B: head and prothorax in lateral view. C: dorsal view. D: ventral view. E: head in anterior view. F: schematic drawing of mouth parts in ventral view. Abbreviations: Ant, antennae; Bp, Bump; Cn, canaliculus; Cly, clypeus; Cd, cardo; Eye, compound eye; Glo, glossa; Lab, labrum; Me, mentum; Pd, pedicellus; Pgl, paraglossa; Plb, labial palpus; Pme, prementum; Pmx, maxillary palpus; Prn, pronotum; Ptg, prothoracic gland; Sc, scapus; St, stipes. Scale bars: A = 5 mm; B = 1 cm.
Thorax: (Fig. 21A–C). Pro-and mesothorax sub-quadrate in cross section, with somewhat flat dorsal and lateral surfaces; ventrally bearing small, short setae. Prothorax about 1.7× longer than wide, slightly convex dorsally, with a central transversal groove; anterolateral margins slightly concave outlining the opening of the prothoracic glands. Pronotum bearing a pair of longitudinal, dorsolateral carinae, running from the gland openings and extending almost to the posterior margin, and bearing a pair of large, anteriorly pointed dorsal spines near the posterior margin, accompanied by smaller spines at their enlarged base. Small granules scattered on the dorsal surface and along the dorsolateral carinae of the pronotum. Profurcasternite slightly wider than long. Mesothorax in dorsal view slightly widening towards the posterior region. Mesonotum 1.8× longer than the pronotum, surface slightly rough, with a pair of longitudinal, dorsolateral carinae extending along the anterior two thirds, with a sparse row of 2 to 3 stout, short spines each. Mesepisternum with longitudinal carinae along the ventral margin; medially bearing large and short, stout rounded granules. Mesobasisterum with a longitudinal median carina. Metathorax and median segment smooth; combined length of both structures approximately 1.4× longer than the mesothorax. Metanotum parallel-sided, slightly longer than the mesonotum and 4–5× longer than the median segment. Metepisternum with a thick longitudinal carina along the ventral margin.
Wings: (Fig. 21D, E). Tegmina distinctly convex, 2.2× longer than wide, reaching tergum I, projecting dorsolaterally into large shoulder pads slightly pointing forward, venation pattern latticed. Costal area with Sc, R, Rs1, Rs2, and Ma veins reaching apical margin; Mp shorter, directed to the Cu margin. Anal area very reduced, A1 short, following the Cu margin, spanning the first third of the length of the tegmina. Hindwings fully developed, reaching or exceeding tergum V. Costal area with Sc vein ending in C margin, spanning around 75% the total length of the costal area; veins R1, Rs1, Rs2, Ma, and MP reaching the apical Cu margin. Anal area tesselate and fan-shaped, with 7 Aa veins and 8 Pa veins gradually decreasing in length.
Fig. 21.
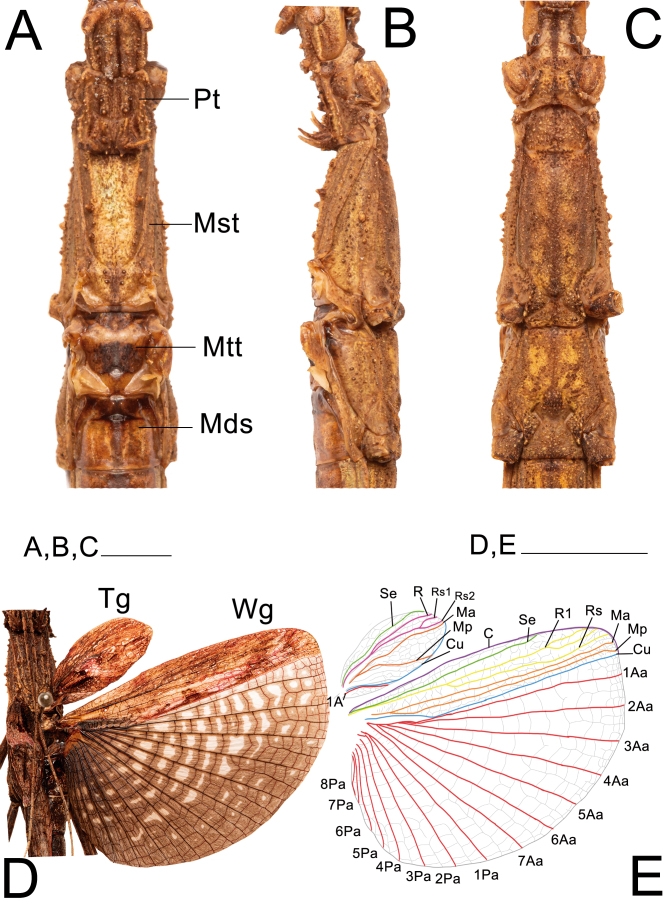
Thorax and wings of ♀ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: dorsal view. B: lateral view. C: ventral view. D: open wings in dorsal view. E: schematic drawing of wing venation. Abbreviations: C, costa vein; Cu cubitus vein; Sc, subcosta vein; R1, radius 1 vein; Rs, radial sector vein; MA, media anterior vein; Mds, median segment; MP, media posterior vein; Mst, mesonotum; Mtt, metanotum; Pt, pronotum; Tg, tegmen; Wg, wing; 1A, first anal vein; 1AA–7AA, first to seventh anterior anal veins; 1PA–8PA, first to fifth posterior anal veins. Scale bars = 1 cm.
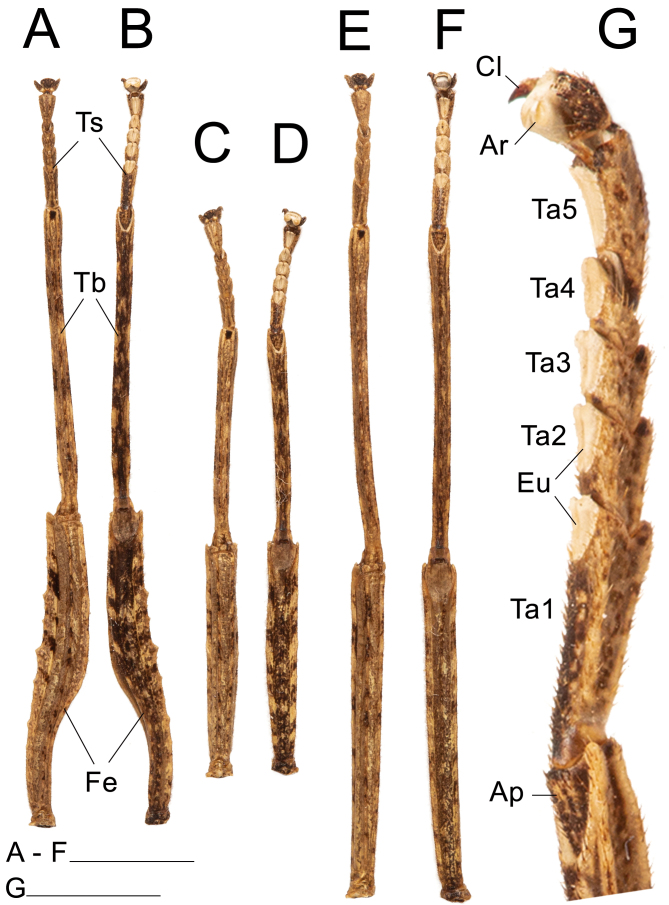
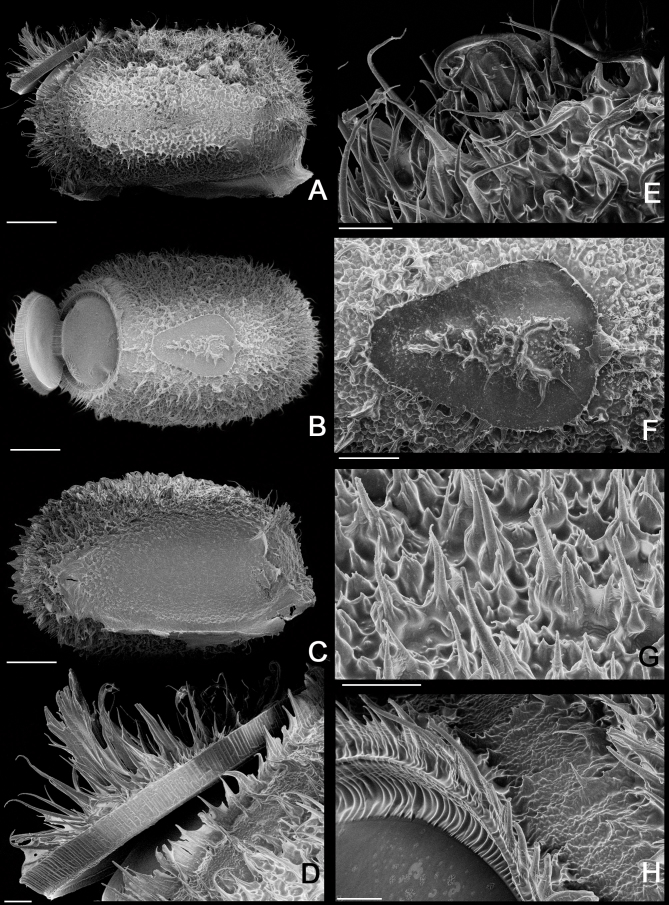
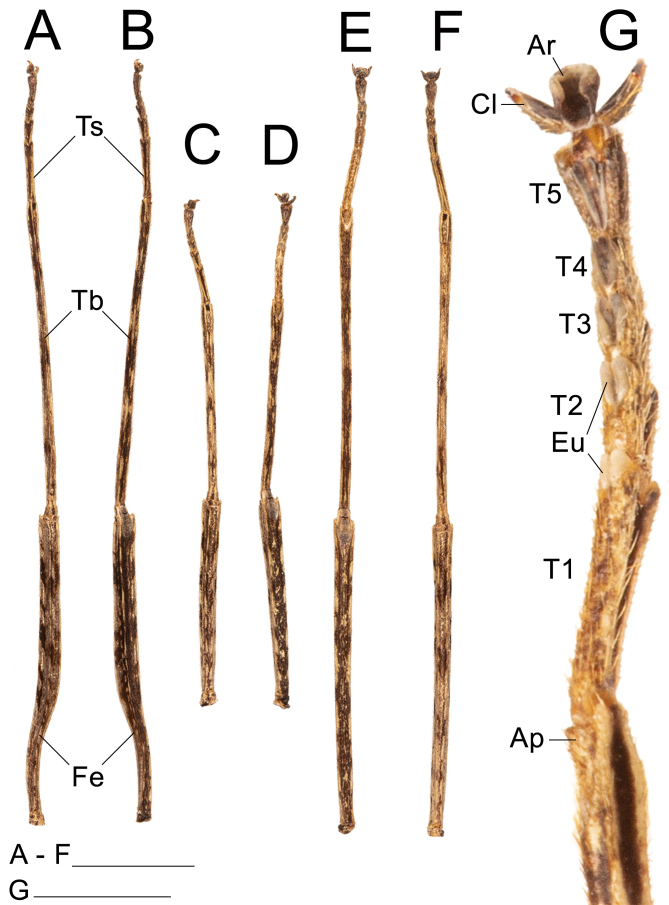
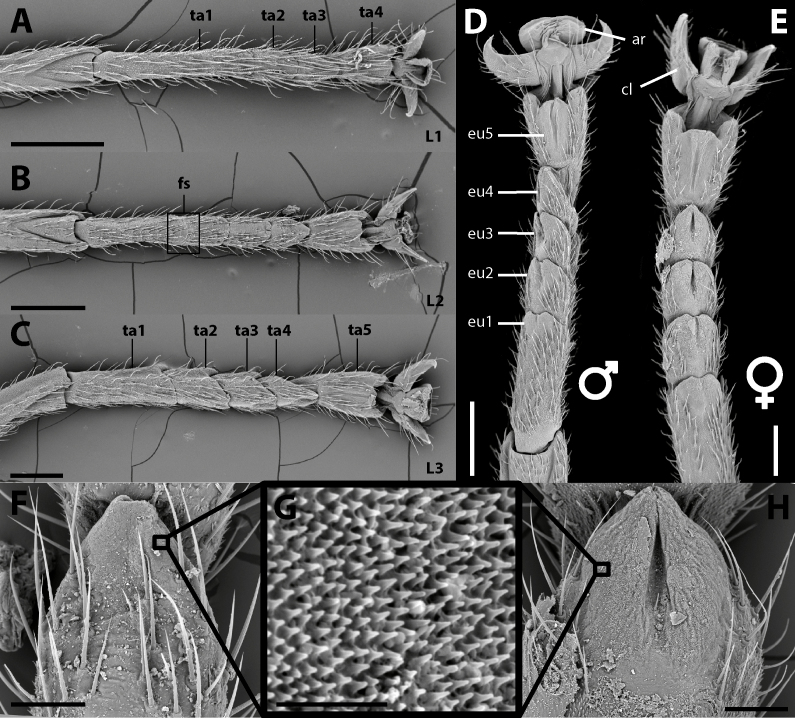
Legs: (Fig. 22). Femora and tibiae rectangular to trapezoidal in cross section, all carinae pronounced bearing one to three rows of short setae. Profemora with basal curvature and laterally compressed, bearing some small acuminate lobes in the ventral-posterior carinae. Area apicalis well developed, densely covered by setae. Tibiae slightly longer than the corresponding femora and 2.5× longer than the corresponding tarsi. Pro-and metabasitarsus slightly longer than the following two corresponding tarsomeres combined; mesobasitarsus relatively shorter, around the same length of the following two tarsomeres combined. Euplantulae strongly developed at the distal ends of tarsomeres 1–4, hemispherical and bilobed. Tarsomere 5 bearing an elongate accessory euplantula. Euplantulae covered with small conical protrusions (nubs). Praetarsus equipped with a large arolium and two curved claws without pectination.
