Introduction
For the majority of patients with end-stage kidney disease (ESKD), kidney transplantation is the optimal treatment.1 Despite the benefits of kidney transplantation over maintenance dialysis, systemic barriers in access to kidney transplantation have resulted in persistent racial and ethnic disparities in transplantation over time.2, 3, 4 In December of 2014, the United Network for Organ Sharing implemented a new kidney allocation system (KAS) with the intent of improving longevity of matching kidneys as well as reducing racial disparities in access to kidney transplantation. As evidenced in 2 studies, the implementation of the new 2014 KAS led to a national 9% decline in overall waitlisting for kidney transplantation and reduced racial disparities in waitlisting5 and transplantation,6 with a 12% lower waitlisting rate among Black versus White patients in the post-KAS era (versus 19% pre-KAS). Under the new KAS, the incentive to waitlist some dialysis patients sooner is lacking because patients no longer receive extra waiting time by getting on the waitlist earlier. Research in the Southeastern United States suggests that both dialysis facility and transplant center behaviors may have changed following KAS implementation.7
A patient’s place of residence can also impact access to kidney transplantation and contribute to racial and ethnic disparities. In a study conducted prior to implementation of the new KAS in 2014, Davis et al.8 reported substantial variation in time on the waitlist across the 58 donor service areas in the United States. More recently, Zhou et al.9 reported that, in the KAS era, geographic disparities in access to deceased donor kidney transplant persisted, with heterogenous rates of deceased donor kidney transplant across United States donor service areas. However, it is unknown whether racial disparities in waitlisting vary across end-stage renal disease networks in the post-KAS era. The details of study methods are shown in the Supplementary Methods.S1,S2 Additional references are cited in the Supplementary References. The STROBE statement can also be found within the Supplementary Materials.
Results
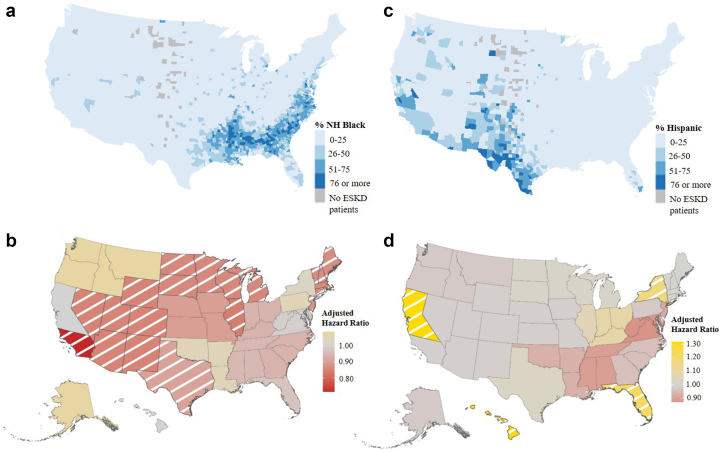
Among the analytical cohort of 407,079 patients (Supplementary Figure S1), majority were male (58%), non-Hispanic White (55%), with a median age at dialysis start of 63 years (interquartile range [IQR]: 52, 71). Patients with kidney failure disproportionately resided in the Southeast (Network 6: 10%), Texas (Network 14: 10%), and Southern California (Network 18: 8%), which corresponds to regions with larger minority patient populations (Network 6: 52% Black, Network 14: 40% Hispanic, and Network 18: 51% Hispanic) (Figure 1a and c). A very small proportion of patients with ESKD lived in the Pacific Northwest (Network 16: 3%) and Northeast (Network 1: 3%), where a vast majority of the patient population is non-Hispanic White (Network 1: 74%, Network 3: 81%) (Supplementary Tables S1 and S2).
Figure 1.
Geographic differences in the population of patients with ESKD and access to the kidney transplant waiting list among Black and Hispanic White patients. Percentage of (a) Black and (c) Hispanic patients with ESKD among all incident ESKD patients in the study cohort, by county. Adjusted hazard ratio of waitlisting among (b) Black and (d) Hispanic White versus non-Hispanic White patients, by ESRD Network. Note: Diagonal White line indicates statistical significance. ESKD, end-stage kidney disease; ESRD, end0stage renal disease.