Fig. 22.
Left legs of ♀ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: front leg in dorsal view. B: front leg in ventral view. C: mid leg in dorsal view. D: mid leg in ventral view. E: hind leg in dorsal view. F: hind leg in ventral view. G: front tarsus in lateral view. Abbreviations: Ap, area apicalis; Ar, arolium; Cl, claw; Eu, euplantula; Fe, femur; Ta1–Ta5, tarsomere; Tb, tibia; Ts, tarsus. Scale bars: A–F = 1 cm; G = 5 mm.
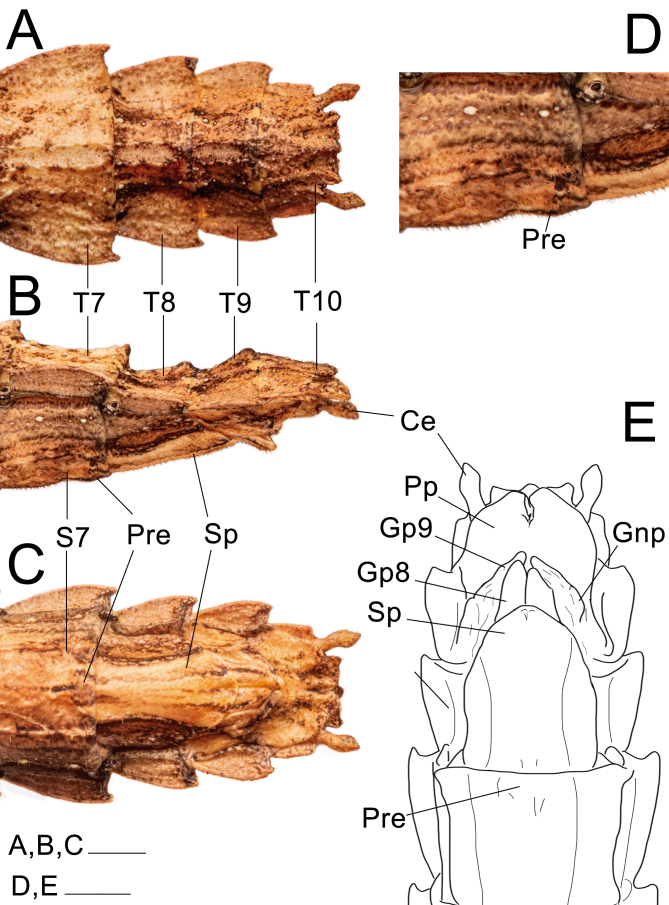
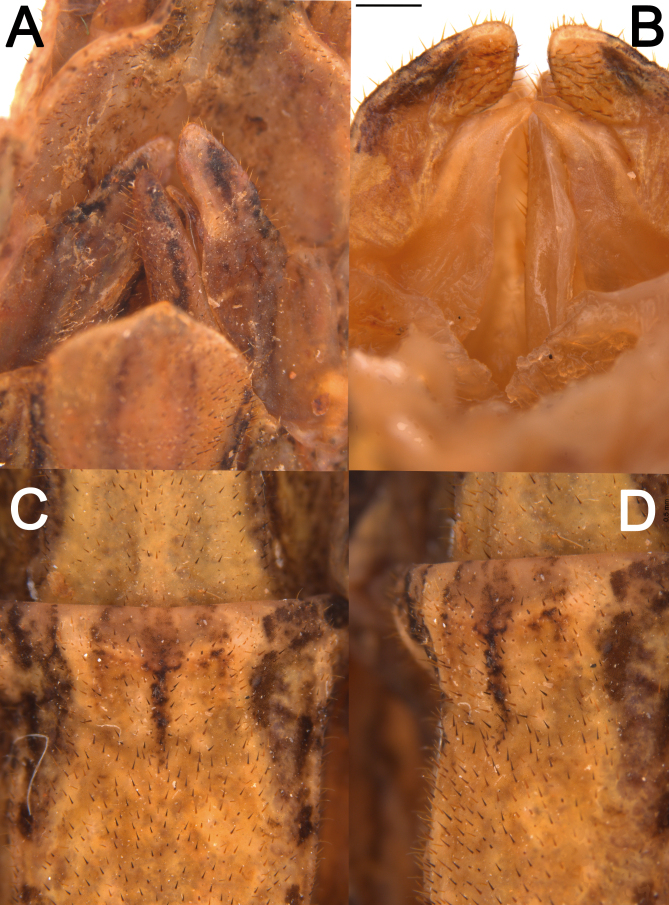
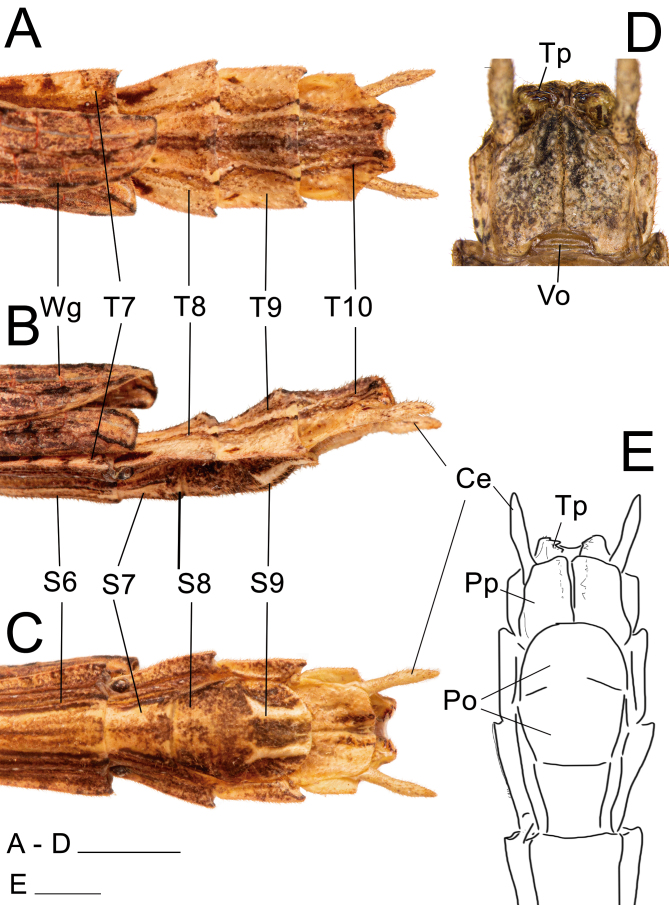
Abdomen: (Figs. 23 and 24). Approximately 1.2× longer than the combined length of the head, thorax and median segment. Dorsoventrally flattened, mainly towards the posterior margin, ventrally bearing short dark setae. Terga VIII–IX with a smooth medial hump near the posterior margin and X with a short and discrete carina near the posterior margin. Posterior margin of tergum X crenate. Sternum VIII (subgenital plate) barely reaching the median length of IX, slightly longer than wide. Epiprocts small, triangular, hidden under tergum X in dorsal view. Paraprocts wide, both briefly separated from each other near the posterior margin. Cerci clavate, apex conical. Praeopercular organ wide, short, shown as a wide elevation preceded by a slight, thin transversal depression and followed by a glabrous thicker depression continuous with the anterior margin. Gonapophyses VIII wide, conical in ventral view, not totally covered by the subgenital plate; surface similar to that of surrounding body areas and similarly bearing setae; gonapophyses IX fused with gonoplac for most of its length, then short, slightly thin, conical, shiny, bearing few setae apically, hidden from ventral view atop gonapophyses VIII and gonoplac. Gonoplac well developed, dorsoventrally flattened, surface similar to that of gonapophyses VIII, longer than both gonapophyses, apically elliptical. Gonangulum absent.
Fig. 23.
Posterior region of the abdomen of ♀ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: dorsal view. B: lateral view. C: ventral view. D: 7th sternum in lateral view. E: schematic drawing in ventral view. Abbreviations: Ce, cerci; Gnp, gonaplac; Gp8, 8th gonapophyses; Gp9, 9th gonapophyses; Pre, preopercular organ; Pp, paraproct; Sp, subgenital plate; S7, 7th sternum; T7–T10, terga. Scale bars: A–C = 1 cm; D, E = 5 mm.
Fig. 24.
Detail of genital structures of ♀ Xerosoma canaliculatum from Aracruz, Espírito Santo, Brazil. A: detail of gonapophyses, gonoplac and subgenital plate in ventral view. B: detail of gonapophyses and gonoplac in dorsal view. C: praeopercular organ in ventral view. D: praeopercular organ in lateral view. Scale bars = 5 mm.
Variations: Some individuals presented a slightly longer and more acute subgenital plate, especially those from Espírito Santo and Bahia states (northern known range of the species) in comparison with those of São Paulo state in the southern range. The cerci also vary from more to less pointed. The holotype bears a slightly rounder cerci (Fig. 17D). The hindwings can be more or less marked with tessellate black stripes and vary slightly in length.
Color: Body mostly stained with irregular shades of brown, complemented by black and white spots and may have green, yellowish or reddish stains. Posterior region of the head, prothorax and mesothorax light brown or yellowish to creamish, lighter than the surrounding areas. Head may have a dorsal longitudinal black line; lateral region of the head, prothorax and mesothorax bearing a dark brown band. Metathorax dorsally black. Abdomen dorsally darker in the first 5 segments covered by the wings and laterally brownish; terga VII–VIII with lateral black spots. Antennae similar in color as the rest of the body, becoming lighter from the 7th antennomere. Costal area of tegmina irregularly brownish with lighter and darker spots and anal region with black spots; ventrally reddish basally. Hindwings with costal area similar to the tegmina and including a red stain basally; anal area with light, translucent cells centrally and near the costal area, area near veins brownish, veins dark brown to blackish. Legs in different shades of brown similar to the body, but with black spots mainly on the anterior femora.
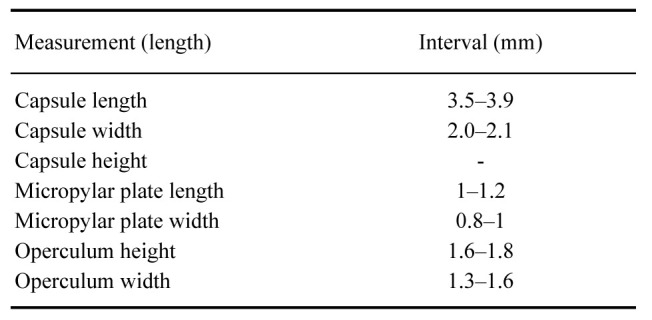
Eggs: (Figs. 25, 26). As for the genus. Elongater than those of X. nannospinus. Micropylar plate rounded to rectangular or triangular. Capsule with medium size hairy setae, smaller than those of X. nannospinus.
Fig. 25.
Egg of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: dorsal view. B: lateral view. C: ventral view. D: anterior view showing the operculum. E: micropylar plate in dorsal view. F: operculum in dorsal view. G: schematic drawing of the micropylar plate. Abbreviations: Op, operculum; Mp, micropylar plate. Scale bars: A–D = 2 mm; F = 2 mm; E, G = 2 mm.
Fig. 26.
Egg of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil under scanning electron micrographs (SEM). A: lateral view. B: dorsal view. C: ventral view. D: operculum in lateral view. E: micropylar cup in lateral view. F: micropylar plate in dorsal view. G: detail of capsule surface. H: operculum rim. Scale bars:A, B, C = 500 μm; D, E, F, G, H = 100 μm.
Male: Body size of 53–67 mm, thinner, less rough, and less granulated than the female; fully winged (Figs. 27–31). Measurements are given in table 4.
Table 5.
Measurements (mm) of 10 eggs of Xerosoma canaliculatum
Head: (Fig. 27). Similar to that of the female, except: with fewer and less pronounced granulations; eyes larger. Ocelli reduced; antennae longer, almost as long as the body; both maxillary and labial palps more elongated; fewer setae on the mesal surface of the lacinia; galea shorter and thicker basally, with the area bearing the microtrichia projected (Fig. 28E); microtrichia of the galea similar, but with shorter patches than those of the female; galealobulus shorter and thicker. Antennal bump on the 13th antennomere.