Among the 19% (n = 75,156) of patients who were waitlisted for transplant during the 5-year study period, the median time to waitlist was 22 months (IQR: 10–36 months). The median time from dialysis initiation to waitlist was statistically different by race/ethnicity group, with White patients having a shorter median time to waitlist of 20 months (IQR: 7–34 months) compared with Black (median: 25 months; IQR: 14–39 months) and Hispanic (median: 25 months; IQR: 14–39 months) patients. A total of 136,621 (34%) patients died during the follow-up period, with a higher proportion of deaths among White patients (40%) compared with Black (28%) and Hispanic White (23%) patients (Supplementary Table S3 and Supplementary Figure S2). A total of 24,228 patients (6%) were preemptively waitlisted, with the largest proportion (41%) among non-Hispanic White patients and a median age of 57 years (IQR: 47, 65). Network 1 in the Northeast had the highest proportion of preemptively waitlisted patients (12%) with the lowest proportion in Network 8 of the Southeast (3%) (Supplementary Table S3). Additional clinical and socioeconomic characteristics and waitlisting status of patients overall and stratified by race and ethnicity are shown in Supplementary Tables S1 and S3.
In unadjusted Cox PH models, Black (vs. White) patients (hazard ratio [HR]: 0.87, 95% CI: 0.72, 1.06) had lower access to the waitlist, whereas Hispanic (vs. White) patients (HR: 1.05, 95% CI: 0.90, 1.23) had slightly greater access to the waitlist (Table 1). After adjustment for selected demographic, clinical, and socioeconomic factors, there was a modest disparity in waitlisting among Black (vs. White) patients (HR: 0.92, 95% CI: 0.89, 0.95), and Hispanic (vs. White) patients were significantly more likely to be waitlisted (HR: 1.05, 95% CI: 1.03, 1.11) (Table 1).
Table 1.
Crude and adjusted hazard ratios for time from dialysis initiation to placement on the deceased donor kidney transplant waitlist, overall and by end-stage renal disease network, 2015–2018 followed through March 13, 2020
| End-stage renal disease Network | Hazard ratios for time from dialysis start to waitlisting by Network (patients starting dialysis January 1, 2015 to December 31, 2018, followed through March 13, 2020) |
|||
|---|---|---|---|---|
| NH-Black to NH-White patient HRs |
Hispanic White to NH-White patient HRs |
|||
| Crude HR (95% CI) | Adjusteda HR (95% CI) | Crude HR (95% CI) | Adjusteda HR (95% CI) | |
| Overall | 0.87 (0.72, 1.06) | 0.92 (0.89, 0.95) | 1.05 (0.90, 1.23) | 1.07 (1.03, 1.11) |
| 1 (CT, MA, ME, NH, RH, VT) n = 13,066 (3%) | 0.91 (0.72, 1.16) | 0.87 (0.77, 0.98) | 1.12 (0.91, 1.37) | 1.01 (0.99, 1.24) |
| 2 (NY) n = 23,568 (6%) | 0.98 (0.77, 1.24) | 1.03 (0.95, 1.11) | 1.08 (0.87, 1.33) | 1.17 (1.05, 1.30) |
| 3 (NJ) n = 11,732 (3%) | 0.83 (0.65, 1.05) | 0.88 (0.79, 0.99) | 0.86 (0.72, 1.03) | 0.91 (0.80, 1.04) |
| 4 (DE, PA) n = 17,622 (4%) | 1.04 (0.86, 1.24) | 1.05 (0.97, 1.14) | 1.00 (0.80, 1.25) | 0.98 (0.82, 1.17) |
| 5 (DC, MD, VA, WV) n = 23,310 (6%) | 1.00 (0.79, 1.26) | 0.99 (0.91, 1.06) | 1.03 (0.80, 1.32) | 0.87 (0.73, 1.03) |
| 6 (GA, NC, SC) n = 39,434 (10%) | 0.86 (0.70, 1.05) | 0.94 (0.88, 1.00) | 1.12 (0.92, 1.35) | 0.96 (0.81, 1.14) |