Fig. 27.
Head and prothorax of ♂ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: 12th and 13th antennomeres. B: head and prothorax in lateral view. C: dorsal view. D: ventral view. E: head in anterior view. F: schematic drawing of the mouth parts in ventral view. Abbreviations: Ant, antennae; Bp, Bump; Cn, canaliculus; Cly, clypeus; Cd, cardo; Eye, compound eye; Glo, glossa; Lab, labrum; Me, mentum; Oc, oceli; Pd, pedicellus; Pgl, paraglossa; Plb, labial palpus; Pme, prementum; Pmx, maxillary palpus; Prn, pronotum; Ptg, protoraxic gland; Sc, scapus; St, stipes. Scale bars = 1 cm.
Fig. 28.
Mouth parts of Xerosoma canaliculatum from Aracruz, Espírito Santo, Brazil. A, B: maxilla of ♀ in ventral view, right maxilla dissected in B. C, D: mandibles of ♀ opened to show internal view. E: internal (dorsal) view of maxilla of ♂. F: two left side maxillae of two ♀, right one in internal view and left one in external (ventral) view. G, H: detail of the apex of the galea apex, left maxilla of ♀, antero-internal view (G) and antero-internal view at right side and dorsal view at left side (H). Scale bars: A–F = 5 mm; G–H = not to scale.
Thorax: (Fig. 29). Similar to that of the female, except: thinner and more elongate; surface less granulated; ventral setae thinner, longer, and lighter in color; pair of spines on the pronotum thinner and bearing only few granules basally; profurcasternite longer than wide.
Wings: (Fig. 29). Similar to that of females, except: slightly elongated. Also presenting Rs subdivided into Rs1 and Rs2, reaching the tegmina apical costal area.
Fig. 29.
Thorax and wings of ♂ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: dorsal view. B: lateral view. C: ventral view. D: open wings in dorsal view. E: schematic drawing of wing venation. Abbreviations: C, costa vein; Cu cubitus vein; Sc, subcosta vein; R1, radius 1 vein; Rs, radial sector vein; MA, media anterior vein; Mds, median segment vein; MP, media posterior vein; Mst, mesonotum; Mtt, metanotum; Pt, pronotum; Tg, tegmen; Wg, wing; 1A, first anal vein; 1AA–7AA, first to seventh anterior anal veins; 1PA–8PA, first to fifth posterior anal veins. Scale bars = 1 cm.
Legs: (Fig. 30). Similar to those of the female, except: slightly longer and more slender, profemora only gently compressed laterally with a weaker basal curvature. Tibiae slightly longer than the corresponding femora and 2.5× longer than the corresponding tarsi. Pro-and metabasitarsus slightly shorter than the respective following four tarsomeres combined; about the same length as the respective following three tarsomeres combined. Euplantulae strongly developed at the distal ends of tarsomeres 1–4, hemispherical and bilobed. Tarsomere 5 bearing an elongated accessory euplantula. Euplantulae covered with small conical protrusions (nubs). Praetarsus equipped with a large arolium and two curved claws without pectination.
Fig. 30.
Left legs of ♂ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: front leg in dorsal view. B: front leg in ventral view. C: mid leg in dorsal view. D: mid leg in ventral view. E: hind leg in dorsal view. F: hind leg in ventral view. G: front tarsus in lateral view. Abbreviations: Ap, area apicalis; Ar, arolium; Cl, claw; Eu, euplantula; Fe, femur; Ta1—Ta5, tarsomere; Tb, tibia; Ts, tarsus. Scale bars: A–F = 1 cm; G = 5 mm.
Abdomen: (Fig. 31). Dorsally glabrous under wings and bearing keels in the posterior, exposed region, but in comparison to the female ventral setae are thinner and longer, light in color. Similar to that of the female, except: approximately 1.5× longer than the combined length of the head, thorax, and median segment; terga II–VI equal in length, parallel-sided, about 1.8× longer than wide; VII slightly shorter than VI; VII–X keeled, VII slightly longer than wide; VIII–IX slightly wider than long, convex; X slightly wider than long, slightly tapering from the mid-length to the posterior margin. Terga VIII–IX bearing a small medial round hump near the posterior margin pointing backwards. Cerci elongated, slightly flattened laterally from middle to apex. Thorn pads along the posterior ventral margin of tergum X with few short, strong dark thorns. Anterior portion of sternum IX short, not surpassing the posterior end of VIII; poculum (posterior portion of sternum IX) dorsoventrally flattened, posterior margin truncated.
Fig. 31.
Posterior region of the abdomen of ♂ Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: dorsal view. B: lateral view. C: ventral view. D: detail of vomer and paraprocts in ventral view. E: schematic drawing in ventral view. Abbreviations: Ce, cerci; Po, poculum; Pp, paraproct; S6–S9, sternum; Tp; thorn pads; T7–T10, terga; Vo, vomer; Wg, wing. Scale bars: A, B, C, E = 1 cm; D = 5 mm.
Color: Similar to that of females, except: more frequently brown; fewer individuals greenish; mesonotum with a more contrasting wide, longitudinal beige band than that of the female.
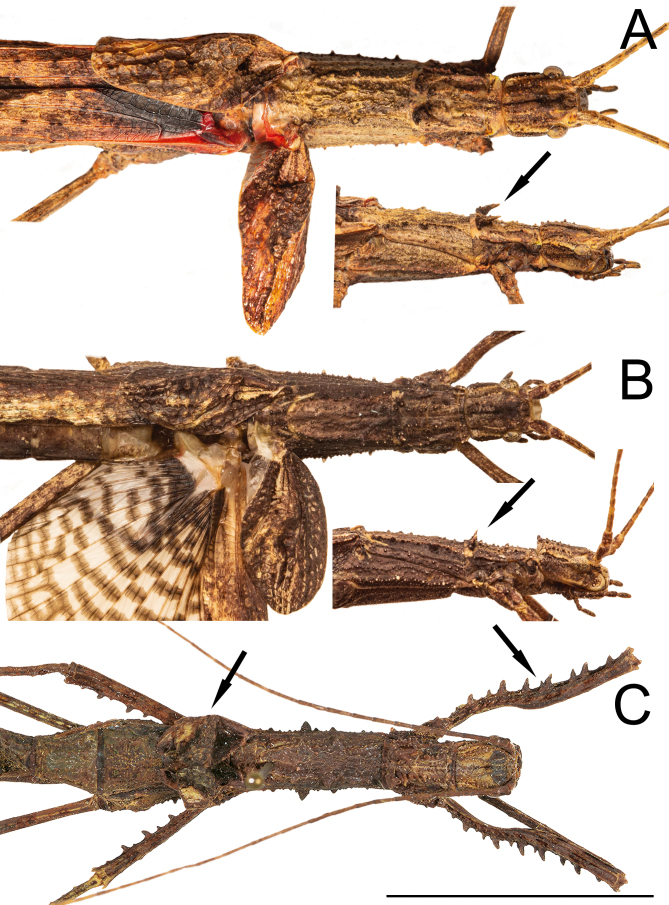
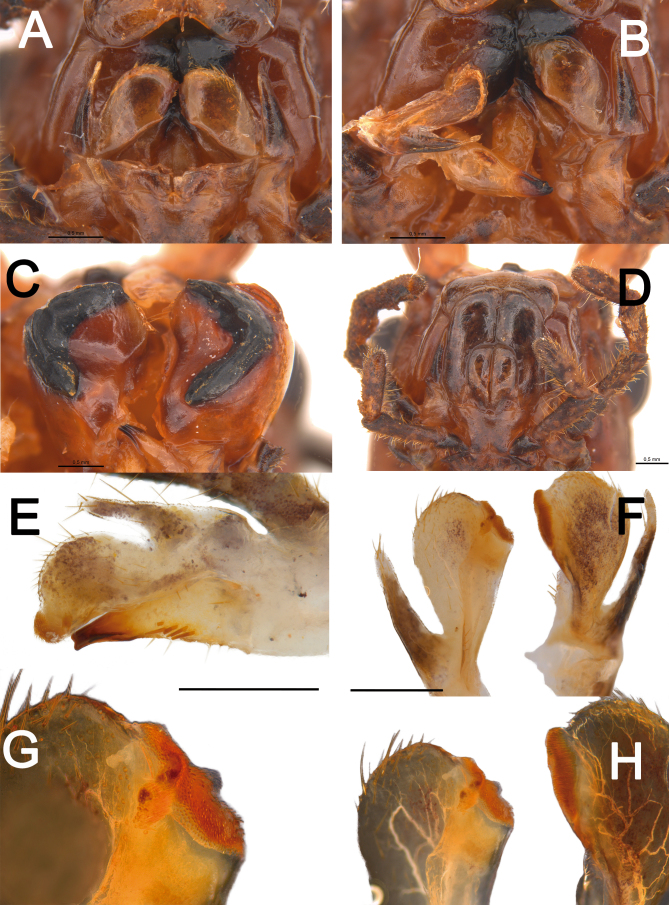
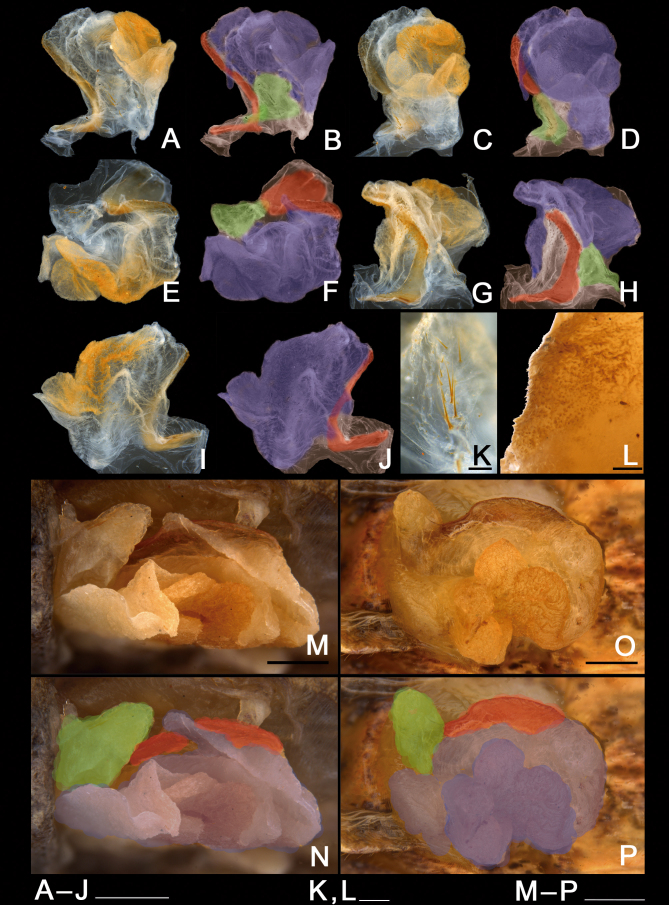
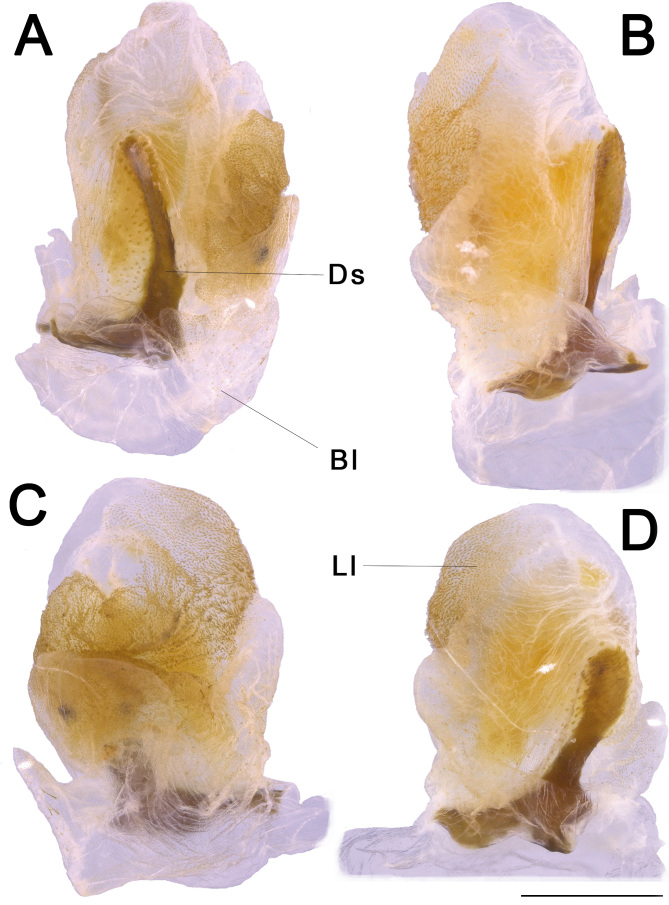
Genitalia (phallic organ): (Figs. 32–33). Dorsal sclerite elongated, bending towards the left; posterior margin from its wide attachment area connected to the dorsal wall of the body and continuing to the left, slightly curved towards the right side (Fig. 33A). Both the membrane covering the right side of the wall of the genitalia and the dorsal sclerite surface itself are dorsally covered with circular sclerotized granules. Longitudinal lobe with around three large folding parts (Fig. 32B). Great area of the longitudinal lobe in the central and right sides densely covered with minute, spiny conical setae (Fig. 32L), conferring a yellow appearance to the membrane in a cleared genitalia (Fig. 32A–J). Right side of the lobe basally bearing around a dozen large, straight, long bristles varying in size (similar to those in Fig. 32K). Basal (left) lobe short and folding within the longitudinal lobe in untreated genitalia and before dissection (Fig. 32M–P), bearing around ten large, straight, long bristles (similar to those of the longitudinal lobe) of different sizes at the ventro-lateral right edge (Fig. 32K).