| 7 (FL) n = 27,575 (7%) | 0.94 (0.78, 1.13) | 0.97 (0.90, 1.05) | 1.20 (1.02, 1.41) | 1.23 (1.11, 1.37) |
| 8 (AL, MS, TN) N = 24,393 (6%) |
0.97 (0.81, 1.14) | 0.95 (0.87, 1.03) | 1.26 (0.95, 1.65) | 0.89 (0.69, 1.14) |
| 9 (IN, KY, OH) n = 31,438 (8%) | 0.88 (0.72, 1.08) | 0.94 (0.87, 1.03) | 1.21 (1.01, 1.45) | 1.09 (0.91, 1.30) |
| 10 (IL) n = 17,558 (4%) | 0.83 (0.68, 1.00) | 0.84 (0.76, 0.93) | 1.13 (0.97, 1.32) | 1.06 (0.93, 1.20) |
| 11 (MI, MN, ND, SD, WI) n = 24,856 (6%) | 0.73 (0.55, 0.96) | 0.86 (0.78, 0.94) | 1.06 (0.84, 1.35) | 1.02 (0.90, 1.16) |
| 12 (IA, KS, MO, NE) n = 15,596 (4%) | 0.82 (0.67, 1.00) | 0.90 (0.82, 1.00) | 1.12 (0.89, 1.37) | 0.99 (0.83, 1.17) |
| 13 (AK, LA, OK) n = 17,786 (4%) | 0.96 (0.81, 1.15) | 1.05 (0.96, 1.15) | 1.07 (0.87, 1.32) | 0.93 (0.74,1.16) |
| 14 (TX) n = 42,162 (10%) | 0.88 (0.73, 1.07) | 0.91 (0.84, 0.98) | 0.98 (0.85, 1.12) | 1.03 (0.94, 1.11) |
| 15 (AZ, CO, NM, NV, UT, WY) n = 19,558 (5%) | 0.82 (0.67, 1.01) | 0.85 (0.75, 0.97) | 0.91 (0.79, 1.06) | 0.99 (0.91, 1.07) |
| 16 (AK, ID, MT, OR, WA) n = 11,905 (3%) | 1.03 (0.77, 1.36) | 1.09 (0.93, 1.27) | 1.05 (0.86, 1.28) | 0.98 (0.85, 1.12) |
| 17 (Northern CA) N = 15,018 (4%) |
1.05 (0.88, 1.26) | 1.00 (0.91, 1.10) | 1.50 (1.29, 1.75) | 1.32 (1.22, 1.43) |
| 18 (Southern CA) n = 30,593 (8%) | 0.67 (0.54, 0.84) | 0.71 (0.64, 0.80) | 0.93 (0.77, 1.12) | 0.99 (0.93, 1.06) |
CI, confidence interval; ESRD: end-stage renal disease; HR, hazard ratio; NH, non-Hispanic.
Models adjusted for categorical age, sex, cause of kidney failure, body mass index ≥35 mg/g2, congestive heart failure, arteriosclerotic heart disease, cardiovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer, current tobacco use, primary health insurance, and pre-ESKD nephrology care
Adjusted HRs for Black patients were lower across 13 of the 18 end-stage renal disease networks (HR range: 0.71–1.09) compared with Hispanic patients. The strongest racial disparities in waitlisting among Black patients with ESKD were found in Network 15 (HR: 0.85, 95% CI: 0.75, 0.97), Network 10 (HR: 0.84, 95% CI: 0.76, 0.93), Network 11 (HR: 0.86, 95% CI: 0.78, 0.94), and Network 18 (HR: 0.71, 95% CI: 0.64, 0.80) (Table 1 and Figure 1b). The Networks with the highest proportion of Black patients with ESKD resided in the Southeast (Network 6: 52%, Network 8: 47%, Network 5: 46%) (Supplementary Table S2). However, there was not a statistically significant racial disparity in waitlisting among Black patients in these Southeastern Networks (Network 6: HR: 0.94, 95% CI: 0.88, 1.00; Network 8: HR: 0.95, 95% CI: 0.87, 1.03; and Network 5: HR: 0.99, 95% CI: 0.91, 1.06) (Table 1 and Figure 1b). Hispanic (vs. White) patients were statistically significantly more likely to be waitlisted in 3 Networks, including Network 2 (HR: 1.17, 95% CI: 1.05, 1.30), Network 7 (HR: 1.23, 95% CI: 1.11, 1.37), and Network 17 (HR: 1.32, 95% CI: 1.22, 1.43). Network 5 (HR: 0.87, 95% CI: 0.73, 1.03) and Network 8 (HR: 0.89, 95% CI: 0.69, 1.14) had the strongest racial disparities among Hispanic patients, but were not statistically significant (Table 1 and Figure 1d).