Fig. 32.
Male genitalia of Xerosoma canaliculatum from Aracruz, Espírito Santo, Brazil. A–L: genitalia treated with KOH. M–P: untreated genitalia attached to the body. B, D, F, H, J: same images as respective previous. N, P: same image as respective above, all artificially colored for structure visualization: red, dorsal sclerite; purplish blue, longitudinal lobe; green, basal lobe. A–B: lateral view from left side. C–D: ventral view. E–F: posterior view. G–H: dorsal view I–J: lateral view from right side. K: detail of setae of the basal lobe. L: detail of minute setae of the longitudinal lobe. M–N: genitalia of specimen preserved in alcohol attached to the body in ventral view. O–P: genitalia of recently dead specimen attached to the body in ventral view. Scale bars = 5 mm.
Fig. 33.
Male genitalia of Xerosoma canaliculatum from Reserva Vale, Espírito Santo, Brazil treated with KOH and expanded. A: latero-dorsal view. B: lateral view. C: ventral view. D: dorsal view. Abbreviations: Ds, dorsal sclerite, Ll, longitudinal lobe, Bl, basal lobe. Scale bar = 5 mm.
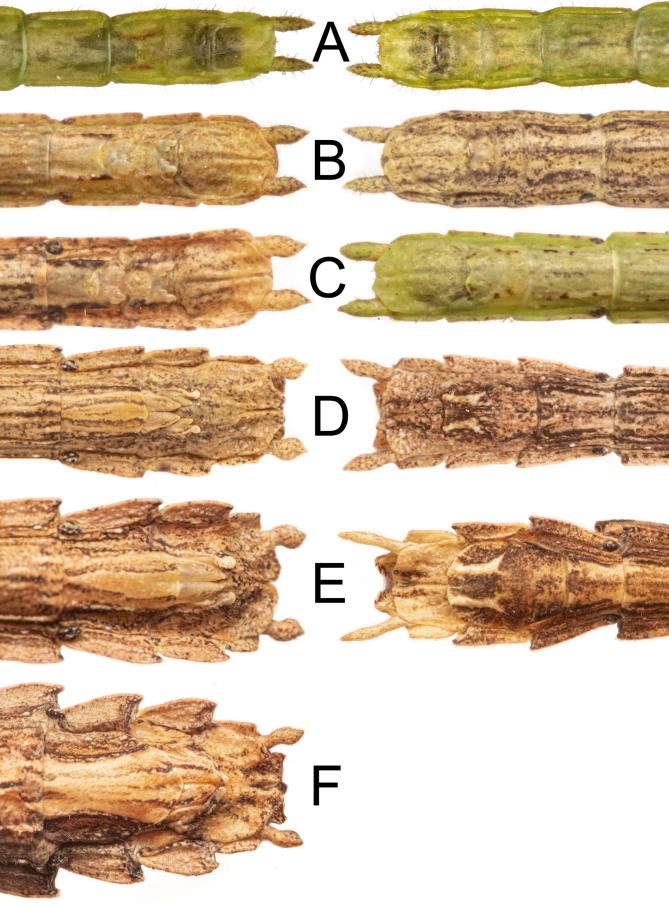
Nymphs: (Fig. 34). First instar nymphs with proportionately large head, smooth body with only very few granules and scattered tiny dark bristles; body green with scattered small black spots and reddish eyes. Nymphs throughout ontogeny gain the adults usual brownish color and the rough and granulate surface with acute spines. Females take 6 instars to become adults, while males take 5 instars (Fig. 35). Nymphs exhibiting sexual dimorphism since the first instar, males bearing a shortened sternum IX (poculum) and females with a shortened sternum VIII (subgenital plate) and budded gonapophyses and gonoplac, similar to Canuleius similis Redtenbacher 1906 (Ghirotto 2021). Gonapophysis IX with the same size as other valves until subadult stage. First instar nymphs with four tarsomeres and inconspicuous euplantulae (Fig. 36). Euplantulae of second instar nymphs larger and with four tarsomeres, but basitarsus showing a faint fissure. Nymphs from the third instar onwards with five tarsomeres. Euplantulae of all instars covered with a nubby microsculpture (Fig. 36).
Fig. 34.
Live nymphs of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: first instar in lateral view. B: first instar in dorsal view. C: third instar of ♂. D: fifth instar of ♀. Scale bars = 1 cm.
Fig. 35.
Ventral view of the posterior region of the abdomen of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil showing development in all instars for both sexes (left ♀, right ♂). A: first instar. B: second instar. C: third instar. D: fourth instar. E: fifth instar. F: sixth instar. Image not to scale.
Fig. 36.
Tarsal morphology of different stages. A: First instar nymph. B: Second instar nymph. C: Third instar nymph. D: Male. E: Female. F: Euplantula 4 of second instar nymph. G: Attachment microstructure on the euplantulae of nymphs and adults. H: Euplantula 4 of the adult female. Abbreviations: ar, arolium; cl, claw; eu 1-5, euplantulae 1 to 5; fs, fissure; L1-3, first to third instar nymphs; ta1-5, tarsomeres 1 to 5. Scale bars: A–E = 500 μm; F = 50 μm; G: 10 μm; H = 150 μm.
Natural history and biological aspects of Xerosoma canaliculatum
Geographic distribution: Xerosoma canaliculatum is recorded from five Brazilian states: Bahia, Espírito Santo, Minas Gerais, Rio de Janeiro, and São Paulo. Habitat: Populations of Xerosoma canaliculatum are recorded from altitudes ranging from 14 meters above sea level (Bertioga, São Paulo state) to 1295 meters (Parque Nacional do Caparaó, Minas Gerais state). They inhabit distinct kinds of phytophysiognomies in the Atlantic Forest, such as Restingas Altas (coastal sandbank forests) (Fig. 37), lowland ombrophilous forests, and submontane ombrophilous forests. In Bertioga, specimens were sighted from near the ground resting in the herbaceous stratum to the forest understory at 6 meters above the ground.
Fig. 37.
Habitat of X. canaliculatum. A: Parque Nacional de Itatiaia, Rio de Janeiro, Brazil B: Reserva Natural SESC Bertioga, São Paulo, Brazil. Images not to scale.
Ecdysis: (Fig. 38). Reared individuals remained about 14 to 18 days in each instar. The duration of the ecdysis processes were approximately 30 minutes. During this process, it was possible for individuals to recover lost appendages (Fig. 39).
Fig. 38.
Xerosoma canaliculatum from Bertioga, São Paulo, Brazil, nymph under ecdysis process from the second to the third instar. A: nymph in second instar, right before molting. B: nymph under ecdysis process (molting). C: freshly molted third instar nymph. Scale bars = 1 cm.
Fig. 39.
Leg regeneration of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: second instar nymph with lost right leg III. B: third instar nymph starting leg regeneration. C: fourth instar nymph with semi-regenerated leg. D, E: ♂ adult (D) with fully regenerated leg with four tarsi instead of five (E). Images not to scale.
Seasonality and longevity: This species appears to be perennial since individuals in all instars were observed during samplings in the rainy period. The lifespan of individuals in captivity and in a controlled environment ranged between 6 and 8 months, with around 3 to 4 months in the nymphal stage and 3 to 4 months in the adult stage. Egg incubation time was approximately 4 to 6 months.
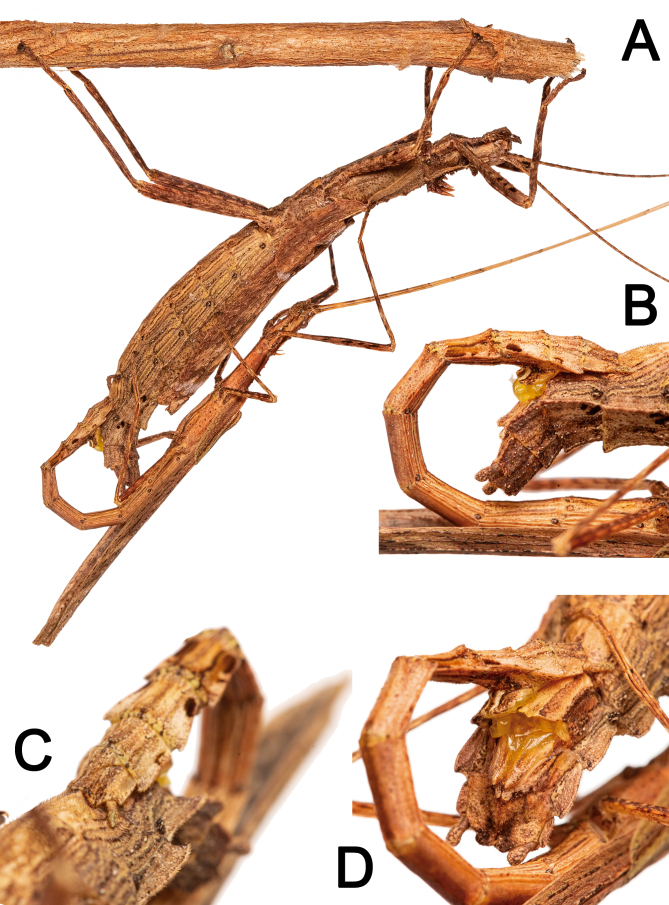
Movement: This species is strictly nocturnal, remaining immobile during the day unless disturbed. Some individuals observed feeding or foraging at night were reencountered during daylight resting in situ (Fig. 40) more than 2 meters away from the feeding site (Fig. 40A). Both in nature and in captivity, they rested during the day in different positions and on several parts of the plants, such as leaves, branches, trunks, dead leaves, or other plant material available (Figs. 40, 41). The most common resting position was with the first pair of legs extended concealing the head, the second pair usually facing forward, flexed at an angle of 45°, and the third pair extended very close to the abdomen. (Figs. 40, 44A). During nocturnal activity most nymphs and adults hanged from the underside of leaves, while some adults, mainly males, were observed on the upper side of leaves, with some males eventually flying away.
Fig. 40.
Xerosoma canaliculatum nymphs from Bertioga, São Paulo, Brazil resting in situ. A: nymph resting on an Araceae food plant stalk. B: nymph resting on an Araceae food plant petiole. C: nymph resting on dead bromeliad leaves. Images not to scale.
Fig. 41.
Xerosoma canaliculatum from Bertioga, São Paulo, Brazil, adults resting on branches and bark. A: two ♀ on a branch. B: ♀ on tree bark. Images not to scale.
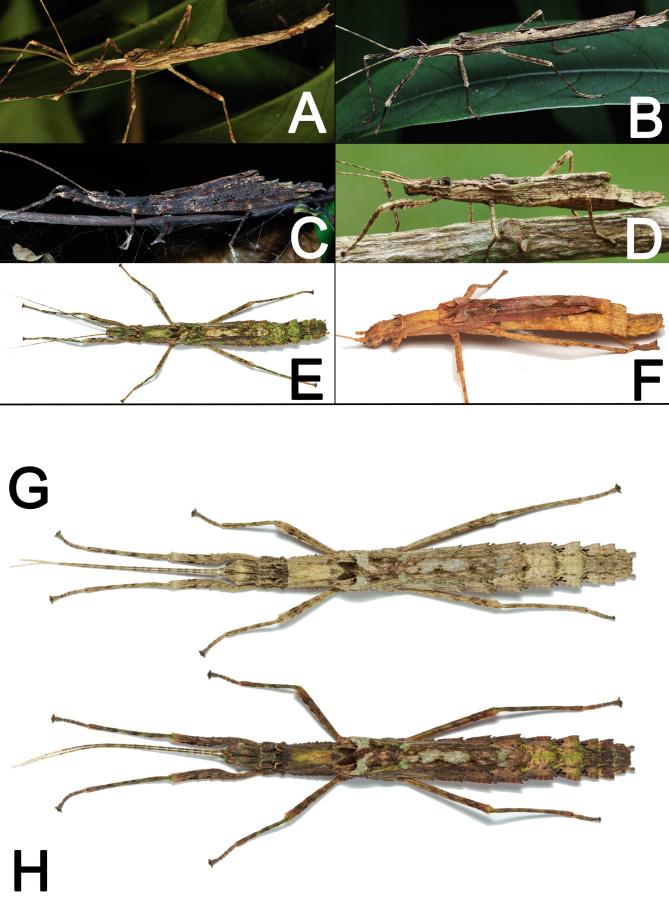
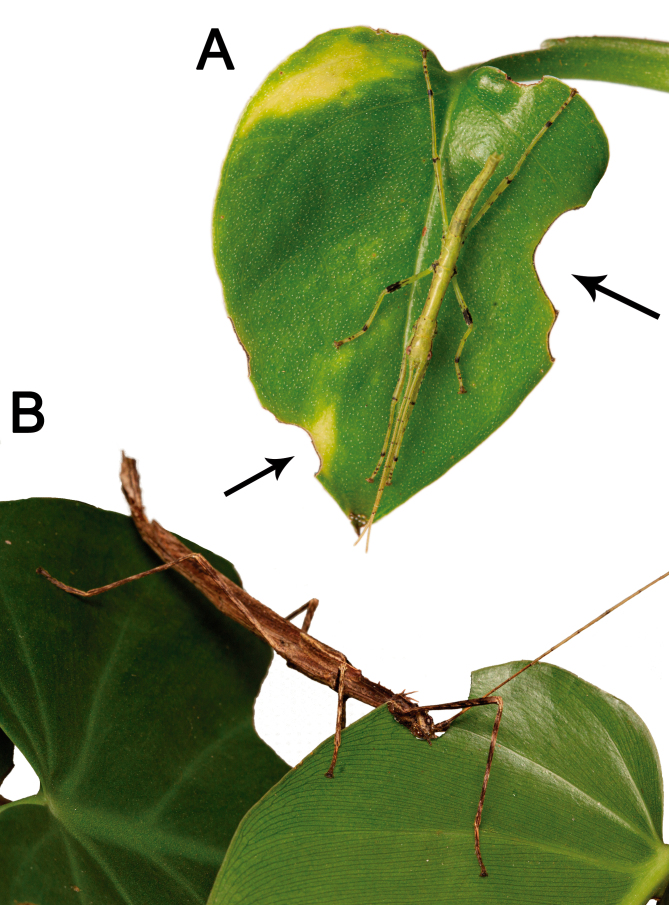
Camouflage: Xerosoma are visually very similar to dry branches, exhibiting different patterns of colors to match their surroundings (Fig. 42A–F). During the day, the insects tend to hide next to thicker branches or bark pieces when available, while at night individuals forage. Active individuals frequently stop moving when exposed to a flashlight. Specimens also vary in color according to the time of day or brightness of the environment, matching tones with their surroundings and perfecting their crypsis (Fig. 42G, H).
Fig. 42.
Adults of Xerosoma canaliculatum from Bertioga, São Paulo (A, E, G, H), from Aracruz, Espírito Santo (F), from Jardim Botânico, Rio de Janeiro (C), and Parque Nacional de Itatiaia, Rio de Janeiro (B, D), in different color patterns. A, B: distinct males. C–F: distinct females. G, H: same female during daylight (G) and during the night (H). Images not to scale.
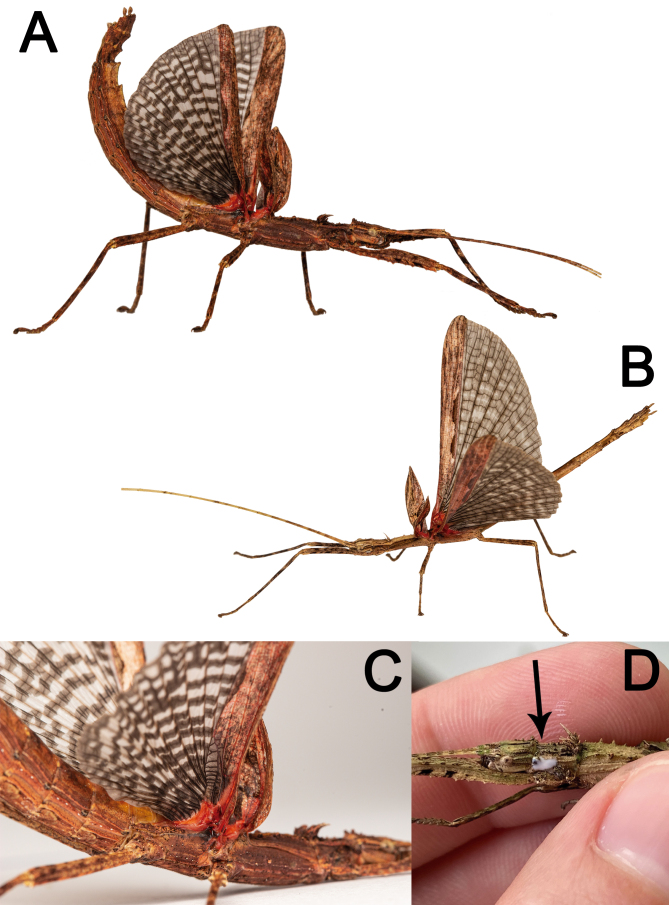
Secondary defense mechanisms: Nymphs mostly exhibit thanatosis when slightly disturbed, falling immobile to the ground with legs held close to the body. When intensely disturbed, i.e., grasped, nymphs always expel an irritating substance from their prothoracic glands. The substance is expelled in a microjet and quickly volatilizes (Fig. 43D). The smell is minty, sour in taste, and causes mild irritation in the airways when inhaled by a person, resulting in coughing or sneezing. Early-stage nymphs frequently autotomize legs when disturbed, probably to minimize damage from an attack (Bedford 1978).
Adults bear red spots at the base of their wings, which can be opened as a deimatic display when they are disturbed. Males and females fully extend their wings, lift their bodies, and curve up their abdomen, while swaying from side to side or running away (Fig. 43). The red color of the wing together with the black and white streak of the membranous area likely create an intimidating pattern of color and shape for visual predators. Whilst males were sometimes observed flying away when disturbed, females never tried to fly in response to a disturbance; but, they are likely to dampen a fall if dropped while running away (Video S1).
Fig. 43.
Xerosoma canaliculatum from Bertioga, São Paulo, Brazil showing deimatic display (A–C) and irritating volatile chemical compound expelled by the prothoracic gland (D). A: ♀. B: ♂. C: detail of ♀ wing base. C: ♀ grabbed by a researcher, arrow showing the chemical compound. Images not to scale.
Food plants: In situ, most specimens of Xerosoma canaliculatum were found in epiphytic Araceae plants, which are common in several Atlantic Forest areas, especially in the trunks of trees, in several distinct locations. Furthermore, whole generations could be bred with only Araceae as their food plant. In captivity, they fed well on several Araceae plant species (i.e., Monstera adansonii, Monstera deliciosa, Xanthosoma violaceum, Epipremnum aureum, Thaumatophyllum bipinnatifidum, Philodendron spp., and others), but also accepted other plants such as the exotic Jalapa mirabilis and the Neotropical Bougainvillea glabra, both Nyctaginaceae, as well as the native Ludwigia sp. (Onagraceae) and Comelina spp. (Comelinaceae). To feed on the leaves, the insects chew from the outside to the inside along the leaf margin, in semicircles (Fig. 34). They usually feed one to three times each night.
Fig. 44.
Feeding habits of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A: nymph resting on an eaten Epipremnum aureum (Araceae) leaf. B: ♂ adult feeding on a Philodendron (Araceae) leaf. Image not to scale.
Mating behavior: While mating pairs were commonly observed in the rearing cage, only one courtship could be observed and filmed. When the male perceived the female, it moved back-and-forth palpating its partner with its antennae, palps, and abdomen. Then it twisted its abdomen to touch the female ventrally. These movements were alternate and were repeated a few times. The entire process took about one hour. The female eventually refused the male, pushing it away with its mid and hind legs (Video S2).
When the male is accepted by the female, it uses its thorn pads to attach to the female’s preopercular organ. After fixation, the male extroverts its phallic organ which penetrates the female. This process always occurs at night, when individuals are active, but can extend into the day, with the mating pair remaining immobile (Fig. 45). Males remain on the back of females throughout the entire process, which lasts about eight to ten hours.
Fig. 45.
Copulation process of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil (photographed in captivity). A: mating pair in lateral view, smaller male atop the female. B: contact detail in lateral view. C: contact detail in ventro-lateral view. D: contact detail in posterior view. Image not to scale.
In situ, adult males were observed flying, or about to fly, hanging on random plants, shaking their heads and antennae, probably trying to find a female.
Oviposition: The eggs of X. canaliculatum are glued to the substrate, close to food plants, and individually or most frequently in batches of 4–14 eggs organized in two alternating rows (Fig. 46). In captivity, each observed ♀ laid around 100–200 eggs. The female holds the eggs with the gonapophysis and presses them against the substrate with the cerci for some seconds, making slight lateral movements of its abdomen. A batch of seven eggs was glued in about 20 minutes. In Bertioga, an egg batch was spotted among roots of epiphytic plants in the bark of a tree bearing several Araceae food plants, where young nymphs were previously observed the night before (Fig. 47).
Fig. 46.
Oviposition process and egg batch of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil. A, B: female gluing eggs on tree bark. C: glued freshly laid eggs. Image not to scale.
Fig. 47.
Xerosoma canaliculatum eggs found in situ in Bertioga, São Paulo, Brazil. A: Base of a tree in the Atlantic Forest covered with X. canaliculatum food plants (Araceae epiphytes), arrow showing location of eggs. B: eggs glued to the tree bark and epiphyte root. Image not to scale.
Ecological relations: One ♀ nymph in Bertioga at night was parasitized by a fly (Forcipomyia sp.: Ceratopogonidae: Diptera) attached to its antenna (Fig. 48).
Fig. 48.
Parasite fly (Forcipomya sp.) feeding on the antenna of a fourth instar female nymph of Xerosoma canaliculatum from Bertioga, São Paulo, Brazil.
DISCUSSION
Evaluation of morphological traits for phylogenetic application
The wing venation and structures are poorly recorded among Phasmatodea. Although there are some early approaches to establishing wing venation as a feature for phylogenetic reconstructions (e.g., Ragge 1955; Beier 1955 1968; Bradler 2009), recent descriptions often lack this important feature. The few studies including wing venation for this purpose are often focused on rather distant lineages of Phasmatodea. For example, in the case of Phylliidae the information about the wing veins is considered useful for taxonomy (Cumming et al. 2020a b c 2021a b). Here, we provide a description and illustration of Xerosoma canaliculatum wing venation. By comparing the tegmina of Xerosoma canaliculatum and X. nannospinus sp. nov. to other species, we could observe that R branches off to Rs, and Rs1 and Rs2, which is not the case for some other documented Pseudophasmatoidea such as Prisopus (Bradler 2009). This feature is apparently present in only a few species, as in the literature only one of almost 90 Phasmatodea species studied by Bradler (2009) shows it, interesting a representative from the Oriophasmata clade (Bradler 2009). Furthermore, the Rs sector exhibits typical characteristics for species with large tegmina (Bradler 2009). We also confirm anal veins 1–7 sharing the same base as a possible autapomorphy for the order. The medial veins are parallel and distant from the edge like in other Pseudophasmatidae (e.g., Bradler 2009). The venation of wings is widely used in the taxonomy of other Polyneoptera groups (e.g., Orthoptera) and may prove to be useful for Phasmatodea as well. However, the data for stick insect wing venation is still scarce and prevents a comparison among related taxa. Consequently, to overcome this obstacle, we provide in this study information about the wing venation in order to make future comparisons easier.
Bradler (2009) found the very well developed and long galealobulus to be an apomorphy for Pseudophasmatidae. In this study, we confirm this character state for Xerosoma and therefore the subfamily Xerosomatinae. The male genitalia of Xerosoma are somewhat simple if compared to those of other phasmid species like Ceroys and Canuleius (Heteronemiidae Rehn 1904) (e.g., Crispino 2022; Ghirotto 2021) and show similarities with other Xerosomatinae (Chiquetto-Machado and Cancello 2021). Chiquetto-Machado and Cancello (2021) analyzed the male genitalia of several Pseudophasmatidae representing all three recognised sublineages: Pseudophasmatinae, Stratocleinae, and Xerosomatinae. The very elongated dorsal sclerite running along most of the genitalia length, together with the absence of other sclerites, and the presence of broad membranous lobes is shared among the Xerosomatinae representatives Metriophasma pericles (identified as Agrostia sexmaculata in Chiquetto-Machado and Cancello 2021), Creoxylus (e.g., Heleodoro and Rafael 2019), Isagoras (e.g., Heleodoro 2022), Paragrostia, Prexaspes, and Xerosoma. The male genitalia of these taxa differ from that of representatives of other Pseudophasmatidae subfamilies, which have additional sclerites as well as a broad, not elongated dorsal sclerite in most species, or at least the dorsal sclerite not running along most of the genitalia length in few species (Chiquetto-Machado and Cancello 2021). Since Xerosomatinae was recovered as a monophyletic group by molecular data (see supplementary additional file 1, fig. S1 in Bank and Bradler 2022), the mentioned characteristics of genitalia shared with Xerosoma could be used to reconstruct apomorphies of the Xerosomatinae lineage in the future.