Conclusions
Our study findings bring to the forefront a correspondence between networks with a small population of Black residents with ESKD and larger racial disparities in waitlisting. In adjusted analyses, Black patients with ESKD were approximately 8% less likely to be waitlisted compared with White patients with ESKD, but this effect varied from 29% less likely to 9% more likely, with the strongest Black versus White racial disparities found in Southern California, Western (AZ, CO, NM, NV, UT, and WY) and Midwestern (IL, MI, MN, ND, SD, and WI) networks. There is clustering of Black patients with ESKD residing in the Southeastern United States. However, racial disparities in waitlisting were not observed among Black patients in the networks covering this region. Although we are unlikely to capture all factors contributing to the structure of a neighborhood, specifically racial segregation and neighborhood poverty, our results in this more recent era post-KAS suggest there have been improvements in racial disparities in some geographic regions.S3 Neighborhood composition is largely impacted by a longstanding history of systemic racism in the United States, and is a root cause of inequitable access to healthcare among Black patients disproportionately impacted by ESKD. The same trends between population size and disparities in waitlisting were not observed among the Hispanic population. Consistent with previous results,S6 Hispanic patients were more likely to be placed on the waiting list compared with non-Hispanic patients in overall adjusted analyses. Small racial disparities in access to the waitlist among Hispanic patients with ESKD were observed within networks across the Southeast, although these were not statistically significant. These results suggest that strategies to eliminate racial and ethnic disparities in access to the waiting list may need to be targeted by geographic region.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors’ work was supported by the National Institute on Minority Health and Health Disparities through grant U01MD010611 and the National Institute of Diabetes and Digestive and Kidney Diseases through grant R01DK122701. The data reported here have been supplied by the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. Preliminary results from this work was presented at Health Service Research Day on May 5, 2022, in Atlanta, Georgia, USA.
Footnotes
Supplementary Methods.
Supplementary Figure S1. Data cohort selection to examine geographic differences in racial disparities in waitlisting in the United States.
Supplementary Figure S2. Cumulative incidence between starting dialysis and being waitlisted for kidney transplant or dying prior to waitlisting, accounting for competing risks.
Supplementary Table S1. Characteristics of adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 (N=407,079), overall and by race/ethnicity.
Supplementary Table S2. Patient race/ethnicity among adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 (N=407,079), overall and stratified by end-stage renal disease network.
Supplementary Table S3. Waitlisting and death events across geographic, demographic, and clinical characteristics among adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 followed through 3/13/2020 (N=407,079).
SUPPLEMENTARY REFERENCES.
STROBE Statement.
Supplementary Material
Supplementary Methods
Supplementary Figure S1. Data cohort selection to examine geographic differences in racial disparities in waitlisting in the United States.
Supplementary Figure S2. Cumulative incidence between starting dialysis and being waitlisted for kidney transplant or dying prior to waitlisting, accounting for competing risks.
Supplementary Table S1. Characteristics of adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 (N=407,079), overall and by race/ethnicity.
Supplementary Table S2. Patient race/ethnicity among adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 (N=407,079), overall and stratified by end-stage renal disease network.
Supplementary Table S3. Waitlisting and death events across geographic, demographic, and clinical characteristics among adult ESKD patients who initiated dialysis in the United States at time of ESKD start, 2015-2018 followed through 3/13/2020 (N=407,079).
SUPPLEMENTARY REFERENCES
STROBE Statement
References
- 1.Wolfe R.A., Ashby V.B., Milford E.L., et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/nejm199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Salter M.L., Liu X., Bae S., et al. Fractures and subsequent graft loss and mortality among older kidney transplant recipients. J Am Geriatr Soc. 2019;67:1680–1688. doi: 10.1111/jgs.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelrod D.A., Guidinger M.K., Finlayson S., et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299:202–207. doi: 10.1001/jama.2007.50. [DOI] [PubMed] [Google Scholar]
- 4.Schold J.D., Gregg J.A., Harman J.S., Hall A.G., Patton P.R., Meier-Kriesche H.U. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760–1767. doi: 10.2215/cjn.08620910. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Melanson T.A., Plantinga L.C., et al. Racial/ethnic disparities in waitlisting for deceased donor kidney transplantation 1 year after implementation of the new national kidney allocation system. Am J Transplant. 2018;18:1936–1946. doi: 10.1111/ajt.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni S., Ladin K., Haakinson D., Greene E., Li L., Deng Y. Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation system. JAMA Surg. 2019;154:618–625. doi: 10.1001/jamasurg.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patzer R.E., Di M., Zhang R., et al. Referral and evaluation for kidney transplantation following implementation of the 2014 national kidney allocation system. Am J Kidney Dis. 2022;80:707–717. doi: 10.1053/j.ajkd.2022.01.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis A.E., Mehrotra S., McElroy L.M., et al. The extent and predictors of waiting time geographic disparity in kidney transplantation in the United States. Transplantation. 2014;97:1049–1057. doi: 10.1097/01.tp.0000438623.89310.dc. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S., Massie A.B., Luo X., et al. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18:1415–1423. doi: 10.1111/ajt.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.