Interestingly, in the ontogeny of females of Xerosoma canaliculatum, the gonapophyses IX, which become very reduced in adults, are large—as large as the other valves in nymphal stages, even in the subadult stage. The gonapophysis IX could have been recently reduced in an evolutive scenario to adapt to a change in oviposition towards gluing the eggs. This hypothesis also arises from the fact that several other related taxa (i.e., Acanthoclonia (Robertson et al. 2018), Agrostia (authors pers. obs.), Creoxylus, Isagoras, and Pachyphloea (Murcia et al. 2019)) present a simple oviposition. That is, they drop or bury eggs without gluing them.
Functional importance of the tarsi
The attachment organs on the tarsi are used for locomotion and physical attachment besides engagement of the claws with rough substrates (Büscher and Gorb 2021). While the arolia are smooth in all Euphasmatodea (Büscher et al. 2018a b 2019), the euplantulae carry microstructures that are assumed to be of functional importance in the different environments settled by phasmids (Büscher et al. 2018a). This enables the deduction of a few aspects of the life history of Xerosoma. Both adult males and females have nubby attachment microstructures on their euplantulae (sensu Büscher et al. 2018a), which are shown to be adaptations to micro-rough substrates (Bußhardt et al. 2012; Büscher and Gorb 2019). Although similar structures are found in the Xerosomatinae Metriophasma (Büscher et al. 2018), the common natural substrates for these two lineages still need to be elucidated. Furthermore, it is necessary to know the euplantula microstructures of more Xerosomatinae and their habitats so as to solve this issue (see Büscher and Gorb 2017). Nevertheless, the attachment microstructures of X. canaliculatum are not sexually dimorphic. Sexual dimorphism in the attachment organs would indicate a difference in the importance of attachment organs and their function between the sexes. Such differences can for example originate from scrambling competition among males that fly and compete for sedentary females, as described for Phyllium philippinicum Hennemann, Conle, Gottardo, and Bresseel 2009 (Boisseau et al. 2022).
There is a fifth euplantula present on the tarsi of X. canaliculatum. The presence of an euplantula on the fifth tarsomere is apparently of evolutionary relevance and has been analyzed in some taxa (Vallotto et al. 2016; Büscher et al. 2019). Gottardo et al. (2015) hypothesized that a shift in the habitat preference during the postembryonic development of Eurycantha calcarata Lucas 1868 was responsible for the occurrence of a fifth euplantula on the tarsi of adults, which are supposed to be ground-associated. This could explain why a fifth euplantula is more frequently found in species that are somewhat associated with the ground or that lay their eggs into the soil (Büscher et al. 2019). However, Xerosoma species are not particularly associated with the ground. Probably the presence of the fifth euplantula in these species is more likely a result of the composition of twig diameters present in the respective habitats and more frequently found in ground-dwelling species because of the respective twig diameter composition. The elongation of the length of the friction increasing part of the tarsal chain can increase the attachment ability on twigs with a diameter that can be embraced by the tarsus (Büscher and Gorb 2019; Büscher et al. 2020a). Xerosoma can embrace twigs with smaller diameters quite often (Figs. 30, 31) and the Araceae food plants provide quite consistent diameters along the length of the plants that can be well engaged by the tarsi. The attachment forces of another species with a similar microstructure on the euplantulae have been measured on a representative of Araceae (i.e., Epipremnum aureum) and compared to other plants (Burack et al. 2022). In comparison with plant substrates with less smooth surfaces, nubby euplantulae show a relatively low attachment to this aracean plant. However, the same study showed that nubby euplantulae are less affected by contamination than smooth ones. During the ontogeny of X. canaliculatum no change in microstructure could be observed, indicating that nymphs and adults probably settle similar habitats, unlike E. calcarata (Gottardo et al. 2015), which dwell on leaves as nymphs and inhabit the grounds as adults. This correlates with a nubby attachment microstructure on the tarsi of the nymphal stages and smooth attachment structures on the tarsi of the adults in E. calcarata. In X. canaliculatum, the attachment microstructure of all stages is nubby (Fig. 36). Nevertheless, there is a noteworthy difference in the number of tarsomeres and consequently in the number of attachment pads. The first nymphal stage has only four tarsomeres and inconspicuous euplantulae. As the size of the euplantulae increases with every instar, the number of tarsomeres itself increases from the first to the third instar. In the second instar, a fissure is visible in the basitarsus (Fig. 36B), which differentiates this tarsomere in the third instar into two distinct tarsomeres, each of them with an euplantula. The increase in size and number of attachment organs is not surprising, as stick insects need to cope with increasing size during the postembryonic development. As the weight of the insects scales with a power of three, the area of the attachment pads scales with a power of two (Labonte et al. 2016 2019). Therefore, although it is expected that the attachment pads will over-proportionally increase in size, it is surprising that the number of tarsomeres and attachment pads increase as well. Nevertheless, the number of tarsomeres in first instar nymphs is not recorded in most species. There are only a few studies showing first instar nymphs that allow the assessment of this feature (e.g., Vallotto et al. 2016; Chiquetto-Machado and Albertoni 2017; Ghirotto 2021; Ghirotto et al. 2022).
Apparently all of these studies show four-segmented tarsi in first instar nymphs, except for Ghirotto (2021), in which the tarsus of Canuleius similis Redtenbacher 1906 first instar nymphs was shown to have five tarsomeres. In first instar nymphs, the first two tarsomeres appear to be fused. The fact that many phasmid species have four tarsomeres in the first instar is particularly interesting when considering the regeneration of autotomized legs (Fig. 39E). Autotomized legs are reported to have only four tarsomeres after regeneration (Maginnis 2006; Büscher et al. 2019) although almost all Euphasmatodea possess five tarsomeres in the adult stage. As the basic pattern of Euphasmatodea includes five tarsomeres in the adults and freshly hatched nymphs have only four tarsomeres, the reduced number of tarsomeres could be a return to the original amount after reset during autotomy. This could suggest a hox gene regulation of the limbs and the induction of a “new” leg at a later life stage (such as for the antennipedia –tarsi which are regenerated instead of antennae on the head in many phasmids). A deep investigation of the genetic regulation of this feature would be interesting and could contribute to reconstructing the evolution of the tarsal morphology in phasmids.
Functional importance of the egg
The egg-gluing characteristic of X. canaliculatum allows females to lay eggs close to food plants (Fig. 46), reducing the effort of newly hatched nymphs to find food. It was also observed that X. nannospinus sp. nov. glue their eggs (Pedro H. Martins pers. comm.), and although X. michaelis eggs are not known, it is likely that they are also glued. Some other Xerosomatinae representatives also glue eggs, such as Metriophasma Uvarov 1940 (Robertson et al. 2018), Olinta Redtenbacher 1906 (Y. Bellanger, 2022, pers. comm.), Periphloea Redtenbacher 1906, and Prexaspes Stål 1875 species (pers. obs.). However, some other Xerosomatini whose eggs are known, such as Acanthoclonia Stål 1875, Creoxylus Audinet-Serville 1838, Xera Redtenbacher 1906, and Xylospinoides Zompro 2004 (Zompro 2004a), do not glue their eggs, but simply drop or bury them. The plesiomorphic condition of oviposition for Euphasmatodea is to drop eggs (Robertson et al. 2018). Egg-gluing not only associates the offspring with the food plant, but also aids in sustaining suitable environmental conditions for the embryonic development (Büscher et al. 2020b c). It requires glue-producing organs in contrast to piercing or inserting eggs into plant material or the soil as done by many stick insect species for a similar purpose and could, therefore, be considered more costly (Goldberg et al. 2015). At least in the phasmid lineages examined in this regard (see Büscher et al. 2020b), colleterial glands are absent and the glue is probably produced by follicle cells, which are also involved in eggshell production. This could explain the glue coverage of the entire eggshell visible in figure 26. The entire egg is mantled in a film of secretion, with the film getting thicker towards the substrate. If the glue is not very viscous and hardens fast after egg deposition, this enables adjustment to the surface profile (see Fig. 43B), and, also supported by the hairy surface of the egg, increases the attachment of the egg to the substrate (Büscher et al. 2020b c). Another presumed cost for the oviposition technique of Xerosoma is the required synchronization of egg maturation and the increased risk of predation due to the higher density of hatchlings at the same time (Goldberg et al. 2015). The gluing of several eggs distant from the ground at once is a potential strategy used by phasmids against parasitoids (Goldberg et al. 2015; Robertson et al. 2018). Most known egg parasitoids of phasmids are flightless wasps, which live close to the forest floor (Goldberg et al. 2015). Furthermore, gluing eggs in batches reduces the stochastic predation risk of a single egg and increases the chance that at least a few hatchlings survive. The surface of the X. canaliculatum and X. nannospinus sp. nov. eggs are covered by spine-like outgrowths that potentially hinder the locomotion of parasitoids (Rebora et al. 2022) and particularly the operculum is protected by a structure that hinders an eventual parasitoid to access the most vulnerable part of the egg (Fig. 26D). Another factor that assists in the gluing process of Xerosoma and other egg-gluing phasmids is the reduced gonapophysis, which holds and positions the eggs during the gluing.
While some Damasippus, Dinelytron, and Prexaspes species glue their eggs individually and Damasippus discoidalis glue them in pairs side by side (pers. obs.), other Occidophasmata also glue their eggs in batches, as is the case for Metriophasma pericles (pers. obs.), Prisopus sacratus (Conle et al. 2020), P. ohrtmanni (Costa-Lima 1938), and different Prisopus species (pers. obs.) (apparently all Prisopus species glue their eggs in batches, different from what was reported by Robertson et al. (2018) (pers. obs.)). For the above mentioned species that glue their eggs in batches this occurs in a single row, with one egg tightly glued in front of the other, whilst Xerosoma glues them alternately in a pair of rows, indicating that this technique appears to be unique of Occidophasmata representatives. The egg batches glued in a single row can be fixed to thin branches, while a pair of rows take up more lateral space and would be placed on wider surfaces, such as trunks and bark, which are the preferred resting places for Xerosoma canaliculatum adult females.
Natural history and behavioral aspects
Xerosoma canaliculatum is specialized in Araceae plants, which are usually consumed by only a few animals (Canto et al. 2004). In the same areas where Xerosoma canaliculatum occurs, few other herbivores were observed feeding on the Araceae plant, as was the case of another stick insect, Metriophasma pericles (pers. obs.). This implies that Xerosoma and other phasmids like Metriophasma could face only scarce competition in a successful evolutionary diet specialization scenario in the Atlantic Forest.
The mating behavior of Phasmatodea is poorly recorded. Although we provide only a single, interrupted (thus incomplete) observation of a mating behavior, this already sheds light into the subject: the female rejection indicates that males have to convince the female to copulate, which is the basis for a sexual courtship (Eberhard 1985). It is very likely that phasmids in fact present a courtship, which can be complex, involve pheromones and distinct behaviors, and in which both sexes take part. However, as outlined above, this interaction does not impact the tarsal morphology as in winged phasmids species in which the males compete with each other (Boisseau et al. 2022).
Taxonomic implications
The genus Xerosoma currently has three species and is the type of its tribe (Xerosomatini) and subfamily (Xerosomatinae) of the Pseudophasmatidae. Xerosoma species share a common overall morphology, and due to the poor sampling of several Brazilian regions, especially the South and the Northeast, it is expected that further sampling will reveal new Xerosoma species. With a better characterization of its morphology, we intend to facilitate future assessments of the taxonomic position of other Xerosomatinae, a subfamily that includes 3 tribes, 16 genera and 108 species (Brock et al. 2022) and has monophyly partially supported by molecular data (Bank and Bradler 2022). This study establishes a basis for the taxonomic characterization of Xerosomatinae. Just like several groups in Phasmatodea (Bradler 2009), Xerosomatinae likely have taxonomic inconsistencies. For example, some genera currently assigned to Prexaspini (also in Xerosomatinae) are apparently more similar to Xerosoma than to the type of that tribe, Prexaspes Stål, 1875. A careful taxonomic review is then necessary in order to solve these issues in most taxa, especially because most of them were described long ago and are still poorly characterized. Much of the diversity of Xerosomatinae occurs in the Amazon Forest with several species known only from females (Brock et al. 2022).
The egg illustrated by Kaup (1871) (Fig. 1C) for X. canaliculatum (of the junior synonym D. neptunus) is certainly not correctly associated with the specimen and shows a different morphology by the rounded shape, the angle of operculum of 90°, the rough, reticulated and glabrous surface, and the rounded micropylar plate. The illustrated egg is more similar to the eggs of Isagoras Stål 1875 (Prexaspini) and Pseudophasma Kirby 1896 (Pseudophasmatini) than those of Xerosoma. Therefore, we clarify that the egg illustrated by Kaup does not belong to any Xerosoma species.
CONCLUSIONS
Xerosoma species live in the Atlantic Forest, one of the most impacted biomes by human activities. Therefore, knowing these insects in a detailed and integrated manner can be the first step to planning conservation strategies in a timely manner so as to reduce the risk of local extinction (see for example Honan 2008). The population’s awareness of the need to conserve biodiversity can be boosted by scientific dissemination strategies. In this context, the results of this study were disseminated on social media and in the classroom with children. Most or all aspects of the biology of most Phasmatodea species are still unknown. To the best of our knowledge, this is the first study to address the morphological details and natural history of Xerosoma. With this work, we expect to provide a basis for a proper diagnosis of Xerosomatinae and encourage future studies on this group since there is still much to be discovered about this lineage of Neotropical stick insects. Furthermore, we intend to inspire studies on Brazilian phasmids as well as research on Phasmatodea using an integrative approach.
Supplementary materials
Projeto Phasma in 16 January 2022, Santa Teresa, BR-ES, BR. Xerosoma michaelis. Uma iniciativa de Ciência Cidadã do Projeto Phasma! Registro enviado por Felipe Afonso no Face. iNaturalist.
Secondary defense mechanisms of Xerosoma canaliculatum.
Xerosoma canaliculatum -Sexual behaviour.
Acknowledgments
This work and the new species name were registered with ZooBank under urn:lsid:zoobank.org:pub:74D54C76-0E10-4744-86E5-D20A858C254. We are grateful to José Paulo L. Guadanucci (UNESP Rio Claro) for the technical support; Pedro Martins, D. Magalhães and H. Filho for fieldwork efforts in the Northeast revealing the new species; Eric Engelking, Pedro Machado, and Silvia Kamakuza for the fieldwork assistance; Marcelo Bokermann for allowing visits and receiving us at SESC Bertioga; Gabriel Ruschi for allowing visits and receiving us at Estação Biologia Marinha Augusto Ruschi (EBMAR); Oskar Conle, Frank Henneman, and Pablo Riquelme for providing type data on X. michaelis and collaborating with the field trip to Parque Nacional do Itatiaia; Harald Bruckner and Susanne Randolf (NMW) for providing access to NMW collection; Bernardo Egito for providing photos of live specimens; Felipe Afonso for providing photos of live X. michaelis; Yannick Bellanger for providing information on Olinta bubastes; Eliana Cancello and Pedro Dias for reviewing an early draft of the manuscript; and Paul Brock and the anonymous reviewer for contributing to improving the quality of the manuscript. We are also thankful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the grants provided to PWE (20/16576-2), VMG (2021/03458-4), and PCB (2019/22833-0 and BIOTA 2021/05986-8); and to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the grants provided to PCB (306400/2022-7 and PROTAX #441119/2020-4). PWE is especially grateful to Lucas Almeida for all the support in the development of this study.
Footnotes
Authors’ contributions: Definition of the scope of the study: PWE and PCB. Literature review: PWE, THB, and VMG. Photographs and videos: PWE, VMG, EBC, RAH, THB, and PABAN. Illustrations: PWE, VMG, and RAH. Morphological descriptions: PWE, VMG, EBC, and RAH. Captivity breeding of specimens: PWE, VMG, EBC, and PABAN. Writing and revision: PWE, VMG, EBC, RAH, PABAN, THB, and PCB. Funding acquisition: PWE and PCB.
Competing interests: The authors declare that they have no conflict of interest.
Consent for publication: Not applicable.
Availability of data and materials: Not applicable.
Ethics approval consent to participate: The activities were carried out in accordance with the ethical standards established by the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) under license SISBIO 68409-6.
References
- Araujo FF, Garraffoni ARS. 2012. Sinopse dos Phasmatodea (Insecta) descritos para o Brasil. EntomoBrasilis 5(3):232–237. doi:10.12741/ebrasilis.v5i3.229.
- Bank S, Bradler S. 2022. A second view on the evolution of flight in stick and leaf insects (Phasmatodea). BMC Ecol Evol 22(1):1–17. doi:10.1186/s12862-022-02018-5. . [DOI] [PMC free article] [PubMed]
- Bedford GO. 1978. Biology and Ecology of the Phasmatodea. Annu Rev Entomol 23:125–149. doi:10.1146/annurev.en.23.010178. 001013.
- Beier M. 1955. Embioidea und Orthopteroidea. In: Dr. H. G. Bronns Klassen und Ordnung des Tierreichs 5 (III). Akademische Verlagsgesellschaft Geest & Portig K.-G.(6):1–304. doi:10.5962/bhl.title.14134.
- Beier M. 1968. Phasmida (Stab-oder Gespenstheuschrecken). In: Handbuch der Zoologie IV. Walter de Gruyter & Co. (2)(2) (9):1–56.
- Blanchard. 1840. In: Castelnau [Ed.]. Histoire naturelle des insectes. Orthoptères, Néuroptères, Hémiptères, Hymenoptères, Lépidoptères et Diptères 1:18. doi:10.5962/bhl.title.59226.
- Boisseau RP, Büscher TH, Klawitter LJ, Gorb SN, Emlen DJ, Tobalske BW. 2022. Multi-modal locomotor costs favor smaller males in a sexually dimorphic leaf-mimicking insect. BMC Ecol Evol 22(1):1–18. doi:10.1186/s12862-022-01993-z. . [DOI] [PMC free article] [PubMed]
- Bradler S. 2009. Die Phylogenie der Stab-und Gespenstschrecken (Insecta: Phasmatodea). Species, Phylogeny and Evolution -SPE. doi:10.17875/gup2009-710.
- Bradler S, Buckley TR, Foottit RG, Adler PH. 2018. Biodiversity of Phasmatodea. Insect Biodiversity: Science and Society (2):281–313. doi:10.1002/9781118945582.ch11.
- Bradley JC, Galil B. 1977. The taxonomic arrangement of the Phasmatodea with keys to the subfamilies and tribes. P Entomol Soc Wash 79(2):202.
- Bragg PE. 2001. Phasmids of Borneo. Kota Kinabalu, Sabah: Natural History Pub. 645. ISBN: 9838120278.
- Brock PD. 1998. Catalogue of type-specimens of Stick-and Leaf-Insects in the Naturhistorisches Museum Wien (Insecta: Phasmida). 13(5):42–57.
- Brock PD, Büscher T, Baker E. 2022. Phasmida Species File Online. Version 5.0/5.0. Available at: http://www.phasmida.speciesfile. org. Accessed in Oct. 2022.
- Bußhardt P, Wolf H, Gorb SN. 2012. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 115(3):135–141. doi:10.1016/j.zool.2011.11.002. . [DOI] [PubMed]
- Burack J, Gorb SN, Büscher TH. 2022. Attachment Performance of Stick Insects (Phasmatodea) on Plant Leaves with Different Surface Characteristics. Insects 13(10):952. doi:10.3390/insects13100952. . [DOI] [PMC free article] [PubMed]
- Burmeister H. 1840. In: Audinet Serville, histoire naturelle des Orthoptéres. Paris 1839. 8. verglichen mit H. Burmeister, Handbuch d. Entomologie. II. Bd. 2. Abth. 1. Hälfte (vulgo Orthoptera). Berlin 1838. 8. vom Verfasser des Letzteren. Zeitschrift für die Entomologie (Zeitsch. Entom.) 2(1840):1–82.
- Büscher TH, Becker M, Gorb SN. 2020a. Attachment performance of stick insects (Phasmatodea) on convex substrates. J Exp Biol 223(17):jeb226514. doi:10.1242/jeb.226514. . [DOI] [PubMed]
- Büscher TH, Buckley TR, Grohmann C, Gorb SN, Bradler S. 2018a. The evolution of tarsal adhesive microstructures in stick and leaf insects (Phasmatodea). Front Ecol Evol 6:69. doi:10.3389/fevo.2018.00069.
- Büscher TH, Grohmann C, Bradler S, Gorb SN. 2019. Tarsal attachment pads in Phasmatodea (Hexapoda: Insecta). Zoologica, Schweizerbart Science Publishers 164:1–94. ISSN: 0044-5088.
- Büscher TH, Gorb SN. 2017. Subdivision of the neotropical Prisopodinae Brunner von Wattenwyl, 1893 based on features of tarsal attachment pads (Insecta, Phasmatodea). ZooKeys (645):1. doi:10.3897/zookeys.645.10783. . [DOI] [PMC free article] [PubMed]
- Büscher TH, Gorb SN. 2019. Complementary effect of attachment devices in stick insects (Phasmatodea). J Exp Biol 222(23):jeb209833. doi:10.1242/jeb.209833. . [DOI] [PubMed]
- Büscher TH, Gorb SN. 2021. Physical constraints lead to parallel evolution of micro-and nanostructures of animal adhesive pads: a review. Beilstein J Nanotech 12(1):725–743. doi:10.3762/bjnano.12.57. . [DOI] [PMC free article] [PubMed]
- Büscher TH, Kryuchkov M, Katanaev VL, and Gorb SN. 2018b. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J R Soc Interface 15(143):20180281. doi:10.1098/rsif.2018.0281. . [DOI] [PMC free article] [PubMed]
- Büscher TH, Lohar R, Kaul MC, Gorb SN. 2020c. Multifunctional adhesives on the eggs of the leaf insect Phyllium philippinicum (Phasmatodea: Phylliidae): solvent influence and biomimetic implications. Biomimetics 5(4):66. doi:10.3390/biomimetics5040066. . [DOI] [PMC free article] [PubMed]
- Büscher TH, Quigley E, Gorb SN. 2020b. Adhesion performance in the eggs of the Philippine leaf insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects 11(7):400. doi:10.3390/insects11070400. . [DOI] [PMC free article] [PubMed]
- Cala-Riquelme F. 2021. Autodesk Sketchbook: An application that minimizes time and maximizes results of taxonomic drawing. Zootaxa 4963(3):577–586. doi:10.11646/zootaxa.4963.3.10. . [DOI] [PubMed]
- Canto A, Parra‐Tabla V, García‐Franco JG. 2004. Variations in leaf production and floral display of Anthurium schlechtendalii (Araceae) in response to herbivory and environment. Funct Ecol 18(5):692–699. doi:10.1111/j.0269-8463.2004.00886.x.
- Chiquetto-Machado PI. 2018. Redescription of the Brazilian stick insect Pseudophasma cambridgei Kirby (Phasmatodea: Pseudophasmatidae), with first description of the female and egg. Austral Entomol 57:392–402. doi:10.1111/aen.12287.
- Chiquetto-Machado PI, Albertoni FF. 2017. Description of the female, egg and first instar nymph of the stick insect Paraphasma paulense (Phasmatodea: Pseudophasmatidae) from Southeast Brazil. Journal of Orthoptera Research 26(2):91–101. doi:10.3897/jor.26.20180.
- Chiquetto-Machado PI, Cancello EM. 2021. Cladistic analysis of Paraphasma (Phasmatodea: Pseudophasmatidae) highlights the importance of the phallic organ for phasmid systematics. Zool J Linnean Soc-Lond 193(1):158–198. doi:10.1093/zoolinnean/zlab004.
- Cotterill FPD, Foissner W. 2009. A pervasive denigration of natural history misconstrues how biodiversity inventories and taxonomy underpin scientific knowledge. Biodivers Conserv 19:291–303. doi:10.1007/s10531-009-9721-4. . [DOI] [PMC free article] [PubMed]
- Cliquennois N. 2021. Ordre des Phasmatodea (Phasmes). In: Les Insectes du Monde. Biodiversité. Classification. Clés de détermination des familles. Aberlenc HP, Versailles M and Plaissan. Éditions Quae & Museo. Chapter: 18:1848.
- Conle OV, Hennemann FH, Bellanger Y, Lelong P, Jourdan T, Valero P. 2020. Studies on neotropical Phasmatodea XX: A new genus and 16 new species from French Guiana. Zootaxa 4814(1):1–136. doi:10.11646/zootaxa.4814.1.1. . [DOI] [PubMed]
- Costa-Lima AM. 1938. Insetos do Brasil. 1° Tomo. Escola Nacional de Agronomia, Série Didática 194(2).
- Crispino EB. 2022. Estudo morfológico e caracterização das espécies de Ceroys Serville, 1838 (Phasmatodea: Heteronemiidae: Heteronemiinae: Pygirhynchini) de Mata Atlântica. Dissertação (Mestrado em Sistemática, Taxonomia Animal e Biodiversidade). Museu de Zoologia da Universidade de São Paulo. São Paulo, SP, 145 pp. doi:10.11606/D.38.2021.tde-18022022-165246.
- Crispino EB, Chiquetto-Machado PI, Engelking PW, Cancello EM. 2020. Contributions to the knowledge of Canuleius Stål (Phasmatodea: Heteronemiidae): taxonomy, morphology and notes on the biology of two species. Zootaxa 4743:511–535. doi:10.11646/zootaxa.4743.4.3. . [DOI] [PubMed]
- Crispino EB, Ghirotto VM, Engelking PW. 2022. Contributions to the knowledge of Ceroys (Miroceroys) Piza, 1936 (Phasmatodea: Heteronemiidae): two new mossy stick insects from the Atlantic Forest of Brazil. Zootaxa 5134(1):34–60. doi:10.11646/zootaxa. 5134.1.2. . [DOI] [PubMed]
- Cumming RT, Bank S, Le Tirant S, Bradler S. 2020. Notes on the leaf insects of the genus Phyllium of Sumatra and Java, Indonesia, including the description of two new species with purple coxae (Phasmatodea, Phylliidae). ZooKeys 913:89–126. doi:10.3897/zookeys.913.49044. . [DOI] [PMC free article] [PubMed]
- Cumming RT, Le Tirant S, Teemsma SN, Hennemann FH, Willemse L, Büscher TH. 2020. Lost lovers linked at long last: elusive female Nanophyllium mystery solved after a century of being placed in a different genus (Phasmatodea, Phylliidae). ZooKeys 969:43–84. doi:10.3897/zookeys.969.56214. . [DOI] [PMC free article] [PubMed]
- Cumming RT, Bank S, Bresseel J, Constant J, Le Tirant S, Dong Z, Sonet G, Bradler S. 2021. Cryptophyllium, the hidden leaf insects-descriptions of a new leaf insect genus and thirteen species from the former celebicum species group (Phasmatodea, Phylliidae). ZooKeys 1018:1–179. doi:10.3897/zookeys.1018.61033. . [DOI] [PMC free article] [PubMed]
- Cumming RT, Thurman JH, Youngdale S, Le Tirant S. 2020. Walaphyllium subgen. nov., the dancing leaf insects from Australia and Papua New Guinea with description of a new species (Phasmatodea, Phylliidae). ZooKeys 939:1–28. doi:10.3897/zookeys.939.52071. . [DOI] [PMC free article] [PubMed]
- Cumming RT, Tirant LS, Büscher TH. 2021. Resolving a century-old case of generic mistaken identity: polyphyly of Chitoniscus sensu lato resolved with the description of the endemic New Caledonia Trolicaphyllium gen. nov. (Phasmatodea, Phylliidae). Zookeys 1055:1–41. doi:10.3897/zookeys.1055.66796. . [DOI] [PMC free article] [PubMed]
- Delfosse E, Cliquennois N, Depraetere M, Robillard T. 2019. Catalogue des types de la collection de phasmes du Muséum national d'Histoire naturelle de Paris (Insecta, Phasmatodea). Zoosystema 41(11):193. doi:10.5252/zoosystema2019v41a11.
- Eberhard WG. 1985. Sexual selection and animal genitalia. Harvard University Press. Cambridge, Massachusetts. doi:10.4159/harvard.9780674330702.
- Ghirotto VM. 2021. Unmasking a master of camouflage: The rich morphology, taxonomy, and biology of the Brazilian stick insect Canuleius similis (Phasmatodea: Heteronemiidae), with general considerations on phasmid genitalia. Zool Anz 292:30–57. doi:10.1016/j.jcz.2021.02.009.
- Ghirotto VM, Crispino EB, Engelking PW, Neves PABA, Góis J, Chiquetto-Machado PI. 2022. Arumatia, a new genus of Diapheromerinae stick insects (Insecta, Phasmatodea) from Brazil, with description of five new species and reassessment of species misplaced in Australian genera. Eur J Taxon 827:1–85. doi:10.5852/ejt.2022.827.1849.
- Goldberg J, Bresseel J, Constant J, Kneubühler B, Leubner F, Michalik P, Bradler S. 2015. Extreme convergence in egg-laying strategy across insect orders. Sci Rep-UK 5(1):1–7. doi:10.1038/srep07825. . [DOI] [PMC free article] [PubMed]
- Gottardo M, Vallotto D, Beutel RG. 2015. Giant stick insects reveal unique ontogenetic changes in biological attachment devices. Arthropod Struct Dev 44(2):195–199. doi:10.1016/j.asd.2015.01. 001. . [DOI] [PubMed]
- Gray GR. 1835. Synopsis of the species of insects belonging to the family of Phasmidae. London, Longman, Rees, Orme, Brown, Green, and Longman, pp. 26–27. doi:10.5962/bhl.title.8697.
- Heleodoro RA. 2022. The first two cases of antisymmetry in the male genitalia of Phasmatodea reveal a new species of Isagoras Stål, 1875 (Phasmatodea: Pseudophasmatidae: Xerosomatinae) from the Brazilian Atlantic Forest. Zool Anz 296:161–178. doi:10.1016/j.jcz.2021.12.006.
- Heleodoro RA, Rafael JA. 2019. Is the Phasmatodea male genitalia useful for systematics? A case study in Creoxylus and Prexaspes (Insecta: Phasmatodea) from the Brazilian Amazon Basin. Zool Anz 278:66–79. doi:10.1016/j.jcz.2018.11.003.
- Helm C, Treulieb S, Werler K, Bradler S, Klass KD. 2011. The male genitalia of Oxyartes lamellatus –phasmatodeans do have complex phallic organs (Insect: Phasmatodea). Zool Anz 250:223–245. doi:10.1016/j.jcz.2011.04.005.
- Honan P. 2008. Notes on the biology, captive management and conservation status of the Lord Howe Island Stick Insect (Dryococelus australis) (Phasmatodea). J Insect Conserv 12:399–413. doi:10.1007/s10841-008-9162-5.
- Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ. 2015. Seven shortfalls that beset largescale knowledge of biodiversity. Annu Rev Ecol Evol S 46:523–549. doi:10.1146/annurev-ecolsys-112414-054400.
- Kaup JJ. 1871. Neue Phasmidae. Berliner Entomologische Zeitung. Entomologischer Verein in Stettin 15(41):1:23, 15:23, pl 1:23. OCLC: 8441524.
- Kirby WF. 1890. On the employment of the names proposed for genera of Orthoptera, previous to 1840, chapter 55. The Scientific Proceedings of the Royal Dublin Society 6:572. LCCN: 16023985.
- Kirby WF. 1904. A synonymic catalogue of Orthoptera. 1. Orthoptera Euplexoptera, Cursoria et Gressoria (Forficulidae, Hemimeridae, Blattidae, Mantidae, Phasmidae). British Museum (Natural History). Department of Zoology 1:414–416. doi:10.5962/bhl. title.6745.
- Labonte D, Clemente CJ, Dittrich A, Kuo CY, Crosby AJ, Irschick DJ, Federle W. 2016. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. P Natl Acad Sci 113(5):1297–1302. doi:10.1073/pnas.1519459113. . [DOI] [PMC free article] [PubMed]
- Labonte D, Struecker MY, Birn-Jeffery AV, Federle W. 2019. Shear-sensitive adhesion enables size-independent adhesive performance in stick insects. P R Soc B 286(1913):20191327. doi:10.1098/rspb.2019.1327. . [DOI] [PMC free article] [PubMed]
- Madeira-Ott T, Thyssen PJ, Costa J. 2020. Phasmatodea (Arthropoda, Insecta) in Brazil: Status, New Record, and Proposal for Using Molecular Tools to Assist in Species Identification. Neotrop Entomol 49(6):916–922. doi:10.1007/s13744-020-00798-3. . [DOI] [PubMed]
- Maginnis TL. 2006. The costs of autotomy and regeneration in animals: a review and framework for future research. Behav Ecol 17(5):857–872. doi:10.1093/beheco/arl010.
- Murcia A, Cadena-Castaneda OJ, Noriega J, García A. 2019. New species of Pachyphloea Redtenbacher, 1906 (Phasmida: Pseudophasmatidae: Xerosomatinae) with comments on Grylloclonia Zompro, 2004 n. syn. Zootaxa 4623(3):545–554. doi:10.11646/zootaxa.4623.3.6. . [DOI] [PubMed]
- iNaturalista. 2022. (Inaturalist) Comisión Nacional para el Conocimiento y Uso de la Biodiversidad Available at: http://www.naturalista.mx. Accessed in Oct., 2022.
- Otte and Brock. 2005. Phasmida Species File. Catalog of Stick and Leaf Insects of the world 342.
- Pinto LP, Bedê L, Paese A, Fonseca M, Paglia A, Lamas I. 2006. Mata Atlântica Brasileira: os desafios para conservação da biodiversidade de um hotspot mundial. Biologia da conservação: essências. São Carlos: RiMa, pp. 91–118.
- Ragge DR. 1955. The wing‐venation of the order Phasmida. T Roy Ent Soc London 106(9):375–392. doi:10.1111/j.1365-2311.1955. tb01272.x.
- Rebora M, Salerno G, Piersanti S, Saitta V, Gorb E, Gorb SN. 2022. Mechanical interaction between the egg parasitoid Anastatus bifasciatus (Hymenoptera: Eupelmidae) and its host egg. Front Mech Eng 8:966429. doi:10.3389/fmech.2022.966429.
- Redtenbacher J. 1906. Die Insektenfamilie der Phasmiden. Wilhelm Engelmann I–VI (1):131–145. Openlibrary edition: OL24245859M.
- Rehn JAG. 1904. Studies in the family Phasmidae. P Acad Nat Sci Phila 56:91–101. BioStor: https://biostor.org/reference/3354.
- Robertson JA, Bradler S, Whiting MF. 2018. Evolution of oviposition techniques in stick and leaf insects (Phasmatodea). Front Ecol Evol 6:216. doi:10.3389/fevo.2018.00216.
- Simon S, Letsch H, Bank S, Buckley TR, Donath A, Liu S, Bradler S. 2019. Old World and New World Phasmatodea: phylogenomics resolve the evolutionary history of stick and leaf insects. Front Ecol Evol 7:345. doi:10.3389/fevo.2019.00345.
- Sellick CJT. 1997. Descriptive terminology of the phasmid egg capsule, with an extended key to the phasmid genera based on egg structure. Syst Entomol 22:97–122. doi:10.1046/j.1365-3113.1997.d01-30.x.
- Serville A. 1831. Annales des Sciences naturelles. Zool Paris 22:61.
- Serville A. 1838[1839]. Histoire naturelle des Insectes. Orthoptères. Librairie Encyclopédique de Roret, Paris 274. Openlibrary edition: OL20599260M.
- Shelford R. 1909. Biologia Centrali-Americana: zoology, botany and archaeology. Insecta Orthoptera. In: Godman et al. 2:375. doi:10.5962/bhl.title.730.
- Stål C. 1875a. Recensio Orthopterorum. Revue critique des orthoptères décrits par Linné, De Geer et Thunberg. Stockholm, P.A. Norstedt & Söner 105p. ISSN: 0284-7280.
- Stål C. 1875b. Öfversigt af Kongliga. Vetenskaps-Akademiens Förhandlingar 32:99.
- Stål C. 1875c. Bihang till Kongliga Svenska Vetenskaps-Akademiens Handlingar 3(14):20.
- Tabarelli M, Pinto LP, Silva JMC, Hirota MM, Bedê LC. 2005. Desafios e oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 1:1.
- Vallotto D, Bresseel J, Heitzmann T, Gottardo M. 2016. A black-and-red stick insect from the Philippines-observations on the external anatomy and natural history of a new species of Orthomeria. ZooKeys (559):35. doi:10.3897/zookeys.559.6281. . [DOI] [PMC free article] [PubMed]
- Weidner VH. 1966. Mitteilungen aus dem Hamburger Zoologischen Museum und Institut 63:232.
- Westwood JO. 1859. Catalogue of the orthopterous insects in the collection of the British Museum. Part I. Phasmidae. 103. URN:oclc:record:1041810609.
- Zompro O. 2001. The type-material of the insect order Phasmatodea, described by Johann Jacob KAUP (Insecta, Phasmatodea). Senckenbergiana biologica 81(1/2):138, fig. 13.
- Zompro O. 2004a. Revision of the genera of the Areolatae, including the status of Timema and Agathemera (Insecta, Phasmatodea). Abhandlungen des Naturwissenschaftlichen Vereins in Hamburg 112:311–323.
- Zompro O. 2004b. Johann Jakob Kaup as the founder of phasmatodean ootaxonomy. Kaupia 13:93.
- Zompro O. 2004c. Darmstädter Beiträge zur Naturgeschichte 13:93.
- Zompro O. 2012. Phasmatodea. In: Rafael JA, Melo GAR, Carvalho CJB, Casari SA, Constantino, R. (Eds.), Insetos do Brasil, Diversidade e Taxonomia. Holos Editora, Ribeirão Preto, São Paulo, pp. 289–295.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Projeto Phasma in 16 January 2022, Santa Teresa, BR-ES, BR. Xerosoma michaelis. Uma iniciativa de Ciência Cidadã do Projeto Phasma! Registro enviado por Felipe Afonso no Face. iNaturalist.
Secondary defense mechanisms of Xerosoma canaliculatum.
Xerosoma canaliculatum -Sexual behaviour.